Abstract
In the present study, we investigated whether apigenin significantly affects tumor necrosis factor-α (TNF-α)-induced production and gene expression of MUC5AC mucin in airway epithelial cells. Confluent NCI-H292 cells were pretreated with apigenin for 30 min and then stimulated with TNF-α for 24 h or the indicated periods. The MUC5AC mucin gene expression and mucin protein production were measured by reverse transcription - polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Apigenin significantly inhibited MUC5AC mucin production and down-regulated MUC5AC gene expression induced by TNF-α in NCI-H292 cells. To elucidate the action mechanism of apigenin, effect of apigenin on TNF-α-induced nuclear factor kappa B (NF-κB) signaling pathway was also investigated by western blot analysis. Apigenin inhibited NF-κB activation induced by TNF-α. Inhibition of inhibitory kappa B kinase (IKK) by apigenin led to the suppression of inhibitory kappa B alpha (IκBα) phosphorylation and degradation, p65 nuclear translocation. This, in turn, led to the down-regulation of MUC5AC protein production in NCI-H292 cells. Apigenin also has an influence on upstream signaling of IKK because it inhibited the expression of adaptor protein, receptor interacting protein 1 (RIP1). These results suggest that apigenin can regulate the production and gene expression of mucin through regulating NF-κB signaling pathway in airway epithelial cells.
Keywords: Airway, Mucin, Apigenin
INTRODUCTION
Airway mucus plays a pivotal role in defense against invading pathogenic microorganisms, chemicals and particles. The protective function of airway mucus is attributed to the viscoelasticity of mucins. However, any abnormality in the quality or quantity of mucins not only cause altered airway physiology but may also impair host defenses often leading to severe airway pathology as exemplified in chronic bronchitis, cystic fibrosis, asthma, and bronchiectasis (Voynow and Rubin, 2009). Therefore, we suggest it is valuable to find the possible activity of controlling (inhibiting) the excessive mucin secretion (production) by various medicinal plants. We have tried to investigate the possible activities of some natural products on mucin secretion and/or prodcution from airway epithelial cells. As a result of our trial, we previously reported that several natural products affected mucin secretion and/or production from airway epithelial cells (Heo et al., 2007; Heo et al., 2009; Lee et al., 2011). According to many literatures in the field of natural product pharmacology, apigenin (4′,5,7-Trihydroxyflavone) was reported to be a nontoxic and nonmutagenic flavonoid and have diverse biological activities including antioxidative, anti-inflammatory and anticancer effects (Illek and Fischer, 1998; Yin et al., 2001; McVean et al., 2002; Paoletti et al., 2009; Zhong et al., 2010; Clere et al., 2011; Zhou et al., 2011). Also, apigenin was isolated as the active component from Selaginellae Herba (Selaginella tamariscina Spring, Selaginellaceae), the major medicinal plant of Pyunkang-hwan (Pyunkang-tang), an herbal medicinal preparation used for controlling the hypersecretion of airway mucus observed in bronchitis, tonsiltis and pneumonitis in folk medicine (unpublished data). In our previous study, we demonstrated that apigenin inhibited epidermal growth factor (EGF)- or phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC protein and gene expression (Kim et al., 2012) and wogonin, another flavonoid, affected TNF-α-induced NF-κB signaling pathway in MUC5AC mucin producing NCI-H292 cells (Sikder et al., 2014). However, to the best of our knowledge, there are no reports about the potential effect of apigenin on mucin gene expression and production stimulated by a proinflammatory cytokine, TNF-α, from airway epithelial cells. Among the twenty one mucin genes which were identified, MUC5AC has been known as a major type of airway gel-forming mucin because it is highly expressed in the goblet cells (Takeyama et al., 2000) and is regulated by proinflammatory cytokines (Song et al., 2003). MUC5AC gene expression and protein production can be stimulated by treatment of TNF-α (Fischer et al., 1999; Shao et al., 2003; Song et al., 2003). Therefore, in this study, we checked whether apigenin significantly affects gene expression and production of airway mucin induced by TNF-α from NCI-H292 cells, a human pulmonary mucoepidermoid cell line, which are frequently used for the purpose of elucidating intracellular signaling pathways involved in airway mucin production and gene expression (Li et al., 1997; Takeyama et al., 1999; Shao et al., 2003).
