Abstract
Non-thermal atmospheric-pressure plasma, also named cold plasma, is defined as a partly ionized gas. Therefore, it cannot be equated with plasma from blood; it is not biological in nature. Non-thermal atmospheric-pressure plasma is a new innovative approach in medicine not only for the treatment of wounds, but with a wide-range of other applications, as e.g. topical treatment of other skin diseases with microbial involvement or treatment of cancer diseases. This review emphasizes plasma effects on wound healing. Non-thermal atmospheric-pressure plasma can support wound healing by its antiseptic effects, by stimulation of proliferation and migration of wound relating skin cells, by activation or inhibition of integrin receptors on the cell surface or by its pro-angiogenic effect. We summarize the effects of plasma on eukaryotic cells, especially on keratinocytes in terms of viability, proliferation, DNA, adhesion molecules and angiogenesis together with the role of reactive oxygen species and other components of plasma. The outcome of first clinical trials regarding wound healing is pointed out.
Keywords: Angiogenesis, Cell surface molecules, Cell viability, Non-thermal atmospheric-pressure plasma, Plasma-cell interaction, Reactive oxygen species, Wound healing
INTRODUCTION`
The number of patients with chronic infected wounds has been reported to increase constantly (Strausberg et al., 2007; Werdin et al., 2009; Günther and Machens 2014). In Germany, about 4.5 to 5 million people are concerned from chronic, non-healing wounds (Werdin et al., 2009). Many of them with vascular disease or diabetes suffer from chronic venous leg or foot ulcers due to a lack of proper wound healing. In consequence, they lose functional ability leading to a poor quality of life and to long-term hospitalization. Chronic wounds are not only a medical problem but also psychologically relevant. Conventional treatments are time consuming, and therefore very expensive, treatment costs in Germany are more than 5 billion € each year (Werdin et al., 2009).
Chronic inflammation with persistence of various bacteria including biofilm formation is a hallmark of the non-healing wounds. Bacterial concentrations exceeding 105 or 106 bacteria colony-forming units per gram of tissue have been shown to impair wound healing. In the majority of cases Staphylococcus aureus was identified in chronic wounds which caused together with the methicillin-resistant Staphylococcus aureus (MRSA) 20% to 50% of cases (Werdin et al., 2009). In Korea, the prevalence of community-associated MRSA infection is still low (Park et al., 2009); however, the prevalence of those MRSA strains in healthcare settings is increasing and currently accounts for up to 70% in most tertiary care hospitals (Kim et al., 2003a; Cha et al., 2005, 2010). MRSA infections contribute significantly to patient morbidity and mortality.
The initial step in the management of any chronic wound is cleaning them to eliminate excessive bacterial burden and necrotic tissue. Antimicrobial strategies are then used to remove or kill bacteria together with stimulation of patient’s general health or the wound’s physical environment (Daeschlein, 2013; Kramer et al., 2013). In chronic wound care antiseptics are effective and well tolerated. They play an important role in the treatment of wound infection; however, especially in treating infections by multidrug-resistant strains such as MRSA they have limitations. New concepts and strategies controlling wound inflammation and thus improving chronic wound care are strongly needed. One of these promising strategies is the application of physical non-thermal atmospheric-pressure plasma (Lloyd et al., 2010).
HOW IS NON-THERMAL ATMOSPHERIC-PRESSURE PLASMA DEFINED?
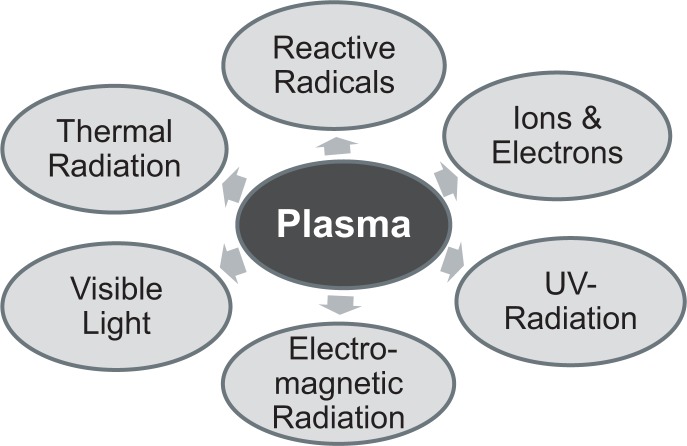
Physical plasma has been considered as the fourth state of matter and is defined as a completely or partly ionized gas. Irvine Langmuir (1928) was the first who named ionized gas “plasma”. In plasmas electrons, positive and negative ions, neutral atoms, and neutral or charged molecules can be identified. It is further characterized by its temperature, different types of radiation (e.g. UVB), and by electric fields (Fig. 1). Plasmas can be seen in daily life, e.g. as lightning in thunderstorms, northern lights, neon lights or plasma displays.
Fig. 1.
Composition of non-thermal atmospheric-pressure plasmas.
Plasmas can be “thermal/hot” and “non-thermal/cold”. Thermal plasma is nearly fully ionized while non-thermal plasma is only partly ionized. Generating plasma artificially, it can be ignited at low or atmospheric pressure by adding energy to a gas, e.g. air, argon or helium. In a variety of different fields plasmas are applied. Plasma applications are found in technology and industry, e.g. in vehicle construction or metallurgy (von Woedtke et al., 2013).
The generation of plasma at atmospheric pressure with temperatures of about 30 to 40°C was the basis for treating living cells, tissues and other heat sensitive material. A new field, “Plasma Medicine”, combining plasma physics with life science and medicine developed rapidly (von Woedtke et al., 2014). New plasma sources and devices were introduced for different applications.
PLASMA SOURCES FOR CELL AND TISSUE RESEARCH
At least three different principles of generating non-thermal plasmas at atmospheric pressure have been developed for biomedical applications (Weltmann et al., 2008; Hähnel et al., 2010; Ehlbeck et al., 2011; Wu et al., 2011; Bussiahn et al. 2013):
Plasma Jets
Corona discharge plasma sources
Dielectric barrier discharge (DBD) plasma sources
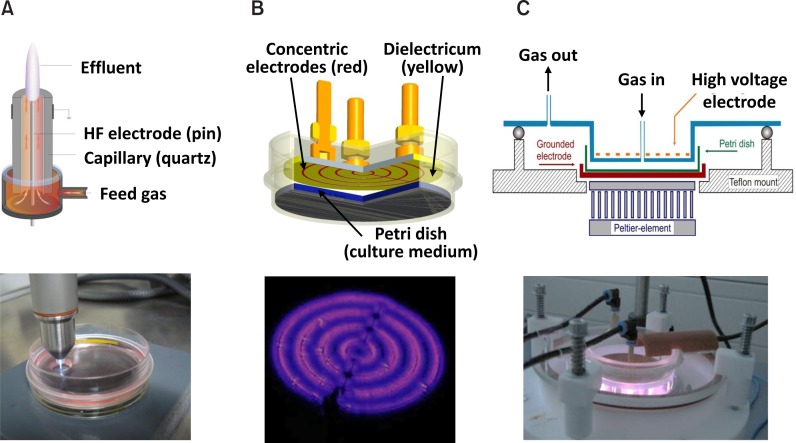
Our group has been working with experimental plasma sources belonging to two of these principles, the plasma jet kINPen 09 (principle 1; Fig. 2A), surface and volume barrier discharge (DBD) plasma sources (principle 3; Fig. 2B, C). All these plasma sources were developed at the Leibniz Institute for Plasma Science and Technology Greifswald e.V. (INP). Argon (kINPen 09, surface DBD, volume DBD), argon-oxygen mixtures (kINPen 09) or ambient air (surface DBD) were used as operating gas. Technical data of these plasma sources are listed in Table 1. Energy output as sign for the power of a plasma source is lowest for the surface DBD with argon as process gas and highest for the volume DBD. Energy output is directly associated with inducing lethal or non-lethal effects on cells or microorganisms.
Fig. 2.
Scheme of plasma sources used and the burning plasmas. (A) Plasma jet kINPen 09; (B) Surface DBD and (C) Volume DBD. All plasma sources were developed and built in the Leibniz Institute for Plasma Science and Technology (INP) in Greifswald, Germany.
Table 1.
Technical data of the different plasma sources
| Parameter | kINPen | Surface DBD | Surface DBD | Volume DBD |
|---|---|---|---|---|
| Process Gas | Argon | Ambient Air | Argon | Argon |
| Voltage | 2–6 kV | 10 kV | 3.5 kV | 9–10 kV |
| Applied Frequency | 1.1 MHz | 20 kHz | 21 kHz | 33 kHz |
| Plasma on/off-time | --- | 0.413/1.223 s | 0.413/1.223 | --- |
| Gas flow (Argon) | 3.8 sl/min | --- | 0,5 sl/min | 0.5 sl/min |
| Energy per min | < 60 J | 18 J | 8.25 J | 360 J |
NON-THERMAL ATMOSPHERIC-PRESSURE PLASMA AND MICROORGANISMS
Non-thermal atmospheric-pressure plasma was found to inactivate very effectively different microorganisms (Hong et al., 2009; Hähnel et al., 2010; Kim et al., 2011a; Zimermann et al., 2011; Matthes et al., 2012; Daeschlein et al. 2012a; Li et al., 2013) and is able to remove biofilms (Joshi et al., 2010; Alkawareek et al., 2012; Fricke et al., 2012; Julak and Scholtz, 2013; Matthes et al., 2013). Even multidrug resistant skin and wound pathogens are susceptible to Non-thermal atmospheric-pressure plasma (Maisch et al., 2012; Daeschlein et al., 2014). Complete inactivation of various bacteria including the methicillin-resistant Staphylococcus aureus (MRSA) was reported by Alkawareek et al. (2014). All this led to the hypothesis that plasma might be an alternative solution for antiseptic treatment of chronic infected wounds (Kramer et al., 2013) or disinfection of surgical instruments or catheters (Polak et al., 2012; Robert et al., 2013; Sung et al., 2013).
Indeed, in terms of wound healing studies in experimental animals (Ermolaeva et al., 2011; Nastuta et al., 2011; Yu et al., 2011; García-Alcantara et al., 2013; Nasruddin et al., 2014) and humans (Isbary et al., 2012, 2013a; Heinlin et al., 2013b; Brehmer et al., 2014) demonstrated first positive effects. Recently, also other skin diseases came into focus for treatment with plasma, e.g. Morbus Hailey-Hailey (Isbary et al., 2011), pruritus (Heinlin et al., 2013a), atopic eczema (Emmert et al., 2013), psoriasis (Klebes et al., 2014).
