Abstract
The high mortality rates associated with cancer reflect the metastatic spread of tumor cells from the site of their origin. Metastasis, in fact, is the cause of 90% of cancer deaths. Therefore, considerable effort is being made to inhibit metastasis. In the present study, we screened ketotifen for anti-migratory and anti-invasive activities against MDA-MB-231 breast cancer and HT-1080 fibrosarcoma cancer cells. Cancer cell migration and invasion were measured using multi-well chambers. Additionally, western blots were used to examine the effects of ketotifen on the expressions of CDC42, Rho, Rac, and matrix metalloproteinase 9 (MMP-9). The results showed that ketotifen dose-dependently suppressed the migration and invasion of MDA-MB-231 and HT-1080 cells. Ketotifen also suppressed the expressions of CDC42, Rac, and Rho, which, significantly, are involved in MDA-MB-231 and HT-1080 cancer cell migration. Moreover, ketotifen suppressed the expression and activity of MMP-9, which is involved in degradation of the extracellular matrix leading to invasion. The overall data suggested that ketotifen suppresses the migration and invasion of MDA-MB-231 and HT-1080 cancer cells via inhibition of CDC42, Rac, Rho, and MMP-9 expression.
Keywords: Ketotifen, Migration, Invasion, MDA-MB-231, HT-1080
INTRODUCTION
Metastasis is the migration of cancer cells from their origin to distant locations within the body for continued growth (Valastyan and Weinberg, 2011). In fact, the development of metastatic lesions at sites distant from that of the primary tumor is the cause of death of 90% of cancer patients (Mendoza and Khanna, 2009).
Due to the importance of metastasis, many studies on its mechanism have been completed, or are underway, in attempts to develop a therapy protocol that can modulate its effects (Mazzocca and Carloni, 2009; Jung et al., 2012). To achieve this goal, precise coordination of cell movement and matrix remodeling are required (Friedl and Wolf, 2003).
The various stages in metastasis include tumor cell invasion of basement membranes and the surrounding tissue, intravasation into blood vessels, survival there, and extravasation and/or growth at different organ sites (Bravo-Cordero et al., 2012). Cell migration can be schematized into five separate steps: 1) lamellipodium extension at the leading edge, 2) formation of new focal adhesion complexes, 3) secretion of surface protease to the extracellular matrix (ECM) contacts and focalized proteolysis, 4) cell-body contraction by actomyosin complexes, and 5) tail detachment (Parri and Chiarugi, 2010). Lamelipodium extension, the first step in cell migration, involves CDC42 and Rac (Parri and Chiarugi, 2010). Cell-body contraction by actomyosin complexes, meanwhile, involves Rho (Friedl and Wolf, 2003). And in the proteolytic degradation of ECM during tumor invasion and metastasis, matrix metalloproteinase-9 (MMP-9) has been long recognized as a key enzyme (Xu et al., 2010).
Pharmaceutical industries have invested huge amounts of R&D money in cancer therapeutics but mostly, these R&D efforts did not give success such as new and novel anticancer drugs (Gupta et al., 2013). These difficult in drug development requires alternative efforts including drug repositioning (Elliott, 2012). So, we evaluated anti-migratory and anti-invasive effects of drugs which is deposited from several years of research on pruritus. We found that ketotifen has anti-migratory and anti-invasive effects.
Ketotifen is a first-generation antihistamine with store-operated Ca2+ channel antagonist properties (Fig. 1) (Franzius et al., 1994; Zhang and Berger, 2003). As a calcium influx blocker, ketotifen can induce cell death in an activation-enhanced manner in leukemia cells, mast cells, and breast cancer cells (Gommerman and Berger, 1998; Soboloff and Berger, 2002; Soboloff et al., 2002; Zhang et al., 2002). Ketotifen also reverses MDR1-mediated multidrug resistance in human breast cancer cells in vitro and alleviates cardiotoxicity induced by doxorubicin in vivo (Zhang and Berger, 2003).
Fig. 1.
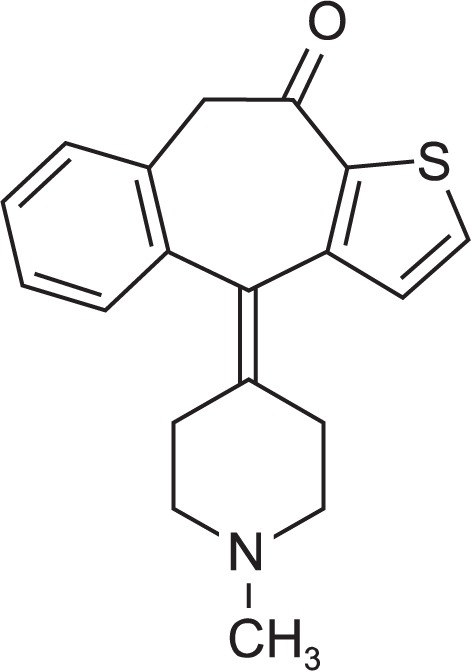
Structure of Ketotifen.
