Abstract
Photobiomodulation utilizes monochromatic (or quasimonochromatic) light in the electromagnetic region of 600∼1000 nm for the treatment of soft tissues in a nondestructive and nonthermal mode. It is conceivable that photobiomodulation is based upon the ability of the light to alter cell metabolism as it is absorbed by general hemoproteins and cytochrome c oxidase (COX) in particular. Recently it has been suggested radiation of visible and infrared (IR) activates retrograde signaling pathway from mitochondria to nucleus. In this review, the role of COX in the photobiomodulation will be discussed. Further a possible role of water as a photoreceptor will be suggested.
Keywords: Photobiomodulation, Hemoprotein, Cytochrome c oxidase, Retrograde signaling, Water
INTRODUCTION
A famous experiment carried out by Otto Warburg uncovered the enzyme responsible for the critical step of cellular respiration (Lane, 2006), which converts the energy conserved in foods to ATP the cell can use. The enzyme he identified is cytochrome oxidase (COX), which is a major player in cellular energy metabolism. To extract energy from exogenous molecules, the cells break down ingested glucose to more simple molecules via glycolysis process. Then, pyruvate, a C3 compound enters the energy-producing subcellular organelle, mitochondria through pyruvate transporter compartmentalized to mitochondrial outer membrane (Divakaruni et al., 2013). The captured electrons from sugar compounds can produce ATP with the aid of oxygen. Cytochrome c oxidase controls the last step of this process, which commonly called oxidative phosphorylation (Lane, 2006; Karu, 2008).
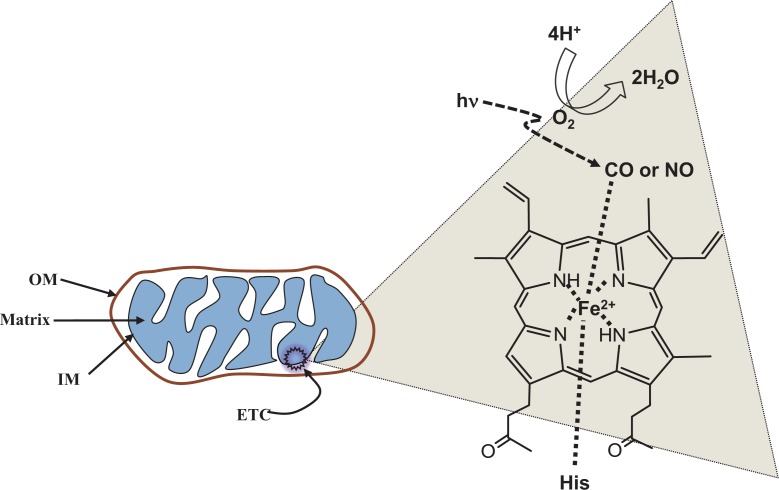
The method that Warburg used to discover is rather intriguing since he has exploited two seemingly unrelated things; carbon monoxide (CO) and light (Lane, 2006). While CO can block cellular respiration by binding and inhibiting COX in place of oxygen, a flash of light can displace the gas molecule. Thus oxygen can bind to the enzyme again and utilize it to produce ATP (Fig. 1). Cells frequently use CO and nitric oxide (NO) to block cellular respiration (Moncada and Bolaños, 2006; Zuckerbraun et al., 2007).
Fig. 1.
Heme-iron axial ligands. Histidine in COX protein and CO or oxygen can be axial ligands. Lights can displace gas molecules with oxygen, which functions as an electron acceptor to make water. Electron transport chain (ETC) located to inner membrane of mitochondria.
Both gas molecules are constantly synthesized in the body via enzymatic catalysis (Kim et al., 2006). Interestingly evidences suggested that those enzymes (i.e., mitochondrial NO synthase, heme oxygenase-1) are also compartmentalized in the mitochondria (Slebos et al., 2007; Zaobornyj and Ghafourifar, 2012). With regards to the existence of gas-producing enzyme in the specific organelles, Moncada (2006) has proposed the idea that slowing respiration by binding of NO instead of oxygen to COX is to divert oxygen elsewhere in cells and tissues. Thus, the strategy may prevent falling down of oxygen levels to a certain extent in the neighboring cells. In fact, it has been demonstrated that NO blocks respiration in endothelial cells, which helps to deliver oxygen to smooth muscle cells in the blood vessel.
