Abstract
Feeding is a rhythmic behavior that consists of several component cycle types. How the timing of these cycles changes over a complete feeding sequence is not well known. To test the hypothesis that cycle frequency/duration changes as a function of time spent feeding, we examined complete feeding sequences in six infant pigs, using EMG of mylohyoid (MH) and thyrohyoid (TH) as cycle markers. We measured the instantaneous frequency of sucking and of swallowing cycles in 19 sequences. Each sequence contained three qualitatively distinctive phases of sucking frequency. Phase 1 started with cycles at a very high frequency and quickly dropped to a more constant level with low variation, which characterized phase 2. Phase 3 had a steady level of frequency but was interspersed with a number of high- or low-frequency cycles. Each phase differed from the others in patterns of within-phase variation and among-phase variation. Phase 2 had the least variation, and phase 3 had the largest range of frequencies. The number of sucks per swallow also differed among phases. These patterns, which characterize normative feeding, could indicate a physiologic basis in satiation. In human infant clinical studies, where data collection is often limited, these results indicated the utility of collecting data in different phases. Finally, these results can be used as a template or pattern with which to assess clinically compromised infants.
Keywords: Pediatric, Infant, Feeding, Animal model, Deglutition, Deglutition disorders
Introduction
Adult mammalian feeding is rhythmic behavior that consists of several component behaviors [18, 19]. The relationships among these components, which include stage 1 intraoral transport, mastication, stage 2 intraoral transport, and swallowing (or the pharyngeal swallow), are complex and incompletely understood [18–20]. The biomechanics and physiology of many of these individual behaviors are well studied and known [10, 11, 14, 18]. However, the coordination among these components and how that coordination changes over the course of a meal, or feeding session, is less well known.
Normal infant suckling is a good model for exploring the timing of behaviors over a complete feeding session, in part because it includes fewer components and is a simpler behavior [3, 7, 16, 32, 35]. Unlike the multiple types of cycles in adult feeding, infant feeding has essentially two types of cycles: suck cycles, which include intraoral transport, and suck-swallow cycles, when one bolus is transported through the oral cavity and the previous bolus passes through the oropharynx into the esophagus [31].
The biomechanics and muscle activity patterns, as well as basic information on the neural control of infant feeding, are well documented [6, 15, 29–31]. Several authors have described various problems with infant feeding [1, 7, 26, 27, 33]. Yet, most of this work focuses on isolated swallows or a few cycles after feeding on one bite. Seldom is an entire sequence characterized and tested against null hypotheses. Arvedson [1], one of the few researchers to consider this problem, states:
Some infants may take a few minutes to “warm up.” If the feeding observation is stopped after 5 min, an erroneous impression might be made. On the other hand, an infant may start out well, become disorganized, and show signs of fatigue as the feeding progresses.
This statement indicates the importance of a quantified basis and understanding of normal timing and changes across a feeding sequence, to measure what aspects of feeding are disordered in an infant that aspirates or has other feeding difficulties. Therefore, in our study we determined what the normal rhythm and timing is in suck and suck-swallow cycles throughout a feeding sequence. To do this, we examined 19 complete sequences obtained from six infant pigs to determine if (1) the rate of sucking, (2) the rate of swallowing, and (3) the number of sucks per swallow change over the course of a complete feeding session in a normal feeding animal. We used EMG signals as markers of cycle timing because they have a very fine time resolution.
Our null hypothesis was that sucking and swallowing rates were constant over a feeding sequence. However, Arvedson’s perspective [1] and earlier work on infant pigs [15] suggested an alternative of changing rates over a feeding session or sequence. Understanding normal timing and changes across a feeding sequence with a quantified basis could lead to a better understanding on general feeding behavior.
Methods
Animal Model
This study included six infant pigs (Sus scrofa) that were 2–3 weeks old and weighed 3.0–5.5 kg (Tom Morris Farms, Reisterstown, MD). At this age and size, the animals were comparable to 6–12-month-old human infants as judged by tooth eruption, weaning status, and skeletal development [3, 32, 35].
The developmental patterns of feeding infants are remarkably similar across most species of mammals [13, 16]. Infant pigs have been used in previous studies of normal feeding and swallowing neurophysiology, and thus a large body of comparable data exists for comparison with the results of this study [4, 12, 17, 21, 22]. The data used here were collected as control data in other studies of dysphagia [8, 9, 23, 24]. The methods used are described in detail in those articles and briefly reviewed here. All work was done with Johns Hopkins University IACUC approval (approval No. SW10M212).
The infant pigs were trained to feed from a standard baby bottle, fitted with a special elongated pig nipple (Nasco, Fort Atkinson, WI). Presurgery control data were collected to ensure that the kinematics was not impacted by electrode placement. Under general anesthesia (2–5 % isoflurane) and aseptic conditions, the suprahyoid and infrahyoid muscles were exposed via a midline submandibular incision and individually identified following an atlas of pig anatomy [28]. Fine-wire bipolar electromyographic (EMG) electrodes were inserted into many muscles; however, only signals from the mylohyoid (MH) and thyrohyoid (TH) muscles were used to identify suck and suck-swallow cycles, respectively. Here on in, we refer to suck-swallow cycles as “swallow cycles” for convenience. It should be noted that in every swallow cycle, a suck with its subsequent transport of milk occurs in addition to the swallow [31]. A postmortem was performed at the end of experiments to confirm the position and state of the electrodes.