MATERIALS AND METHODS
Materials
All the chemicals and reagents used in this study including apigenin (purity: 95.0%) were purchased from Sigma (St. Louis, MO, USA) unless otherwise specified. Anti-NF-κB p65 (sc-8008), anti-IkBα (sc-371), anti-actin (sc-8432) and anti-p84 (sc-98783) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-specific anti-p65 (serine 536, #3036S) and phospho-specific anti-IκBα (serine 32/36, #9246), anti-phospho-IKKα/β (Ser176/180, #2687) antibodies were purchased from Cell signaling Technology Inc. (Danvers, MA, USA). Goat Anti-rabbit IgG (#401315) or Goat Anti-mouse IgG (#401215) was used as the secondary antibody (Calbiochem, Carlsbad, CA, USA).
NCI-H292 cell culture
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 units/mL), streptomycin (100 μg/mL) and HEPES (25 mM) at 37ºC in a humidified, 5% CO2/95% air, water-jacketed incubator. For serum deprivation, confluent cells were washed twice with phosphate-buffered saline (PBS) and recultured in RPMI 1640 with 0.2% fetal bovine serum for 24 h.
Treatment of cells with apigenin
After 24 h of serum deprivation, cells were pretreated with varying concentrations of apigenin for 30 min and treated with TNF-α (10 ng/mL) for 24 h in serum-free RPMI 1640. Apigenin was dissolved in dimethylsulfoxide and treated in culture medium (final concentrations of dimethylsulfoxide were 0.5%). The final pH values of these solutions were between 7.0 and 7.4. Culture medium and 0.5% dimethylsulfoxide did not affect mucin gene expression and production from NCI-H292 cells. After 24 h, cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA and protease inhibitor cocktail (Roche Diagnostics, IN, USA) and collected to measure the production of MUC5AC protein (in 24-well culture plate). The total RNA was extracted for measuring the expression of MUC5AC gene (in 6-well culture plate) by using RT-PCR. For western blot analysis, cells were treated with apigenin for 24 h and then treated with TNF-α for the indicated periods.
MUC5AC mucin analysis
MUC5AC airway mucin production was measured by ELISA. Cell lysates were prepared with PBS at 1:10 dilution, and 100 μL of each sample was incubated at 42ºC in a 96-well plate, until dry. Plates were washed three times with PBS and blocked with 2% bovine serum albumin (BSA)(fraction V) for 1 h at room temperature. Plates were again washed three times with PBS and then incubated with 100 μL of 45M1, a mouse monoclonal MUC5AC antibody (1:200) (NeoMarkers, CA, USA), which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 h, the wells were washed three times with PBS, and 100 μL of horseradish peroxidasegoat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 h, plates were washed three times with PBS. Color reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) peroxide solution and stopped with 1 N H2SO4. Absorbance was read at 450 nm.
Total RNA isolation and RT-PCR
Total RNA was isolated by using Easy-BLUE Extraction Kit (INTRON Biotechnology, Inc. Kyung-Ki-do, Korea) and reverse transcribed by using AccuPower RT Premix (BIONEER Corporation, Daejeon, Korea) according to the manufacturer’s instructions. 2 mg of total RNA was primed with 1 μg of oligo (dT) in a final volume of 50 μL (RT reaction). 2 μL of RT reaction product was PCR amplified in a 25 mL by using Thermorprime Plus DNA Polymerase (ABgene, Rochester, NY, USA). Primers for MUC5AC were (forward) 5′-TGA TCA TCC AGC AGG GCT-3′ and (reverse) 5′-CCG AGC TCA GAG GAC ATA TGG G-3′. As quantitative controls, primers for Rig/ S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, were used. Primers for Rig/S15 were (forward) 5′-TTC CGC AAG TTC ACC TAC C-3′ and (reverse) 5′-CGG GCC GGC CAT GCT TTA CG-3′. The PCR mixture was denatured at 94ºC for 2 min followed by 40 cycles at 94ºC for 30 s, 60ºC for 30 s and 72ºC for 45 s. After PCR, 5 μL of PCR products were subjected to 1% agarose gel electrophoresis and visualized with ethidium bromide under a transilluminator.
