Abstract
Surveillance studies are useful to evaluate how a new medicinal product performs in everyday treatment and how the patient who takes it feels and functions, thereby determining the benefit/risk ratio of the drug under real-life conditions. Prolonged-release melatonin (PRM; Circadin) was approved in Europe for the management of primary insomnia patients age 55 years or older suffering from poor quality of sleep. With traditional hypnotics (e.g. benzodiazepine-receptor agonists), there are concerns about rebound insomnia and/or withdrawal symptoms. We report data from a postmarketing surveillance study in Germany on the effects of 3 weeks of treatment with PRM on sleep in patients with insomnia during treatment and at early (1–2 days) and late (around 2 weeks) withdrawal. In total, 653 patients (597 evaluable) were recruited at 204 sites (mean age 62.7 years, 68% previously treated with hypnotics, 65% women). With PRM treatment, the mean sleep quality (on a scale of 1–5 on which 1 is very good and 5 is very bad) improved from 4.2 to 2.6 and morning alertness improved from 4.0 to 2.5. The improvements persisted over the post-treatment observation period. Rebound insomnia, defined as a one-point deterioration in sleep quality below baseline values, was found in 3.2% (early withdrawal) and 2.0% (late withdrawal). Most of the patients (77%) who used traditional hypnotics before PRM treatment had stopped using them and only 5.6% of naive patients started such drugs after PRM discontinuation. PRM was well tolerated during treatment and the most frequently reported adverse events were nausea (10 patients, 1.5%), dizziness, restlessness and headache (five patients each, <1%). There were no serious adverse events and no adverse events were reported after discontinuation. The persisting treatment effect and very low rebound rate suggest a beneficial role of sleep–wake cycle stabilization with PRM in the treatment of insomnia.
Keywords: hypnotics, prolonged-release melatonin, rebound insomnia, withdrawal symptoms
Introduction
Circadin, a prolonged-release formulation containing 2 mg of melatonin (PRM), is an innovative treatment for primary insomnia in patients aged 55 years or older with sleep problems characterized by poor quality of sleep. Circadin was launched in Europe in 2008. It is the first drug to be approved in a new class of sleep medications (melatonin receptor agonists) and has a different mode of action from the traditional benzodiazepines and benzodiazepine-like hypnotic drugs (modulators of GABA-A receptors). In randomized-controlled clinical trials versus placebo, it showed improvements in quality of sleep, morning alertness, and quality of life as well as of other sleep and daytime parameters over treatment periods of 3 weeks–6 months (Garfinkel et al., 1995; Lemoine et al., 2007; Wade et al., 2007, 2008, 2010, 2011; Luthringer et al., 2009). After drug cessation, there were no signs of rebound insomnia or withdrawal symptoms after 3 weeks or 6 months of treatment (Lemoine et al., 2007; Luthringer et al., 2009; Wade et al., 2011).
With traditional sleep medications, there are concerns about rebound insomnia and/or withdrawal symptoms (Roth et al., 2001; Wilson et al., 2010). Rebound insomnia is defined as a worsening of the insomnia parameters below pretreatment values and withdrawal symptoms appearing as adverse effects, both occurring after discontinuation of treatment. Rebound may be interpreted as worsening of a patient’s symptoms and as the appearance of withdrawal symptoms related to physical dependency by the physician. This may lead both the patient and the physician to restart hypnotic intake. It is logically apparent with such a connection that resuming medication increases the risk of long-term use and may form an essential prerequisite in the cause of drug dependence (Hajak et al., 1998). Randomized clinical trials, although essential for registration purposes, often fail to reflect the real-life usage of a drug in the general population, including the real-life risk of rebound and occurrence of withdrawal symptoms (Hajak et al., 1994) that could be detected in noninterventional studies (Hajak and Bandelow, 1998). Ultimately, it is how a new medicinal product performs in everyday life and how the patient who takes it feels and functions that determines the real benefit/risk ratio of the drug.
