Abstract
The ACE2/Ang-(1-7)/MAS axis of the renin-angiotensin system has emerged as a pathway of interest in treating both cardiovascular disorders and cancer. The MAS protein is known to bind to and be activated by Ang-(1-7); however mechanisms of this activation are just starting to be understood. Whereas there are strong biochemical data regarding regulation and activation of the AT1 and AT2 receptors, with models of how Ang II binds each receptor, fewer studies have characterized MAS. We characterize the MAS promoter and provide a potential feedback mechanism that would compensate for the MAS degradation following activation by Ang-(1-7). Analysis of ENCODE data for the MAS promoter revealed potential epigenetic control by KRAB/KAP1. A proximal promoter construct for the MAS gene was repressed by the SOX proteins SRY, SOX2, SOX3, and SOX14, of which SRY is known to interact with the KRAB domain. The proteins KRAB/KAP1 can both be tyrosine nitrated, causing the dissociation of the KAP-1 protein, and thus a potential loss of epigenetic control. Activation of MAS can lead to an increase in nitric oxide, suggesting feedback mechanisms of MAS on its own promoter. These results present a more complete view of MAS regulation and for the first time suggest biochemical outcomes for nitration to the KRAB domain.
Keywords: MAS, Ang-(1-7), Tyrosine nitration, KRAB, KAP1, SOX
Introduction
Originally discovered as a potential oncogene [1], MAS has also recently been identified as a receptor of angiotensin (Ang) peptides [2,3] and thus a critical component of the renin-angiotensin system (RAS). Little is known about the mechanism of Ang peptide activation of MAS. Previously mutagenesis and modeling approaches to the activation of angiotensin receptors AT1 and AT2 have yielded an understanding of how peptide binding alters the structure of the receptors [4]. The details of Ang peptides binding to MAS are currently unknown. It is known that Ang-(1-7) binds to the G-protein coupled receptor (GPCR) MAS, activating intracellular signaling through nitric oxide synthase [5], Akt [5], GSK3β [6], SHP-1 [7] and phosphorylation of numerous proteins including many involved in insulin signaling [8]. Overall, activation of MAS results in actions antagonist to those of the Ang II-activated AT1 receptor [9].
MAS is expressed in cardiac tissue, and knockout mice exhibit alterations in the cardiovascular system [10]. Most studies have addressed the role of MAS activation in cardiac myocytes [10,11], fibroblasts [12] and the kidney [13,14]. Additional studies have addressed its role in testis [15–16], ovaries [17–19], skeletal muscle [20], brain neurotransmitter uptake [21], and memory formation [22,23]. Surprisingly, a detailed analysis has yet to be performed on promoter conservation and regulation of the MAS gene, addressing the local transcriptional control mechanisms. We previously showed that the proximal promoter of the MAS gene had the potential to be repressed by the human HMG box containing protein SRY (hSRY), a Y-chromosome gene only found in males [24]. The Ang-(1-7)/ACE2/MAS axis of the RAS was only recently identified, and there are many aspects of the axis still missing from the literature. Here, we detail MAS gene promoter conservation and regulation, proposing a novel feedback mechanism through nitration of the KRAB domain and KAP1 protein, potentially altering epigenetic regulation of the MAS gene. This is the first report of how nitration to the KRAB domain alters its biochemistry, with numerous impacts in cancer and cardiovascular disease.
Methods
MAS promoter conservation and regulation
ECR browser analysis [25] was performed on the MAS gene promoter for human sequence relative to Pan troglodytes (panTro3), Rhesus macaque (rheMac2), Canis familiaris (canFam2), Bos Taurus (bosTau6), Mus musculus (mm10), Rattus norvegicus (rn4), and Monodelphis domestica (monDom5) with conservation identified as 100 bases of length with a minimum of 90% homology. The ENCODE data [26] for the MAS promoter was visualized using the human genome browser (http://genome.ucsc.edu/ENCODE/) with the GRCh37/hg19 build. Cloning of the proximal promoter for the MAS gene and the hSRY pEF-1 vectors was previously described [24]. SOX genes from humans were cloned following PCR as described in Table 1 using Phusion Hot-Start II (Thermo-Fisher). Luciferase assays for the SOX constructs on the MAS pGL3 promoter were performed as previously published [24]. MAS promoter binding by Sry was determined using 5′biotin labeled probe 5′TTATTCCAATTCAACAATTTTCATGGCTT (Sry binding site underlined), located at −98 bases from the ATG site for MAS. A control element 5′-CATACTGCGGGGGTGATTGTTCAGGATCATAC TGCG-3′ (Philips DNA [27]) that Sry is known to bind, was used as a positive control for DNA binding. Sry protein was produced by expression of pGEX-4T vector containing only the HMG box of Sry with an additional GST tag or from a pET28 vector containing full length human SRY with a 6x-His tag. Proteins were concentrated using either Ni- or glutathione-Sepharose (GE Life Science) depending on the tag used for purification, and then dialyzed into molecular grade water. 10 μM antisense and sense DNA probes were annealed in 80 μL primer annealing buffer (10mM Tris, 1mM EDTA, 100mM NaCl, pH 8.0) by heating to 95°C for five minutes, cooling to 65°C for 10 minutes, 55°C for 10 minutes and then held at 23°C for 30 minutes. Each protein was mixed with 500 fmols DNA probe and analyzed on non-denaturing 6% TBE PAGE. Gels were transferred to Biodyne B Nylon Membrane (Thermo) and biotin probe measured with the LightShift Chemiluminescent EMSA kit (Thermo). Control binding experiments used the LightShift EMSA optimization and control kit (Thermo). Densitometry was performed using ImageJ (http://rsbweb.nih.gov/ij/).
Table 1. Cloning primers and PCR amplification conditions for the SOX genes.