Preparation of nuclear and cytosolic extracts
NCI-H292 cells (confluent in 150 mm culture dish) were pretreated for 24 h at 37ºC with 20 μM of apigenin and then stimulated with TNF-α (50 ng/mL) for 30 min. After the treatment, the cells were harvested using 3 x trypsin-EDTA solution and then centrifuged in a microcentrifuge (1,200 rpm, 3 min, 4ºC). The supernatant was discarded and the cell pellet was washed by suspending in PBS. The cytoplasmic and nuclear protein fractions were extracted using NE-PER® nuclear and cytoplasmic extraction reagent (Thermo-Pierce Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Both extracts were stored at −20ºC. Protein content in extract was determined by Bradford method.
Preparation of whole cell extract
After the treatment of the cells with apigenin, media were aspirated and the cells were washed with cold PBS. The cells were collected by scraping and centrifuged at 3,000 rpm for 5 min. The supernatant was discarded. The cells were mixed with RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) for 30 min with continuous agitation. The lysate was centrifuged in a microcentrifuge at 14,000 rpm for 15 min at 4ºC. The supernatant was used or immediately stored at −80ºC. Protein content in extract was determined by Bradford method.
Detection of proteins by western blot analysis
Cytosolic, nuclear and whole cell extracts containing proteins (each 20–60 mg as protein) were subjected to 7–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto the polyvinylidene difluoride (PVDF) membrane. The blots were blocked using 5% skim milk and probed with appropriate primary antibody in blocking buffer overnight at 4ºC. The membrane was washed with PBS and then probed with the secondary antibody conjugated with horseradish peroxidase. Immunoreactive bands were detected by an enhanced chemiluminescence kit (Pierce ECL western blotting substrate, Thermo Scientific, Waltham, MA, USA).
Statistics
Means of individual group were converted to percent control and expressed as mean ± S.E.M. The difference between groups was assessed using one-way ANOVA and Holm-Sidak test as a post-hoc test. p<0.05 was considered as significantly different.
RESULTS
Effect of apigenin on TNF-α-induced MUC5AC mucin production and gene expression