The aim of this postmarketing surveillance study was to investigate discontinuation, withdrawal, and rebound effects of Circadin (PRM) in a heterogeneous population with insomnia and being treated by physicians practicing in outpatient and under prescribing conditions according to the Summary of Product Characteristics. Sleep quality and morning alertness during treatment and at early (within days) and late (within weeks) withdrawal were assessed. Rebound insomnia was measured as a worsening of sleep quality and/or morning alertness after discontinuation of treatment below pretreatment levels. Withdrawal symptoms were evaluated by comparing the incidence of adverse events (AEs) during treatment and after discontinuation of treatment.
Patients and methods
Conduct of the study
This prospective, referenced, cohort study of PRM in insomnia was approved by the Ethics Committee of the University of Regensburg, Germany. The study was notified pursuant to Art 67 §6 German Drug Law (Arzneimittelgesetz) to the National Association of Statutory Health Insurance Physicians and the German Federal Institute for Drugs and Medical Devices.
There was no onsite monitoring during the study and no special requirements of treatment or diagnostic procedures. The data were checked for plausibility and adherence to the observational protocol. Missing data related to mandatory parameters were queried, but left missing if queries remained unanswered.
Patients were informed by the physician about the study and provided their written consent to have data stored and processed in accordance with the applicable law for data protection.
Patient population
The sample size as agreed with the European authorities was 625 patients. With an estimated dropout rate of 20%, at least 500 patients were expected to have an evaluable withdrawal period. The patients were adult outpatients with insomnia requiring a pharmacological intervention, as diagnosed by the treating physician. Patients for whom a PRM treatment was prescribed and who were scheduled for a routine visit after treatment could be included in the study. The use of PRM was based solely on patients’ medical needs, as judged by the physician, reflecting current medical practice. The patients were instructed to take one PRM 2 mg tablet 2 h before bedtime for a duration of 3 weeks, as recommended in the Circadin (RAD Neurim Pharmaceuticals Ltd, Reading, Berkshire, UK) Summary of Product Characteristics.
Patients were asked to come back ∼2 weeks after discontinuation of the PRM treatment, as part of a routine follow-up of a new drug treatment. For the documentation of results, case report forms with documentation were provided for baseline (treatment start) and follow-up comprising the data collection during treatment, at early withdrawal (within 2 days of treatment discontinuation), or at late withdrawal (∼2 weeks after treatment discontinuation).
Parameters
At baseline, the following parameters were recorded: sex, age, height, weight, severity and duration of insomnia, concomitant illnesses, concomitant medication (including the most recent insomnia treatment), and reason for starting PRM treatment, PRM start date, and patient-reported quality of sleep and morning alertness. At the post-treatment visit, the following parameters were measured: duration of and compliance with PRM intake, date and reason for discontinuation, concomitant sleep medication during and after PRM treatment, quality of sleep, morning alertness, and AEs. For sleep quality, morning alertness, and AEs, the patients had to provide separate assessments of the time during ongoing treatment, the first 2 days after discontinuation (early withdrawal), and 2 weeks after discontinuation of PRM (late withdrawal) (Fig. 1).
Fig. 1.
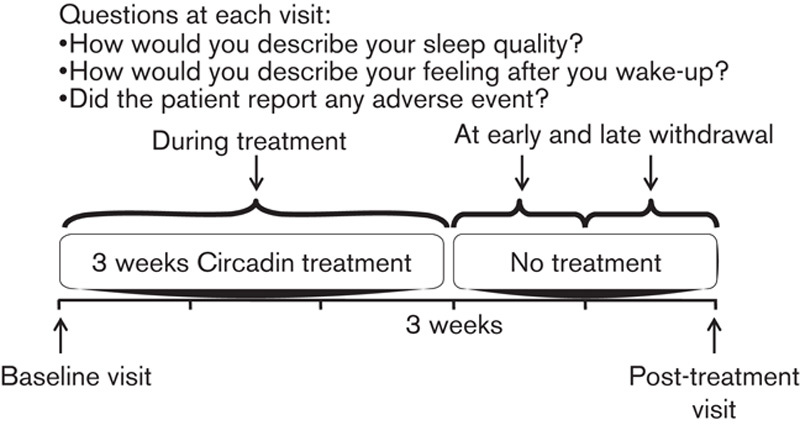
Study design.