The entire surgical procedure lasted 2–4 h. Following recovery from anesthesia and after quadrupedal posture, the animals were offered milk every hour until they were able to feed normally. Postoperatively, the following medications were administered and were continued for the duration of the experiment: ampicillin (0.16 mL of 250 mg/mL) and buprenorphine (0.17 mL of 0.3 mg/mL) twice daily and meloxicam (0.1 mL of 5 mg/mL) once daily.
Feeding Sessions
During the feeding sessions, each pig stood, without restraint, and fed from a bottle that was warmed in a hot water bath for approximately 2 min to ensure standardized temperature. The pig determined the duration of the feeding and the amount of milk ingested; all animals were permitted to feed until they voluntarily stopped feeding. The milk was a commercial pig milk replacer (Land O Lakes Solustart pig milk replacer, St. Paul, MN). Throughout the feeding session, EMG signals were recorded at 10 kHz on a Powerlab 30/16 (ADInstruments, Colorado Springs, CO). Feeding sessions were 4 h apart and lasted from 3 to 10 min, with an average of 6 min.
Data Analysis
Timing of cycles was determined from the raw EMG signals recorded from the mylohyoid (MH, suck cycles) and thyrohyoid (TH, suck-swallow cycles, referred to as “swallow” cycles here) muscles following Thexton et al. [31]. The EMG signals were used a cycle markers, not as measures of a particular kinematic movement. These muscles are frequently monitored in human studies for a similar purpose of identifying the time of the swallow or of jaw movement [5]. EMG signals are more accurate than corresponding kinematic measures because the EMG is recorded at 10 kHz, rather than at 30 or 60 Hz that is used for fluoroscopic imaging data. The MH is active with each suck cycle, including the suck-swallow cycles. The TH is active only in the cycles that contain a pharyngeal swallow. The start of the cycle was manually determined from the onset of MH EMG and marked in each sequence using LabChart® 7 (ADInstruments, Colorado Springs, CO). Sucking frequency was an “instantaneous” frequency and calculated as the inverse of the time from the beginning of MH activity in one cycle to the beginning of MH activity in the next cycle. Similarly, swallow frequency was also “instantaneous” and calculated as the inverse of the time between pharyngeal swallows, as marked by the TH activity. The number of suck, or oral transport, cycles per pharyngeal swallow was calculated by counting the number of suck cycles (MH activity) between swallow cycles (TH activity). The data set consisted of 19 sequences, which included 17,678 suck cycles and 2,839 swallow cycles.
Three analyses were performed. In the first two, the unit of analysis for suck and swallow frequency analyses was the cycle, indexed at the time it occurred in the feeding sequence. Each sequence was analyzed by fitting a linear model (regression) to the (1) instantaneous suck frequency and (2) instantaneous swallow frequency, as a function of time. The coefficients (slope and intercept) of these regressions measured change in frequency or sucks per swallow (slope) and the starting frequency (intercept). A subsequent analysis used the slope and intercept of each sequence for a comparison among the slopes; thus, the unit of analysis became a sequence. There were two hierarchical levels of variation that were measured in these analyses. The first is the standard error (SE) of slope and intercept as calculated in the initial linear models, and measured the variation of slope or intercept within a sequence. For the slope, this indicated the goodness of fit of the line to the data. A low SE means that there was relatively little variation in frequency around the line that described the change in frequency within the sequence, and a high SE means that there was greater variation in frequency around the slope. Because the sequence became the unit of analysis, the variation among sequences was a different measure of variation. Thus, we also generated a measure of variation among the slopes across the 19 sequences in the data set. This was indicated as error bars in the figures and measured variation among individuals or sequences.
The final analysis (3) calculated the number of sucks per swallow in each sequence. For the number of sucks per swallow, the unit of analysis was a swallow cycle.
Initial exploration of the raw data revealed obvious qualitative changes over time within each sequence (Fig. 1). These differences were characterized as phases and were analyzed using distinct subsets of the data. The phases were visually identified by two different authors (RZG, EGN). We tested for differences among phases with ANOVA, using individual as a random factor. All statistical analyses were performed using SYSTAT 13 (SYSTAT Software, Inc., Chicago, IL).