Apigenin inhibited TNF-α-induced MUC5AC mucin production. The amounts of MUC5AC mucin in the cells of apigenintreated cultures were 100 ± 8%, 368 ± 28%, 225 ± 13%, 153 ± 13% and 98 ± 8% for control, TNF-α 10 ng/mL only, TNF-α plus apigenin 5 μM, TNF-α plus apigenin 10 μM and TNF-α plus apigenin 20 μM, respectively (Fig. 1A). MUC5AC gene expression induced by TNF-α was also inhibited by pretreatment with 10 μM and 20 μM of apigenin (Fig. 1B). Cell viability was checked by sulforhodamine B (SRB) assay and there was no cytotoxic effect of apigenin, at 5, 10 and 20 μM concentration (data were not shown).
Fig. 1.
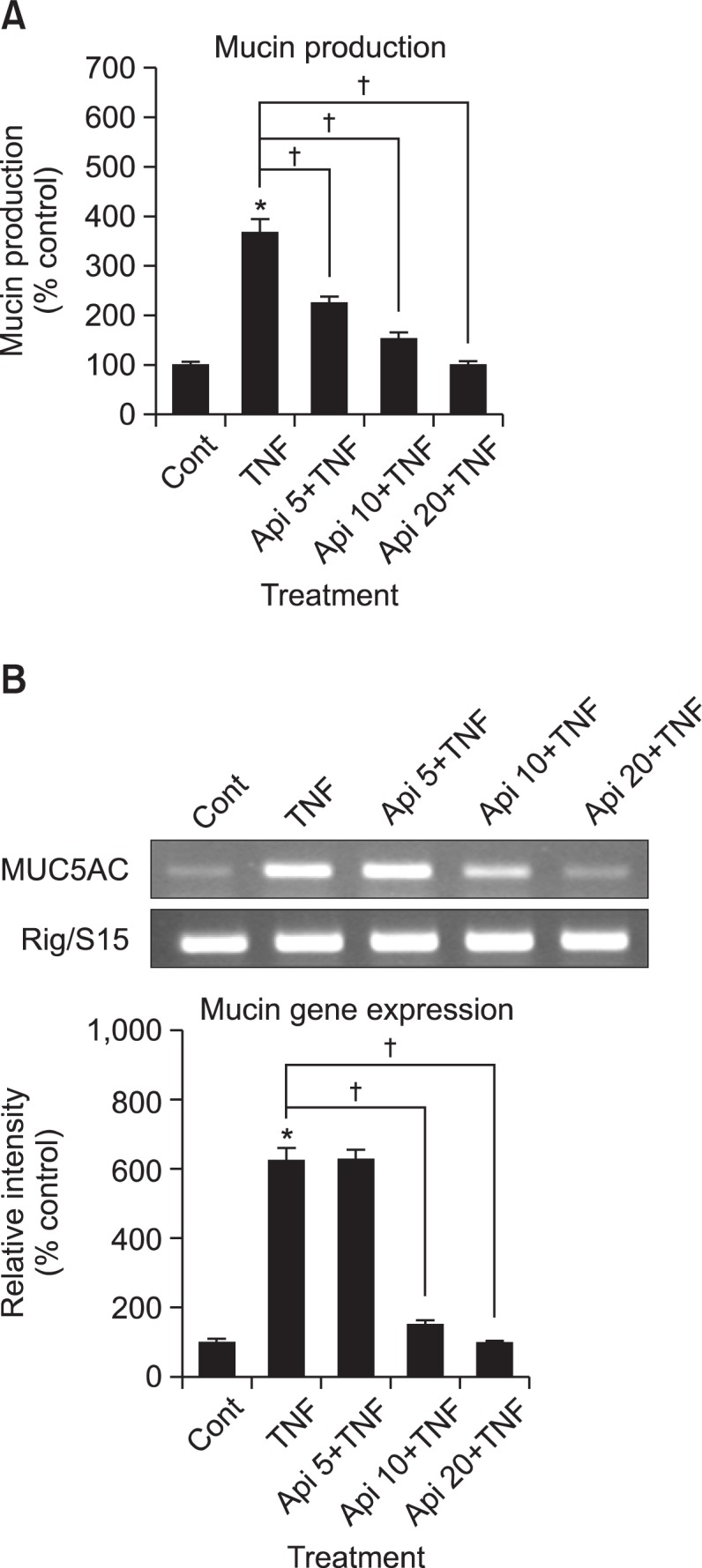
Effect of apigenin on TNF-α-induced MUC5AC mucin production and gene expression. NCI-H292 cells were pretreated with various concentrations of apigenin (5, 10 and 20 μM) for 30 min and then stimulated with TNF-α (10 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Three independent experiments were performed and the representative data were shown. Each bar represents a mean ± S.E.M. of 3 culture wells in comparison with that of control set at 100% (A). MUC5AC mucin gene expression was measured by RT-PCR. As quantitative control, Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, was used. Three independent experiments were performed and the representative data were shown (B). *significantly different from control (p<0.05). †significantly different from TNF-α alone (p<0.05). (cont: control, Api: apigenin, TNF: TNF-α, concentration unit is μM).
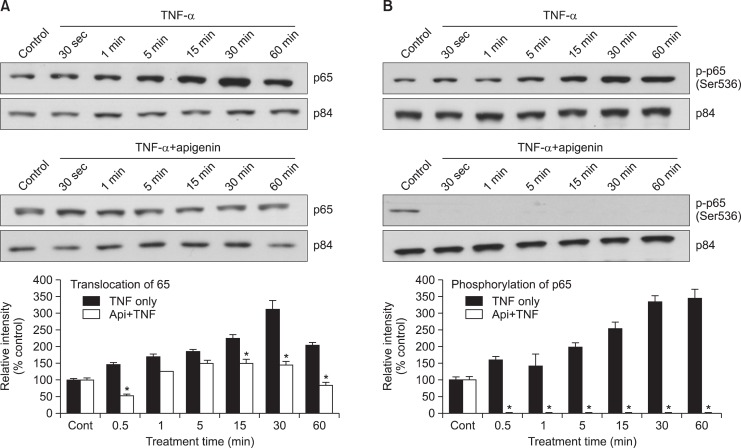
Effect of apigenin on TNF-α-induced nuclear translocation of NF-κB p65 and phosphorylation of p65
To determine the effect of apigenin on NF-κB activation followed by TNF-α treatment, we examined whether apigenin inhibits TNF-α-induced nuclear translocation of NF-κB p65. As shown in Fig. 2A, nuclear translocation of NF-κB p65 by TNF-α was inhibited by pretreatment with 20 μM of apigenin. In the nuclear fraction of the cells treated with TNF-α only, there was an increase in nuclear translocation of p65 gradually and reached optimal level at 30 min. However, in the cells treated with apigenin plus TNF-α, the level of p65 was gradually decreased as compared to the cells treated with TNF-α only. Next, transcriptional activity of p65 largely depends on its phosphorylation. For this reason, we investigated the effect of apigenin on TNF-α-induced phosphorylation of p65. As shown in Fig. 2B, TNF-α-induced phosphorylation of p65 was gradually increased and reached optimal level at 30 min. However, apigenin completely blocked the phosphorylation of p65.