Sleep quality was classified as 1=very good, 2=good, 3=fair, 4=bad, and 5=very bad. Morning alertness was classified as 1=completely alert, 2=alert, 3=fair, 4=tired, and 5=very tired. An improvement or deterioration in sleep quality or morning alertness was defined as a change (decrease) of at least one point from baseline.
AEs were coded according to MedDRA (version 12.0).
Statistical analysis
The all-patients-treated set (APTS) was defined as all patients who had at least one intake of PRM. The APTS data set was used for the safety analysis. The full-analysis set (FAS) was defined as all patients in the APTS with post-treatment documentation of sleep quality or morning alertness or AE.
The efficacy and safety data were analyzed using descriptive statistics (mean, SD, minimum, first quartile, median, third quartile and maximum, or the frequency distribution, depending on the type of value). To evaluate rebound insomnia with the parameters sleep quality and morning alertness, two-sided 95% confidence intervals (CIs) were calculated. All analyses were carried out using statistical analysis system (SAS, version 8.2, North Caroline, USA).
Results
Patient population
The study was carried out from July 2008 to April 2009 at 204 sites in Germany. During that period, 653 patients were recruited. As one patient was not treated with PRM, the APTS comprised 652 patients. As 55 patients had no post-treatment data, the FAS comprised 597 patients (Fig. 2).
Fig. 2.
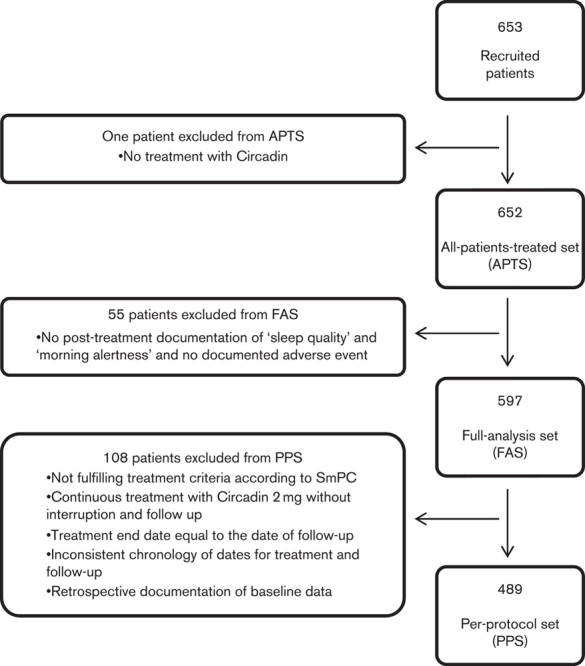
Overall study patients’ disposition. APTS, all-patients-treated set; FAS, full-analysis set; PPS, per-protocol set; SmPC, summary of product characteristics.
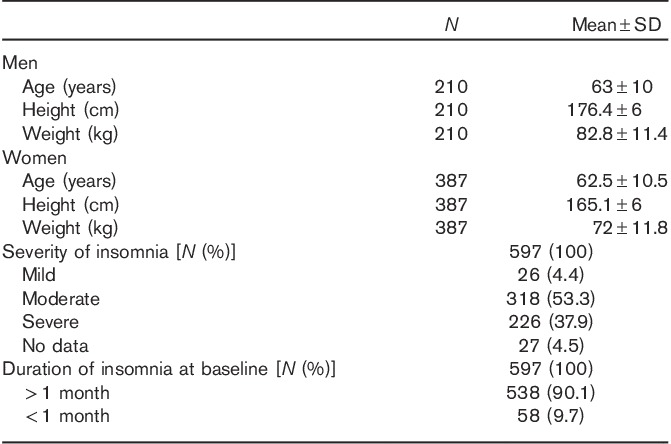
Almost two-thirds of the patients in the FAS were women (65%). The patients’ mean±SD age was 62.7±10.3 years. Sixty (10.1%) patients were younger than 55 years old. The mean±SD height was 176.4±6.0 cm for men and 165.1±6.0 cm for women. The mean±SD body weight was 82.8±11.4 kg for men and 72.0±11.8 kg for women (Table 1).