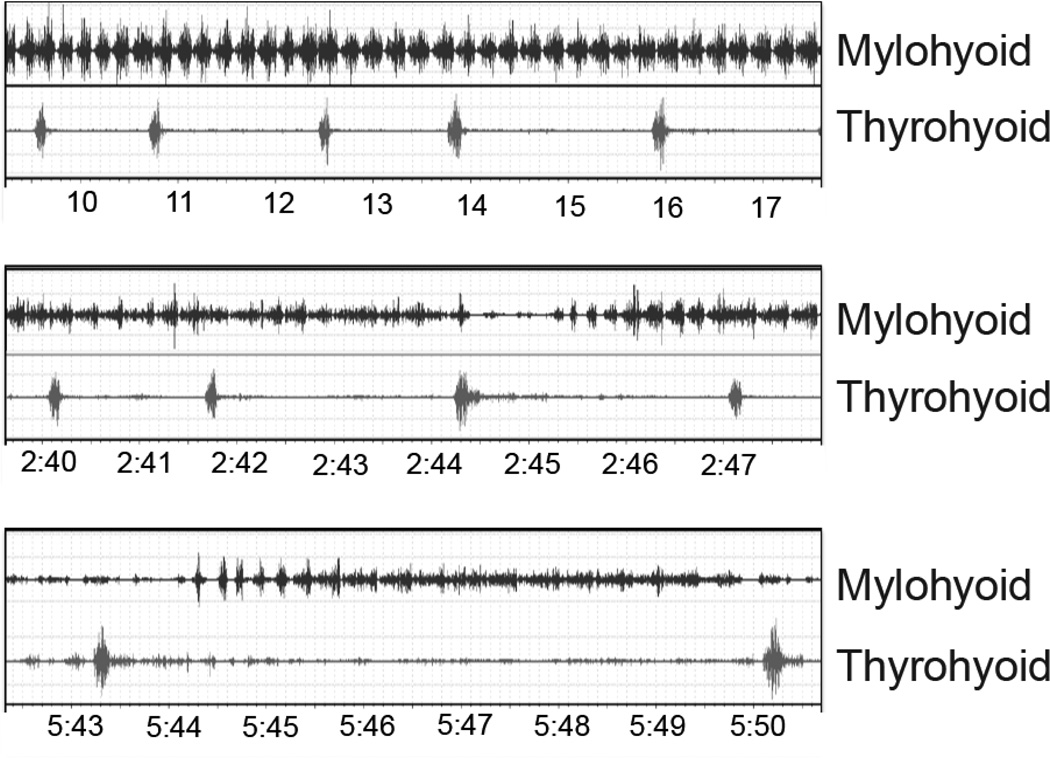
Fig. 1.
Raw EMG data from three phases (time in minutes:seconds). Each panel is roughly 8 s in duration and all are the same scale. The mylohyoid bursts in each suck cycle; the thyroid hyoid bursts in each swallow cycle. The top panel is a sample from phase 1, the middle is a sample from phase 2 and the bottom is a sample from phase 3
Results
Within-Sequence Variation
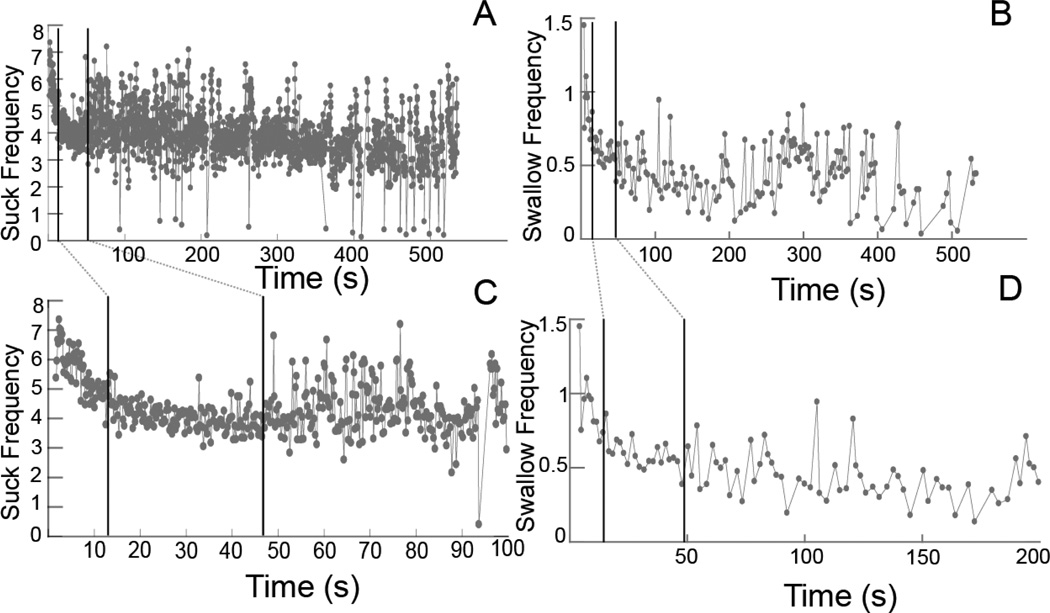
The average feeding sequence lasted 307.0 s and ranged from 145.9 to 731.7 s (Table 1). Each of the feeding sequences in these data contained three qualitatively distinctive phases of sucking frequency. The phases were characterized by changes in slope and variation for the model fit to frequency vs. time in sequence (Fig. 2). Phase 1 started with a very high frequency and quickly dropped to a more constant level with low variation. Reaching this constant level constituted the beginning of phase 2. Phase 3 did not have a systematic change in frequency over time; however, it had a relatively small number of occurrences of very high or low frequency cycles. The break between phase 1 and phase 2 was the point at which the frequency leveled off into a relatively constant value. The break between phase 2 and phase 3 was the occurrence of the first either very-high- or very-low-frequency cycle (> 1.0 Hz). The timing of the breaks among the phases of the suck cycles was used to divide the sequence of swallow cycles and the change in sucks per swallow over time. The phases also lasted variable amounts of time, with, on average, phase 1 shorter than phase 2, which was shorter than phase 3 (Table 1).