Fig. 2.
Effect of apigenin on TNF-α-induced nuclear translocation of NF-κB p65 and phosphorylation of p65. NCI-H292 cells were either untreated or pretreated with 20 μM apigenin for 24 h at 37°C and then stimulated with TNF-α (50 ng/mL) for the indicated periods. Nuclear protein extracts were prepared and resolved on 10% SDS-PAGE, transferred onto a PVDF membrane, probed with antibody against p65 (A) or phospho-specific p65 (Ser 536) antibody (B). The results shown are the representative of three independent experiments. To ensure equal protein loading, the membrane was reprobed with anti-p84 antibody. *significantly different from TNF-α alone at each time point (p<0.05, Student’s t-test) (cont: control, Api: apigenin, TNF: TNF-α).
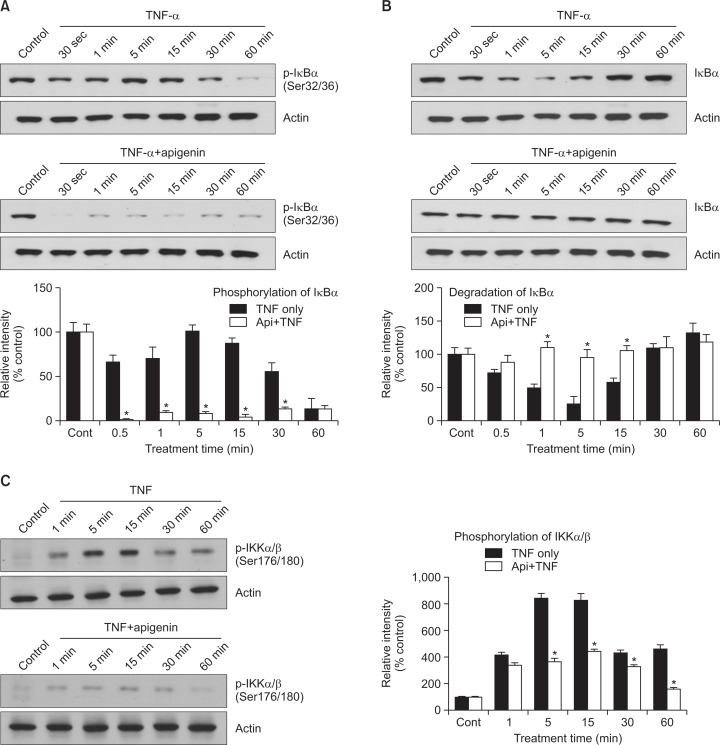
Effect of apigenin on TNF-α-induced phosphorylation of IκBα, degradation of IκBα and phosphorylation of IKKα/β
Apigenin appears to affect the phosphorylation and degradation of IκBα which is required for NF-κB dimerization and maximal activation of transcription (Fig. 3A, B). NF-κB activation involves the phosphorylation of inhibitory kappa B alpha (IκBα) by inhibitory kappa B kinases (IKKs), resulting in IκBα degradation. As a result, NF-κB subunits are released and translocated to the nucleus. Therefore, we investigated the effect of apigenin on the phosphorylation of IκBα. As shown in Fig. 3A, TNF-α increased the phosphorylation of IκBα to its maximal level at 5 min. However, preincubation of NCIH292 cells with apigenin prior to TNF-α exposure dramatically attenuated the phosphorylation of IκBα. On the other hand, IκBα degradation is required for the activation of NF-κB. Consequently, we determined whether apigenin inhibits TNF-α-induced NF-κB activation by inhibition of IκBα degradation. As shown in Fig. 3B, at 5 min after treatment, TNF-α showed the maximal induction of IκBα degradation. However, preincubation of NCI-H292 cells with apigenin prior to TNF-α exposure suppressed the degradation of IκBα. For most agents that activate NF-κB through a common pathway based on phosphorylation, proteaseome mediated the degradation of IκB. The key regulatory step in this pathway involves the activation of IkB kinase (IKK) complex. Activation of IKK depends on phosphorylation. Therefore, we investigated whether apigenin inhibits the TNF-α-induced activity of IKKα/β. As shown in Fig. 3C, TNF-α activated the IKKα/β, although apigenin suppressed its activation by regulating the serine 176/180 phosphorylation of IKKα/β.