The mechanisms by which plasma exerts its promising wound healing effects are still under investigation. Additionally to antibacterial effects plasma has also consequences for all other cells important for closing a wound. Here, we will review some effects of plasma, which are important regarding wound healing.
GENERAL EFFECTS OF NON-THERMAL ATMOSPHERIC- PRESSURE PLASMA ON WOUND RELATING SKIN CELLS
Effects of plasma were extensively investigated in vitro by using different types of cells in monolayer. Wound relating cells are keratinocytes, fibroblasts, epithelial and endothelial cells, but also inflammatory cells, especially in terms of chronic infected wounds. Studies were either done with cell lines or primary cells. The Greifswald group mainly deals with effects of plasma on keratinocytes (Haertel et al., 2011, 2012a, 2013a, 2013b; Blackert et al., 2013; Schmidt et al., 2013a, 2013b; Straβenburg et al., 2013; Straβenburg, 2014; Wende et al., 2014), namely the HaCaT cell line (Boukamp et al., 1988). Other groups focus either on epithelial cells (Kieft et al., 2004; Kalghatgi et al., 2011a, 2011b; Hoentsch et al., 2012), endothelial cells (Kalghatgi et al., 2010), ocular keratocytes (Brun et al., 2012), fibroblasts (Shashurin et al., 2010) or immune cells (Shi et al., 2008; Haertel et al., 2012b; Bekeschus et al., 2013a, 2013b, 2014; Bundscherer et al., 2013a, 2013b). For plasma treatment the different groups used various plasma sources. Basically, two principally different plasma sources were utilized: plasma jets and dielectric barrier discharge plasma sources. Up to now no general standardization of the different plasma sources with regard to technical data, quantification of generated free radicals or emission of radiation exists, which is, however, strongly demanded. Only in Germany a first “General requirements for plasma sources in medicine” is just published (DIN SPEC 91315, 2014), which were presented at the 5th International Conference on Plasma Medicine (ICPM5) by Mann et al. (2014). In that, simple and generally applicable biological (inactivation of microorganisms, cytotoxicity and detection of chemical species in liquids) and physical test methods (temperature, thermal capacity, optical emission spectrometry, UV-irradiation, gas emission, and leakage current) are proposed. These are basic criteria, which should be helpful to identify plasma sources for potential therapeutic applications. By using such standards plasma sources will achieve higher acceptance for dermatological and other medical applications. Taking all this into account, it is very difficult to compare the results of different laboratories published till now in terms of plasma treatment times/plasma doses which induce stimulating or lethal effects on cells or tissues. However, despite the use of different cell types or different plasma sources the following general plasma-treatment-time-dependent/plasma-dose-dependent effects were observed in all studies:
- Plasma membrane alteration,
- Induction of intracellular reactive oxygen radicals,
- Mitochondrial damage
- Induction of apoptosis and necrosis with decrease of cell viability and cell death,
- Increase or decrease of cell proliferation
- Increase or decrease of cell migration and
- DNA breakdown with cell cycle arrest.
All these effects are not only dependent on plasma treatment time, but also on the process gas (ambient air, argon, helium), the treatment regimen (direct, indirect), the time of investigation after plasma exposure, the cell type and whether the cells were treated in suspension (immune cells) or as adherent cell monolayer (e.g. keratinocytes, fibroblasts).
It is very important to distinguish between plasma-induced lethal and plasma-induced stimulating effects on cells. The following statement is generally accepted:
- Short plasma treatment times/low plasma doses have stimulating effects (increase of proliferation and migration, induction of DNA repair) and
- Long plasma treatment times/high plasma doses induce lethal effects (cell death by apoptosis, stop of proliferation, DNA damage, cell cycle arrest).
The first reaction pattern is strongly demanded for improving wound healing, the latter properties can be used for treating cancer cells.
In the following sections of “GENERAL EFFECTS OF NON-THERMAL ATMOSPHERIC-PRESSURE PLASMA ON WOUND RELATING SKIN CELLS” we will describe effects of plasma on viability, apoptosis and proliferation, on DNA and on the role of reactive radicals.
Viability, apoptosis and proliferation
As already mentioned above, despite different physical parameters general effects on cell viability of plasma-treated cells are very similar. Determination of viability gives first information about the power of a given plasma treatment. Thinking about wound healing, microorganisms should be killed without harming keratinocytes or fibroblasts. For this reason HaCaT cells were treated with a broad range of plasma intensity/plasma treatment times, ranging from short to longer plasma exposure, to find plasma treatment times which do not induce lethal effects on keratinocytes (Haertel et al., 2011, 2012a, 2013a, 2013b; Blackert et al., 2013; Straβenburg et al., 2013; Straβenburg, 2014; Wende et al., 2014).
Comparing the plasma sources and treatment regimen main differences can be identified in the treatment time necessary to induce 50% cell death (Table 2). Treating cells directly with plasma, all the plasma components shown in Fig. 1 are relevant for the subsequent effects on the cells. In contrast, if cells are only exposed to plasma-treated medium (=indirect treatment), any effects on cells due to the different kinds of radiation are excluded. Similar results on viability after plasma treatment have also been reported by others for different other cell types, as e.g. immune cells (Shi et al., 2008; Haertel et al., 2012b; Bundscherer et al., 2013b; Bekeschus et al., 2103b), epithelial cells (Hoentsch et al., 2012, 2014; Kalghatgi et al., 2011a, 2011b, 2012), endothelial cells (Kalghatgi et al., 2010), fibroblasts (Lopes et al., 2013).
Table 2.
Relation between plasma sources, treatment regimens and treatment times which cause a reduction of viability of HaCaT keratinocytes of about 50% 24h after plasma exposure (experiments in RPMI 1640)
| kINPen 09 Argon | Surface-DBD Air | Surface-DBD Argon | Volume-DBD Argon | |
|---|---|---|---|---|
| Direct Plasma Treatment | ||||
| Cells in Suspension | 10 s | < 1 min | > 5 min | Ø |
| Adherent Cells | Ø | 5 min | 10 min | 10 s |
| Indirect Plasma Treatment | ||||
| Cells in Suspension | Ø | < 1 min | < 5 min | Ø |
| Adherent Cells | 1 min | Ø | Ø | 10 s |
| Direct Plasma Treatment with medium exchange | ||||
| Cells in Suspension | Ø | 1 min | Ø | Ø |
| Adherent Cells | Ø | > 20 min | Ø | > 1 min |
The working gas alone, argon or helium often used by others (Kieft et al., 2004; Shashurin et al., 2010; Brun et al., 2012), and short exposure times of cells to plasma were without any influence on cell viability. For any treatment regimen applied to the cells we observed that effects on cell viability were treatment-time-dependent. Direct and indirect plasma treatment caused very similar effects (Haertel et al., 2012a), thereby, major effects of any radiation emitted by plasma, e.g. UV radiation can be excluded. Viability is much improved if the medium is changed immediately after plasma treatment (Haertel et al., 2012a; Blackert et al., 2013).
An important factor for cell viability is the surrounding medium in which the cells are treated and cultured further. HaCaT cell number decreased in RPMI 1640 medium much more than in IMDM (Wende et al., 2014).The reason for this difference is the diverse composition of the culture media, which will be discussed later together with reactive oxygen species.
Mechanisms of reduced/enhanced cell viability can be reduction/promotion of cell proliferation or induction/prevention of apoptosis and/or necrosis. Indeed, by using the kINPen 09 or the surface DBD with ambient air reduction of HaCaT cell proliferation was detected, which correlated well with decrease of viability (Straβenburg, 2014; Wende et al., 2014). Otherwise, HaCaT cells on plasma-modified collagen films showed an increased proliferation (Garcia et al., 2010). Endothelial cell or fibroblast cell proliferation is enhanced by nonthermal plasma through release of fibroblast growth factor-2 or -7 (Kalghatgi et al., 2010; Ngo et al., 2014). Non-thermal plasma can induce apoptosis (Kim et al., 2011b; Haertel et al., 2012a, 2013b; Blackert et al., 2013; Duval et al., 2013; Wende et al., 2014). However, plasma induction of apoptosis measured by using Annexin V and propidium iodide (PI) was not observed after short plasma treatments. After longer plasma treatments apoptosis in HaCaT cells was still seen 24 h after plasma treatment (Blackert et al., 2013; Haertel et al., 2013b; Wende et al., 2014). To see apoptotic processes investigations have to be done early after treatment (e.g. 30 min to 4 h after plasma treatment). This is underlined by induction of early apoptosis (Annexin V/PI) in rat primary immune cells that was highest 4h after direct treatment with surface DBD/air and was found to be reduced after 24 h and 48 h (Haertel et al., 2012b). Duval et al. (2013) detected more Annexin V/PI positive cells in Jurkat cells (a T-cell line) 24 h after plasma treatment compared to 8 h post treatment. These opposed results might be explained by the use different plasma sources and different cells. Another possibility to analyze apoptosis is the disruption of active mitochondria as distinctive feature of early apoptosis including changes in the mitochondrial membrane potential (Mito-MP). These changes can be measured by a lipophilic, cationic dye (JC-1) (Salvioli et al., 1997). Plasma treatment of HaCaT cells with the kINPen 09 induced changes in the Mito-MP 60 min after treatment. Four and 24 h after treatment only weak changes were observed.
In conclusion, if the plasma dose applied to the cells is high enough, cell death as result of more than one process is induced. At least induction of apoptosis/necrosis and reduction of proliferation due to cell cycle arrest (see under “Influence on DNA”) play significant roles.