Table 1.
Sequence and phase lengths (in seconds) for 19 sequences
| Total sequence | Phase 1 | Phase 2 | Phase 3 | |
|---|---|---|---|---|
| Minimum | 145.9 | 4.6 | 12.9 | 68.1 |
| Maximum | 731.7 | 40.3 | 397.6 | 483.7 |
| Average | 307.0 | 14.8 | 82.4 | 209.8 |
Fig. 2.
Suck and swallow frequencies over time. A, B Suck frequency and swallow frequency, respectively, against time for an entire feeding sequence, from when the animal started until it stopped feeding. C, D Details over a shorter period of time (100 and 200 s) for A and B. The dark vertical lines in each graph represent the breaks between phases. Linear models were fit to each phase for each sequence. The values of the slopes and intercepts from these models were subsequently analyzed
Frequency of Suck Cycles
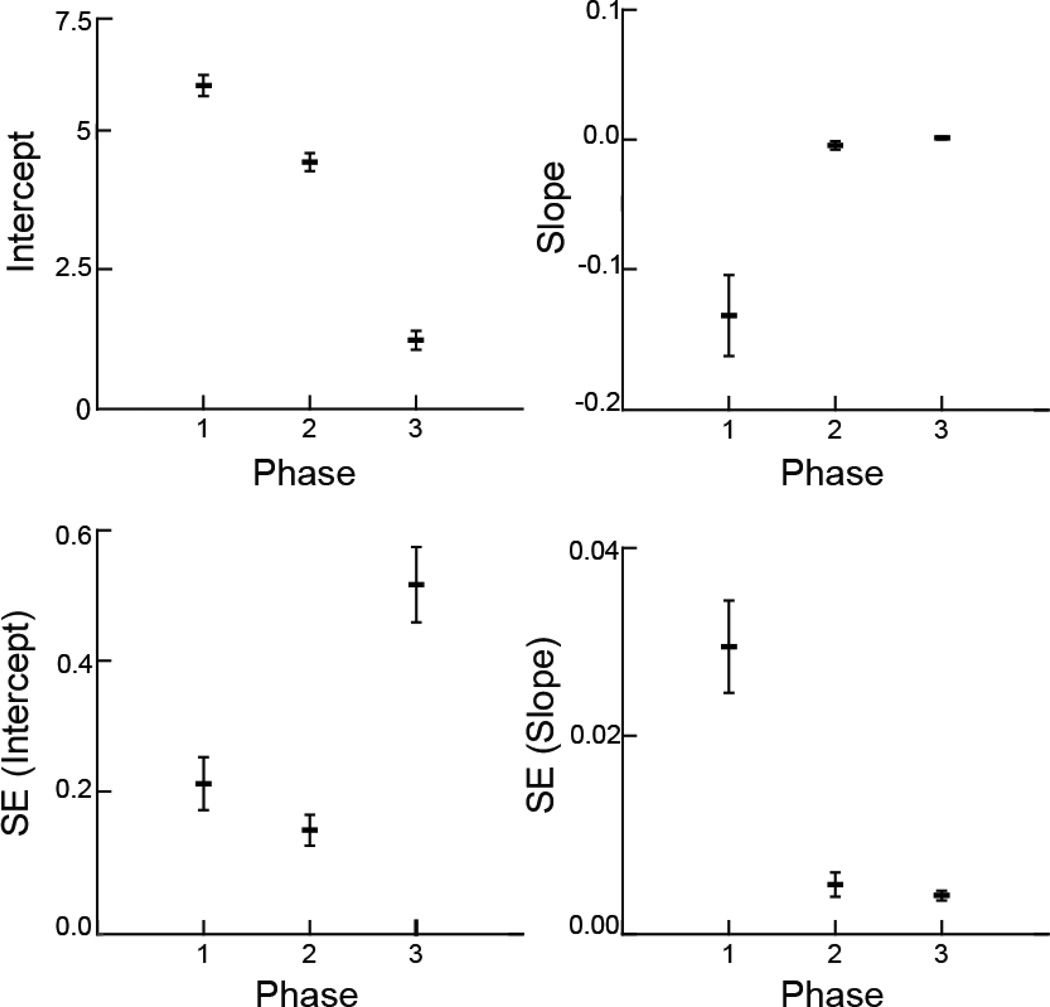
The three phases had different slopes, intercepts, and SEs of slopes and intercepts (Fig. 3A–D). The slopes, indicating change in sucking frequency, were negative in phase 1 and different from phases 2 and 3 (p < .001). The animals started sucking rapidly, but the frequency then dropped. Phases 2 and 3 had slopes that were not different from 0 (p > .9), indicating no change in frequency during these phases. The intercepts for each phase of the model decreased from phase 1 to phase 2 to phase 3 (p < .001). This indicates that at the start of each phase, the animals sucked at a lower rate than in the prior phase. The SE (slope) was different in phase 1 (p < .001), indicating variation change in sucking frequency in that phase compared to that in phases 2 and 3, which were not different (p > .97). The SE (intercept) was much higher in phase 3 (p < .001), with phases 1 and 2 not different (p > .45).