Coincidently, light has long been considered to augment wound healing (Mester et al., 1971; Conlan et al., 1996; Reddy et al., 2001; Whelan et al., 2003; Ells et al., 2004). So far, however, the molecular basis for the therapeutic potential of light has only recently uncovered. At long wavelengths in the infrared (IR) spectrum region, photons may penetrate about several centimeters into the skin (Schindl et al., 2000; Lane, 2006). More recently Dr. Karu (2008) suggested that near-IR radiation may modulate cellular respiration and retrograde signaling from mitochondria to nucleus. This opens new modalities of therapeutics by adopting light, photobiomodulation (PBM).
WHAT IS “PHOTOBIOMODULATION”
Laser PBM, also called as low-energy laser therapy, manifests the utilization of monochromatic or quasimonochromatic low-level light to induce mainly non-thermal and non-invasive photochemical activities (Mester et al., 1985; Schindl et al., 2000; Desmet et al., 2006). Some investigators are skeptical for this multidisciplinary field since they deeply believe electromagnetic light must ionize biological systems it affects. Or they thought of the light signals are so weak to induce significant biological effects (Karu, 2008).
However, it has been demonstrated that PBM could stimulate or inhibit a wide variety of cellular processes, thereby modulating cell functions (Mester et al., 1985; Schindl et al., 2000; Desmet et al., 2006). Santana-Blank et al. (2013) recently reported a singular low-energy IR pulsed laser device with high-frequency ultrasound and near-IR radiation could successfully adopted to treat patients who suffered from advanced and progressive neoplasia. Morphological study revealed that the PBM triggers the selective activation of programmed cell death only in neoplastic cells, but not in peripheral tissues, which is further supported by the subsequent data obtained from Tanaka’s group (2010). Even intriguingly, magnetic resonance imaging data showed the alteration of water content in the tumor may act as an early diagnostic predicator of tumor response (Santana-Blank et al., 2013).
Other investigations demonstrate that PBM can activate regenerative responses, alone or associated with stem cell therapy (Desmet et al., 2006; Karu, 2008). Recent effects also focused on the dentistry (Carroll et al., 2014), degenerative neuronal disorders (Wong-Riley et al., 2005; Ying et al., 2008; Quirk et al., 2012), and metabolic syndromes (Reddy et al., 2001). Therefore, the advent of PBM is likely to cover a wide spectrum of clinical applications. The finding that irradiation of external electromagnetic energy can increase and substitute for endogenous ATP production, provide a theoretic background to account for the potential of PBM.
THERAPEUTIC POTENTIALS OF PBM
One of the most studied PBM would be an arena of wound healing. Low-density red laser light therapy was first introduced to accelerate would healing in 1970’s (Mester et al., 1971). The light therapy has been applied to a variety of pathological conditions such as wound healing and dermatologic, neurologic diseases (Desmet et al., 2006). It further extended their boundary to blood disorders and musculoskeletal diseases and pain (Abergel et al., 1984; Lee et al., 1996; Wedlock et al., 1996; Yaakobi et al., 1996; Rochkind et al., 2013). Red laser therapy was known to affect cellular processes without significant thermal effects.
Zhang et al. (2003) have reported that irradiation of red light (628 nm) resulted in expression shift of 111 genes in HS27 human fibroblasts. They also grouped those genes into 10 functional categories. Most genes seem to play roles in the cell proliferation and suppression of cell death. Besides, they also promote the production of extracellular matrix proteins, all of which are important factors in wound healing. Overall, these genes orchestrated a prolonged increase in mitochondrial ATP production and cellular stress resistance (Lane, 2006). Energy transduction and production of ATP is largely relied on electron transport chain in mitochondria. The protein complex in the chain has a lot of metal clusters including iron and copper. In most cases, the iron is incorporated in iron-sulfur cluster or heme cofactors found in electron transport chain.
Researchers studied that near IR therapy protects from methanol poisoning (Eells et al., 2003), which can injure the retina and optic nerve. A metabolic toxicant of methanol, formic acid binds and inhibits COX activity. In this animal model, irradiation of near IR restores the function of retina. Retinitis pigmentosa was shown to be attenuated by near IR phototherapy via inhibiting apoptosis in retinal cells.
HEMOPROTEINS
Heme cofactors are found in many proteins (Kim et al., 2006; Munro et al., 2009; Lee et al., 2013). Hemoproteins play various roles in oxygen transport, energy transduction, gene regulation, de novo steroid and lipid biosynthesis, cellular signaling and gene expression (Table 1). Diverse physiological functions performed by hemoproteins make heme as one of the most versatile of protein cofactors (Munro et al., 2009).
