Abstract
In the present study, we found that the natural compound arctigenin inhibited hydrogen peroxide-induced reactive oxygen species (ROS) production in rat primary astrocytes. Since hemeoxygenase-1 (HO-1) plays a critical role as an antioxidant defense factor in the brain, we examined the effect of arctigenin on HO-1 expression in rat primary astrocytes. We found that arctigenin increased HO-1 mRNA and protein levels. Arctigenin also increases the nuclear translocation and DNA binding of Nrf2/c-Jun to the antioxidant response element (ARE) on HO-1 promoter. In addition, arctigenin increased ARE-mediated transcriptional activities in rat primary astrocytes. Further mechanistic studies revealed that arctigenin increased the phosphorylation of AKT, a downstream substrate of phosphatidylinositol 3-kinase (PI3K). Treatment of cells with a PI3K-specific inhibitor, LY294002, suppressed the HO-1 expression, Nrf2 DNA binding and ARE-mediated transcriptional activities in arctigenin-treated astrocyte cells. The results collectively suggest that PI3K/AKT signaling pathway is at least partly involved in HO-1 expression by arctigenin via modulation of Nrf2/ARE axis in rat primary astrocytes.
Keywords: Arctigenin, Astrocyte, Hemeoxygenase-1, PI3K/AKT, Nrf2/ARE axis
INTRODUCTION
Astrocytes, the major non-neuronal cells in the brain, play a pivotal role by interacting with neurons to provide structural, metabolic, and trophic support (Dallerac et al., 2013). In particular, astrocytes maintain a homeostatic environment for neurons by controlling oxidative stress. Various antioxidant enzymes are prominently expressed in astrocytes, enabling efficient detoxification and protection against reactive oxygen species (ROS) (Ryter et al., 2006; de Vries et al., 2008; Vargas and Johnson, 2009).
Hemeoxygenase-1 (HO-1) is the rate limiting-enzyme that catalyzes the oxidation of heme to carbon monoxide (CO), biliverdin, and free iron (Fe2+). HO-1 belongs to phase II antioxidant enzymes that are mainly involved in detoxification and cytopoprotection mechanisms of astrocytes, the expression of which is controlled by Nrf2-ARE signaling pathways (Otterbein et al., 2003). Considering that brain is highly vulnerable to oxidative stress, development of agents that can control oxidative stress by modulating HO-1 expression is considered to be a potential therapeutic approach for treatment of various neurodegenerative diseases (Syapin, 2008).
Arctigenin, the phenylpropanoid dibenzylbutyrolactone lignan isolated from the seeds of Arctium lappa, has been reported to have a variety of pharmacological activities including antitumor, anti-viral, anti-inflammatory, diuretic, and detoxifying effects (Kou et al., 2011; Li et al., 2014; Shi et al., 2014). In addition, arctigenin decreased H2O2-induced ROS production and elevated antioxidant capacity of the skeletal muscles (Wu et al., 2014). Recent studies have demonstrated on the neuroprotective effects of arctigenin in the brain. Arctigenin protected cortical neurons from glutamate-induced toxicity by binding to kainate receptor (Jang et al., 2002). Arctigenin exerted protective effects in ischemic stroke through inhibition of neuroinflammation, and ameliorated memory impairment in Alzheimer’s disease mouse models (Fan et al., 2012; Zhu et al., 2013).
Despite previous studies, the antioxidant effects of arctigenin in brain glial cells have not been reported and detailed molecular mechanism remains obscure. In this study, we found that arctigenin inhibited ROS production and increased HO-1 expression in rat primary astrocytes. Further mechanistic studies revealed that PI3K/AKT signaling pathway is involved in HO-1 expression by arctigenin via modulation of Nrf2/ARE axis. Our data collectively suggest that arctigenin may have therapeutic potential for various neurodegenerative diseases that are accompanied by oxidative stress.
MATERIALS AND METHODS
Reagents
Arctigenin was purchased from Enzo Life Sciences (Farmingdale, NY, USA). The chemical structure of arctigenin is as shown in Fig. 1A. All reagents used for cell culture were purchased from Thermo Scientific (Fremont, CA, USA). Antibodies against HO-1, Nrf-2, c-Jun, and lamin A were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Antibodies against phospho- or total forms of ERK, JNK, p38, and AKT were obtained from Cell Signaling Technology (Danvers, MA, USA). All reagent used for RT-PCR were purchased from Promega (Madison, WI, USA).