Table 1.
Baseline characteristics of the study population
Concomitant diseases were reported by 400 (67.0%) patients, the most frequent being hypertension in 251 (42.0%) patients. Diseases affecting more than 10% of patients included diseases of lipid metabolism in 119 (19.9%) patients, diabetes mellitus in 69 (11.6%), and coronary heart disease in 65 (10.9%). Concomitant medication for conditions other than insomnia was taken by 520 (87.1%) patients. The most frequent ones were antihypertensives in 144 (24.1%) patients, antidepressants in 123 (20.6%), lipid-lowering drugs in 100 (16.8%), and antidiabetic drugs in 59 (9.9%).
At baseline, 318 (53.3%) patients had moderate insomnia and 226 (37.9%) had severe insomnia. The majority of patients (538 patients, 90.1%) had a documented duration of insomnia of more than 1 month (Table 1). In total, 406 (68.0%) patients had been treated previously for insomnia. The most frequently prescribed medications were zopiclone (127 patients, 21.3%) and zolpidem (126 patients, 21.1%).
The most frequent reasons for starting PRM treatment were insomnia (289 patients, 48.4%) and lack of efficacy of previous insomnia treatment (99 patients, 16.6%). The incidence of other reasons was less than 5%. The mean±SD treatment duration in the study was 24.9±11.7 days. In total, 460 (77.1%) patients received a treatment of 3 weeks or longer, whereas 133 (22.3%) patients took the medication for less than 3 weeks (no data for four patients). The most frequent reasons for premature discontinuation were not specified (14.2%), patient’s wish (3.0%), lack of efficacy (2.3%), and AE (2.2%). The mean±SD time between treatment discontinuation and the post-treatment visit was 15.4±10.8 days. Patient compliance with medicine was high, with 506 (84.8%) patients reporting daily intake during a 3-week period, 55 (9.2%) patients reporting 1–3 tablets not taken, and only eight (1.3%) patients reporting four or more missed tablets.
Efficacy (full-analysis set, N=597)
Sleep quality
During treatment, the percentage of patients with good sleep quality rated 1–3 (very good, good, fair) increased from 42 (7.1%) patients at baseline to 462 (77.4%) patients (Fig. 3a). The mean±SD sleep quality rating during treatment improved from 4.2±0.6 at baseline to 2.6±1.1 during treatment. Sleep quality improved during treatment by at least one point from baseline in 486 (81.4%) patients. For 90 (15.1%) patients, there was no change in sleep quality, whereas 17 (2.8%) patients and two (0.3%) patients reported a worsening of sleep quality of one or two points, respectively.
Fig. 3.
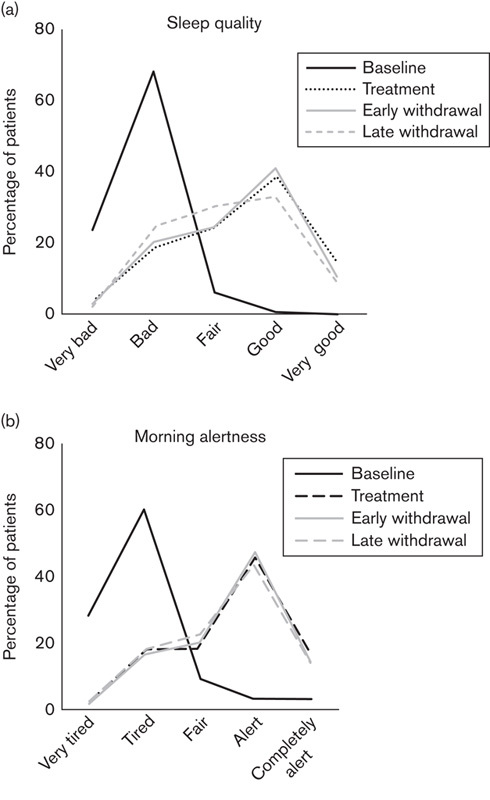
Sleep quality and morning alertness: (a) percentage of patients with very good, good, fair, bad, and very bad sleep quality at baseline, during treatment, at immediate, and at late withdrawal. (b) Percentage of patients who felt completely alert, alert, fair, tired, and very tired at baseline, during treatment, at immediate, and at late withdrawal.