Reactive oxygen and nitrogen species and induction of intracellular reactive oxygen species
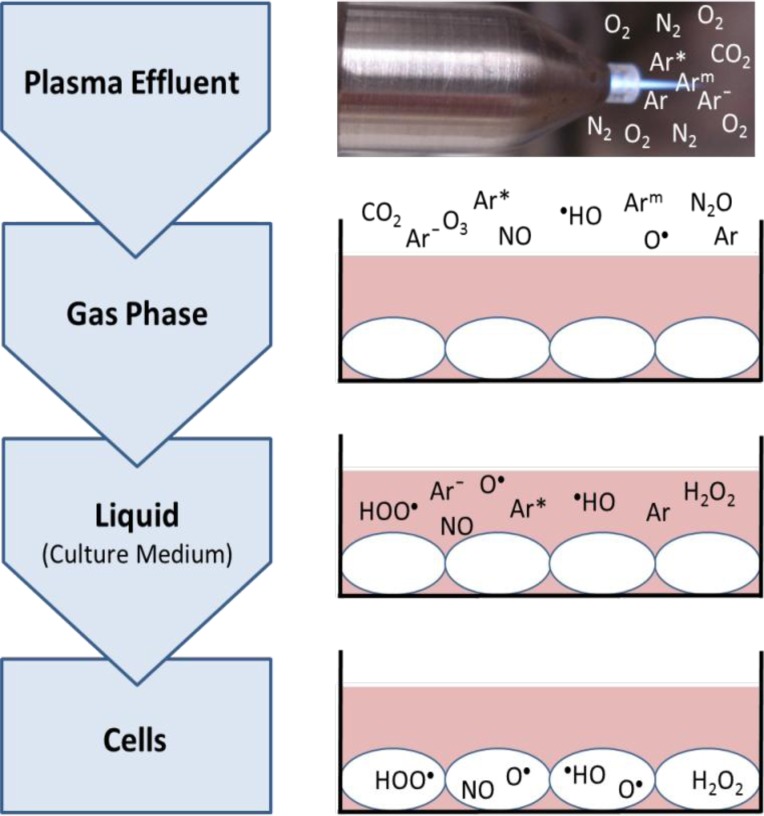
As already mentioned plasma emits several kinds of radiation and is further characterized by reactive oxygen and nitrogen species (ROS and RNS, Schaper et al., 2009; Schmidt-Bleker et al., 2014; Oehmigen, 2014). Among them, e.g. ozone/O3 (Reuter et al., 2012b), nitric oxide/NO (Pipa et al., 2012), atomic oxygen/O (Reuter et al., 2012a), and hydroxyl radical /·HO (Winter et al., 2014) were detected. These reactive species exert lots of effects on cells which can be positive or even negative (Table 3). After treatment of cells in culture medium reactive species are detectable in the gas phase over the cells as well as in the culture medium (Fig. 3). By using an argon plasma jet the effluent is surrounded by ambient air, thereby in the gas phase are not only argon atoms but also species built from ambient air. Furthermore, all species can also enter the cells possibly by diffusion or can induce new species within the cells (Fig. 3). These can be detected as intracellular ROS (iROS) by different fluorescent dyes (DAF-2: Arjunan et al., 2011a; H2DCFDA: Brun et al., 2012; Haertel et al., 2012a; CM-H2DCFDA: Haertel et al., 2013b; carboxy-H2DCFDA: Leduc et al., 2010; Ma et al., 2014).
Table 3.
Examples of possible effects of reactive oxygen and nitrogen radicals (for review see Dröge, 2002)
| Positive effects | Negative effects as “oxidative stress” |
|---|---|
| Signal transduction (NO) | Cell wall damage (ROS) |
| Stimulation of angiogenesis (NO) | Oxidation of DNA and proteins (O) |
| Influence on immune cells | Oxidation of lipids in cell bilayers (HO) |
| Proliferation of keratinocytes | Influence on cell respiration (O3) |
| Smooth muscle relaxation (NO) | |
| Control of ventilation | |
| Antimicrobial effects (H2O2) |
Fig. 3.
Principle way of reactive oxygen and nitrogen species from an argon plasma jet over the gas phase and liquid into the treated cells. Since plasma jets are open systems the effluents are surrounded by ambient air with its gases N2, O2 and CO2. Some species are exemplified shown. Species can enter the cell possibly by diffusion or can induce new species within the cells.
Ozone being a neutral oxygen species is known to inactivate microorganisms (e.g. bacteria, viruses, fungi, yeast and protozoa), to stimulate oxygen metabolism and to activate the immune system. Thereby, it is widely used not only in food industry but also in medicine (Kim et al., 2003b). Ozonized water is used e.g. in dental medicine. Furthermore, medical ozone is also applied in the treatment of various diseases as e.g. circulatory disorders, macular degeneration, viral diseases or rheumatism (for review see Bocci et al., 2009, Elvis and Ekta, 2011). For local application ozone seems to be useful in the treatment of infected wounds (Białoszewski and Kowalewski, 2003). However, ozone has also disadvantages, mainly due to its potential of oxidation, peroxidation or generation of free radicals. As a component of plasma ozone may contribute to the effects observed in vitro after plasma treatment. This question was addressed by Kalghatgi et al. (2012) and our group (Haertel et al., 2013b) by using either mammalian breast epithelial cells (MCF10A) or human keratinocytes (HaCaT) in culture. For that, first we measured the concentration of ozone accumulated in the gas phase over the cells in medium during a 300 s treatment cycle (energy input about 9 J/cm2) with DBD/air plasma in a closed system. A concentration of about 100 ppm which is 1000 times higher than the maximum allowable concentration (MAC) was detected. The DBD plasma source used by Kalghatgi et al. (2012) caused ozone concentrations of 182 ppm (4.65 J/cm2) and of 30 ppm (1.95 J/cm2) within 15s. Exposure of HaCaT cells to 300s DBD/air plasma resulted in a decrease of viable cells to about 20% while 100 ppm ozone did not significantly reduce cell viability (Haertel et al., 2013b). A concentration of 1000 ppm led to a reduction of about 50% of cell viability. Viability of cells was not analyzed by Kalghatgi et al. (2012); however, they found no DNA damage by ozone compared to DBD treatment. Ozone itself does not play a role in mediating the observed effects of plasma on HaCaT keratinocytes or breast epithelial cells in culture.
To clarify whether other reactive oxygen species (ROS) have direct effects on viability, HaCaT cells were treated with 100 μM hydrogen peroxide (H2O2), a concentration which can be measured in the liquid after 300 s treatment with DBD/air. In liquids H2O2 can act with oxygen (O2) to hydrogen peroxide radicals (HOO·), which then can form protons (H+) and super-oxide radicals (O2·). After exposure of HaCaT to H2O2 viability of cells was significantly decreased to 38%, which was very similar to that of plasma treated cells (Haertel et al., 2013b). These results clearly demonstrate that hydrogen peroxide itself or ROS built from it and not ozone is responsible for plasma-induced effects on cell viability. We further have to take into consideration that ROS can interact with components of the culture media and oxidize them.
To investigate whether or not these species penetrate from plasma over the liquid into the treated cells or whether intracellular ROS are induced, fluorescent dyes as already mentioned above were used to detect ROS intracellularly. Both mechanisms cannot be distinguished by measuring intracellular ROS by using CM-H2DCFDA, but this method gives a general indication of the oxidation state of the cells following plasma treatment. By using this dye H2O2, peroxynitrite anion (ONOO−), and hydroxyl radical (HO·), as well as alkylperoxyl and hydroxyl peroxyl radicals (ROO·, HOO·) can be detected. A plasma-treatment time dependent increase of iROS was found after exposure of HaCaT cells or human primary keratocytes to plasma (Brun et al., 2012; Haertel et al., 2013b; Straβenburg et al., 2013). Hydrogen peroxide (100 μM) and DBD/air treatment for 300 s caused similar results (Haertel et al., 2013b). Addition of a radical scavenger, e.g. N-acetyl cysteine (NAC), or a pre-treatment decreased the proportion of cells with enhanced iROS (Brun et al., 2012; Blackert et al., 2013) and completely blocked phosphorylation of H2AX after non-thermal plasma treatment of breast epithelial cells (Kalghatgi et al., 2012) underlying the crucial role of ROS for plasma-induced effects.
The effects of plasma on cells are significant dependent on the surrounding liquids. Various culture media differ in their composition markedly and thereby determine the extent of plasma effects considerably (Wende et al., 2014). They differ in their composition of sugars, amino acids, vitamins and buffer systems and they are characterized by different radical scavenging capacities. HEPES buffered media (e.g. IMDM) exerted highest scavenging activity (Wende et al., 2014). Therefore, it is not surprising that cells treated with plasma in IMDM survive better than after treatment in RPMI 1640 medium. HEPES in IMDM medium could scavenge ROS produced in the medium during plasma treatment. Hence, intracellular ROS concentration is reduced and oxidative effects within the cells, e.g. oxidation of DNA, are less. Addition of fetal calf serum (FCS) or antibiotics to culture media were found to be of minor importance (Wende et al., 2014).
Plasma and DNA
Since plasma components can enter the cells it is not surprising that also cell organelles including mitochondria or nuclei with its DNA are influenced. DNA damages can be base damages, deoxyribose modifications, single strand breaks (SSBs) or double strand breaks (DSBs) and DNA protein cross-links. Some of these damages can be repaired by the cells; however, DSBs are lethal to them. In this process reactive oxygen species play a central role and as we have demonstrated, ROS are detectable within the cells after plasma treatment. Hence, if the oxidative stress is high enough all four DNA bases can be oxidized by ROS (e.g. 8-hydroxy-2′-deoxyguanosine or N6-etheno-2′-deoxyadenosine) (Goetz and Luch, 2008). Different methods are used to recognize and detect changes in the DNA. First of all, the Comet assay as single cell gel electrophoresis detects single strand breaks (Singh et al., 1988). By using the neutral version of this test single but also double strand breaks are detectable. If double strand breaks occurred, this is followed by phosphorylation of the histone H2AX. This newly phosphorylated protein, γ-H2AX, is thereby a novel biomarker for DNA double-strand breaks (Kuo and Yang, 2008).