Fig. 1.
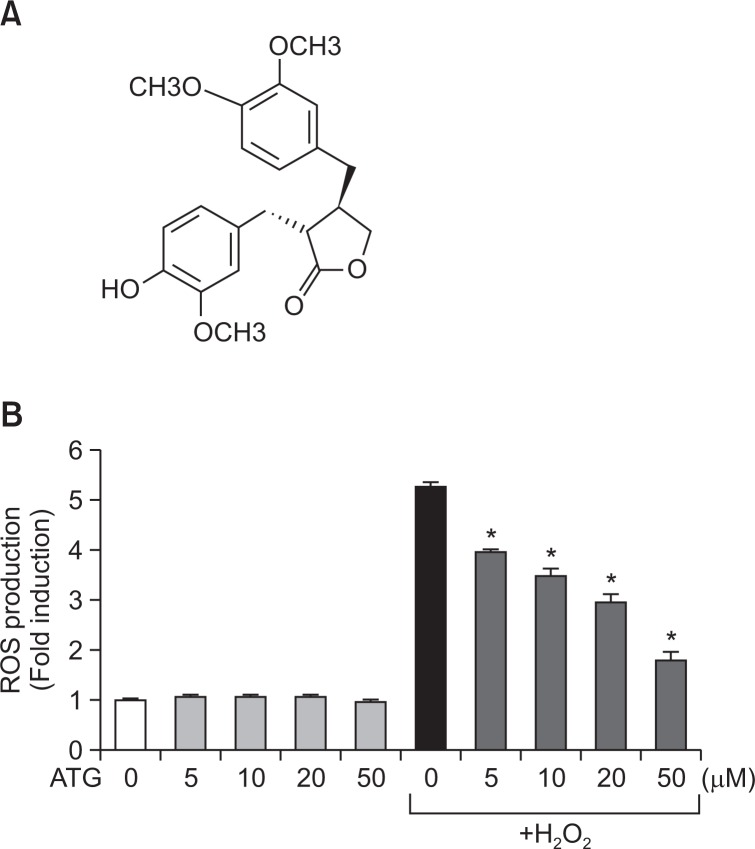
Antioxidant effects of arctigenin in rat primary astrocytes. (A) Chemical structure of arctigenin. (B) Rat primary astrocyte cells were treated with 5 to 50 μM arctigenin for 1 h, followed by treatment of H2O2 (500 μM) for 30 min. The intracellular ROS levels were then measured by the DCF-DA method as described in the Materials and Methods section. The data are expressed as the mean ± S.E.M. of three independent experiments. *p<0.05, significantly different from H2O2-treated samples. ‘ATG’ indicates arctigenin.
Rat primary astrocyte cell culture
Rat primary astrocytes cultures were prepared from mixed glial cultures using a previous method with modifications (Park et al., 2011b). In brief, after cortices were dissected from 2-day-old rats, cells were dissociated by pipetting and resuspended in minimal essential medium containing 10% fetal bovine serum, streptomycin (10 μg/mL), penicillin (10 U/mL), 2 mM glutamine, and 10 mM HEPES. Cell suspensions were plated on poly-D-lysine (1 μg/mL)-coated T75 flasks and incubated for 7-10 days. After the primary cultures reached confluence, the culture flasks were shaken at 280 rev/min for 16 h to remove microglia and oligodendrocytes. The purity of the astrocyte-enriched cultures (>95%) were confirmed by staining with antibodies against the astrocyte-specific marker glial fibrillary acidic protein.
Measurement of intracellular ROS levels
The intracellular accumulation of ROS was measured with H2DCF-DA (Sigma-Aldrich) by modifying a previously reported method (Lee and Kim, 2011). In brief, astrocyte cells were stimulated with H2O2 for 1 h and stained with 20 μM H2DCF-DA in PBS for 30 min at 37°C. DCF fluorescence intensity was measured at the 488 nm excitation and 535 nm emission on a fluorescence plate reader.