T(A) = temperature of annealing for PCR. In some constructs a secondary digest was performed which cleaved only the control vector not containing an insert.
| SOX gene | Sox group | DNA Source | Right Primer | Left Primer | T(A) °C | Restriction enzymes for cloning | Secondary digest | pEF-1 vector frame |
|---|---|---|---|---|---|---|---|---|
| SOX2 | B1 | DNASU: HsCD00329522 | TCGGAATTCCCACATGTGTGAGAGGGGCA | AATGGATCCAGCATGGACAACATGATGGAGACG | 60.5 | BamHI and EcoRI | - | A |
| SOX3 | B1 | Thermo: MHS6278-202857278 | AAAGAATTCTCCGATGTGGGTCAGCGGCA | ATAGGATCCGGAATGCGACCTGTTCGAGAGA | 59.8 | BamHI and EcoRI | - | A |
| SOX4 | C | DNASU: HsCD00299790 | TTGGAAGATATCGTAGGTGAAAACCAGGTTGGAGATG | TTCCAAGGATCCGCCATGGTGCAGCAAACCAAC | 60.5 | BamHI and EcoRV | EcoRI | B |
| SOX5 | D | DNASU: HsCD00442638 | GATGCGGCCGCGTTGGCTTGTCCTGCAATATGGTTTTCACTG | ACCGGATCCACCATGTCTTCCAAGCGACCAGCCTC | 64.4 | BamHI and NotI | EcoRI | C |
| SOX6 | D | DNASU: HsCD00080539 | TTGGAAGCGGCCGCGTTGGCACTGACAGCCTC | TTCCAAGAATTCGAAATGGGAAGAATGTCTTCCAAG | 56.3 | EcoRI and NotI | BstXI | C |
| SOX9 | E | DNASU: HsCD00074606 | TCGGAATTCCCAAGGTCGAGTGAGCTG | AATGGATCCATGGATCTCCTGGACCCC | 56.7 | BamHI and EcoRI | - | A |
| SOX14 | B2 | DNASU: HsCD00436531 | GATGCGGCCGCCATGGCCGTAGCGTGGGC | ACCGGATCCACCATGGCCAAACCTTCAGACCAC | 64.2 | BamHI and NotI | - | C |
| SOX15 | G | DNASU: HsCD00075337 | TTGGAAGCGGCCGCGAGGTGGGTTAGGGGCATGGG | TTCCAAGGATCCGCCATGGCGCTACCAGGCTCC | 65.4 | BamHI and NotI | EcoRI | C |
| SOX17 | F | DNASU: HsCD00303132 | TCGGAATTCCACGTCAGGATAGTTGCAGTAA | AATGGATCCATGGGCAGCCCGGATG | 57.5 | BamHI and EcoRI | - | A |
| SOX30 | H | DNASU: HsCD00005630 | TTGGAAGCGGCCGCATCCCTGAGCACTTTTTCTTCTTCC | TTCCAAGAATTCGCCATGGAGAGAGCCAGACCC | 60.2 | EcoRI and NotI | EcoRV | C |
Tyrosine nitration of ZNF274 and KAP-1 proteins
The ZNF274 and KAP1 proteins were expressed and purified as previously published [28]. The co-pull down experiment was performed by freshly binding GST-tagged ZNF274 protein lysate to 40 μL of glutathione sepharose (GE Healthcare) in BB500 (500mM NaCl, 20mM Tris, 10% glycerol, pH 7.9). This sample was incubated for one hour at room temperature while rotating on a LabQuake rotator. Samples were centrifuged at 16,000 × g for one minute, liquid aspirated, and washed with BB750 (750mM NaCl, 20mM Tris, 10% glycerol, pH 7.9) four times and BB500 three times. The glutathione sepharose was then resuspended in 500 μL BB500 and 10 μg of KAP1 or nitrated KAP1 (nKAP1) protein added. KAP1 was nitrated by adding 2 μL of peroxynitrite (Cayman Chemicals) to 10 μg purified KAP1 protein. The KAP1 or nKAP1 protein was incubated with the ZNF274 bound to glutathione for one hour at room temperature on the rotator. This was washed three times with BB750, twice with BB500, and once with BB250 (250mM NaCl, 20mM Tris, 10% glycerol, pH 7.9). Samples for preformed ZNF274/KAP1 complex were nitrated after the KAP-1 protein was bound by adding 2 μL of peroxynitrite to the protein bound glutathione sepharose suspended in 30 μL BB500. After centrifuging the peroxynitrite into the samples, they were incubated at room temperature for five minutes and washed once with BB500 and once with BB250. All washed beads were then resuspended in 20 μL 5X Laemmli sample buffer and boiled for 10 minutes to remove proteins from the beads. Samples were run on SDS PAGE and transferred to nitrocellulose. Membranes were blocked in blocking buffer (5% milk in PBS-T) for one hour at room temperature, followed by incubation with polyclonal anti-nitrotyrosine antibody (Cayman Chemicals) in blocking buffer for one hour at room temperature. Membranes were washed four times with PBS-T, incubated in anti-Rabbit antibody congregated with HRP in blocking buffer for one hour at room temperature, washed four times with PBS-T, and imaged with SuperSignal West Pico Chemiluminescent substrate (Thermo-Fisher) on X-ray film. Amino acids in Sry critical to binding KRAB were determined by site directed mutagenesis of the pET28 full length human Sry (expressed and purified as above). Mutations were incorporated into primers, vector amplified with those primers using Phusion Hot Start II (Thermo) and vectors ligated together using T4 ligase. All constructs were sequence confirmed. GST pull down assays for Sry by the GST-KRAB-O construct were performed similarly to KRAB-KAP pull down experiments. Western blot for the His tag was performed by transferring the gel to a nitrocellulose membrane, blocking in 5% milk, probing with the His-probe (G-18, Santa Cruz Biotechnology), washed in PBS-T, treated with secondary donkey anti-Goat HRP antibody, washed in PBS-T, and chemiluminescence measured on film.