There are several groups in the plasma community who detected DNA damages after plasma treatment by using different methods (γ-H2AX: Kalghatgi et al., 2011a, 2011b; 8-hydroxy-2′-deoxyguanosine (8-OHdG): Brun et al., 2012; Comet Assay: Blackert et al., 2013; Steinbeck et al., 2013; Morales-Ramirez et al., 2013; Straβenburg et al., 2013; Straβenburg, 2014; Wende et al., 2014). In our group the Comet Assay and detection of changed DNA bases, namely guanine to 8-OHdG and N6-etheno-2-deoxyadenosine, were used to define DNA damages (Blackert et al., 2013; Wende et al., 2014; Straβenburg et al., 2013; Straβenburg, 2014;). By using different plasma sources, the kINPen 09 (Wende et al., 2014), surface-DBD (Blackert et al., 2013) and volume-DBD (Straβenburg et al., 2013) treatment time-dependent DNA changes in HaCaT cells were detected 1 h and 24 h after plasma exposure. After short treatment cycles the induced DNA changes observed after 1 h of plasma exposure were no longer detectable after 24 h. Under these conditions viability of treated cells was not significantly influenced, thereby; this is a clear indication for DNA repair. Preliminary results give advice for induction of repair mechanisms (Straβenburg, 2014). As a consequence of double strand breaks in epithelial cells a plasma dose- or rather a time-dependent increase of γ-H2AX was detected 1 h after treatment with DBD plasma, which was completely blocked by the intracellular ROS scavenger NAC (Kalghatgi et al., 2011b). Similar results were reported for osteoblast-like cells (Steinbeck et al., 2013). γ-H2AX was analyzed 1 h after plasma treatment and short-term treatment was found to be negative in inducing phosphorylation of H2AX.
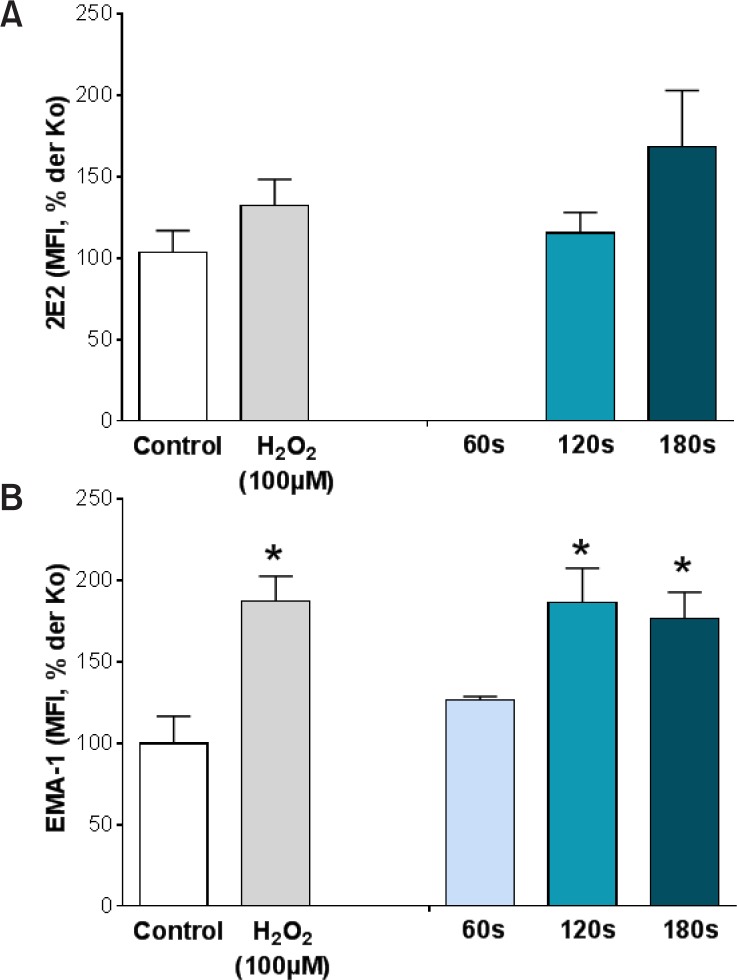
DNA base changes were observed after exposing HaCaT cells to the plasma jet kINPen 09 (Fig. 4). Flow cytometry was used to detect binding of corresponding antibodies, EMA-1 for N6-etheno-2′-desoxyadenosine and 2E2 for 8-hydroxy-2′-deoxyguanosine. While N6-etheno-2′-desoxyadenosine was found to be significantly increased by hydrogen peroxide and kINPen 09 treatment for at least 120 s (Fig. 4A), 8-hydroxy-2′-deoxyguanosine was only slightly enhanced after hydrogen peroxide and 180 s kINPen 09 exposure (Fig. 4B). These different results might be due to the fact that 8-hydroxy-2′-deoxyguanosine is the result from oxidation, while the DNA adduct N6-etheno-2′-deoxyadenosine arises from reaction of DNA with lipid peroxidation products (Taghizadeh et al., 2008). Lipid peroxidation can be the result of plasma treatment due to ROS generation. Transient increased expression of 8-hydroxy-2′-deoxyguanosine was also seen by Brun et al. (2012) in ocular keratocytes after exposing them to plasma.
Fig. 4.
Detection of oxidized DNA bases, namely (A) 8-hydroxy-2′-deoxyguanosine (8-OHdG) and (B) N6-etheno-2-deoxyadenosine after treatment of HaCaT keratinocytes with the kINPen 09 or hydrogen peroxide (100 μM). 24 h after exposure of cells to plasma they were fixed and stained with the antibodies 2E2 for 8-OHdG for and EMA-1 for N6-etheno-2′-desoxyadenosine. Binding of antibodies was detected by using flow cytometry. Mean fluorescence intensities (MFI) are expressed as percentage of that of untreated control cells. Mean ± SEM, *p<0.05 vs. untreated control cells.
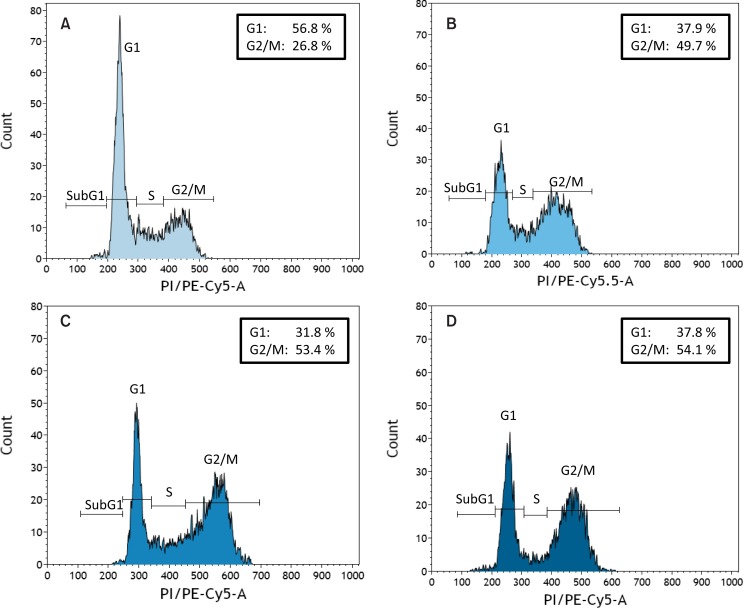
Cell cycle analyses after plasma treatment give additional indication for influences on DNA. In HaCaT keratinocytes a G2/M phase arrest was detected after treating the cells with plasma. All plasma sources used induced comparable effects, however, in dependence on the plasma source with different plasma treatment times (Blackert et al., 2013; Straβenburg et al., 2013; Straβenburg, 2014; Wende et al., 2014). Representative examples for a G2/M arrest of HaCaT keratinocytes are shown in Fig. 5. HaCaT cells remained untreated (Fig. 5A), were either treated with the kINPen 09 for 60s (Fig. 5B), SDBD for 120 s (Fig. 5C) or V-DBD for 20 s (Fig. 5D). G2/M arrest seems to be a typical sign after a given plasma treatment, since also others observed such a phenomenon not only in keratinocytes (Volotskova et al., 2012a) but particularly in different types of cancer cells (Vandamme et al., 2012; Volotskova et al., 2012a; Arndt et al., 2013; Köritzer et al., 2013). A cell cycle arrest in the G2/M phase gives cells time for DNA repair. Oxidative DNA damages will be detected, deleted and replaced by the DNA base excision repair (BER) pathway. In preliminary studies with HaCaT keratinocytes we looked for two enzymes (Ogg1 and APE-1) belonging to this repair pathway. By using the western blot technique, Ogg1, responsible for excision of 8-hydroxy-2′-deoxyguanosine, was found to be enhanced after kINPen 09 treatment of HaCaT cells (Kurth, 2013). These results are underlined by Brun et al. (2012) who demonstrated an increase of Ogg1 2 to 24h after a 2 min plasma treatment. In contrast, the repair enzyme APE-1 (apurinic/apyrimidinic endonuclease-1) was found to be reduced in HaCaT cells after kINPen 09 treatment (Kurth, 2013). This enzyme is the major human repair enzyme for abasic sites and incises the phosphodiester backbone 5′ to the lesion to initiate a cascade of events aimed at removing the AP moiety and maintaining genetic integrity (Hadi et al., 2000). Up to now it is not clear whether the repair mechanism stops after deleting of 8-hydroxy-2′-deoxyguanosine by Ogg1.
Fig. 5.
Cell cycle analysis of HaCaT keratinocytes which stayed either untreated (A) or were treated with the kINPen 09 for 60s (B), surface DBD for 120 s (C) or volume DBD for 20 s (D). Analysis was done 24 h after exposure to plasma. Representative histograms with indication of the percentage of cells detected in the G1 and G2/M phase are shown. Plasma treatment induced independent of the plasma source used a typical G2/M.
SPECIFIC EFFECTS OF NON-THERMAL ATMOSPHERIC- PRESSURE PLASMA ON WOUND RELATING SKIN CELLS
Plasma and angiogenesis
Angiogenesis is a physiological process not only in embryogenesis but also in wound healing. Especially in chronic infected wounds aberrant angiogenesis is evident. In addition, growth and spread of solid tumors is dependent on formation of new blood vessels, which should be inhibited for a successful treatment. For improving wound healing angiogenesis should be promoted. Formation of new blood vessels is stimulated by a lack of oxygen and different endogenous proangiogenic factors. Among them are not only growth factors (VEGF, EGF, FGF) and cytokines (e.g. IL-1, 2, 6, 8; TNF, TGF) but also ROS and NO. Since plasma generates different ROS and NO, it was hypothesized that plasma should be able to stimulate angiogenesis. There are different methods to demonstrate an influence on the angiogenic process. Established in vitro methods use endothelial cells to measure simply their proliferation, migration or their ability to form tubes. Indeed, non-thermal plasma increased endothelial cell proliferation either by release of fibroblast growth factor-2 release (FGF-2), which is a promoter of angiogenesis (Kalghatgi et al., 2010) or by production of NO (Arjunan and Clyne, 2011a). Enhanced tube formation by using primary porcine aortic endothelial cells was found by Arjunan et al. (2012), who reported that particularly hydroxyl radicals and hydrogen peroxide seem to be responsible for the observed effects (Arjunan and Clyne, 2011b).