Table 1.
Representatives of hemoproteins and their function, location
| Hemoprotein | Function | Subcellular localization |
|---|---|---|
| Succinate dehydrogenase | Electron transport (complex II) | Mitochondria inner membrane (IM) |
| Cytochrome bc1 complex | Electron transport (complex III) | Mitochondria IM |
| Cytochrome c | Electron transport | Intermembrane space |
| COX | Electron transport (complex IV) | Mitochondria IM |
| Cyp11A1 | Steroid biosynthesis | Mitochondria matrix |
| Hemoglobin | Gas-binding | Cytosol |
| Myoglobin | Gas-binding | Cytosol |
| Neuroglobin | Gas-binding | Cytosol |
| Cytoglobin | Gas-binding | Cytosol |
| NOS | NO signaling | Cytosol, membrane |
| Soluble guanylyl cyclase | NO and calcium signaling | Cytosol |
| Hap1 | Transcription factor | Nucleus |
| Bach1 | Transcription factor | Cytosol/nucleus |
| Rev-erbα | Transcription factor | Nuclear |
| DGCR8 | miRNA processing | Nuclear |
| mPer2 | Circadian rhythm | Cytosol/nuclear |
| Cytochrome b5 | Electron carrier | Cytosol |
| P450 cytochromes | Xenobiotics metabolism | ER* membrane, cytosol |
| Prostaglandin synthase | Signaling (COX-1, COX-2) | ER |
| myeloperoxidase | Microbicide | Lysosome-like azurophil granule |
| Catalase | Antioxidant | peroxisome |
| Thyroperoxidase | Thyroid hormone synthesis | Plasma membrane |
| Ferric reductase | Iron transport | Plasma membrane |
| Lactoperoxidase | Microbicide | Secreted |
| Ligninase | Nutrient breakdown | Secreted |
| Heme-containing chloroperoxidase | Haloperoxidase | Secreted |
| Peroxidasin | Extracellular matrix synthesis | Secreted |
| HRG-3 | Heme mobilization | Secreted |
Korolnek, T. and Hamza I. (2014) Like iron in the blood of the people: the requirement for heme trafficking in iron metabolism. Front. Pharmacol. 5, 126 (modified).
ER: endoplasmic reticulum.
A wide variety of heme axial ligands are known (Munro et al., 2009). These ligands are very important for the modulation of heme iron reduction potential; thereby the capability of the cytochromes to take parts in the redox reactions. Ligands to heme iron can be removed or replaced by other ligands during catalytic cycles of certain hemoproteins to carry out their functions (i. e., light replace NO with oxygen). Thus, alteration of the protein environment, along with the changes in axial ligands to the heme iron resulted in considerable variations in heme iron reduction potential and in the activity of hemoproteins. So far, there is a wealth of structural data available for hemoproteins depending on their redox states and ligand-bound forms.
Probably hemes are the most easily identifiable cofactors since their conjugated porphyrin structures give rise to electronic transitions in the visible region and strong red color (Munro et al., 2009). The factors for determining the absorption spectra for heme proteins are unique, for example, redox state, the nature of the ligands to the heme iron, and the heme iron spin-state. Characteristic feature of the absorption spectra for heme proteins are shown in Table 2. The spectra for various hemoproteins are ranging from visible to near IR or IR region at 800–2,500 nm (Munro et al., 2009).
Table 2.
Principal UV/Vis characteristics of some hemoproteins
| Enzyme | Status | Absorption wavelength (nm) |
|---|---|---|
| Bovine COX | Fully oxidized | 425, 597 |
| Fully reduced | 444, 604 | |
| Compound A (CO) | 588–590 (+CO), 608–612 (−CO) | |
| Horseradish peroxidase | Ferric | 402–404, 495–500 |
| Ferrous | 437, 556–560 | |
| Yeast cytochrome c peroxidase | Ferric | 408, 507, 647 |
| Ferrous | 438, 560 |
Rich, P. R. and Iwaki, M. (2007) A comparison of catalytic site intermediates of cytochrome c oxidase and peroxidases. Biochemistry (Moscow) 72, 1047–1055 (modified).