Fig. 3.
Effect of apigenin on TNF-α-induced phosphorylation of IκBα, degradation of IκBα and phosphorylation of IKKα/β. NCI-H292 cells were incubated with 20 μM apigenin for 24 h and treated with 50 ng/mL TNF-α for the indicated periods. Cytoplasmic extracts were fractionated and then subjected to western blot analysis using phospho-specific IκBα (Ser 32/36) antibody (A) or antibody against anti-IκBα (B). NCI-H292 cells were incubated with 20 μM apigenin for 24 h and treated with 25 ng/mL TNF-α for the indicated periods. Whole cell lysates (100 μg) were prepared and then subjected to western blot analysis using phospho-specific IKKα/β (Ser 176/180) antibody (C). The results shown are the representative of three independent experiments. Equal protein loading was evaluated by β-actin levels. *significantly different from TNF-α alone at each time point (p<0.05, Student’s t-test) (cont: control, Api: apigenin, TNF: TNF-α).
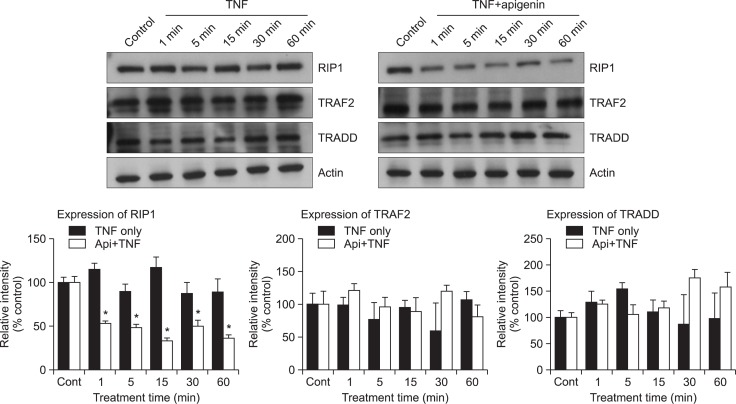
Effect of apigenin on adaptor proteins of TNF receptor (TNFR) 1 signaling
Binding of TNF-α to TNFR1 induces receptor trimerization and recruitment of several downstream signaling proteins to their cytoplasmic domains (Hsu et al., 1995). TNFR1 interacts with the signaling protein TNFR1-associated death domain protein (TRADD), which recruits the adaptor protein, TNF receptor-associated factor 2 (TRAF2) (Hsu et al., 1996), receptor interacting protein (RIP1) (Stanger et al., 1995) and Fas associated protein with death domain (FADD) (Chinnaiyan et al., 1995). Subsequently, RIP1 is mainly polyubiquitinated and induces the recruitment to the TNFR1 and activation of IKK complex. For this reason, we investigated whether apigenin affects the adaptor protein expression of TNFR1 signaling pathway. As shown in Fig. 4, it was found that apigenin affected the expression of RIP1.
Fig. 4.