Fig. 3.
Mean and variation of the coefficients and standard errors from linear models of suck frequency vs. time. The unit of analysis was the sequence, with a sample size of 19. The horizontal bars are the average for each response variable, and the vertical lines are the standard error around that average, a measure of variation among the 19 sequences. The intercept and slope are from the linear models fit to the frequency of sucks in each sequence. The SE (intercept) and SE (slope) are the within-sequence standard errors from each of the 19 linear models
Frequency of Swallow Cycles
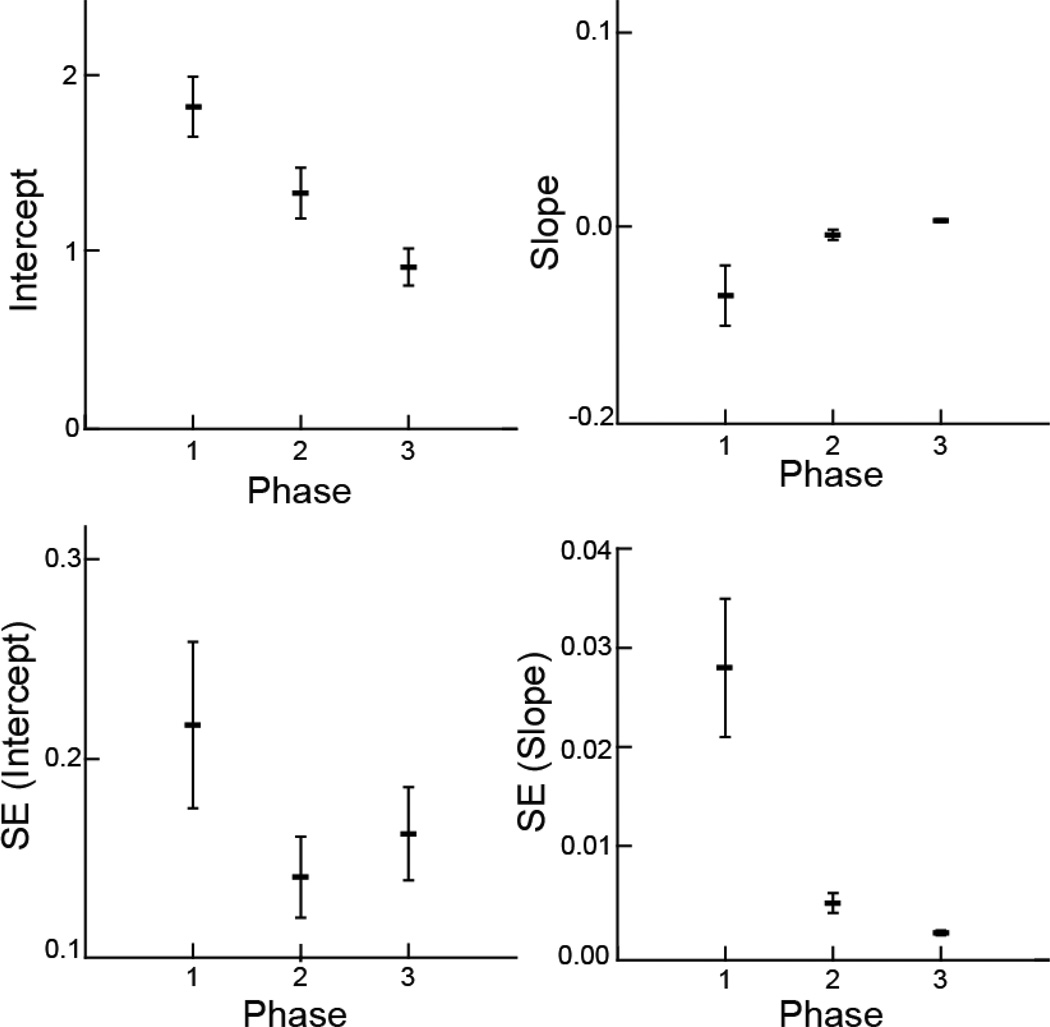
The three phases were different in slopes, intercepts, and SEs of slopes and intercepts (Fig. 4A–D) for swallow frequency over time, following much the same pattern as for sucking frequency over time. The slopes, indicating change in swallowing frequency, were negative in phase 1. The animals started swallowing very frequently, but the frequency then dropped in value. Phases 2 and 3 had slopes that were not different from 0 (p > .82), indicating no change in frequency during these phases, but both were different from phase 1 (p < .05). The intercepts for each phase of the model decreased from phase 1 to phase 2 to phase 3 (p < .001). This indicates that at the start of each phase, the animals swallowed at a lower rate than in the prior phase. The SE (slope) was different in phase 1 from that in phases 2 and 3 (p < .001), indicating more variation in swallowing frequency in phase 1 than in phases 2 and 3, which were not different from each other (p > .86). The SE (intercept) was higher in phase 1 (p < .05).
Fig. 4.