RT-PCR
Rat primary astrocytes (2.5×105 cells on a 6 well plate) were treated with arctigenin, and total RNA was extracted with TRI reagent (Invitrogen). For RT-PCR, total RNA (1 μg) was reverse-transcribed in a reaction mixture that contains 1 U RNase inhibitor, 500 ng random primers, 3 mM MgCl2, 0.5 mM dNTP, and 10 U reverse transcriptase (Promega). The synthesized cDNA was used as a template for PCR reaction using GoTaq polymerase (Promega) and primers, as below. 5′-TGT CAC CCT GTG CTT GAC CT-3′ and 5′-ATA CCC GCT ACC TGG GTG AC-3′ for HO-1; 5′-GTG CTG AGT ATG TCG TGG AGT C-3′ and 5′-ACA GTC TTC TGA GTG GCA GTC A-3′ for GAPDH.
Western blot analysis
Cells were appropriately treated and total cell lysates were prepared, as previously described (Jung et al., 2010). Proteins (20–100 μg) were heated with 4×SDS sample buffer and separated by SDS-PAGE gel electrophoresis and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies (1:1000) and then, horseradish peroxidase-conjugated secondary antibodies (1:2000 dilution in TBST; New England Biolabs, Ipswich, MA) were applied and the blots were developed using an enhanced chemiluminescence detection kit (Thermo Fisher Scientific, Waltham, MA).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from astrocytes as previously described (Woo et al., 2003). The double-stranded DNA oligonucleotides containing the ARE consensus sequences (Promega) were end-labeled by [γ-32P] ATP. Five micrograms of the nuclear proteins were incubated with 32P-labeled ARE probes on ice for 30 min and resolved on a 5% acrylamide gel as previously described (Woo et al., 2003).
Transient transfection and luciferase assays
Rat primary astrocytes were plated in 12 well plate at the density of 2.5×105 cells, and transfected with 0.5 μg of plasmid DNA using ConvoyTm Platinum transfection reagent (CellTA-Gen, Korea). To determine the effect of arctigenin on ARE promoter activity, cell were treated with arctigenin and incubated for 16 h prior to harvesting cells and luciferase assay was performed as previously described (Park et al., 2007).
Statistical analysis
Unless otherwise stated, all experiments were performed with triplicate samples and repeated at least three times. The data are presented as mean ± S.E.M. and statistical comparisons between groups were performed by using one-way analysis of variance, followed by Newman-Keuls test. A p value < 0.05 was considered significant.
RESULTS
Arctigenin inhibited ROS production in H2O2-treated astrocytes
To determine the antioxidant capacity of arctigenin, we measured intracellular ROS scavenging activity of arctigenin in H2O2-treated astrocyte cells. We found that arctigenin significantly inhibited H2O2-induced ROS production in a dose-dependent manner (Fig. 1B). MTT assay data showed that arctigenin was not cytotoxic at least up to 100 μM (data not shown). The results suggest the strong antioxidant effects of arctigenin in rat primary astrocytes.
Arctigenin increased the expression of antioxidant enzyme HO-1 in astrocytes
To investigate the molecular mechanism underlying antioxidant effects of arctigenin, we examined the effect of arctigenin on the expression of HO-1, which plays a critical role as an antioxidant defense factor in the brain. Western blot and RTPCR analyses showed that arctigenin increased HO-1 expression at the protein and mRNA levels (Fig. 2). We observed that 5–20 μM of arctigenin upregulated HO-1 expression in a concentration-dependent manner (Fig. 2A, C). In addition, arctigenin (20 μM) induced HO-1 mRNA and protein expression at 1 h, the level of which was increased at least up to 6 h (Fig. 2B, D).
Fig. 2.
Effect of arctigenin on HO-1 expression in rat primary astrocytes. Cells were treated with various concentration of arctigenin for 6 h (A, C) or incubated with 20 μM arctigenin for the indicated time points (B, D). The HO-1 protein and mRNA levels were determined by western blot and RT-PCR analyses. The data are representative of three independent experiments. Quantification data are shown at the bottom of each panel. Values are the mean ± S.E.M. of three independent experiments. *p<0.05, compared with the control group.