Effect of apigenin on adaptor proteins of TNF receptor (TNFR) 1 signaling. NCI-H292 cells, either untreated or pretreated with 20 μM apigenin for 24 h, were treated with 25 ng/mL TNF-α for the indicated periods. Whole cell lysates were prepared and analyzed by western blotting using antibodies against RIP1, TRAF2 and TRADD. The results shown are representative of three independent experiments. Equal protein loading was evaluated by β-actin. *significantly different from TNF-α alone at each time point (p<0.05, Student’s t-test) (cont: control, Api: apigenin, TNF: TNF-α).
DISCUSSION
Pathological hypersecretion of airway mucus is one of the major symptoms associated with severe pulmonary inflammatory diseases (Voynow and Rubin, 2009). There might be dual ways in order to get rid of excessive mucus from the airway; 1) removing the mucus by physical methods, i.e, aspiration after dilution of mucus, and 2) suppression of secretion and/or production of mucus by pharmacological methods. However, clinically, the physical method induces the irritation of the airway luminal wall and stimulates the hypersecretion of mucus through a reflex mechanism.
Therefore, the pharmacological methods for regulating mucin secretion and/or production have become an important approach to control the hypersecretion of airway mucus (Heo et al., 2007; Heo et al., 2009; Lee et al., 2011; Kim et al., 2012). Secretion of airway mucin can be induced by diverse compounds (Voynow and Rubin, 2009). Whereas glucocorticoidal compounds inhibit the hyperproduction and/or hypersecretion of airway mucins, they have various limitations in their application to pharmacotherapy of human diseases involving pulmonary mucus hypersecretion (Sprenger et al., 2011). An alternative approach for controlling airway mucus hypersecretion is to regulate the excessive mucin secretion and/or production using natural compounds derived from various medicinal plants that have been used in folk medicine for the management of pulmonary inflammatory diseases.
Modulation of inflammatory reactions would appear to be the most appropriate course of action for treating diverse pulmonary diseases including asthma, cystic fibrosis and chronic obstructive pulmonary diseases. While it would be quite difficult to achieve this modulation with conventional drugs, flavonoids, a group of natural compounds found in many medicinal plants, appear to be uniquely fitted to this purpose based on their ability to affect multiple converging signaling pathways (Romano et al., 2013). Apigenin is a nontoxic and nonmutagenic flavonoid found in multitude of medicinal plants, fruits and vegetables and has diverse biological activities including antioxidative, anti-inflammatory and anticancer effects (Illek and Fischer, 1998; Yin et al., 2001; McVean et al., 2002; Paoletti et al., 2009; Zhong et al., 2010; Clere et al., 2011; Zhou et al., 2011). On the other hand, TNF-α was reported to induce MUC5AC gene expression in normal human airway epithelial cells (Song et al., 2003) and the secretion of mucin from guinea pig tracheal epithelial cells (Fischer et al., 1999). Shao and his colleagues reported that TNF-α converting enzyme (TACE) provoked MUC5AC mucin expression in cultured human airway epithelial cells (Shao et al., 2003). When airway inflammatory diseases are exacerbated, the level of TNF-α is increased in sputum of patients (Takeyama et al., 1999; Cohn et al., 2002). Based upon these reports, we investigated apigenin significantly affects the production and gene expression of airway mucin induced by TNF-α. As can be seen in results, apigenin suppressed the expression of MUC5AC mucin gene and the production of MUC5AC mucin protein, induced by TNF-α (Fig. 1). This result suggests that apigenin can regulate mucin gene expression and production of mucin protein induced by TNF-α, by acting on airway epithelial cells. However, it is not fully elucidated how apigenin manifested its activity on MUC5AC production, we investigated whether apigenin affects TNF-α-induced NF-κB signaling in NCI-H292 cells.
Through binding its receptor, TNF-α activates several intracellular signal transduction cascades among which the NF-κB pathway is of pivotal importance. NF-κB is a heterodimer composed of p65, p50 and IκBα subunits present in the cytoplasm as an inactive state. In response to various stimuli, the IκBα subunit is phosphorylated and degraded, thereby facilitating the translocation of p50–p65 heterodimer to the nucleus. The p50–p65 acts as a transcription factor regulating the expression of numerous genes including MUC5AC (Li and Verma, 2002). In the present study, we demonstrate that apigenin suppressed the expression of RIP1, one of the adaptor proteins of TNF receptor and TNF-α-induced phosphorylation of IKKα/β. This, in turn, inhibits the phosphorylation and degradation of IκBα which is required for NF-κB dimerization and maximal activation of transcription. Subsequently, the phosphorylation and nuclear translocation of NF-κB p65 and transcriptional activation of MUC5AC mucin gene are inhibited. Recently, similar action of apigenin on NF-κB pathway in pancreatic cancer cells was reported by Wu and his colleagues (Wu et al., 2014). However, to the best of our knowledge, this is the first report on the action of apigenin on TNF-α-induced airway MUC5AC mucin gene expression and production through affecting NF-κB signaling pathway.