Results
Transcriptional regulation of the MAS promoter
Little has been published about the regulation of the MAS promoter. To characterize this promoter we began by analyzing evolutionary conserved regions (ECRs) and known ENCODE transcription factor binding sites in the MAS promoter. Analysis of the MAS receptor gene promoter with ECRs revealed 2 highly conserved regions across a diverse range of mammalian species (Figure 1A, sites A and B). At these two sites (Site A at −15,894 to −16,116 from the ATG of MAS and Site B from −17,924 to −18,122), no known transcription factors bind (Table 2). Interestingly, BLAST analysis of the ECR sites against the RefSeq database of mRNA sequences revealed site A to have high homology to a previously identified transcript variant of the MAS1 gene in Odobenus rosmarus (Walrus, Accession codes XM_004401116.1 and XM_004401115.1), Ceratotherium simum simum (White Rhinoceros, XM_004440536.1 and XM_004440535.1), and Felis catus (Cat, XM_003986692.1 and XM_003986691.1) in the 5′ UTR. This suggests a high probability of a secondary transcriptional start site at this location (site A). The ECR site B has previously been identified in a cDNA library (HY016251.1) from the human testis; it is not associated with the MAS transcript, but is likely a non-coding RNA.
Figure 1. Regulation of MAS promoter.
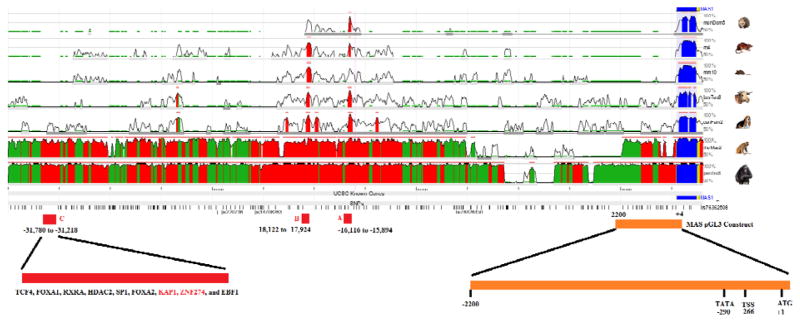
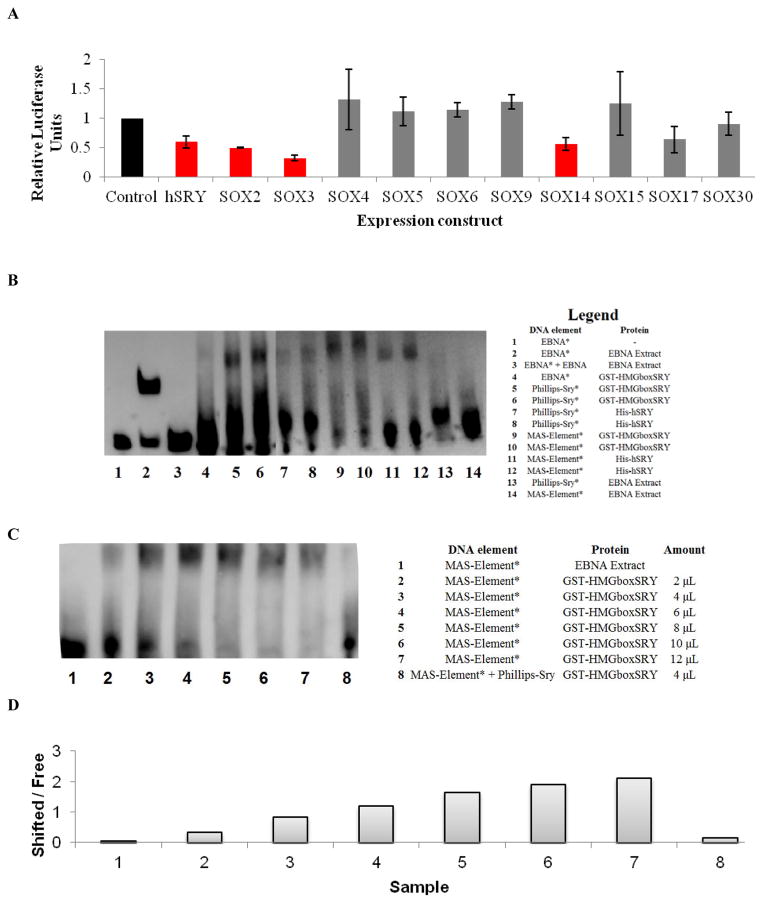
Conservation of the MAS promoter in multiple mammalian species. Red represents regions of high conservation, green repetitive elements, blue coding segments, and yellow the UTR sequence as determined with the ECR browser. Known SNPs are shown, with each black line representing an SNP. Three promoter sites (A, B, and C) were identified as highly conserved. Site C contains no known SNPs and also several transcription factor binding sites as identified in ENCODE. The proximal promoter for MAS (orange) was cloned into a pGL3 luciferase reporter vector. The proposed transcriptional start site (TSS) for humans based on primate UTR sequence analysis has a TATA box located 24 bases upstream the TSS.
Table 2. ENCODE transcription factors binding 5′ of the MAS gene.