Arctigenin increased the nuclear translocation and DNA binding of Nrf2/c-Jun to ARE, and ARE-mediated transcriptional activities in rat primary astrocytes
We performed EMSA to determine whether arctigenin increases nuclear factor binding to ARE on HO-1 promoter. As shown in Fig. 3A, arctigenin increased ARE-nuclear protein binding complex. We have recently demonstrated that Nrf2 and c-Jun bind to ARE and coordinately regulate HO-1 expression in astrocytes (Park et al., 2011b; Park and Kim, 2014). Thus, we examined the effects of arctigenin on Nrf-2 and c-Jun translocation to nucleus. Western blot analysis showed that arctigenin increased the protein amount of Nrf2 and c-Jun in nuclear extracts of astrocyte cells (Fig. 3B). In addition, arctigenin increased ARE-mediated transcriptional activities as shown by ARE-luc reporter gene assay (Fig. 3C). The data suggest that arctigenin increases HO-1 gene expression by enhancing the transcription factor Nrf2/c-Jun binding to ARE on HO-1 promoter.
Fig. 3.
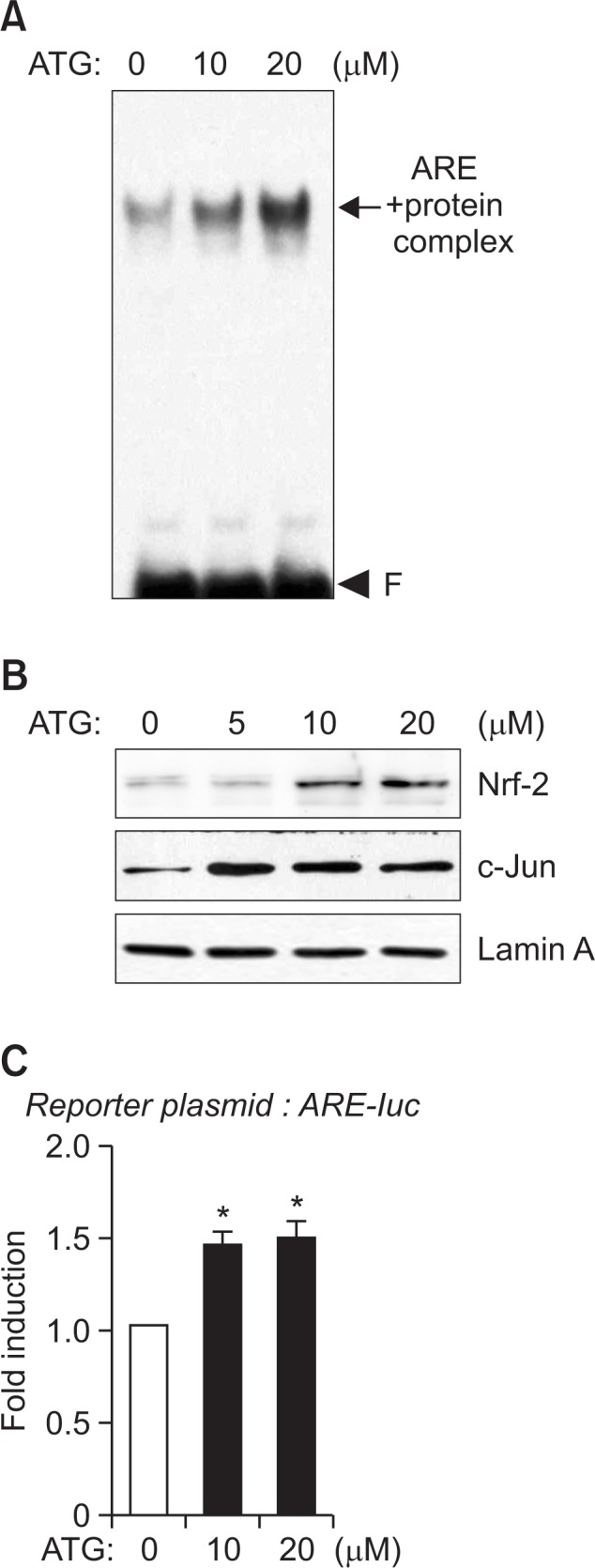
Effect of arctigenin on DNA binding and nuclear translocation of Nrf2 and c-Jun, and ARE-mediated transcriptional activities. The nuclear extracts were isolated from cells treated with 10 or 20 μM of arctigenin for 3 h. (A) The extracts were incubated 32P-labled with ARE probe. The arrow indicates the ARE-nuclear protein complex. ‘F’ indicates free probe. (B) The nuclear extracts used for EMSA were assessed by immunoblot using antibodies against Nrf-2 or c-Jun. The data are representative of three independent experiments. (C) Primary astrocytes were transfected with ARE-luc reporter plasmid and treated with arctigenin. After 16 h, cells were harvested, and luciferase assays were performed. Values correspond to the mean ± S.E.M of three independent experiments. *p<0.05, compared with control cells.