In our group more complex models like the rat aortic ring assay (AOR assay) and the in-ovo chick embryo chorioallantoic membrane assay (CAM assay) were used to measure the influence of non-thermal atmospheric-pressure plasma on the formation of new microvessels (Haertel et al., 2014). Either Matrigel-embedded aortic rings from LEW.1W or WOKW rats or chick embryo chorioallantoic membranes were indirectly treated with the plasma jet kINPen 09. Surprisingly, angiogenic response to plasma was found to be differentially influenced, depending on the models used and on the rat strain in the AOR test. In the CAM assay we found stimulation of angiogenesis, which could be quantified by fractal dimension and vessel area (Haertel et al., 2014). This effect was comparable to that observed with VEGF, a growth factor which secretion is stimulated by plasma e.g. from keratinocytes (Barton, 2013). Sprouting of microvessels from rat aortic rings was dependent on the rat strain used and either inhibited (WOKW) or not influenced (LEW.1W). It is difficult to explain this result, however, for mice it has been reported that the genetic background plays an essential role for VEGF-stimulated vessel sprouting in the aortic ring assay (Zhu et al., 2003).
Besides growth factors, cytokines, ROS and NO angiogenesis is fundamentally influenced by adhesion molecules, especially by integrin expression on endothelial cells mediating cell-matrix interaction. Non-thermal plasma is known to modify integrins on fibroblasts, keratinocytes and immune cells and thereby possibly also on endothelial cells. Future work should concentrate on the influence of plasma on the different key players influencing angiogenesis.
Plasma and cell surface molecules
In wound healing, cell adhesion plays a critical role for proliferation of cells as fibroblasts, keratinocytes and endothelial cells and their migration into the wound area. Cell adhesion is mediated by specialized molecules located on the cell surface which can be divided into cell-cell and cell-matrix adhesion molecules. These molecules are responsible for cell adhesion or detachment, for cell migration, cell signaling, growth and differentiation (Lauffenburger and Horwitz, 1996) and should be influenced by plasma according to the requirements.
Cell detachment often observed after treating cells with plasma (Stoffels et al., 2003; Kieft et al., 2004; Haertel et al., 2011; Hoentsch et al., 2012, 2014) provides a potent indication for the role of cell adhesion molecules, especially cell-matrix molecules. Integrins are transmembrane adhesion receptors which consist of a α- and a β-subunit. They mediate binding of cells to components of the extracellular matrix (ECM) and thereby, they are also responsible for cell migration. The role of integrin expression on fibroblasts, epithelial cells and HaCaT keratinocytes was underlined by investigations of Shashurin et al. (2010), Volotskova et al. (2012b) and our group (Haertel et al. 2011, 2012a, 2013a, 2013b). Treatment of adherent fibroblasts with a plasma jet was found to reduce expression of integrin β1 and αv on the cell surface (Shashurin et al., 2010). It is concluded that this is the original cause for cell detachment and reduced cell migration which was observed under similar conditions. In contrast, β1 integrin intensity was reported to be increased after treating mouse fibroblasts by a plasma jet although migration rate of fibroblasts was found to be significantly reduced (Volotskova et al., 2012b). Analysis of αv integrin revealed no change in intensity. On the other hand, increased migration of a mouse fibroblast line after plasma treatment was reported by Ngo et al. (2014), however, without referring to adhesion molecules. Our group analyzed the expression of several integrins on the surface of HaCaT keratinocytes. For plasma effects it was very important whether the cells were treated as monolayer or as cell suspension. Cells in suspension are neither connected to each other by cell adhesion molecules (CAM’s) as e.g. E-cadherin, nor to a matrix by integrins. In contrast, cells in monolayer are attached to a matrix and to surrounding cells. Therefore, they are not as sensitive to external influences as cells in suspension. Indeed, while cell number after treating HaCaT cells in suspension is already significantly reduced by a 20 s treatment cycle (Haertel et al., 2012a), monolayers can be exposed to surface DBD for 120 s before cell number is reduced (Haertel et al., 2013b). Integrin β1 was found to be up-regulated on HaCaT cells treated as suspension and as monolayer, however, treatment time to reach this result was longer for monolayers (120 s vs. 300 s). For regulation of integrin α2 an opposite behavior was detected after surface DBD treatment. While it was decreased on suspended cells, it increased on adherent cells. Stoffels et al. (2003) postulated that additionally to integrins, also cell-cell adhesion must be disturbed during cell detachment caused by plasma treatment. Indeed, we found a remarkable reduction of E-cadherin on HaCaT keratinocytes after kINPen 09 or surface DBD treatment (Haertel et al., 2011, 2012a). However, this result was only observed if the cells were treated in suspension. Treating a monolayer of HaCaT keratinocytes with plasma E-cadherin expression was not influenced (Haertel et al., 2013a, 2013b).
Detailed investigation of a greater panel of integrins after exposing HaCaT cells to surface DBD in monolayer revealed, in addition to an increase of α2 and β1 integrin, also an enhanced intensity for α5, α6 and β3 (Haertel et al., 2013b). The subunit α4 was never influenced and α3 and αv were slightly decreased (not significant). Regulation of β1 and αv integrin on HaCaT cells by plasma is in accordance with changes of those molecules on fibroblasts reported by Volotskova et al. (2012b). The observed effects of plasma on integrins were not mediated by ozone, but by reactive oxygen species as demonstrated for hydrogen peroxide (Haertel et al., 2013b). Only very high ozone concentrations (about 1800 ppm) increased integrin α2 comparable to surface DBD (300 s). As already mentioned during a 300 s treatment cycle with the surface DBD/air plasma about 100 ppm ozone was detected, which is 1000 times higher than the maximum allowable concentration (MAC).
The relevance of non-thermal atmospheric-pressure plasma for treating chronic infected wounds is not only given by its antimicrobial effects and stimulation of proliferation and migration of wound relating skin cells but also by its influence on cell adhesion receptors. Activation or inhibition of integrin receptors by plasma may provide an excellent means of influencing wound healing. In particular, down-regulation of the integrin receptor α5β1 in chronic wounds (Widgerow, 2013) could be enhanced by plasma. In contrast, αvβ6 is induced in chronic wounds and at least αv was decreased by plasma, however, not significantly. As demonstrated, plasma seems to be able to counteract the deleterious effects in chronic wounds in terms of integrin expression.
SUMMARY OF DETECTED IN VITRO EFFECTS OF NON-THERMAL ATMOSPHERIC-PRESSURE PLASMA
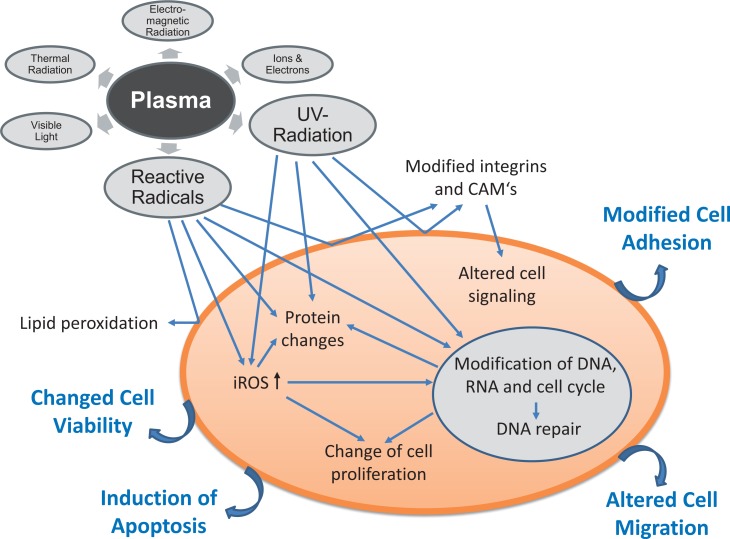
Figure 6 summarizes the effects of plasma on eukaryotic cells and tries to demonstrate some interplay between plasma components e.g. reactive radicals or UV radiation and resulting effects. Effects on different levels of the cells were recognized. First target is the cell membrane with its lipids and all embedded receptor proteins or enzymes. Lipid peroxidation and modification of cell adhesion molecules were observed resulting e.g. in an altered cell migration and cell signaling. Reactive molecules reach the cells possibly by diffusion, but they can also be induced within the cells and can thereby exert their effects e.g. on proteins. UV radiation and reactive radicals are further able to influence the DNA leading to a change of cell proliferation or induction of apoptosis. All these effects are dependent on the plasma dose/plasma treatment time and thereby both stimulating and deleterious effects are possible.
Fig. 6.
Schematic summary of plasma effects on eukaryotic cells. Some interplay between plasma components e.g. reactive radicals or UV radiation and resulting effects are depicted. Effects on different levels of the cells were recognized.
NON-THERMAL ATMOSPHERIC-PRESSURE PLASMA AND FIRST CLINICAL TRIALS REGARDING WOUND HEALING
Meanwhile, a good compatibility of plasma on skin has been reported. Plasma treatment of wounded pig skin, which closely resembles human skin, did not cause any toxic effects on the skin. Effective and fast blood coagulation was observed (Dobrynin et al., 2011). The authors concluded that plasma treatment is safe for living intact and wounded skin in plasma doses several times higher than required for inactivation of bacteria. Human skin physiology parameters were influenced by plasma, however, without damaging the skin or skin functions, indicating the safety of plasma under in vivo conditions (Fluhr et al., 2012). First clinical studies confirmed that plasma treatment was well tolerated, painless and without side effects (Isbary et al., 2010, 2012, 2013a; Daeschlein et al., 2012b; Emmert et al., 2013; Brehmer et al., 2014). However, future studies are needed to exclude long-term side effects. Regarding promotion of wound healing by plasma first clinical results are promising (Isbary et al., 2013b). Decrease of bacterial load in chronic wounds as presumption for an improved wound healing was shown in randomized controlled trials by using the atmospheric-pressure plasma jet MicroPlaSter plasma torch (Isbary et al., 2010, 2012). From a retrospective study of the same group it was concluded that wound healing may be accelerated by plasma, particularly for chronic venous ulcers (Isbary et al., 2013c). The plasma jet kINPen med® entails no risk for humans in terms of temperature increase, UV radiation or free radical formation and reduced bacterial load (Lademann et al., 2013). A different plasma device, the PlasmaDerm® VU-2010 device (CINOGY GmbH, Duderstadt, Germany) generating plasma by dielectric barrier discharge has also been shown to decrease bacterial load effectively in patients with chronic venous leg ulcers with more than 50% ulcer size reduction (Brehmer et al., 2014).