Table shows the transcription factor identified, the location of the sequence bound in ChIP on chr6 (showing both the 5′ and 3′ base locations), the location of the sequence relative to the ATG of MAS (both 5′ and 3′), the signal intensity (high of 1000), the cell type the transcription factor was associated in, and the lab that identified the binding. KAP1 binding was not detected in K562b cells (red).
| Transcription factor | chr6 position | chr6 position | relative to ATG of MAS | relative to ATG of MAS | Signal | cell type | lab |
|---|---|---|---|---|---|---|---|
| GATA | 160,327,593 | 160,327,783 | −205 | −395 | 754 | SH-SY5Y | Stanford |
| PAX5-C20 | 160,325,587 | 160,325,810 | −2,178 | −2,401 | 424 | GM-12878 | Hudson Alpha |
| PAX5-N19 | 160,325,577 | 160,325,836 | −2,152 | −2,411 | 455 | GM-12878 | Hudson Alpha |
| JunD | 160,319,976 | 160,320,235 | −7,753 | −8,012 | 226 | HepG2 | Stanford |
| ZNF263 | 160,319,370 | 160,319,992 | −7,996 | −8,618 | 1000 | T-Rex-HEK293 | Stanford |
| EBF | 160,318,810 | 160,318,885 | −9,103 | −9,178 | 401 | GM-12878 | Stanford |
| EBF-(C-8) | 160,318,752 | 160,318,987 | −9,001 | −9,236 | 146 | GM-12878 | Hudson Alpha |
| SRF | 160,301,004 | 160,301,004 | −26,984 | −26,984 | 166 | K562 | Hudson Alpha |
| FOXA2_(SC-6554) | 160,299,821 | 160,300,044 | −27,944 | −28,167 | 125 | HepG2 | Hudson Alpha |
| EBF | 160,299,817 | 160,300,080 | −27,908 | −28,171 | 91 | GM-12878 | Stanford |
| FOXA1_(SC-101058) | 160,299,813 | 160,300,082 | −27,906 | −28,175 | 228 | HepG2 | Hudson Alpha |
| EBF_(C-8) | 160,296,469 | 160,296,719 | −31,269 | −31,519 | 759 | GM-12878 | Hudson Alpha |
| ZNF274 | 160,296,351 | 160,296,734 | −31,254 | −31,637 | 191 | NT2-D1 | Stanford |
| KAP-1 | 160,296,310 | 160,296,770 | −31,218 | −31,678 | 736 | U2OS | Stanford |
| KAP-1 | 160,296,310 | 160,296,770 | −31,218 | −31,678 | 701 | HEK293(b) | Stanford |
| KAP-1 | 160,296,310 | 160,296,770 | −31,218 | −31,678 | None | K562b | Stanford |
| FOXA2_(SC-6554) | 160,296,228 | 160,296,411 | −31,577 | −31,760 | 618 | HepG2 | Hudson Alpha |
| HDAC2_(SC-6296) | 160,296,223 | 160,296,472 | −31,516 | −31,765 | 657 | HepG2 | Hudson Alpha |
| SP1 | 160,296,223 | 160,296,502 | −31,486 | −31,765 | 265 | HepG2 | Hudson Alpha |
| FOXA1_(C-20) | 160,296,219 | 160,296,452 | −31,536 | −31,769 | 724 | HepG2 | Hudson Alpha |
| FOXA1_(SC-101058) | 160,296,215 | 160,296,458 | −31,530 | −31,773 | 983 | HepG2 | Hudson Alpha |
| RXRA | 160,296,216 | 160,296,475 | −31,513 | −31,772 | 343 | HepG2 | Hudson Alpha |
| TCF4 | 160,296,208 | 160,296,591 | −31,397 | −31,780 | 96 | HepG2 | Stanford |
| CTCF_(SC-5916) | 160,284,383 | 160,284,598 | −43,390 | −43,605 | 316 | H1-hESC | Hudson Alpha |
| SMC3_(ab9263) | 160,284,358 | 160,284,616 | −43,372 | −43,630 | 760 | GM-12878 | Stanford |
Based on data from ENCODE transcription factor binding (Table 2), there are several clusters of known transcription factor binding sites in the MAS promoter. One site in particular (Site C on Figure 1A from −31,218 to −31,780) contains known binding by ZNF274, KAP1, SP1 and HDAC2, to name a few, suggesting epigenetic control for the MAS gene. For the proximal promoter, based on primate MAS mRNA, high conservation is found from −266 to the A of the ATG start codon of MAS. This site is the longest known 5′ UTR in primate MAS genes, and we suggest that it is potentially the major transcriptional start site (TSS). A TATA box is located 24 bases from this TSS at −290. To begin to characterize this proximal promoter of MAS, we previously cloned from +4 to −2200 into the pGL3 luciferase vector and showed that hSRY repressed this construct [24]. Human SRY is a member of the SOX family. To see if this repression is conserved in the SOX family, an individual member of each SOX subgroup was cloned. The SOXA group contains SRY, the SoxB1 group both SOX2 and SOX3, the SoxB2 group SOX14, the SoxC group SOX4, the SoxD group SOX5 and SOX6, the SoxE group SOX9, the SoxF group SOX17, the SoxG group SOX15, and the SoxH group SOX30. In addition to SRY repression of the MAS construct, SOX2, SOX3 and SOX14 also repressed the proximal promoter of MAS (Figure 2A). The most probable SOX binding sequence was identified at −98 from the ATG of MAS. This DNA sequence could be bound by various SRY proteins but not a control EBNA lysate (Figure 2B). Sry protein concentration increased the amount of shifted DNA (Figure 2C–D). Unlabeled DNA out-competed the SRY binding, showing specificity in DNA-protein complex (Figure 2C).
Figure 2.
A) Using the MAS promoter construct, members of the SOX genes were tested for ability to regulate the MAS promoter regulation of luciferase. Error bars represent the standard error of the mean with an n of 3. The bars in red (hSRY, SOX2, SOX3, and SOX14) were statistically different from the control while those in gray were not, based on student’s t-test with p≤0.05. B) Gel shift assays of various DNA elements (EBNA=control, Philips=known Sry element, MAS=−98 to −62 of the MAS promoter sequence, and * indicated when the DNA probe had a biotin tag bound) with different protein constructs. C) Varying the concentration of Sry lysate increased the amount of shifted DNA (top) relative to the free DNA probe (bottom) that was outcompeted with DNA that did not contain a biotin tag (sample 8). D) Densitometry of the free and shifted bands confirms the concentration dependent shift.