Swallow variables vs. phase. Mean and variation of the coefficients and standard errors from linear models of swallow frequency vs. time. The unit of analysis was sequence, with a sample size of 19. The horizontal bars are the average for each response variable, and the vertical lines are the standard error around that average, a measure of variation among the 19 sequences. The intercept and slope are from the linear models fit to the frequency of sucks in each sequence. The SE (intercept) and SE (slope) are the within-sequence standard errors from each of the 19 linear models
Sucks per Swallow
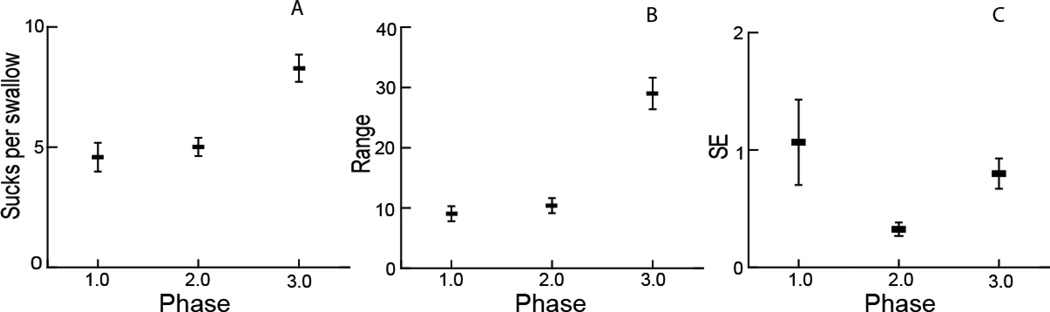
The sucks per swallow had distinct patterns for each phase (Fig. 5A–C). Measured over each phase for the entire data set, the average number of sucks per swallow was highest in phase 3 (x̅ = 8.14, p < .005), with the sucks per swallow in phase 1 (x̅ = 3.98) and phase 2 (x̅ = 4.57) not being statistically different from each other. The range and SE for sucks per swallow showed different patterns of variation. The range of values over the sequences, i.e., the difference between the smallest number of sucks per swallow and the largest number of sucks per swallow, was highest in phase 3 and different from that in phases 1 and 2 (p < .001) although phases 1 and 2 were not different from each other. The SE of sucks per swallow was significantly lower in phase 2, indicating less variation in this measure for phase 2 relative to the others (p < .005).
Fig. 5.
Sucks per swallow. A Average sucks per swallow within each phase. B Range of sucks per swallow within each phase. C Standard error within each phase
Discussion
Limitations of this Study
We studied the naturally occurring time-related changes in the rates of suckling and swallowing. Six bottle-fed pigs generated some 20,000 cycles of sucking and swallowing. A consistent finding from these cycles was that the rates of sucking differed between “early,” “mid,” and “later” suckling periods within a feeding sequence. While there could obviously be some random (or even systematic) variation in individual suck volumes, the general suck volume trends over substantial numbers of “early” periods are likely to be the same; the same argument would also apply to mid and late periods.
In normal feeding animals, various changes in cycle characteristics occurred as a function of time, in each feeding sequence. The changes in cycle defined three phases, and although we recognize that this was a relatively simple distinction, it was one that made a relatively straightforward quantitative analysis possible. We considered using other strategies such as fitting a complex curve or sampling a smaller time window, such as 2–3 s at the beginning and end. None of these options, however, would have captured the complexity of changes that we were able to quantify and test using the three-phase method.
Three Phases of Feeding Frequency
Across all animals and sequences, there was an initial phase of high-frequency sucking cycles that decreased in frequency over the first 520 s of feeding. The variation around this change in frequency was high within each sequence. This pattern also held for the changes in swallow frequency. The animals next reached a steady state of sucking, with no significant change in frequency, i.e., slopes of zero for phase 2 and phase 3. The variation around these regression lines, as measured by the standard error (SE), was minimal compared to that of phase 1. Phase 3 was defined as the start of very-low-frequency cycles, or cycles of more than 1–2 s in duration. The difference between phase 2 and phase 3 was in the value of the frequency of suck cycles vs. time, as measured by the intercept. It was significantly lower in phase 3, although with more variation, both within and between sequences. This reflects the finding that phase 3 contained extreme value cycles. These cycles were scattered throughout phase 3, so that while there was not a significant slope change, the overall effect was to lower the average frequency, as measured by the intercept for a horizontal line. In the case of swallow cycles, the patterns for phase 2 and phase 3 were similar to those for suck cycles.
The results from the analysis of sucks per swallow also differed from our null hypothesis. The number of sucks per swallow was the same in phases 1 and 2, even though the rates of sucking changed. In phase 3 there were more sucks per swallow. The two measures we used to capture the patterns of variation in sucks per swallow range and standard error (SE) produced two different patterns. The range, which measures extremes, was much higher in phase 3 than in phases 1 and 2. This is consistent with, and a likely result of, of the definition of phase 3 as the time when extreme values of cycle frequency started. The range of sucks per swallow was the same in phases 1 and 2. The SE of sucks per swallow, which measured within-sequence variability, indicated that despite these extreme values, phase 3 had the same amount of variation around the mean number of sucks per swallow as did phase 1. Phase 2, however, had much less variation in the number sucks per swallow across a sequence. This again supports the qualitative assessment that phase 2 is a more stable phase than either the beginning or the end.