CONCLUSIONS
Taken together, non-thermal atmospheric-pressure plasma can support wound healing by its antiseptic effects, by stimulation of proliferation and migration of wound relating skin cells, by activation or inhibition of integrin receptors on the cell surface or by its pro-angiogenic effect. Non-thermal atmospheric-pressure plasma is a new innovative approach not only for the treatment of chronic wounds, but with a wide-range of other applications, as e.g. topical treatment of other skin diseases with microbial involvement or treatment of cancer diseases. Plasma parameters have to be defined for a safe application according to their needs. Norms for the technical devices to allow a standardized treatment of given diseases are very important and strongly needed. This is also the basis for comparison of the outcome of various trials conducted in different clinics. In future, effectivity of plasma treatment has to be demonstrated in controlled, randomized and greater clinical trials.
Acknowledgments
The authors acknowledge Robert Koch, Christiane Meyer and Rüdiger Titze (Leibniz Institute for Plasma Sciences and Technology e.V.) for providing technical support. This study was realized within the joint research project “Campus PlasmaMed” supported by the German Federal Ministry of Education and Research (grant no. 13N9774 and 13N11182) as well as the project “Plasmamedical Research - New pharmaceutical and medical fields of application” funded by the Ministry of Education, Science and Culture of the State of Mecklenburg-Western Pomerania and the European Union, European Social Fund (grant number: AU 11 038; ESF/IV-BMB35-0010/13).
None of the authors has to declare any conflict of interest including financial and other relationships.
REFERENCES
- Alkawareek MY, Algwari QT, Laverty G, Gorman SP, Graham WG, O'Connell D, Gilmore BF. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure nonthermal plasma. PLoS One. 2012;7:e44289. doi: 10.1371/journal.pone.0044289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. . Int. J. Antimicrob. Agents. 2014;43:154–60. doi: 10.1016/j.ijantimicag.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Arjunan KP, Clyne AM. A nitric oxide producing pin-to-hole spark discharge plasma enhances endothelial cell proliferation and migration. Plasma Med. 2011a;1:279–293. [Google Scholar]
- Arjunan KP, Clyne AM. Hydroxyl radical and hydrogen peroxide are primarily responsible for dielectric barrier discharge plasma-induced angiogenesis. Plasma Process Polym. 2011b;8:1154–1164. [Google Scholar]
- Arjunan KP, Friedman G, Fridman A, Clyne AM. Nonthermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface. 2012;9:147–157. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S, Wacker E, Li YF, Shimizu T, Thomas HM, Morfill GE, Karrer S, Zimmermann JL, Bosserhoff AK. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol. 2013;22:284–289. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- Barton A. Impact of non-thermal plasma on cell signaling in keratinocytes. 2013. Doctoral Thesis, Ernst-Moritz-Arndt-University of Greifswald, Germany. [Google Scholar]
- Bekeschus S, Kolata J, Müller A, Kramer A, Weltmann KP, Bröker B, Masur K. Differential viability of eight human blood mononuclear cell subpopulations after plasma treatment. Plasma Med. 2013a;3:1–13. [Google Scholar]
- Bekeschus S, Kolata J, Winterbourn C, Kramer A, Turner R, Weltmann KD, Bröker B, Masur K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic Res. 2014;48:542–549. doi: 10.3109/10715762.2014.892937. [DOI] [PubMed] [Google Scholar]
- Bekeschus S, Masur K, Kolata J, Wende K, Schmidt A, Bundscherer L, Barton A, Kramer A, Bröker B, Weltmann KD. Human mononuclear cell survival and proliferation is modulated by cold atmospheric plasma jet. Plasma Process Polym. 2013b;10:706–713. [Google Scholar]
- Białoszewski D, Kowalewski M. Superficially, longer, intermittent ozone therapy in the treatment of the chronic, infected wounds. Ortop Traumatol Rehabil. 2003;5:652–658. [PubMed] [Google Scholar]
- Blackert S, Haertel B, Wende K, von Woedtke T, Lindequist U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (Ha-CaT) J Dermatol Sci. 2013;70:173–181. doi: 10.1016/j.jdermsci.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Bocci V, Borrelli E, Travagli V, Zanardi I. The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29:646–682. doi: 10.1002/med.20150. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer F, Haenssle HA, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, Simon D, Schön MP, Wandke D, Emmert S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial ( NCT01415622) J Eur Acad Dermatol Venereol. 2014 doi: 10.1111/jdv.12490. in press. [DOI] [PubMed] [Google Scholar]
- Brun P, Vono M, Venier P, Tarricone E, Deligianni V, Martines E, Zuin M, Spagnolo S, Cavazzana R, Cardin R, Castagliuolo I, La Gloria Valerio A, Leonardi A. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS One. 2012;7:e33245. doi: 10.1371/journal.pone.0033245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundscherer L, Bekeschus S, Tresp H, Hasse S, Reuter S, Weltmann KD, Lindequist U, Masur K. Viability of human blood leucocytes compared with their respective cell lines after plasma treatment. Plasma Med. 2013a;3:71–80. [Google Scholar]
- Bundscherer L, Wende K, Ottmüller K, Barton A, Schmidt A, Bekeschus S, Hasse S, Weltmann KD, Masur K, Lindequist U. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology. 2013b;218:1248–1255. doi: 10.1016/j.imbio.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Bussiahn R, Lembke N, Gesche R, von Woedtke T, Welt-mann KD. Plasma sources for biomedical applications. Hyg Med. 2013;38:212–216. [Google Scholar]
- Cha HY, Kim HO, Jin JS, Lee JC. Emergence of vancomycin-intermediate Staphylococcus aureus from predominant methicillin-resistant S. aureus clones in a Korean hospital. J Microbiol. 2010;48:533–535. doi: 10.1007/s12275-010-0062-5. [DOI] [PubMed] [Google Scholar]
- Cha HY, Moon DC, Choi CH, Oh JY, Jeong YS, Lee YC, Seol SY, Cho DT, Chang HH, Kim SW, Lee JC. Prevalence of the ST239 clone of methicillin-resistant Staphylococcus aureus and differences in antimicrobial susceptibilities of ST239 and ST5 clones identified in a Korean hospital. J Clin Microbiol. 2005;43:3610–3614. doi: 10.1128/JCM.43.8.3610-3614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J. 2013;10(Suppl. 1):9–14. doi: 10.1111/iwj.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeschlein G, Napp M, von Podewils S, Lutze S, Emmert S, Lange A, Klare I, Haase H, Gümbel D, von Woedtke T, Jünger M. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Process Polym. 2014;11:175–183. [Google Scholar]
- Daeschlein G, Scholz S, Ahmed R, Majumdar A, von Woedtke T, Haase H, Niggemeier M, Kindel E, Brandenburg R, Welt-mann KD, Jünger M. Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. J Dtsch Dermatol Ges. 2012b;10:509–515. doi: 10.1111/j.1610-0387.2012.07857.x. [DOI] [PubMed] [Google Scholar]
- Daeschlein G, Scholz S, Ahmed R, von Woedtke T, Haase H, Niggemeier M, Kindel E, Brandenburg R, Weltmann KD, Jünger M. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J Hosp Infect. 2012a;81:177–183. doi: 10.1016/j.jhin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- DIN SPEC 91315. “General requirements for plasma sources in medicine”. 2014. DIN Deutsches Institut für Normung e.V., Beuth Verlag Berlin, June 2014. http://www.spec.din.de/cmd?level=tpl-art-detailansicht&committeeid=0&artid=203493369&languageid=en&bcrumblevel=2.