The anti-migratory and anti-invasive activities of ketotifen and its relevant mechanisms, however, are as yet unreported. Therefore, in the present study, we examined the effects of ketotifen on the migration and invasion of HT-1080 and MDA-MB-231 cancer cells. We demonstrated that ketotifen has anti-migratory and anti-invasive effects against HT-1080 and MDA-MB-231 cells and that those effects are mediated by suppression of the expression and activity of CDC42, Rho and Rac, and MMP-9.
MATERIALS AND METHODS
Reagents
Chemicals and reagents were purchased from Sigma-Aldrich Co., unless specified otherwise. All of the aqueous solutions were stored in a deep freezer before use.
Cell culture
The human sarcoma cell line, HT-1080 (ATCC CCL-121), was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) (Rasheed et al., 1974; Geiser et al., 1989). The cells were cultured in Roswell Park Memorial Institute medium (RPMI)-1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS, WelGENE), streptomycin (100 μg/ml) and penicillin (100 U/ml). The human breast cancer cell line, MDA-MB-231 (ATCC CRM-HTB-26), was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) (Cailleau et al., 1974). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with L-glutamine (2 mM), streptomycin (100 μg/ml), penicillin (100 U/ml). and sodium pyruvate (1 mM). The cells were incubated at 37°C in a humidified atmosphere containing 10% CO2. The cells were washed twice in serum-free RPMI-1640 for HT-1080 and DMEM for MDA-MB-231 and incubated in serum-free RPMI-1640 and DMEM for 18 hours before the respective experiments.
Cell migration assay
Migration assays were performed using a multi-well chamber (Neuroprobe Inc. Gaithersburg, MD, USA) coated with 10 μg/ml fibronectin as a chemoattractant (Park et al., 2013). Briefly, HT-1080 cells were suspended in DMEM at 1×106 cells/ml, and a 25 μl aliquot of this suspension was placed in the upper well of the chamber. Next, the aliquot was separated from the 3% serum (in the lower well) by an 8 μm polyhydrocarbon filter. After 37°C incubation for 4 h, non-migrated cells on the upper surface of the membrane were scraped off, and the migrated cells on the lower surface were stained by Diffquick, and subsequently counted under five randomly chosen high-power (400×) fields.
Cell invasion assay
Invasion assays were performed using a 24-well Transwell unit with polycarbonate filters (diameter: 6.5 mm, pore size: 8.0 mm; Corning Costar). Briefly, a fixed number of cells (5×104 cells/chamber) were used for each invasion assay. The lower and upper parts of the Transwell were coated with 20 ml of a 1:2 mixture of Matrigel:DMEM. The cells were plated on the Matrigel-coated Transwell in the presence of various concentrations of ketotifen. The medium in the lower chambers contained 0.1 mg/ml of bovine serum albumin (BSA). After 37°C incubation for 24 h, cells invading the lower surface of the membrane were fixed with methanol, stained with hematoxylin and eosin (H&E) and subsequently counted under five randomly selected high-power (400×) fields.
Western blot analysis
After incubation, the cells were collected and washed twice with cold PBS. They were then lysed in a lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% triton X-100, 2 mM EDTA, 1% DOC (Deoxycholic acid), 0.1% SDS, 1 mM NaVO3, 10 mM NaF, 1 mM DTT) and centrifuged to yield whole-cell lysates. The protein concentration was measured using the Bradford method. Aliquots of the lysates (20-30 μg of protein) were separated on 4-12% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) with a glycine transfer buffer (192 mM glycine, 25 mM Tris-HCl (pH 8.8), 10% MeOH (v/v)). After blocking the non-specific site with 3% non-fat dry milk, the membrane was then incubated with specific primary antibody in 3% BSA at 4°C for overnight. The following primary antibodies were used: anti-β-actin (1:1000, Santa Cruz, CA,USA); anti-MMP-9 (1:1000, Abcam, Cambridge, UK); anti-CDC42 (1:1000, Abcam, Cambridge, UK); anti-Rho (1:1000, Abcam, Cambridge, UK); anti-Rac (1:1000, Abcam, Cambridge, UK). The membrane was further incubated for 60 min with a peroxidase-conjugated secondary antibody (1:5000, Santa Cruz, CA, USA) at room temperature. Immunoactive proteins were detected using the PowerOpti-ECL western blotting detection reagent (Animal Genetics Inc., Gyeonggi, Korea).
Gelatin zymography
Matrix metalloproteinase MMP-9 enzymatic activities were assayed by gelatin zymography (Park and Lee, 2011). Samples of serum-free conditioned medium were electrophoresed on a 10% SDS-polyacryamide gel. After the electrophoresis, the gel was washed in 2.5% Triton X-100 for 1 h and incubated at 37°C for 24 h in activation buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM CaCl2, 0.02% NaN3). After staining with Coomassie brilliant blue R-250, enzymatic activities were detected as clear bands against the blue background.