The improvement in sleep quality persisted beyond the PRM treatment period (Fig. 3a). The mean±SD value was 2.7±1.0 during early withdrawal and 2.8±1.0 during late withdrawal and was therefore only slightly different from the on-treatment values and clearly better than the baseline values. Sleep quality remained improved by at least one point compared with baseline for 478 (80.1%) patients at immediate withdrawal and for 396 (66.3%) patients at late withdrawal.
Morning alertness
The percentage of patients with a rating of morning alertness of 1–3 (completely alert, alert, fair) increased from 87 (14.6%) patients at baseline to 472 (79.1%) patients during treatment (Fig. 3b). The mean±SD morning alertness rating improved from 4.0±0.8 at baseline to 2.5±1.0 during treatment. Morning alertness improved during treatment by at least one point as compared with baseline in 452 (75.7%) patients. For 105 (17.6%) patients, there was no change in morning alertness, whereas 20 (3.4%) patients, four (0.7%) patients, and one (0.2%) patient reported a worsening of sleep quality of one, two, or three points, respectively.
The improvement in morning alertness persisted beyond the PRM treatment period (Fig. 3b). The mean±SD morning alertness rating remained at 2.5±1.0 both during the immediate and during the late withdrawal phase, and morning alertness was improved by at least one point compared with baseline in 459 (76.9%) patients during early withdrawal and in 380 (63.7%) patients during late withdrawal.
Rebound insomnia (full-analysis set, N=597)
Rebound insomnia, predefined as a deterioration in sleep quality by at least one point from baseline, was evident for 19 (3.2%, 95% CI 1.9–4.9) patients at early and for 12 (2.0%, 95% CI 1.0–3.5) patients at late withdrawal. This figure should also be put in perspective with the fact that 19 (3.2%, 95% CI 1.9–4.9) patients showed a deterioration in sleep quality of at least one point compared with baseline during treatment (Fig. 4).
Fig. 4.
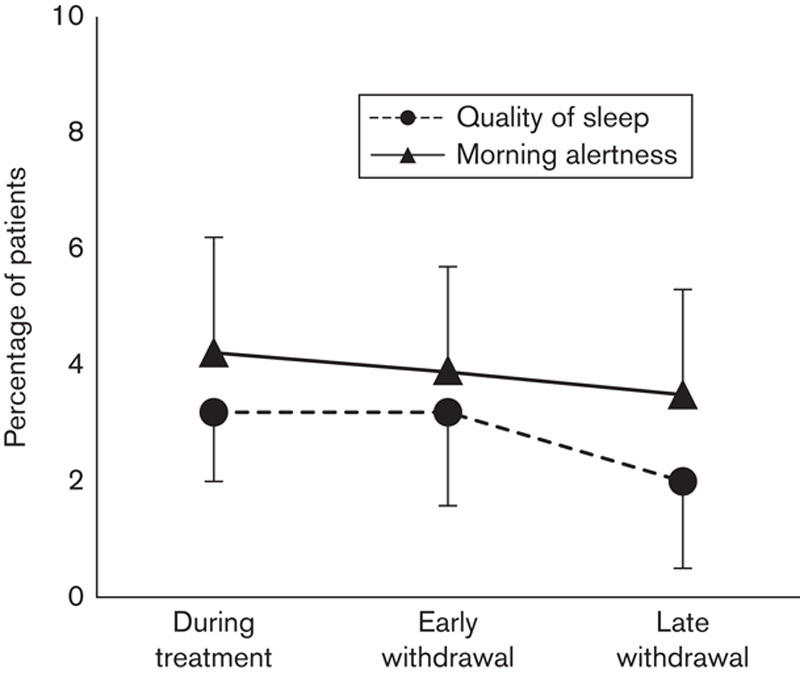
Rebound insomnia: percentage of patients with a deterioration by at least one point from baseline at immediate and late withdrawal in sleep quality or morning alertness. As a comparison, the percentage of patients deteriorating during ongoing treatment is also shown.