Arctigenin increased AKT phosphorylation, and treatment of LY294002 inhibited HO-1 expression by modulating Nrf2/ARE axis
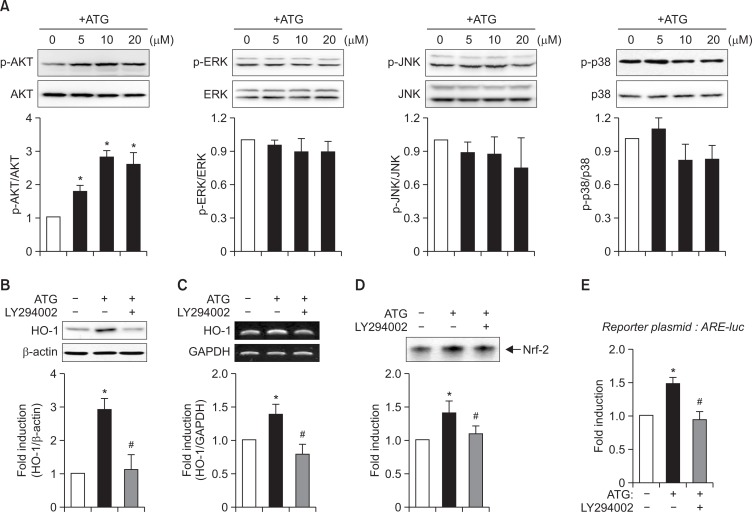
Previous studies reported that HO-1 expression is under the control of MAPK or PI3K signaling pathways in various cell types (Mates, 2000; Chen et al., 2012; Wang et al., 2012). To identify the signaling pathways modulating HO-1 induction by arctigenin, we examined the effects of arctigenin on MAPK and AKT phosphorylation. As shown in Fig. 4A, arctigenin increased AKT phosphorylation, but it did not affect the phosphorylation of three types of MAP kinases. To investigate whether PI3K/AKT pathway is involved in HO-1 upregulation by arctigenin, we examined the effect of LY294002 (a PI3K inhibitor) on HO-1 expression. As shown in Fig. 4B, C, LY294002 diminished arctigenin-induced HO-1 expression at the protein and mRNA levels. In addition, LY204002 suppressed Nrf2 DNA binding and ARE-luc reporter gene activities (Fig. 4D, E). The data suggest that PI3K/AKT signaling pathway is at least partly involved in HO-1 expression by arctigenin by modulating Nrf2/ARE axis in rat primary astrocytes.
Fig. 4.
Role of PI3K/AKT signaling pathway in HO-1 expression in arctigenin-treated astrocyte cells. (A) Cell extracts were prepared from astrocytes treated with arctigenin for 1 h, and subjected to immunoblot analysis using antibodies against phospho- or total forms of AKT or three types of MAP kinases (p38 MAPK, ERK1/2, and JNK). The blots are representative of three independent experiments. Phosphorylation levels of AKT and MAP kinases were quantified by densitometer and expressed as fold induction. Data are the mean ± S.E.M of three independent experiments. *p<0.05, compared with the control. (B, C) Effect of PI3K inhibitor (LY294002) on HO-1 protein and mRNA expressions. Cells were treated arctigenin in the absence of presence of LY294002 (20 μM) for 12 h, and western blot (B) and RT-PCR (C) analyses were performed. The blots are representative of three independent experiments. Quantification data are shown at the bottom panel. (D, E) Effect of LY294002 on Nrf2 DNA binding (D), and ARE-luc activities (E). *p<0.05, compared with control samples. #p<0.05, compared with arctigenin-treated samples.
DISCUSSION
In the present study, we have demonstrated that the natural compound arctigenin inhibited ROS production in H2O2-treated rat primary astrocytes (Fig. 1). We have also demonstrated that arctigenin increased HO-1 expression by enhancing the nuclear translocation and ARE binding of Nrf2 and c-Jun transcription factors (Fig. 2, 3). Finally, PI3K/AKT signaling pathway was identified to be involved in HO-1 expression by modulating Nrf2/ARE axis in arctigenin-treated astrocyte cells (Fig. 4).