Nitration pathway for regulation of the MAS receptor
Binding and activation of the MAS receptor by Ang-(1-7) results in stimulation of nitric oxide production, and in internalization and degradation of MAS [29]. Some tissues such as cardiac myocytes have been shown to desensitize to Ang-(1-7) signaling, likely through this degradation of MAS upon activation. However, renal tissue does not desensitize with treatment of Ang-(1-7), except in the SHR [14]. This suggests that a mechanism might exist to compensate for the increased degradation of MAS, likely involving signaling such as increased nitric oxide following Ang-(1-7) activation of MAS. Perturbations of this compensatory mechanism may be involved in hypertension. Our promoter analysis revealed a high probability of the KRAB/KAP1 complex binding to the promoter of MAS. This complex is associated with transcriptional repression [30]. Studies also suggest that the KRAB domain in ZN432 can be nitrated [31]. Sequence comparisons show the tyrosine known to be nitrated in the KRAB domain of ZN432 is conserved in most KRAB containing proteins such as ZNF274. To determine if proteins such as ZNF274 can be nitrated and how this nitration alters the KRAB/KAP-1 interaction, we utilized glutathione pull down experiments. GST tagged ZNF274 was isolated on glutathione sepharose and pulled KAP-1 out of solution (Figure 3A). Addition of peroxynitrite to the beads caused a reduction of the association of ZNF274 with KAP-1. Surprisingly, the KAP-1 protein was found to be nitrated in these experiments (Figure 3B). Purified nitrated KAP-1 protein had no ability to bind the ZNF274 protein and be pulled out of solution using the glutathione sepharose. This suggests that the nitric oxide induced by the MAS signaling may alter the ability of ZNF274 and KAP-1 complex to form. In the absence of repression by ZNF274/KAP1 complex, the MAS promoter would likely have an increased ability to actively transcribe the Mas gene, resulting in the production of more MAS protein to compensate for the Ang-(1-7) stimulated MAS protein destined for degradation (Figure 3C).
Figure 3. Nitration of KAP-1 and ZNF274 suggests possible feedback mechanism in MAS activation by Ang-(1-7).
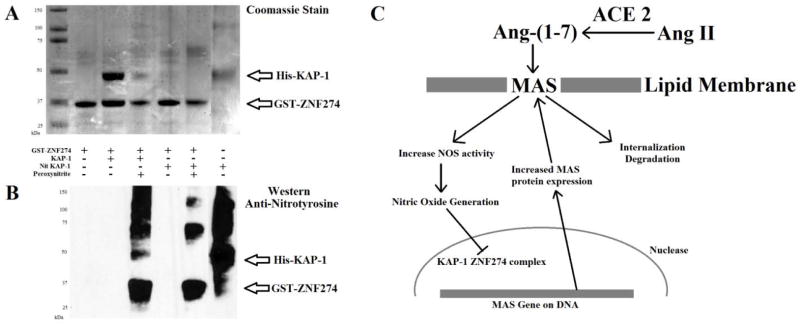
A) Coomassie stain of glutathione sepharose pull down experiments of ZNF274 and KAP-1 with nitration of the protein induced with peroxynitrite. Conditions for each pull down are shown below the gel. B) Western blot with the polyclonal anti-nitrotyrosine antibody of the samples in A. C) Proposed mechanism for maintaining MAS receptor on the cell surface with stimulation by Ang-(1-7). Activation of the receptor results in increased nitric oxide and thus nitration of the KAP-1 ZNF274 proteins preventing their inhibition of the MAS promoter activity.
SRY-KRAB interaction
SRY is known to directly interact with the KRAB domain. To determine how this interaction occurs, SRY protein was mutated at sites in the bridge domain. GST pull downs used the minimal KRAB domain (KRAB-O) with a GST tag to pull out protein from lysates of pET28 empty vector, SRY wild type (WT) or mutated SRY proteins. Coomassie stains of the GST-KRAB-O pull downs show a strong band for the KRAB-O protein, with fainter band seen for SRY in the SRY WT sample (Figure 4A). To confirm that the K136E and K140E mutations altered the binding of SRY to the KRAB protein a western blot of pull downs was performed against the His tag of the SRY protein. The SRY WT band can be seen with only a very faint band for the K136E protein and no band for the K140E protein (Figure 4B). These two polar basic amino acids of SRY most likely contact the two polar acid amino acids of the first helix of the KRAB domain (Figure 4C).
Figure 4.
A) GST pull down experiments of various SRY constructs using the GST-KRAB-O protein. The GST tag alone did not pull SRY out of solution. Only a band for the hSRY WT can be seen which was further confirmed through western blots against the His tag of SRY (B). C) Models suggesting the critical amino acids for the binding of SRY (gray) to the KRAB domain (yellow) through to polar basic (blue) and polar acidic (red) amino acids while SRY in bound to DNA (cyan).
Discussion
MAS is expressed in testis, kidney, heart, hippocampus, forebrain, piriform cortex and the olfactory bulb in mouse [32]. In many of these same tissues, the male specific gene Sry is expressed in the rat [33]. The Sry gene has been shown to contribute to the elevation of blood pressure in the SHR [34] partially through its regulation of the RAS, producing increased Ang II levels [35,36], and through its role in the sympathetic nervous system [37]. The alterations to Sry in the SHR animal may explain in part how the SHR animal desensitizes with treatment of Ang-(1-7) [14], as an increased prevalence of Sry will recruit additional KRAB domain proteins which when nitrated do not loose interaction with KAP1 (data not shown). Sry is a member of the SOX family of architectural transcription factors. The SOX family of genes is involved in nearly every stage of development and functions in many diseases from cancer to cardiovascular. Most of the SOX genes share similar DNA binding sequences [38], and with hSRY having potential binding and transcriptional control of the MAS promoter, additional SOX proteins may also regulate the MAS gene.