CYTOCHROME C OXIDASE
Oxidative phosphorylation take place in mitochondria composed of four complex (Wong-Riley et al., 2001; Lane, 2006; Karu, 2008). Among them, cytochrome c oxidase (1.9.3.1, COX) is a component of complex IV with metal clusters containing iron and copper. COX is a large transmembrane protein complex found in bacteria and the mitochondria of eukaryotes. It is the last enzyme in the respiratory electron transport chain of mitochondria, receives an electron from each of four cytochrome c molecules, and transfers the electrons to an oxygen molecule, forming 2 moles of water (Fig. 1). Since most of the oxygen consumption in cells are catalyzed by the heme-copper oxidases, their uniqueness in the cellular respiration and energy supply is essential for aerobic organisms. COX catalyzes the reduction of oxygen to water and utilizes the free energy of the redox reaction for proton pumping across the membrane, establishing the electrochemical proton gradient that drives ATP production.
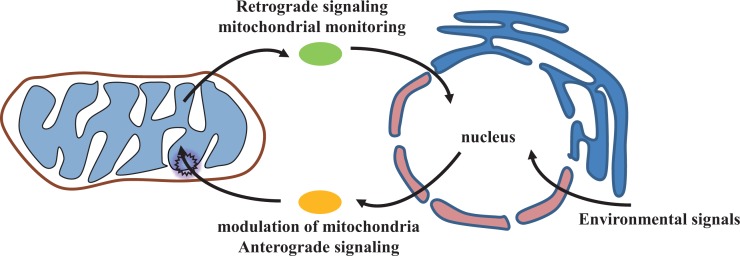
The rationale of retrograde signaling from mitochondria is based upon 2 observations. First, PBM (300–860 nm) can increase in DNA synthesis rate in cultured cells. However, the nucleus does not have chromophores absorbing light in this range of spectrum. Second, cumulating data by this time clearly shown that the photoacceptors are localized to the respiratory chain of mitochondria (Karu, 2008). Therefore it would be reasonable to propose the existence of cellular signaling pathway originated from this organelle. Experimental data also support retrograde signaling can be mediated via mitochondrial membrane potential (MMP), reactive oxygen species (ROS), changes in calcium flow, NO binding to COX (Fig. 1). Impairment of mitochondrial functioning routinely generates signaling mediators described above, which may determine the fate of this organelle. Rapidly growing cells prefer cell death instead of fixing the damaged mitochondria. In contrast, quiescent cells such as neuron try to recover the injured mitochondria by retrograde signaling pathway (Fig. 2). Therefore, it would be a survival strategy adopted by cells or organisms to maintain homeostasis of the quintessential sub-cellular organelle, mitochondria. In line with this notion, Park et al. (2013) reported that repeated thermal therapy by far-IR irradiation improves vascular functions via acute activation of endothelial NO synthase (NOS). The increase of eNOS was accompanied by an increase in intracellular calcium levels. Therefore, they suggested a possible involvement of temperature-sensitive calcium channel, transient receptors potential vannilloid (TRPV) ion channel at the plasma membrane as a photoacceptor. They did not discuss the involvement of retrograde signaling pathway in this setting, it might be possible that NO acts as a mediator for the signaling pathway.
Fig. 2.
Integration of intracellular mitochondrial function rely on both anterograde and retrograde signaling pathway. The anterograde signaling system transduces signals from environments (external and/or internal) to nucleus, activating genomic program to adjust mitochondrial functioning. The retrograde signaling system monitors the function of mitochondria and transduce signals back to the nucleus. Studies on cytoplasmic transducers are actively pursued. See text for details.
As described above, the analysis of the PBM action on DNA and RNA synthesis rate at the wavelength range 330∼860 nm helped conclude that in fact COX acts as a photoacceptor (Karu, 2008). The band peaks of light for the activation of DNA synthesis were identified by analogy with metal-ligand absorption spectra, which span through visible, near IR ranges. This conclusion goes also well with Warburg’s old experimental data (Lane, 2006).
RETROGRADE SIGNALING FROM MITOCHONDRIA: THE FUNCTION OF CELLULAR RESPIRATION
Mitochondria are the central organelle for diverse cellular functions as an integrator of signals originated from outside or inside of the cells (Lee et al., 2013). Many reports proposed that extramitochondrial factors which control the nuclear gene expression for mitochondrial proteins and their biogenesis (Fig. 2). A major cellular communication includes the transduction of extracellular signal to nucleus (Karu, 2008). However, other pathway of cellular communication from mitochondria to the nucleus is known as a mitochondrial retrograde signaling pathway. It is, therefore, not surprising that mitochondria are once free-living organisms accidently residing in eukaryotic common ancestors. The most investigated retrograde signaling pathway is performed using budding yeasts Saccharomyces cerevisiae. Later, the signaling pathway of mitochondria-nucleus crosstalk is also described in mammalian cells, including myocytes and tumor cells (Karu, 2008).