Recent studies have suggested Nrf2-ARE signaling pathways as key therapeutic targets for treatment of neuroinflammation and brain disorders (Syapin, 2008). Moreover, Nrf2 is known as a multi-organ protector (Lee et al., 2005). Nrf2 protects various cell types such as liver, kidney, lung, and brain by increasing ARE-driven detoxification and antioxidant genes, such as HO-1, NQO-1, and γ-glutamate cysteine ligase (Lee et al., 2005). Natural compounds, such as curcumin, sulforaphane, resveratrol, and caffeic acid phenyl ester have been reported to induce HO-1 expression by modulating Nrf2 signaling pathways (Lee and Surh, 2005). Our group recently reported that isoflavone metabolites such as tectorigenin and glycitein exert antioxidant effects in hydrogen peroxide-stimulated rat primary astrocytes by upregulating HO-1 and NQO1 expression (Park et al., 2011b). In addition, we demonstrated that tertiary-butylhydroquinone upregulates HO-1 expression by inducing the coordinate interaction of Nrf2 and c-Jun in astrocytes (Park and Kim, 2014). In the present study, we report for the first time the antioxidant effects of arctigenin in astrocytes, and that Nrf2-mediated HO-1 expression may be involved in antioxidant effects of arctigenin.
There are some studies linking the activation of PI3K/AKT signaling pathways to the up-regulation of phase II antioxidant enzymes (Surh, 2003; Li et al. 2006). Oxidative stress activates PI3K/AKT, which subsequently phosphorylate Nrf2 and facilitate Nrf2 release from its cytosolic inhibitor Keap1. Then, Nrf2 is translocated into the nucleus and binds to ARE, inducing the expression of downstream antioxidant genes (Surh, 2003). In addition, there are reports that LY294002 blocked HO-1 expression through Nrf2 pathway against oxidative stress (Martin et al., 2004; Lim et al., 2008). In support of these previous reports, our present study demonstrated that inhibition of PI3K/AKT by LY294002 blocked Nrf2 activation and subsequent HO-1 expression in arctigenin-treated astrocytes, suggesting that AKT phosphorylation by arctigenin plays an important role in Nrf2 activation.
The therapeutic effects of arctigenin have been reported in the brain and peripheral systems. Arctigenin ameliorates inflammation in lipopolysaccharide-induced acute lung injury in rats via activation of AMPK and suppression of NF-κB signaling pathway (Shi et al., 2014). Arctigenin also attenuated TNBS-induced cytokine expressions by inhibiting PI3K, AKT and IKKβ phosphorylations in mice (Hyam et al., 2013). They suggested arctigenin may ameliorate inflammatory disease by inhibiting PI3K/AKT and polarizing M1 macrophages to M2-like macrophages. Thus, the roles of PI3K/AKT appear to be somewhat different in arctigenin-treated astrocytes and inflammatory macrophage cells. The anti-inflammatory effects of arctigenin have been also reported in Raw264.7 macrophage cells, peritoneal macrophages, and brain microglia (Cho et al., 2004; Kou et al., 2011; Park et al., 2011a). A recent study reported that oral administration of arctigenin lowered blood glucose levels and improved lipid metabolism in ob/ob mice, suggesting the therapeutic potential of arctigenin for type 2 diabetes (Huang et al., 2012). In addition, the neuroprotective activity of arctigenin has been demonstrated in various in vitro and in vivo models of neuronal disorders. Arctigenin improved the movement behavior and upregulated dopamine levels in MPTP-injected mouse, an animal model of Parkinson’s disease (Li et al., 2014). Furthermore, the neuroprotective effects of arctigenin have been reported in cerebral ischemia rats and Alzheimer’s disease mouse models (Fan et al., 2012; Zhu et al., 2013).
Considering that oxidative stress is one of the factors contributing to development of neurodegenerative diseases, the antioxidant effects and HO-1 upregulation by arctigenin may support the therapeutic potential of arctigenin for treatment of various neurodegenerative diseases.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant #2012R1A5A2A32671866).