The proximal promoter of MAS contains numerous potential SOX binding elements as determined with MatInspector analysis. We previously showed that human SRY represses transcriptional activity of the proximal promoter of the MAS gene [24]. We have cloned at least one member of each of the SOX subgroups into mammalian expression vectors and studied the potential for these proteins to regulate the MAS reporter construct. The proteins most homologous to SRY (SOX2, SOX3, and SOX14) repressed the MAS reporter construct similarly to SRY. Since the most homologous genes of Sry behave similarly on the MAS reporter construct, it suggests that these SOX proteins also have the potential to regulate MAS. Additionally, we have shown that SRY, through its HMG box, has the ability to directly bind to a sequence of the MAS promoter.
Given that SRY has the potential to regulate the proximal promoter of MAS, it is of interest that ENCODE data indicates the presence of downstream binding sites for SP1 and ZNF274, both of which are known to interact with SRY [39,40]. ZNF274 contains a KRAB domain which can bind the KAP1 protein in a 1:3 ratio (KRAB:KAP1) with KRAB at the center of the complex [28]. This complex results in repression of transcription [41] and is thought to serve as a master regulator switch in many genes [30]. Many of the sites at which KAP1 functions are located 10 to 100 kb away from the genes they regulate, and regulation at these sites is through epigenetic mechanisms [42]. The zinc finger proteins containing the KRAB domain recruit the KAP1 protein to regions of the genome. The KAP1 protein then recruits epigenetic machinery, including heterochromatin protein 1 (HP1) and histone deacetlyases (HDAC), to the target site [30]. KAP-1 knockout in the forebrain resulted in increased stress response with alterations in spatial memory [43]. MAS knockout models also provided alterations in memory formations and elevated anxiety [23]. Additionally, the MAS gene has been shown to be imprinted in the mouse [44] and in human breast cancer tissues [45]. This may well be influenced by the role of the KAP1 heterochromatin formation. We suggest a working model in which SRY binds to the proximal promoter of MAS and through DNA folding back on itself, the identified element C of the MAS promoter (that has the zinc fingers of ZNF274 bound) is recruited through its interaction with SRY close to the transcriptional start site (Figure 5A). Activation of MAS by Ang-(1-7) results in increased nitric oxide. Showing that the KRAB domain and also KAP1 can be nitrated by increased nitric oxide demonstrates that nitration can block KRAB and KAP1 from directly interacting. This suggests a potential for the nitration signal to decrease KAP1 association to the complex formed on the MAS promoter.
Figure 5.
A) Proposed mechanism for the fold back of element C of the MAS promoter to regulate the transcriptional control of MAS through interaction of ZNF274 and SRY recruiting KAP1. Nitration in the KRAB domain may lead to the differential regulation of the MAS promoter following Ang-(1-7) activation and possible nitration of transcription factors. The hypertensive SHR animal contains an additional copy of Sry which may facilitate additional binding to the promoter sequence thus recruiting additional KRAB domains when compared to the normotensive WKY animal. B) Proposed nuclear mechanism involving nitric oxide repression of the KRAB/KAP1 complex on promoters of multiple RAS genes as a result of activation of MAS and AT2 by Ang peptides.
An intracellular RAS has been shown in the heart, vasculature, brain and kidney [46]. These pathways serve as an intracrine system, with receptors such as AT1 [47], AT2 [48] and MAS [13] on the surface of the nucleus and the production of intracellular Ang peptides including Ang-(1-7) in the cytoplasm. Activation of these receptors leads to signaling in the nucleus through nitric oxide, IP2, ERK1/2, and p38 [49]. Here, we show that nitration of either the KRAB domain or the KAP1 protein results in decreased affinity of the two proteins and thus a potential for altered transcriptional ability at the genomic target sites. The intracellular RAS may serve to facilitate regulation of the RAS gene promoters that have been associated with KAP1 binding including renin (REN), the (pro)renin receptor (ATP6AP2), ACE2, Neprilysin (MME), and MAS based on ENCODE results. The generation and actions of Ang peptides inside a cell may thus regulate long term transitions from heterochromatin to euchromatin for the RAS genes and others, changing the cellular phenotype (Figure 5B).
Activation of MAS by Ang-(1-7) is known to result in modifications to eNOS and nNOS, increasing nitric oxide levels in the cytoplasm [5] and the nucleus [13]. In a high throughput screen of proteins to be nitrated, a KRAB containing protein was identified [31] in a region highly conserved in other KRAB proteins. Of these KRAB domain proteins, one (ZNF273) has been identified in ENCODE to be bound close to the MAS gene. We used purified ZNF274 protein to show that it also has the potential to be nitrated. Further, we demonstrate that its binding partner, KAP1, can be nitrated. Nitration of either of these two proteins, KAP1 or KRAB, resulted in a loss of binding to the other protein. When ZNF274 and KAP-1 complex was preformed, addition of peroxynitrite (nitrating the tyrosines of each protein) caused the proteins to dissociate. The loss of interaction between ZNF274 and KAP1 is expected to lead to a loss of any epigenetic repression machinery associated with KAP1 at the ZNF274 regulated genes. Stimulation of the MAS protein should result in increased nitric oxide, potentially nitrating the ZNF274/KAP1 complex, resulting in dissociation of KAP1 and activation of the transcription of MAS (Figure 3C and Figure 5B). ZNF263 contains homologous sequences to ZNF274, including the nitrated tyrosine. Most of the RAS genes associated with KAP1 binding are also associated with ZNF263, with MAS containing the highest possible signal strength (1000) for ZNF263 binding in the ENCODE dataset. Therefore, it may be of interest in the future to study the ZNF263 protein nitration and the role it serves in regulation of RAS genes. Although we show the ZNF274 and KAP1 proteins can be nitrated in vitro, the degree to which these proteins can be nitrated in vivo has not been determined. Additional studies must be performed to address in vivo nitration. This study suggests the need to look at more targets of tyrosine nitration due to the activation of MAS, as many other proteins may be modified, altering the cell phenotype.