Recently Karu (2008) provides a summary for the mitochondrial retrograde signaling which operates in the irradiated cells. As described above, the retrograde signaling is associated with mitochondrial membrane potential, ROS and calcium mobilization. In some cases biological gases are involved in the signaling pathway by binding to heme center of a certain hemoproteins in general, COX in particular. Mitochondria have their own gas-synthesizing enzymes, mtNOS and heme oxygenase-1 (Slebos et al., 2007; Zaobornyj and Ghafourifar, 2012). The enzymatic activity is localized to the mitochondrial inner membrane where the electron transport chain resides and operates. Binding of the gas molecules produced by PBM to COX hemoprotein can divert the fate of oxygen into ROS formation (Moncada and Bolaños, 2006; Zuckerbraun et al., 2007). Under the condition of metabolic demand, COX hardly has more than 20% of the total control over ATP production in cells, implying that the gas-mediated changes in oxygen consumption can occur without a significant decline of ATP synthesis (Karu, 2008). Thus a window exists at which low level of gas molecules can regulate mitochondrial ROS production without ATP loss. But the spatio-temporal regulation of gas producing enzyme by PBM is not known.
Retrograde signaling pathway transduces the signal via regulation of redox sensitive transcription factors, nuclear factor (NF)-κB or AP-1 (Karu, 2008). In some tumors, reduced production of ATP also linked to the activation of NF-κB via generation of ROS (Chen et al., 2014). Besides, retrograde signaling pathway contributes for the maintenance of mitochondrial homeostasis by controlling fusion-fission events (Karu, 2008).
WATER: A NOVEL PLAYER?
The IR absorption spectrum of water was known as early as 1950’s (Curcio and Petty, 1951). With advances in new technology the absorption spectra of water was more clearly demonstrated (Santana-Blank et al., 2013). However most of the studies were carried out using ordinary liquid water. Recently, experimental evidences show that the intracellular water structure is not the same as that of ordinary bulk water. As mentioned above, the structured water, also termed exclusion zone (EZ) water, is formed at the interface of most hydrophilic surfaces (polymers or biological molecules). This feature of EZ is a solute-free area, extending hundreds of micrometers (Pollack, 2013). Very recently, Pollack (2013) summarized and noted the exclusion zone (EZ), defined as a fourth phase of water. Based upon this water state, a few investigators hypothesize that EZ might be a target of PBM as an energy sinker, which cells may use this to fuel cellular processes (from page 2).
In biological milieu, intracellular proteins may serve as a surface for water structuring. Interestingly, it has been reported that PBM can increase potential energy in the EZ water, which in turn, acts as an energy reservoir. The reserved energy, then, can selectively supply energy demands in cells (Pollack, 2013).
The behavior of cells is one of the current topics in cell biology. Water contents in tumor cells may different from those found in normal cells (Pollack, 2013). Cellular aging and neurodegenerative diseases are at least in part attributed to the impairment of water handling in cells or organisms (Pollack, 2013). If PBM can modulate the behavior of water in some ways, it would affect the integrity of biological molecules including DNA and proteins. It warrants further investigations.
CONCLUSION
PBM including near IR irradiation therapy starts its journey to cell biology and medicine. The molecular target of PBM might be a variety of hemoproteins, but COX was the only target to be investigated actively. In addition, the targets of the PBM may be a simple molecule, water. Two-thirds by volume, 99% by number are water in mammalian cells. But water research is in its embryo state, slowing the better understanding of cell biology.
Acknowledgments
The work was supported by National Research Foundation, Korea 2011-0024407.