Rebound daytime fatigue, predefined as a deterioration in morning alertness by at least one point from baseline, was documented for only 23 (3.9%, 95% CI 2.5–5.7) patients at early withdrawal and for 21 (3.5%, 95% CI 2.2–5.3) patients at late withdrawal. As for sleep quality, these figures must be put into perspective with the 25 (4.2%, 95% CI 2.7–6.1) patients who reported deterioration in morning alertness by at least one point from baseline during treatment (Fig. 4).
Traditional hypnotic drugs use
Among the 597 patients in the study, 406 (68%) patients had used sleep medications before the start of Circadin 2 mg. Concomitant sleep medication other than PRM was taken by 68 (11.4%) patients during treatment and after discontinuation of PRM 138 (23.1%) patients used sleep mediations. With respect to traditional hypnotic drugs use, 276 (45.9% of the 597 patients in the study) had used benzodiazepines or benzodiazepine-like hypnotics before the start of treatment. During the PRM treatment, only 37 (6.2%) patients used traditional hypnotics and 61 (10.2%) patients used such drugs after the treatment (Fig. 5). Thus, 213 (77.7%) of the patients who had used traditional hypnotics before PRM treatment did not use them any more after discontinuation of PRM. Of the 323 patients not using benzodiazepines or benzodiazepine derivatives before the start of PRM treatment, 305 (94.4%) patients were still not using them after discontinuation of PRM and only 18 (5.6%) started using such drugs.
Fig. 5.
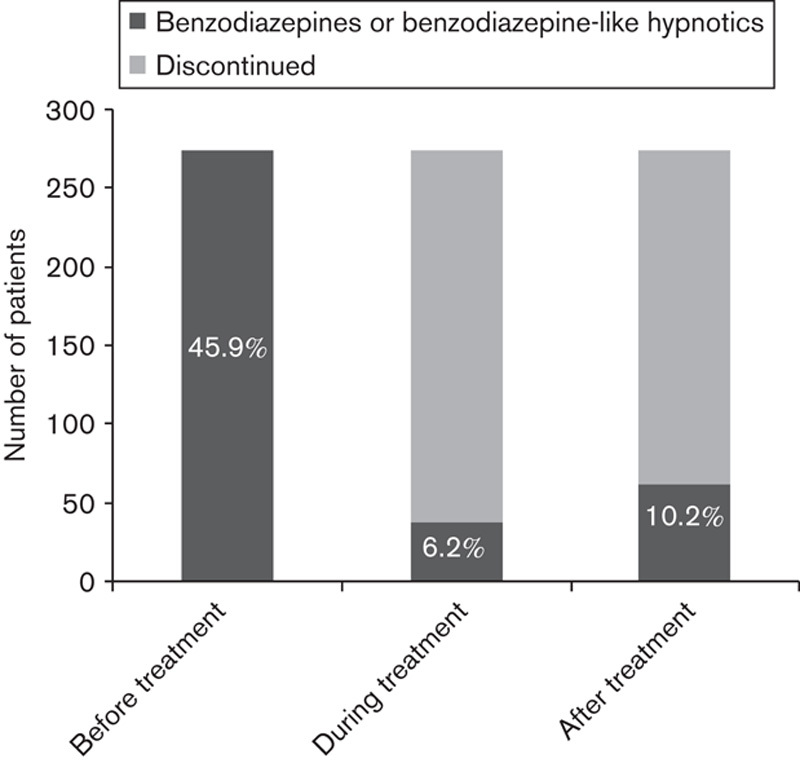
Use of benzodiazepine and benzodiazepine-like hypnotics: number of patients who used traditional hypnotics before, during the course of, and after prolonged-release melatonin treatment. Percentage of patients using traditional hypnotics (out of 597 in the study) is also shown.
Safety (all-patients-treated set, N=652)
During treatment, 46 (7.1%) patients reported 79 AEs, of which 75 were considered by the physician to be possibly or probably related to PRM. The most frequently affected body systems were gastrointestinal disorders (24 patients, 3.7%), psychiatric disorders (20 patients, 3.1%), and nervous system disorders (15 patients, 2.3%). The most frequently reported AEs were nausea (10 patients, 1.5%), dizziness, restlessness, and headache (five patients each, <1%). No serious AEs were reported and no AEs were reported after discontinuation of treatment.