Supplementary Material
Clinical Perspectives.
The ACE2/Ang-(1-7)/MAS axis is critical for the cardiovascular system; however there are currently many limitations in our detailed understanding of the pathway. In this paper we have characterized the promoter of the MAS gene and identified numerous transcription factors associated with gene repression that may be altered by nitric oxide signaling. It shows the potential for tyrosine nitration to alter gene regulation and epigenetics. Overall, this study will serve to clarify details of the regulation and activation of MAS, allowing for better characterization in clinical variations and future drug design.
Acknowledgments
We would like to thank Dulce Caserini and Almir Martins for their help throughout the project.
Funding
Funding for this project was provided by the American Heart Association [11PRE7380033], Ohio Board of Regents, The University of Akron, and National Institutes of Health [5R01CA129833-05].
Footnotes
Author Contributions
JWP performed most experiments and wrote the manuscript; FJR III, HP, YL advised and aided in KRAB/KAP1 experiments; FCA performed MAS promoter analysis; IW cloned and characterized the MAS proximal promoter; FMR and AM advised on MAS regulation and activation. All authors approved the final manuscript.
References
- 1.Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 2.Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature. 1988;335:437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- 3.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prokop JW, Santos RAS, Milsted A. Differential Mechanisms of Activation of the Ang Peptide Receptors AT1, AT2, and MAS: Using In Silico Techniques to Differentiate the Three Receptors. PLoS ONE. 2013;8:e65307. doi: 10.1371/journal.pone.0065307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 6.Gomes ERM, Lara AA, Almeida PW, Guimarães D, Resende RR, Campagnole-Santos MJ, Bader M, Santos RA, Guatimosim S. Angiotensin-(1-7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway. Hypertension. 2010;55:153–160. doi: 10.1161/HYPERTENSIONAHA.109.143255. [DOI] [PubMed] [Google Scholar]
- 7.Gava E, Samad-Zadeh A, Zimpelmann J, Bahramifarid N, Kitten GT, Santos RA, Touyz RM, Burns KD. Angiotensin-(1-7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant. 2009;24:1766–1773. doi: 10.1093/ndt/gfn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verano-Braga T, Schwämmle V, Sylvester M, Passos-Silva DG, Peluso AA, Etelvino GM, Santos RA, Roepstorff P. Time-Resolved Quantitative Phosphoproteomics: New Insights into Angiotensin-(1–7) Signaling Networks in Human Endothelial Cells. J Proteome Res. 2012;11:3370–3381. doi: 10.1021/pr3001755. [DOI] [PubMed] [Google Scholar]
- 9.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. (G)-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 10.Santos RAS, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 11.Dias-Peixoto MF, Ferreira AJ, Almeida PW, Braga VB, Coutinho DC, Melo DS, Gomes Filho A, Melo MB, Greco L, Campagnole-Santos MJ, Lima RF, Santos RA, Guatimosim S. The cardiac expression of Mas receptor is responsive to different physiological and pathological stimuli. Peptides. 2012;35:196–201. doi: 10.1016/j.peptides.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:H2356–H2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 13.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan Z, Wu J, Ma H. Regulation of angiotensin-converting enzyme 2 and Mas receptor by Ang-(1-7) in heart and kidney of spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst. 2011;12:413–419. doi: 10.1177/1470320311402109. [DOI] [PubMed] [Google Scholar]
- 15.Reis AB, Araújo FC, Pereira VM, Dos Reis AM, Santos RA, Reis FM. Angiotensin (1-7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Histol. 2010;41:75–80. doi: 10.1007/s10735-010-9264-8. [DOI] [PubMed] [Google Scholar]
- 16.Xu P, Santos RAS, Bader M, Alenina N. Alterations in gene expression in the testis of angiotensin-(1-7)-receptor Mas-deficient mice. Regul Pept. 2007;138:51–55. doi: 10.1016/j.regpep.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Pereira VM, Reis FM, Santos RA, Cassali GD, Santos SH, Honorato-Sampaio K, dos Reis AM. Gonadotropin stimulation increases the expression of angiotensin-(1--7) and MAS receptor in the rat ovary. Reprod Sci. 2009;16:1165–1174. doi: 10.1177/1933719109343309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95:176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 19.Viana GEN, Pereira VM, Honorato-Sampaio K, Oliveira CA, Santos RA, Reis AM. Angiotensin-(1-7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol. 2011;96:957–965. doi: 10.1113/expphysiol.2011.058453. [DOI] [PubMed] [Google Scholar]
- 20.Prasannarong M, Santos FR, Henriksen EJ. ANG-(1-7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun. 2012;426:369–373. doi: 10.1016/j.bbrc.2012.08.093. [DOI] [PubMed] [Google Scholar]
- 21.Lopez Verrilli MA, Rodriguez Fermepín M, Longo Carbajosa N, Landa S, Cerrato BD, García S, Fernandez BE, Gironacci MM. Angiotensin-(1-7) through Mas receptor up-regulates neuronal norepinephrine transporter via Akt and Erk1/2-dependent pathways. J Neurochem. 2012;120:46–55. doi: 10.1111/j.1471-4159.2011.07552.x. [DOI] [PubMed] [Google Scholar]