These results taken together suggest a distinct character for each of these phases. Phase 1 has a changing value of sucking and swallowing frequency. This is similar to what we previously documented [15], although the earlier study had a more complex design. While the number of sucks per swallow was similar, it was much more variable in this phase than in phase 2. Phase 2 is characterized by a very steady and constant behavior. The SE of sucking and swallowing frequency and the SE of sucks per swallow are all very low, while these measures vary in phases 1 and 3. Finally, phase 3, which always lasted the longest, was characterized by a more variable behavior.
The Physiologic Basis of Changing Frequencies
There are several possible explanations for the physiologic basis of this pattern of change. One of the most likely is the changing levels of satiation. An animal, or infant, that is hungry will start out feeding very quickly and then settle down to a more constant steady feeding as immediate hunger is reduced. As satiation increases, the animals are increasingly distracted and less interested in feeding. This pattern is similar to that of one animal in our previous study that suckled with multiple starts and stops [15]. As satiation increased, the level of interest in feeding decreased until it completely vanished which indicated the end of the feeding sequence. Yet in that study we occasionally observed another pattern that does not support this conclusion. Animals would occasionally “break off” from sucking for a short period of time, up to about 0.5–1.5 s. When they started sucking again, it was always at a higher rate. This pattern appeared to be a series of pairs of phase 1 and phase 2.
An alternate explanation for slowing down or longer cycles in phase 3 could be fatigue. Although sucking and swallowing are not “power” behaviors, such as mastication of hard food, they still require significant muscle activity. Many of the supra- and infrahyoid muscles involved are much smaller, parallel-fibered muscles, some with negligible cross-sectional area [25, 34]. It is possible that over time some of these muscles tire.
Finally, another explanation would be that the nipple is a source of enjoyment for the infants. Based on our observations, it was noted that the infant pigs were reluctant to give up the nipple even at the late stages of the feeding sequence. Thus, even if tired, full, or not interested, they would continue to feed at a slower rate, with small breaks, most likely because the nipple was a source of enjoyment to them. We have occasionally observed animals that play with the nipple, pulling on it, pushing on it with their nose, or other nonfeeding behaviors, rather than walking away from the nipple once their feeding needs were met.
Although these scenarios possibly explain some of the general feeding behaviors in normal-feeding animals, further research is needed to identify the specific source of the changes in frequency that occur during a regular feeding sequence.
Implications of These Results for Clinical Studies
The changes in feeding rates that we documented occurred in normal animals, freely feeding at their own pace. Because this is an animal model, there were no limitations on the amount of data we could collect; essentially, we could get data at every feeding. That is not always true in human clinical studies, particularly those that involve compromised children with feeding concerns. The existence of this variation in feeding is an important consideration for human data collection. If it is not possible to collect a complete sequence, then phase 2 data are likely representative of the neuromuscular and kinematic pattern of feeding that represents the majority of nutrient intake. If measuring the potential upper limit on feeding speed, then phase 1 would provide additional information on potential abilities.
The patterns of variation may also be important for understanding how feeding is compromised. If phase 3 does represent a change due to satiation, a lack of change in frequency or of phase 3, even after a long feeding bout, may suggest a lack of satiation. Thus, these results can be used as a template or pattern against which to compare a simple measure (sucking or swallowing frequency) in clinically compromised infants. Our research has identified individual phase variations to better understand the coordination among several component behaviors, therefore improving our understanding of general feeding behavior.
Acknowledgments
We appreciate Laurie Pipitone for her support during data collection. We also thank Melanie Albano and Kristy Koenig for their work during animal surgery. Stacey L. Lukasik and Regina Campbell-Malone provided thoughtful support and assistance during the whole study. Allan Thexton’s insights improved this article significantly. This study was funded by the National Institutes of Health (DC009980 to RZG).
Footnotes
Conflict of interest None
Contributor Information
Estela M. Gierbolini-Norat, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Shaina D. Holman, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA.
Peng Ding, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Shubham Bakshi, Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, OH, USA.
Rebecca Z. German, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, OH, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University, 4209 SR 44, Rootstown, OH 44272, USA rgerman@neomed.com.