Forty-two patients in the FAS reported AEs during treatment and 13 (1.9%) patients discontinued treatment because of AEs, with nausea (five patients), dizziness (four patients) as well as vomiting, insomnia, and headache (two patients each) being reported in more than one patient. No AEs were reported after discontinuation of treatment.
Discussion
This postmarketing surveillance study investigated the effectiveness and tolerability as well as potential discontinuation effects of a 3-week treatment with PRM under real-life usage in patients with insomnia aged 55 years and older. In total, 652 patients were treated with PRM under normal prescribing conditions. For 597 patients, post-treatment data were recorded during a post-treatment visit. Over 90% of patients had at least moderately severe insomnia with a documented duration of at least 1 month and almost 70% had been treated previously for insomnia. Three-quarters of the patients were treated for at least 3 weeks and reported a high treatment compliance with a daily intake of PRM.
Before PRM treatment, over 80% of the patients reported bad to very bad sleep quality and being tired or very tired in the morning. During the 3-week treatment period, more than 75% of the patients reported sleep quality and morning alertness to have improved to at least fair to good or very good. The mean rating of sleep quality and morning alertness improved by at least one point compared with baseline in more than 75% of patients.
Rebound insomnia was defined as deterioration from baseline of at least one point in sleep quality or morning alertness after treatment discontinuation. This was not observed in this study. Post-treatment values of sleep quality and morning alertness were very similar to the on-treatment values and the number of patients deteriorating from baseline by at least one point was the same after treatment as during treatment. This was true days following withdrawal (early withdrawal) as well as weeks following withdrawal (late withdrawal).
PRM treatment was well tolerated. No serious AEs were reported. The overall incidence of AEs was less than 10%. None of the AEs was reported by more than 1.5% of patients, with the most frequent being nausea (1.5%) and dizziness (<1%). Less than 2% of patients discontinued PRM treatment because of AEs. As no AEs were reported after discontinuation of PRM, there was no evidence for any kind of withdrawal symptoms.
The lasting effect of PRM after cessation of drug intake, that is, patients continued to have benefit beyond the treatment phase, has not been observed with other hypnotic drugs. Melatonin (N-acetyl-5-methoxytryptamine) is a hormone that is produced naturally by the pineal gland at night. It signals darkness and serves to facilitate synchronization of the circadian clock with the ambient day–night cycle. In addition, melatonin is an endogenous sleep regulator that induces sleep-like brain activation patterns (Zisapel, 2007). Studies in totally blind individuals have shown that the time it takes to entrain to the endogenous clock phase is relatively long (weeks to months) and varies considerably between individuals (Lockley et al., 2000; Lewy et al., 2004), and the dissipation of rhythms may also take days or weeks. We therefore propose that besides the soporific effect leading to improvement in sleep per se, PRM therapy may act to reinstate the internal temporal order that appears to dissipate in older age (Czeisler et al., 1992; Mirmiran et al., 1992; Hofman and Swaab, 1994). This notion is compatible with the change in cortisol peak time, improvement in nocturnal blood pressure rhythm, and advance in bedtime hours observed with this formulation (Zisapel et al., 2005; Grossman et al., 2006; Wade et al., 2010, 2011).
Overall, the current data support the positive benefit–risk profile of PRM that has been reported from controlled studies over treatment periods of 3 weeks to 6 months (Garfinkel et al., 1995; Lemoine et al., 2007; Wade et al., 2007, 2008, 2010, 2011; Luthringer et al., 2009). As in the earlier placebo-controlled studies of PRM (Lemoine et al., 2007; Luthringer et al., 2009; Wade et al., 2011), there was no evidence of rebound insomnia or withdrawal symptoms. In another open-label study, treatment was prolonged to 12 months, with no sign of rebound insomnia or withdrawal symptoms (Lemoine et al., 2011). PRM was recommended recently as a first-line therapy in insomnia patients aged 55 years and older (Wilson et al., 2010). In addition, treatment duration was extended from 3 to 13 weeks on 2010. These recommendations remain valid in view of the results obtained in the present postmarketing surveillance study.