- 22.Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci. 2005;29:427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Lazaroni TLN, Raslan AC, Fontes WR, de Oliveira ML, Bader M, Alenina N, Moraes MF, Dos Santos RA, Pereira GS. Angiotensin-(1-7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem. 2012;97:113–123. doi: 10.1016/j.nlm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Prokop JW, Watanabe IK, Turner ME, Underwood AC, Martins AS, Milsted A. From rat to human: regulation of Renin-Angiotensin system genes by sry. Int J Hypertens. 2012;2012:724240. doi: 10.1155/2012/724240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 2004;32:W280–W286. doi: 10.1093/nar/gkh355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ENCODE Project Consortium. A user’s guide to the encyclopedia of DNA elements (ENCODE) PloS Biol. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips NB, Jancso-Radek A, Ittah V, Singh R, Chan G, Haas E, Weiss MA. SRY and human sex determination: the basic tail of the HMG box functions as a kinetic clamp to augment DNA bending. J Mol Biol. 2006;358:172–192. doi: 10.1016/j.jmb.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 28.Peng H, Gibson LC, Capili AD, Borden KL, Osborne MJ, Harper SL, Speicher DW, Zhao K, Marmorstein R, Rock TA, Rauscher FJ., 3rd The Structurally Disordered KRAB Repression Domain Is Incorporated into a Protease Resistant Core upon Binding to KAP-1-RBCC Domain. J Mol Biol. 2007;370:269–289. doi: 10.1016/j.jmb.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Gironacci MM, Adamo HP, Corradi G, Santos RA, Ortiz P, Carretero OA. Angiotensin (1-7) induces MAS receptor internalization. Hypertension. 2011;58:176–181. doi: 10.1161/HYPERTENSIONAHA.111.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar S, Farnham PJ. KAP1 Protein: An Enigmatic Master Regulator of the Genome. J Biol Chem. 2011;286:26267–26276. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem. 2006;354:279–289. doi: 10.1016/j.ab.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the Mouse and Rat Mas Protooncogene in the Brain and Peripheral-Tissues. FEBS Lett. 1995;357:27–32. doi: 10.1016/0014-5793(94)01292-9. [DOI] [PubMed] [Google Scholar]
- 33.Turner ME, Ely D, Prokop J, Milsted A. Sry, more than testis determination? Am J Physiol Regul Integr Comp Physiol. 2011;301:R561–R571. doi: 10.1152/ajpregu.00645.2010. [DOI] [PubMed] [Google Scholar]
- 34.Turner ME, Farkas J, Dunmire J, Ely D, Milsted A. Which Sry Locus Is the Hypertensive Y Chromosome Locus? Hypertension. 2008;53:430–435. doi: 10.1161/HYPERTENSIONAHA.108.124131. [DOI] [PubMed] [Google Scholar]
- 35.Ely D, Boehme S, Dunphy G, Hart M, Chiarappa F, Miller B, Martins AS, Turner M, Milsted A. The Sry3 Y Chromosome Locus Elevates Blood Pressure and Renin-Angiotensin System Indexes. Gend Med. 2011;8:126–138. doi: 10.1016/j.genm.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milsted A, Underwood AC, Dunmire J, DelPuerto HL, Martins AS, Ely DL, Turner ME. Regulation of multiple renin–angiotensin system genes by Sry. J Hypertens. 2010;28:59–64. doi: 10.1097/HJH.0b013e328332b88d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ely D, Milsted A, Dunphy G, Boehme S, Dunmire J, Hart M, Toot J, Turner M. Delivery of sry1, but not sry2, to the kidney increases blood pressure and sns indices in normotensive wky rats. BMC Physiol. 2009;9:10. doi: 10.1186/1472-6793-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolma A, Yan J, Whitington T, Toivonen J, Nitta KR, Rastas P, Morgunova E, Enge M, Taipale M, Wei G, Palin K, Vaquerizas JM, Vincentelli R, Luscombe NM, Hughes TR, Lemaire P, Ukkonen E, Kivioja T, Taipale J. DNA-binding specificities of human transcription factors. Cell. 2013;152:327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Peng H, Ivanov AV, Oh HJ, Lau YFC, Rauscher FJ. Epigenetic Gene Silencing by the SRY Protein Is Mediated by a KRAB-O Protein That Recruits the KAP1 Co-repressor Machinery. J Biol Chem. 2009;284:35670–35680. doi: 10.1074/jbc.M109.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wissmüller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34:1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witzgall R, O’Leary E, Leaf A, Onaldi D, Bonventre JV. The Krüppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proc Natl Acad Sci U S A. 1994;91:4514–4518. doi: 10.1073/pnas.91.10.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyengar S, Ivanov AV, Jin VX, Rauscher FJ, 3rd, Farnham PJ. Functional analysis of KAP1 genomic recruitment. Mol Cell Biol. 2011;31:1833–1847. doi: 10.1128/MCB.01331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobsson J, Cordero MI, Bisaz R, Groner AC, Busskamp V, Bensadoun JC, Cammas F, Losson R, Mansuy IM, Sandi C, Trono D. KAP1-Mediated Epigenetic Repression in the Forebrain Modulates Behavioral Vulnerability to Stress. Neuron. 2008;60:818–831. doi: 10.1016/j.neuron.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 44.Cebra-thomas J, Tsai JY, Pilder SH, Copeland NG, Jenkins NA, Silver LM. Localization of the Mas Protooncogene to a Densely Marked Region of Mouse Chromosome-17 Associated with Genomic Imprinting. Genomics. 1992;13:444–446. doi: 10.1016/0888-7543(92)90267-v. [DOI] [PubMed] [Google Scholar]
- 45.Miller N, McCann AH, O’Connell D, Pedersen IS, Spiers V, Gorey T, Dervan PA. The MAS proto-oncogene is imprinted in human breast tissue. Genomics. 1997;46:509–512. doi: 10.1006/geno.1997.5063. [DOI] [PubMed] [Google Scholar]
- 46.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302:R518–R530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DK, Lança AJ, Cheng R, Nguyen T, Ji XD, Gobeil FJr, Chemtob S, George SR, O’Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 48.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tadevosyan A, Vaniotis G, Allen BG, Hébert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol. 2012;590:1313–1330. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.