Taken together, we suggest it is valuable to find the natural products that have specific inhibitory effects on mucin secretion, production and gene expression-in view of both basic and clinical sciences. Findings in this study suggest apigenin suppresses TNF-α-induced production of mucin through regulating NF-κB signaling pathway in airway epithelial cells and a possibility of using apigenin as a new efficacious mucoregulator for pulmonary diseases, although further studies are essentially required.
Acknowledgments
This research was supported by a grant from Pyunkang Medical Foundation in 2012.
REFERENCES
- Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Clere N, Faure S, Martinez MC, Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem. 2011;9:62–77. doi: 10.2174/187152511796196498. [DOI] [PubMed] [Google Scholar]
- Cohn L, Whittaker L, Niu N, Homer RJ. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp. 2002;248:201–213. [PubMed] [Google Scholar]
- Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:413–422. doi: 10.1165/ajrcmb.20.3.3393. [DOI] [PubMed] [Google Scholar]
- Heo HJ, Lee HJ, Kim YS, Kang SS, Son KH, Seok JH, Seo UK, Lee CJ. Effects of baicalin and wogonin on mucin release from cultured airway epithelial cells. Phytother Res. 2007;21:1130–1134. doi: 10.1002/ptr.2222. [DOI] [PubMed] [Google Scholar]
- Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009;23:1458–1461. doi: 10.1002/ptr.2801. [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, Lee CJ. Phorbol ester or epidermal growth factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012;26:1784–1788. doi: 10.1002/ptr.4650. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, Lee CJ. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011;25:1196–1200. doi: 10.1002/ptr.3362. [DOI] [PubMed] [Google Scholar]
- Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-κappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- McVean M, Weinberg WC, Pelling JC. A p21(waf1)-independent pathway for inhibitory phosphorylation of cyclin-dependent kinase p34(cdc2) and concomitant G(2)/M arrest by the chemopreventive flavonoid apigenin. Mol Carcinog. 2002;33:36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- Paoletti T, Fallarini S, Gugliesi F, Minassi A, Appendino G, Lombardi G. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur J Pharmacol. 2009;620:120–130. doi: 10.1016/j.ejphar.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediated MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA. 2003;100:11618–11623. doi: 10.1073/pnas.1534804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder MA, Lee HJ, Mia MZ, Park SH, Ryu J, Kim JH, Min SY, Hong JH, Seok JH, Lee CJ. Inhibition of TNF-α-induced MUC5AC mucin gene expression and production by wogonin through the inactivation of NF-κB signaling in airway epithelial cells. Phytother Res. 2014;28:62–68. doi: 10.1002/ptr.4954. [DOI] [PubMed] [Google Scholar]
- Song K, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003;278:23243–23250. doi: 10.1074/jbc.M300096200. [DOI] [PubMed] [Google Scholar]
- Sprenger L, Goldmann T, Vollmer E, Steffen A, Wollenberg B, Zabel P, Hauber HP. Dexamethasone and N-acetylcysteine attenuate Pseudomonas aeruginosa-induced mucus expression in human airways. Pulm Pharmacol Ther. 2011;24:232–239. doi: 10.1016/j.pupt.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Lee H, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- Wu DG, Yu P, Li JW, Jiang P, Sun J, Wang HZ, Zhang LD, Wen MB, Bie P. Apigenin potentiates the growth inhibitory effects by IKK-β-mediated NF-κB activation in pancreatic cancer cells. Toxicol Lett. 2014;224:157–164. doi: 10.1016/j.toxlet.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–420. [PubMed] [Google Scholar]
- Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, Baek SJ. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur. J. Cancer. 2010;46:3365–3374. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol Pharmacol. 2011;79:886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]