- Dobrynin D, Wu A, Kalghatgi S, Park S, Shainsky N, Wasko K, Dumani E, Ownbey R, Joshi S, Sensing R, Brooks AD. Live pig skin tissue and wound toxicity of cold plasma treatment. Plasma Med. 2011;1:93–108. [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duval A, Marinov I, Bousquet G, Gapihan G, Starikovskaia SM, Rousseau A, Janin A. Cell death induced on cell cultures and nude mouse skin by non-thermal, nanosecond-pulsed generated plasma. PLoS One. 2013;8:e83001. doi: 10.1371/journal.pone.0083001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlbeck J, Schnabel U, Polak M, Winter J, von Woedtke T, Brandenburg R, von dem Hagen T, Weltmann KD. Low temperature atmospheric pressure plasma sources for microbial decontamination. J Phys D: Appl Phys. 2011;44:013002. [Google Scholar]
- Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert S, Brehmer F, Hänβle H, Helmke A, Mertens N, Ahmed R, Simon D, Wandke D, Maus-Friedrichs W, Daeschlein G, Schön MP, Viöl W. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clin Plasma Med. 2013;1:24–29. [Google Scholar]
- Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova JM, Murashev AN, Selezneva II, Shimizu T, Sysolyatina EV, Shaginyan IA, Petrov OF, Mayevsky EI, Fortov VE, Morfill GE, Naroditsky BS, Gintsburg AL. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol. 2011;60:75–83. doi: 10.1099/jmm.0.020263-0. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Sassning S, Lademann O, Darvin ME, Schanzer S, Kramer A, Richter H, Sterry W, Lademann J. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Exp Dermatol. 2012;21:130–134. doi: 10.1111/j.1600-0625.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- Fricke K, Koban I, Tresp H, Jablonowski L, Schroder K, Kramer A, Weltmann KD, von Woedtke T, Kocher T. Atmospheric pressure plasma: a high-performance tool for the efficient removal of biofilms. PLoS One. 2012;7:e42539. doi: 10.1371/journal.pone.0042539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JL, Asadinezhad A, Pacherník J, Lehocký M, Junkar I, Humpolícek P, Sáha P, Valásek P. Cell proliferation of HaCaT keratinocytes on collagen films modified by argon plasma treatment. Molecules. 2010;15:2845–2856. doi: 10.3390/molecules15042845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alcantara E, López-Callejas R, Morales-Ramírez PR, Peña-Eguiluz R, Fajardo-Muñoz R, Mercado-Cabrera A, Barocio SR, Valencia-Alvarado R, Rodríguez-Méndez BG, Muñoz-Castro AE, de la Piedad-Beneitez A, Rojas-Olmedo IA. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res. 2013;44:169–177. doi: 10.1016/j.arcmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Günther CI, Machens HG. Innovations in wound medicine. Wound Med. 2014;4:9–12. [Google Scholar]
- Hadi MZ, Coleman MA, Fidelis K, Mohrenweiser HW, Wilson DM., 3rd Functional characterization of Ape1 variants identified in the human population. Nucleic Acids Res. 2000;28:3871–3879. doi: 10.1093/nar/28.20.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertel B, Eiden K, Deuter A, Wende K, von Woedtke T, Lindequist U. Differential effect of non-thermal atmospheric-pressure plasma on angiogenesis. Lett Appl NanoBioSci. 2014;3:159–166. [Google Scholar]
- Haertel B, Hähnel M, Blackert S, Wende K, von Woedtke T, Lindequist U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biol Int. 2012a;36:1217–1222. doi: 10.1042/CBI20120139. [DOI] [PubMed] [Google Scholar]
- Haertel B, Straβenburg S, Harms M, Wende K, Lindequist U, von Woedtke T. Biological effects of non-thermal atmospheric-pressure plasma on human HaCaT-keratinocytes. Hyg Med. 2013a;38:198–205. [Google Scholar]
- Haertel B, Straβenburg S, Oehmigen K, Wende K, von Woedtke T, Lindequist U. Differential influence of components resulting from atmospheric-pressure plasma on integrin expression of human HaCaT keratinocytes. BioMed Res Int. 2013b;2013:761451. doi: 10.1155/2013/761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertel B, Volkmann F, von Woedtke T, Lindequist U. Differential sensitivity of lymphocyte subpopulations to non-thermal atmospheric-pressure plasma. Immunobiology. 2012b;217:628–633. doi: 10.1016/j.imbio.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Haertel B, Wende K, von Woedtke T, Weltmann KD, Lind-equist U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Exp Dermatol. 2011;20:282–284. doi: 10.1111/j.1600-0625.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- Hähnel M, von Woedtke T, Weltmann KD. Influence of the air humidity on the reduction of bacillus spores in a defined environment at atmospheric pressure using a dielectric barrier surface discharge. Plasma Process Polym. 2010;7:244–249. [Google Scholar]
- Heinlin J, Isbary G, Stolz W, Zeman F, Landthaler M, Morfill G, Shimizu T, Zimmermann JL, Karrer S. A randomized two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus. J Eur Acad Dermatol Venereol. 2013a;27:324–331. doi: 10.1111/j.1468-3083.2011.04395.x. [DOI] [PubMed] [Google Scholar]
- Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, Landmthaler M, Maisch T, Monetti R, Morfill G, Shimizu T, Steinbauer J, Stolz W, Karrer S. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013b;21:800–807. doi: 10.1111/wrr.12078. [DOI] [PubMed] [Google Scholar]
- Hoentsch M, von Woedtke T, Weltmann KD, Nebe JB. Time-dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J Phys D: Appl Phys. 2012;45:025206. [Google Scholar]
- Hoentsch M, Bussiahn R, Rebl H, Bergemann C, Eggert M, Frank M, von Woedtke T, Nebe JB. Persistent Effectivity of gas plasmaa-treated, long time-stored liquid on epithelial cell adhesion capacity and membrane morphology. PLoS One. 2014;9:e104559. doi: 10.1371/journal.pone.0104559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YF, Kang JG, Lee HY, Uhm HS, Moon E, Park YH. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett Appl Microbiol. 2009;48:33–37. doi: 10.1111/j.1472-765X.2008.02480.x. [DOI] [PubMed] [Google Scholar]
- Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, Klaempfl T, Karrer S, Landthaler M, Stolz W. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167:404–410. doi: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbary G, Köritzer J, Mitra A, Li YF, Shimizu T, Schroeder J, Schlegel J, Morfill GE, Stolz W, Zimmermann JL. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013c;1:36–44. [Google Scholar]
- Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, Bunk W, Monetti R, Zimmermann JL, Pompl R, Stolz W. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- Isbary G, Morfill G, Zimmermann J, Shimizu T, Stolz W. Cold atmospheric plasma: a successful treatment of lesions in Hailey-Hailey disease. Arch Dermatol. 2011;147:388–390. doi: 10.1001/archdermatol.2011.57. [DOI] [PubMed] [Google Scholar]
- Isbary G, Stolz W, Shimizu T, Monetti R, Bunk W, Schmidt HU, Morfill GE, Klämpfl TG, Steffes B, Thomas HM, Heinlin J, Karrer S, Landthaler M, Zimmermann JL. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin Plasma Med. 2013a;1:25–30. [Google Scholar]
- Isbary G, Zimmermann JL, Shimizu T, Li YF, Morfill G, Thomas HM, Steffes B, Heinlin J, Karrer S, Stolz W. Nonthermal plasma-More than five years of clinical experience. Clin Plasma Med. 2013b;1:19–23. [Google Scholar]
- Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of non-thermal dielectric-barrier discharge plasma. Am. J. Infect. Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Julak J, Scholtz V. Decontamination of human skin by low-temperature plasma produced by cometary discharge. Clin Plasma Med. 2013;1:31–34. [Google Scholar]
- Kalghatgi S, Azizkhan-Clifford J. DNA damage in mammalian cells by atmospheric pressure microsecond-pulsed dielectric barrier discharge plasma is not mediated via lipid peroxidation. Plasma Med. 2011a;1:167–177. [Google Scholar]
- Kalghatgi S, Fridman A, Azizkhan-Clifford J, Friedman G. DNA damage in mammalian cells by non-thermal atmospheric pressure microsecond pulsed dielectric barrier discharge plasma is not mediated by ozone. Plasma Process Polym. 2012;9:726–732. [Google Scholar]
- Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng. 2010;38:748–757. doi: 10.1007/s10439-009-9868-x. [DOI] [PubMed] [Google Scholar]
- Kalghatgi S, Kelly C, Cerchar E, Torabi B, Alekseev O, Fridman A, Friedman G, Azizkhan-Clifford J. Effects of nonthermal plasma on mammalian cells. PLoS One. 2011b;6:e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft IE, Broers JL, Caubet-Hilloutou V, Slaaf DW, Ramaekers FC, Stoffels E. Electric discharge plasmas influence attachment of cultured CHO K1 cells. Bioelectromagnetics. 2004;25:362–368. doi: 10.1002/bem.20005. [DOI] [PubMed] [Google Scholar]
- Kim HB, Park WB, Lee KD, Choi YJ, Park SW, Oh M, Kim EC, Choe KW. Nationwide surveillance for Staphylococcus aureus with reduced susceptibility to vancomycin in Korea. J Clin Microbiol. 2003a;41:2279–2281. doi: 10.1128/JCM.41.6.2279-2281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Yousef AE, Khadre MA. Ozone and its current and future application in the food industry. Adv Food Nutr Res. 2003b;45:167–218. doi: 10.1016/s1043-4526(03)45005-5. [DOI] [PubMed] [Google Scholar]
- Kim PY, Kim YS, Koo IG, Jung JC, Kim GJ, Choi MY, Yu ZQ, Collins GJ. Bacterial inactivation of wound infection in a human skin model by liquid-phase discharge plasma. PLoS One. 2011a;6:e24104. doi: 10.1371/journal.pone.0024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Woo KC, Kim GC, Kim KT. Non-thermal-plasma-mediated animal cell death. J Phys D: Appl Phys. 2011b;44:013001. [Google Scholar]
- Klebes M, Lademann J, Philipp S, Ulrich C, Patzelt A, Ulmer M, Kluschke F, Kramer A, Weltmann KD, Sterry W, Lange-Asschenfeldt B. Effects of tissue-tolerable plasma on psoriasis vulgaris treatment compared to conventional local treatment: A pilot study. Clin Plasma Med. 2014 doi: 10.1016/j.cpme.2013.11.002. in press. [DOI] [Google Scholar]
- Köritzer J, Boxhammer V, Schäfer A, Shimizu T, Klämpfl TG, Li YF, Welz C, Schwenk-Zieger S, Morfill GE, Zimmermann JL, Schlegel J. Restoration of sensitivity in chemoresistant glioma cells by cold atmospheric plasma. PLoS One. 2013;8:e64498. doi: 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Lademann J, Bender C, Sckell A, Hartmann B, Münch S, Hinz P, Ekkernkamp A, Matthes R, Koban I, Partecke I, Heidecke CD, Masur K, Reuter S, Weltmann KD, Koch S, Assadian O. Suitability of tissue tolerable plasmas (TTP) for the management of chronic wounds. Clin Plasma Med. 2013;1:11–18. [Google Scholar]
- Kuo LJ, Yang LX. γ-H2AX - A novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–310. [PubMed] [Google Scholar]
- Kurth A. 2013. Einfluss von physikalischem Plasma auf in vitro kultivierte HaCaT-Zellen: Einfluss auf DNA und Reparaturmechanismen. Diploma Thesis, Ernst-Moritz-Arndt-University Greifswald. [Google Scholar]