Acknowledgements
The authors acknowledge and thank Dr Tali Nir, who made a substantial contribution to the conception and design of the study and was involved in preparing the protocol and the interpretation of data and critical review of the manuscript, and Dr Richard Thorstenson (Lundbeck) for participating in study design and data analysis.
The study was funded by Neurim Pharmaceuticals Ltd.
Conflicts of interest
Nava Zisapel is an employee of Neurim. Göran Hajak has received grants for research and honoraria for scientific presentations and scientific advice from Neurim Pharmaceuticals Ltd, Lundbeck GmbH Germany, and Lundbeck Denmark. Lemme Kathrin is an employee of Lundbeck Hamburg, Germany.
References
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, et al. (1992). Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 340:933–936. [DOI] [PubMed] [Google Scholar]
- Garfinkel D, Laudon M, Nof D, Zisapel N. (1995). Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet 346:541–544. [DOI] [PubMed] [Google Scholar]
- Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, et al. (2006). Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med 119:898–902. [DOI] [PubMed] [Google Scholar]
- Hajak G, Bandelow B. (1998). Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16 944 cases. Int Clin Psychopharmacol 13:157–167. [DOI] [PubMed] [Google Scholar]
- Hajak G, Clarenbach P, Fischer W, Haase W, Rüther E. (1994). Zopiclone improves sleep quality and daytime well-being in insomniac patients: comparison with triazolam, flunitrazepam and placebo. Int Clin Psychopharmacol 9:251–261. [DOI] [PubMed] [Google Scholar]
- Hajak G, Clarenbach P, Fischer W, Rodenbeck A, Bandelow B, Broocks A, Rüther E. (1998). Rebound insomnia after hypnotic withdrawal in insomniac outpatients. Eur Arch Psychiatry Clin Neurosci 248:148–156. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. (1994). Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res 651:134–142. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Nir T, Laudon M, Zisapel N. (2007). Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res 16:372–380. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Garfinkel D, Laudon M, Nir T, Zisapel N. (2011). Prolonged-release melatonin for insomnia – an open-label long-term study of efficacy, safety, and withdrawal. Ther Clin Risk Manag 7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Bernert RA, Lefler BJ. (2004). Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: clinical implications. J Biol Rhythms 19:68–75. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. (2000). Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 164:R1–R6. [DOI] [PubMed] [Google Scholar]
- Luthringer R, Muzet M, Zisapel N, Staner L. (2009). The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int Clin Psychopharmacol 24:239–249. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Swaab DF, Kok JH, Hofman MA, Witting W, Van Gool WA. (1992). Circadian rhythms and the suprachiasmatic nucleus in perinatal development, aging and Alzheimer’s disease. Prog Brain Res 93:151–162discussion 162–163. [DOI] [PubMed] [Google Scholar]
- Roth T, Hajak G, Ustün TB. (2001). Consensus for the pharmacological management of insomnia in the new millennium. Int J Clin Pract 55:42–52. [PubMed] [Google Scholar]
- Wade A, Zisapel N, Lemoine P. (2008). Prolonged-release melatonin for the treatment of insomnia: targeting quality of sleep and morning alertness. Aging Health 4:11–21. [Google Scholar]
- Wade AG, Ford I, Crawford G, McMahon AD, Nir T, Laudon M, Zisapel N. (2007). Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin 23:2597–2605. [DOI] [PubMed] [Google Scholar]
- Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N. (2010). Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade AG, Crawford G, Ford I, McConnachie A, Nir T, Laudon M, Zisapel N. (2011). Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response. Curr Med Res Opin 27:87–98. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. (2010). British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 24:1577–1601. [DOI] [PubMed] [Google Scholar]
- Zisapel N. (2007). Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci 64:1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N, Tarrasch R, Laudon M. (2005). The relationship between melatonin and cortisol rhythms: clinical implications of melatonin therapy. Drug Dev Res 65:119–125. [Google Scholar]