REFERENCES
- Chen JH, Huang SM, Tan TW, Lin HY, Chen PY, Yeh WL, Chou SC, Tsai CF, Wei IH, Lu DY. Berberine induces heme oxygenase-1 up-regulation through phosphatidylinositol 3-kinase/AKT and NF-E2-related factor-2 signaling pathway in astrocytes. Int Immunopharmacol. 2012;12:94–100. doi: 10.1016/j.intimp.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Cho MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-alpha inhibition. Int Immunopharmacol. 2004;4:1419–1429. doi: 10.1016/j.intimp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Dallerac G, Chever O, Rouach N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front Cell Neurosci. 2013;7:159. doi: 10.3389/fncel.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Fan T, Jiang WL, Zhu J, Feng Zhang Y. Arctigenin protects focal cerebral ischemia-reperfusion rats through inhibiting neuroinflammation. Biol Pharm Bull. 2012;35:2004–2009. doi: 10.1248/bpb.b12-00463. [DOI] [PubMed] [Google Scholar]
- Huang SL, Yu RT, Gong J, Feng Y, Dai YL, Hu F, Hu YH, Tao YD, Leng Y. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia. 2012;55:1469–1481. doi: 10.1007/s00125-011-2366-3. [DOI] [PubMed] [Google Scholar]
- Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, Han MJ, Kim DH. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol. 2013;708:21–29. doi: 10.1016/j.ejphar.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Jang YP, Kim SR, Choi YH, Kim J, Kim SG, Markelonis GJ, Oh TH, Kim YC. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J Neurosci Res. 2002;68:233–240. doi: 10.1002/jnr.10204. [DOI] [PubMed] [Google Scholar]
- Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, Kim DH, Hyun JW, Shin CY, Kim HS. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010;115:1668–1680. doi: 10.1111/j.1471-4159.2010.07075.x. [DOI] [PubMed] [Google Scholar]
- Kou X, Qi S, Dai W, Luo L, Yin Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int Immunopharmacol. 2011;11:1095–1102. doi: 10.1016/j.intimp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim HS. Inhibitory mechanism of MMP-9 gene expression by ethyl pyruvate in lipopolysaccharide-stimulated BV2 microglial cells. Neurosci Lett. 2011;493:38–43. doi: 10.1016/j.neulet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Li D, Liu Q, Jia D, Dou D, Wang X, Kang T. Protective effect of arctigenin against MPP+ and MPTP-induced neurotoxicity. Planta Med. 2014;80:48–55. doi: 10.1055/s-0033-1360171. [DOI] [PubMed] [Google Scholar]
- Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res. 2008;57:325–331. doi: 10.1016/j.phrs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Park JH, Hong YJ, Moon E, Kim SA, Kim SY. Forsythiae fructus and its active component, arctigenin, provide neuroprotection by inhibiting neuroinflammation. Biomol Ther. 2011a;19:425–430. [Google Scholar]
- Park JS, Jung JS, Jeong YH, Hyun JW, Le TK, Kim DH, Choi EC, Kim HS. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression. J Neurochem. 2011b;119:909–919. doi: 10.1111/j.1471-4159.2011.07395.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim HS. Regulation of hemeoxygenase-1 gene expression by Nrf2 and c-Jun in tertiary butylhydroquinone-stimulated rat primary astrocytes. Biochem Biophys Res Commun. 2014;447:672–677. doi: 10.1016/j.bbrc.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Park JS, Woo MS, Kim DH, Hyun JW, Kim WK, Lee JC, Kim HS. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J Pharmacol Exp Ther. 2007;320:1237–1245. doi: 10.1124/jpet.106.114322. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Shi X, Sun H, Zhou D, Xi H, Shan L. Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammation. 2014 doi: 10.1007/s10753-014-9969-z. [Epub ahed of print] [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21:368–378. doi: 10.1007/s12640-011-9292-5. [DOI] [PubMed] [Google Scholar]
- Woo MS, Jang PG, Park JS, Kim WK, Joh TH, Kim HS. Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutyryl-cAMP in BV2 microglial cells. Brain Res Mol Brain Res. 2003;113:86–96. doi: 10.1016/s0169-328x(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Wu RM, Sun YY, Zhou TT, Zhu ZY, Zhuang JJ, Tang X, Chen J, Hu LH, Shen X. Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol Sin. 2014;35:1274–1284. doi: 10.1038/aps.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, Li C, Hu L, Jiang H, Shen X. Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both beta-amyloid production and clearance. J Neurosci. 2013;33:13138–13149. doi: 10.1523/JNEUROSCI.4790-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]