- Lademann J, Ulrich C, Patzelt A, Richter H, Kluschke F, Klebes M, Lademann O, Kramer A, Weltmann KD, Lange-Asschenfeldt B. Risk assessment of the application of tissue-tolerable plasma on human skin. Clin Plasma Med. 2013;1:5–10. [Google Scholar]
- Langmuir I. Oscillations in ionized gases. Proc Nat Acad Sci USA. 1928;14:627–637. doi: 10.1073/pnas.14.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Leduc M, Guay D, Coulombe S, Leask RL. Effects of non-thermal plasmas on DNA and mammalian cells. Plasma Process Polym. 2010;7:899–909. [Google Scholar]
- Li YF, Taylor D, Zimmermann JL, Bunk W, Monetti R, Isbary G, Boxhammer V, Schmidt HU, Shimizu T, Thomas HM, Morfill GE. In vivo skin treatment using two portable plasma devices: Comparison of a direct and an indirect cold atmospheric plasma treatment. Clin Plasma Med. 2013;1:35–39. [Google Scholar]
- Lloyd G, Friedman G, Jafri S, Schultz G, Fridman A, Harding K. Gas plasma: medical uses and developments in wound care. Plasma Process Polym. 2010;7:194–211. [Google Scholar]
- Lopes BB, Kraft MBPL, Rehder J, Batista FRX, Puzzi MB. The interactions between non-thermal atmospheric pressure plasma and ex-vivo dermal fibroblasts. Procedia Engin. 2013;59:92–100. [Google Scholar]
- Ma Y, Ha CS, Hwang SW, Lee HJ, Kim GC, Lee KW, Song S. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ros stress-response pathways. PLoS One. 2014;9:e91947. doi: 10.1371/journal.pone.0091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch T, Shimizu T, Li YF, Heinlin J, Karrer S, Morfill G, Zimmermann JL. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS One. 2012;7:e34610. doi: 10.1371/journal.pone.0034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M, Tiede R, Ahmed R, Wandtke D, Wurster S, Weltmann KD, Deaschlein G, Emmert S, von Woedtke T. Standards in plasma medicine: development, contents and importance of the first German DIN specification. ICPM5. 2014 2014 May 18–23; Nara, Japan. [Google Scholar]
- Matthes R, Bekeschus S, Bender C, Koban I, Hübner NO, Kramer A. Pilot-study on the influence of carrier gas and plasma application (open resp. delimited) modifications on physical plasma and its antimicrobial effect against Pseudomonas aeruginosa and Staphylococcus aureus. GMS Krankenhaushyg Inter-diszipl. 2012;7:1–7. doi: 10.3205/dgkh000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes R, Bender C, Schlüter R, Koban I, Bussiahn R, Reuter S, Lademann J, Weltmann KD, Kramer A. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS One. 2013;8:e70462. doi: 10.1371/journal.pone.0070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ramírez P, Cruz-Vallejo V, Peña-Eguiluz R, López-Callejas R, Rodríguez-Méndez BG, Valencia-Alvarado R, Mercado-Cabrera A, Muñoz-Castro AE. Assessing cellular DNA damage from a helium plasma needle. Radiat Res. 2013;179:669–673. doi: 10.1667/RR3223.1. [DOI] [PubMed] [Google Scholar]
- Nasruddin Nakajima Y, Mukai K, Rahayu HSE, Nur M, Ishijima T, Enomoto H, Uesugi Y, Sugama J, Nakatani T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plasma Med. 2014 doi: 10.1016/j.cpme.2014.01.001. in press. [DOI] [Google Scholar]
- Nastuta AV, Topala I, Grigoras C, Pohoata V, Popa G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J Phys D: Appl Phys. 2011;44:105204. [Google Scholar]
- Ngo MHT, Liao JD, Shao PL, Weng CC, Chang CY. Increased fibroblast cell proliferation and migration using atmospheric N2/Ar micro-plasma for the stimulated release of fibroblast growth factor-7. Plasma Process Polym. 2014;11:80–88. [Google Scholar]
- Oehmigen K. Plasma-Flüssigkeit-Wechselwirkungen. 2014. Doctoral Thesis, Ernst-Moritz-Arndt-University of Greifswald. [Google Scholar]
- Park SH, Park C, Yoo JH, Choi SM, Choi JH, Shin HH, Lee DG, Lee S, Kim J, Choi SE, Kwon YM, Shin WS. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated bloodstream infections in Korea. Infect Control Hosp Epidemiol. 2009;30:146–155. doi: 10.1086/593953. [DOI] [PubMed] [Google Scholar]
- Pipa AV, Reuter S, Foest R, Weltmann KD. Controlling the NO production of an atmospheric pressure plasma jet. J Phys D: Appl Phys. 2012;45:085201. [Google Scholar]
- Polak M, Winter J, Schnabel U, Ehlbeck J, Weltmann KD. Innovative plasma generation in flexible biopsy channels for inner-tube decontamination and medical applications. Plasma Process Polym. 2012;9:67–76. [Google Scholar]
- Reuter S, Winter J, Schmidt-Bleker A, Schroeder D, Lange H, Knake N, Schulz-von der Gathen V, Weltmann KD. Atomic oxygen in a cold argon plasma jet: TALIF spectroscopy in ambient air with modelling and measurements of ambient species diffusion. Plasma Sources Sci Technol. 2012a;21:024005. [Google Scholar]
- Reuter S, Winter J, Iseni S, Peters S, Schmidt-Bleker A, Dünnbier M, Schäfer J, Foest R, Weltmann KD. Detection of ozone in a MHz argon plasma bullet jet. Plasma Sources Sci Technol. 2012b;21:034015. [Google Scholar]
- Robert E, Vandamme M, Brullé L, Lerondel S, LePape A, Sarron V, Riès D, Darny T, Dozias S, Collet G, Kiedaand C, Pouvesle JM. Perspectives of endoscopic plasma applications. Clin Plasma Med. 2013;1:8–16. [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess Δψ changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Schaper L, Reuter S, Waskoenig J, Niemi K, Schulz-von der Ga-then V, Gans T. The dynamics of radio-frequency driven atmospheric pressure plasma jets. . J. Phys: Conference Series. 2009;162:012013. [Google Scholar]
- Schmidt A, von Woedtke T, Weltmann KD, Masur K. Identification of the molecular basis of non-thermal plasma-induced changes in human keratinocytes. Plasma Med. 2013a;3:15–25. [Google Scholar]
- Schmidt A, Wende K, Bekeschus S, Bundscherer L, Barton A, Ottmüller K, Weltmann KD, Masur K. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free Radic Res. 2013b;47:577–592. doi: 10.3109/10715762.2013.804623. [DOI] [PubMed] [Google Scholar]
- Schmidt-Bleker A, Winter J, Iseni S, Dünnbier M, Weltmann KD, Reuter S. Reactive species output of a plasma jet with a shielding gas device-combination of FTIR absorption spectroscopy and gas phase modelling. J Phys D: Appl Phys. 2014;47:145201. [Google Scholar]
- Shashurin A, Stepp MA, Hawley TS, Pal-Ghosh S, Brieda L, Bronnikov S, Jurjus RA, Keidar M. Influence of cold plasma atmospheric jet on surface integrin expression of living cells. Plasma Process Polym. 2010;7:294–300. [Google Scholar]
- Shi XM, Zhang GJ, Yuan YK, Ma Y, Xu GM, Yang Y. Effects of low-temperature atmospheric air plasmas on the activity and function of human lymphocytes. Plasma Process Polym. 2008;5:482–488. [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low-levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Steinbeck J, Chernets N, Zhang J, Kurpad DS, Fridman G, Fridman A, Freeman TA. Skeletal cell differentiation is enhanced by atmospheric dielectric barrier discharge plasma treatment. PLoS One. 2013;8:e82143. doi: 10.1371/journal.pone.0082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels E, Kieft IE, Sladek REJ. Superficial treatment of mammalian cells using plasma needle. J Phys D: Appl Phys. 2003;36:2908–2913. [Google Scholar]
- Straβenburg S. 2014. Untersuchungen zum Einfluss von physikalischem biological auf in vitro kultivierte Zellen. Doctoral Thesis, Ernst-Moritz-Arndt-University of Greifswald. [Google Scholar]
- Straβenburg S, Greim U, Bussiahn R, Haertel B, Wende K, von Woedtke T, Lindequist U. Comparison of biological effects on human keratinocytes using different plasma treatment regimes. Plasma Med. 2013;3:57–69. [Google Scholar]
- Strausberg J, Lehmann N, Kröger K, Maier I, Schneider H, Niebel W. Changes in secondary care may explain increasing pressure ulcer rates in an University Clinic in Germany. Wound Manag. 2007;5:194–198. [Google Scholar]
- Sung SJ, Huh JB, Yun MJ, Chang BM, Jeong CM, Jeon YC. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J Adv Prosthodont. 2013;5:2–8. doi: 10.4047/jap.2013.5.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh K, McFaline JL, Pang B, Sullivan M, Dong M, Plummer E, Dedon PC. Quantification of DNA damage products resulting from deamination, oxidation and reaction with products of lipid peroxidation by liquid chromatography isotope dilution tandem mass spectrometry. Nat Protoc. 2008;3:1287–1298. doi: 10.1038/nprot.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Le Pape A. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer. 2012;130:2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- Volotskova O, Hawley TS, Stepp MA, Keidar M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci Rep. 2012a;2:636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volotskova O, Stepp MA, Keidar M. Integrin activation by a cold atmospheric plasma jet. N J Phys. 2012b;14:053019. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Woedtke T, Metelmann HR, Weltmann KD. Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contrib Plasma Phys. 2014;54:104–117. [Google Scholar]
- von Woedtke T, Reuter S, Masur K, Weltmann KD. Plasmas for medicine. Phys Rep. 2013;530:291–320. [Google Scholar]
- Weltmann KD, Brandenburg R, von Woedtke T, Ehlbeck J, Foest R, Stieber M, Kindel E. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs) J Phys D: Appl Phys. 2008;41:194008. [Google Scholar]
- Wende K, Straβenburg S, Haertel B, Harms M, Holtz S, Barton A, Masur K, von Woedtke T, Lindequist U. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Biol Int. 2014;38:412–425. doi: 10.1002/cbin.10200. [DOI] [PubMed] [Google Scholar]
- Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence-based management strategies for treatment of chronic wounds. ePlasty. 2009;9:e19. [PMC free article] [PubMed] [Google Scholar]
- Widgerow AD. Chronic wounds: is cellular ‘reception’ at fault? Examining integrins and intracellular signaling. Int Wound J. 2013;10:185–192. doi: 10.1111/j.1742-481X.2012.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Tresp H, Hammer MU, Iseni S, Kupsch S, Schmidt-Bleker A, Wende K, Dünnbier M, Masur K, Weltmann KD, Reuter S. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J Phys D: Appl Phys. 2014;47:285401. [Google Scholar]
- Wu Z, Chen M, Li P, Zhua Q, Wang J. Dielectric barrier discharge non-thermal micro-plasma for the excitation and emission spectrometric detection of ammonia. Analyst. 2011;136:2552–2557. doi: 10.1039/c0an00938e. [DOI] [PubMed] [Google Scholar]
- Yu Y, Tan M, Chen H, Wu Z, Xu L, Li J, Cao J, Yang Y, Xiao X, Lian X, Lu X, Tu Y. Non-thermal plasma suppresses bacterial colonization on skin wound and promotes wound healing in mice. J Huazhong Univ Sci Technol Med Sci. 2011;31:390–394. doi: 10.1007/s11596-011-0387-2. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer V, Schlegel J, Gansbacher B, Anton M. Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys. 2011;44:505201. [Google Scholar]