REFERENCES
- Abergel RP, Meeker CA, Lam TS, Dwyer RM, Lesavoy MA, Uitto J. Control of connective tissue metabolism by lasers: recent developments and future prospects. J Am Acad Dermatol. 1984;11:1142–1150. doi: 10.1016/s0190-9622(84)80194-2. [DOI] [PubMed] [Google Scholar]
- Carroll JD, Milward MR, Cooper PR, Hadis M, Pain WM. Developments in low level light therapy (LLLT) for dentistry. Dent Mater. 2014;30:465–475. doi: 10.1016/j.dental.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Chen WW, Birsoy K, Mihaylova MM, Snitkin H, Stasinski I, Yucel B, Bayraktar EC, Carette JE, Clish CB, Brummelkamp TR, Sabatini DD, Sabatini DM. Inhibition of ATPIF1 ameliorates severe mitochondrial respiratory chain dysfunction in mammalian cells. Cell Rep. 2014;7:27–34. doi: 10.1016/j.celrep.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol. 1996;23:492–496. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Curcio JA, Petty CC. The near infrared absorption spectrum of liquid water. J Optic Soc Am. 1951;41:302–304. [Google Scholar]
- Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, Buchmann EV, Connelly MP, Dovi JV, Liang HL, Henshel DS, Yeager RL, Millsap DS, Lim J, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Clinical and experimental applications of NIR-LED photo-biomodulation. Photomed Laser Surg. 2006;24:121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whela NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Karu TI. Mitochondrial signaling in mammalian cells activated by red and near IR radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- Korolnek T, Hamza I. Like iron in the blood of the people: the requirement for heme trafficking in iron metabolism. Front Pharmacol. 2014;5:126. doi: 10.3389/fphar.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. Power games. Nature. 2006;443:901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- Lee G, Wong E, Mason DT. New concepts in pain management and in the application of low-power laser for relief of cervicothoracic pain syndromes. Am Heart J. 1996;132:1329–1334. doi: 10.1016/s0002-8703(96)90515-3. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Zhang J, Choi AM, Kim HP. Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev. 2013;2013:327167. doi: 10.1155/2013/327167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–535. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Munro AW, Girvan HM, McLean KJ, Cheesman MR, Leys D. Heme and Hemoproteins. In: Warren MJ, Smith AG, editors. Tetrapyrrole: Birth, Life and death. Landes Bioscience and Springer Science; Austin: 2009. pp. 160–183. [Google Scholar]
- Park JH, Lee S, Cho DH, Park YM, Kang DH, Jo I. Far-infrared radiation acutely increases nitric oxide production by increasing Ca(2+) mobilization and Ca(2+)/calmodulin-dependent protein kinase II-mediated phosphorylation of endothelial nitric oxide synthase at serine 1179. Biochem Biophys Res Commun. 2013;436:601–606. doi: 10.1016/j.bbrc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Pollack GH. The fourth phase of water. Ebner & Sons publishers; Seattle: 2013. [Google Scholar]
- Quirk BJ, Desmet KD, Henry M, Buchmann E, Wong-Riley M, Eells JT, Whelan HT. Therapeutic effect of near infrared (NIR) light on Parkinson’s disease models. Front Biosci. 2012;4:818–823. doi: 10.2741/E421. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Stehno-Bittel L, Enwemeka CS. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001;9:248–255. doi: 10.1046/j.1524-475x.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- Rich PR, Iwaki M. A comparison of catalytic site intermediates of cytochrome c oxidase and peroxidases. Biochemistry (Moscow) 2007;72:1047–1055. doi: 10.1134/s0006297907100033. [DOI] [PubMed] [Google Scholar]
- Rochkind S, Geun S, Shainberg A. Phototherapy and nerve injury: focus on muscle response. Int Rev Neurobiol. 2013;109:99–109. doi: 10.1016/B978-0-12-420045-6.00004-3. [DOI] [PubMed] [Google Scholar]
- Santana-Blank L, Rodríguez-Santana E, Santana-Rodríguez KE. Photobiomodulation of aqueous interfaces: finding evidence to support the exclusion zone in experimental and clinical studies. Photomed Laser Surg. 2013;31:461–462. doi: 10.1089/pho.2013.3583. [DOI] [PubMed] [Google Scholar]
- Schindl A, Schindl M, Pernerstorfer-Schön H, Schindl L. Low-intensity laser therapy: a review. J Investig Med. 2000;48:312–326. [PubMed] [Google Scholar]
- Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tanaka Y, Matsuo K, Yuzuriha S, Yan H, Nakayama J. Non-thermal cytocidal effect of infrared irradiation on cultured cancer cells using specialized device. Cancer Sci. 2010;101:1396–1402. doi: 10.1111/j.1349-7006.2010.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlock P, Shephard RA, Little C, McBurney F. Analgesic effects of cranial laser treatment in two rat nociception models. Physiol Behav. 1996;59:445–448. doi: 10.1016/0031-9384(95)02080-2. [DOI] [PubMed] [Google Scholar]
- Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Bai X, Buchmann E, Whelan HT. Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport. 2001;12:3033–3037. doi: 10.1097/00001756-200110080-00011. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int. 1996;59:297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- Ying R, Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Res. 2008;1243:167–173. doi: 10.1016/j.brainres.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaobornyj T, Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol. 2012;303:H1283–1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120:849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]