References
- 1.Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118–127. doi: 10.1002/ddrr.17. [DOI] [PubMed] [Google Scholar]
- 2.Arvedson J, Brodsky L. Pediatric Swallowing and Feeding. 2nd ed. New York: Singular Learning-Thomson; 2002. [Google Scholar]
- 3.Book SA, Bustad LK. The fetal and neonatal pig in biomedical research. J Anim Sci. 1974;38(5):997–1002. doi: 10.2527/jas1974.385997x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell-Malone R, et al. Ontogenetic changes in mammalian feeding: insights from electromyographic data. Integr Comp Biol. 2001;51(2):282–288. doi: 10.1093/icb/icr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi-Fishman G, Sonies BC. Motor strategy in rapid sequential swallowing: new insights. J Speech Lang Hear Res. 2000;43(6):1481–1492. doi: 10.1044/jslhr.4306.1481. [DOI] [PubMed] [Google Scholar]
- 6.Crompton AW, Thexton AJ, German RZ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology. 2008;111:339–349. doi: 10.1016/j.zool.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney AL, Arvedson JC. Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev. 2008;14(2):105–117. doi: 10.1002/ddrr.16. [DOI] [PubMed] [Google Scholar]
- 8.Ding P, et al. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia. 2013;28(3):404–412. doi: 10.1007/s00455-013-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding P, et al. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. Laryngoscope. 123(8):1942–1947. doi: 10.1002/lary.24051. 20013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks HA, Crompton AW, German RZ. Mechanism of intraoral transport in macaques. Am J Phys Anthropol. 1984;65(3):275–282. doi: 10.1002/ajpa.1330650307. [DOI] [PubMed] [Google Scholar]
- 11.Franks HA, et al. Mechanism of intraoral transport in a herbivore, the hyrax (Procavia syriacus) Arch Oral Biol. 1985;30(7):539–544. doi: 10.1016/0003-9969(85)90054-8. [DOI] [PubMed] [Google Scholar]
- 12.German RZ, Crompton AW. Ontogeny of suckling mechanisms in opossums (Didelphis virginiana) Brain Behav Evol. 1996;48(3):157–164. doi: 10.1159/000113194. [DOI] [PubMed] [Google Scholar]
- 13.German RZ, Crompton AW. The Ontogeny of Feeding in Mammals. In: Schwenk K, editor. Feeding: Form, Function and Evolution in Tetrapod Vertebrates. San Diego: Academic Press; 2000. pp. 449–457. [Google Scholar]
- 14.German RZ, et al. Food transport through the anterior oral cavity in macaques. Am J Phys Anthropol. 1989;80(3):369–377. doi: 10.1002/ajpa.1330800310. [DOI] [PubMed] [Google Scholar]
- 15.German RZ, et al. Determinants of rhythm and rate in suckling. J Exp Zool. 1997;278:1–8. doi: 10.1002/(sici)1097-010x(19970501)278:1<1::aid-jez1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.German RZ, Crompton AW, Thexton AJ. The role of animal models in understanding feeding behavior in infants. Int J Orofacial Myology. 2004;30:20–30. [PubMed] [Google Scholar]
- 17.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–1025. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiiemae KM. Feeding in Mammals. In: Schwenk K, editor. Feeding: Form, Function and Evolution in Tetrapod Vertebrates. San Diego: Academic Press; 2000. pp. 411–448. [Google Scholar]
- 19.Hiiemae KM, Crompton AW. Mastication, food transport and swallowing. In: Hildebrand M, et al., editors. Functional Vertebrate Morphology. Cambridge, MA: Harvard University Press; 1985. pp. 262–290. [Google Scholar]
- 20.Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistencies. Dysphagia. 1999;14(1):31–42. doi: 10.1007/PL00009582. [DOI] [PubMed] [Google Scholar]
- 21.Holman SD, et al. Regional variation in geniohyoid muscle strain during suckling in the infant pig. J Exp Zool A Ecol Genet Physiol. 2012;317(6):359–370. doi: 10.1002/jez.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman SD, Campbell-Malone R, et al. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia. 2013;28(2):178–187. doi: 10.1007/s00455-012-9427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holman SD, Waranch DR, et al. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. J Neurophysiol. 2013;110(2):387–396. doi: 10.1152/jn.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holman SD, et al. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs. Laryngoscope. 2014;124(2):436–445. doi: 10.1002/lary.24204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konow N, et al. Regional differences in length change and electromyographic heterogeneity in sternohyoid muscle during infant mammalian swallowing. J Appl Physiol. 2010;109:439–448. doi: 10.1152/japplphysiol.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefton-Greif MA, McGrath-Morrow SA. Deglutition and respiration: development, coordination, and practical implications. Semin Speech Lang. 2007;28(3):166–179. doi: 10.1055/s-2007-984723. [DOI] [PubMed] [Google Scholar]
- 27.Rommel N, et al. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil. 2011;23(10):e401–e408. doi: 10.1111/j.1365-2982.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 28.Sack WO. Pig anatomy and atlas. Ithaca, NY: Veterinary Textbooks; 1982. [Google Scholar]
- 29.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102(2):587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 30.Thexton AJ, et al. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol. 2009;101(3):1386–1393. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol (1985) 2012;112(9):1512–1519. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver ME, Jump EB, McKean CF. The eruption pattern of permanent teeth in miniature swine. Arch Oral Biol. 1969;14(3):323–331. doi: 10.1016/0003-9969(69)90235-0. [DOI] [PubMed] [Google Scholar]
- 33.Weckmueller J, Easterling C, Arvedson J. Preliminary temporal measurement analysis of normal oropharyngeal swallowing in infants and young children. Dysphagia. 2011;26(2):135–143. doi: 10.1007/s00455-010-9283-3. [DOI] [PubMed] [Google Scholar]
- 34.Wentzel SE, Konow N, German RZ. Regional differences in hyoid muscle activity and length dynamics during mammalian head shaking. J Exp Zool A Ecol Genet Physiol. 2011;315(3):111–120. doi: 10.1002/jez.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wykes LJ, Ball RO, Pencharz PB. Development and validation of a total parenteral nutrition model in the neonatal piglet. J Nutr. 1993;123(7):1248–1259. doi: 10.1093/jn/123.7.1248. [DOI] [PubMed] [Google Scholar]