Statistical analysis
All of the data are expressed as percentages of the control and shown as means ± SD. The student’s t-test was used to determine the statistical significance of the value differences between experimental and control groups. Values of p less than 0.05 were considered significant.
RESULTS
Effect of ketotifen on migration of MDA-MB-231 and HT-1080 cancer cells
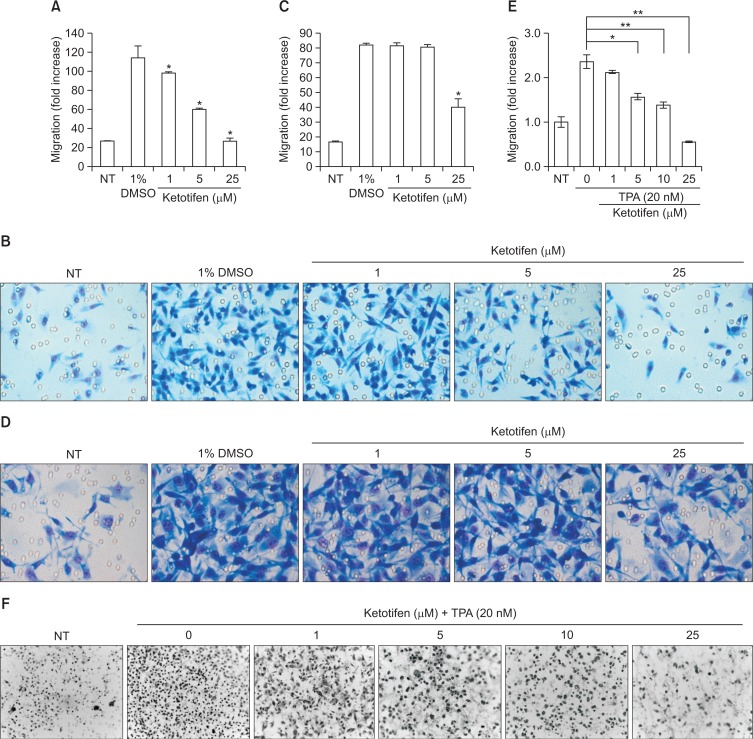
We examined the effects of ketotifen on the migration of MDA-MB-231 and HT-1080 cancer cells known to be highly metastatic. The migration of MDA-MB-231 cells was dose-dependently suppressed by ketotifen (Fig. 2A, 2B); that of HT-1080 cells was suppressed only at 25 μM (Fig. 2C, 2D). The inhibitory effects of ketotifen on MDA-MB-231 cells were stronger than on HT-1080 cells. TPA-induced migration of HT-1080 was much more efficiently suppressed by ketotifen than was FBS-induced migration of the same cells (Fig. 2E, 2F).
Fig. 2.
Ketotifen inhibited the migration of MDA-MB-231 and HT-1080 cells. (A) Effect of ketotifen on MDA-MB-231 cell migration. MDAMB-231 cells (5×104/well) were treated with vehicle or increasing concentrations (1, 5, 25 μM) of ketotifen for 24 h. Cells migrated through the membrane after 6 h of incubation. (B) Photographs of migrated MDA-MB-231 cells. (C) Effect of ketotifen on HT-1080 cell migration. HT-1080 cells (5×104/well) were treated with vehicle or increasing concentrations (1, 5, 25 μM) of ketotifen for 24 h. Cells migrated through the membrane after 6 h of incubation were stained and photographed. (D) Photographs of migrated HT-1080 cells. (E) Effect of ketotifen on migration of TPA-treated HT-1080 cells. HT-1080 cells were treated with vehicle or increasing concentrations (1, 5, 10, 25 μM) of ketotifen and with TPA (20 nM) for 24 h. Cells migrated through the membrane after 6 h of incubation were stained and photographed. (F) Photographs of migrated HT-1080 cells. For the migration assay, the lower-chamber transwells were coated with fibronectin (10 μg/ml). After incubation, the cells on the bottom side of the filter were fixed, stained by Diff-quick, and subsequently counted in four randomly chosen high-power (200×) fields. The lower chambers contained 3% FBS except NT (not treated with 3% FBS). *p<0.05, **p<0.01.
Effect of ketotifen on invasion of MDA-MB-231 and HT-1080 cancer cells
We also examined the effects of ketotifen on the invasion of the same two MDA-MB-231 and HT-1080 cancer cell lines. In the results, ketotifen dose-dependently suppressed the invasion of MDA-MB-231 cells (Fig. 3A, 3B) as well as the TPA-induced invasion of HT-1080 cells (Fig. 3C, 3D).
Fig. 3.
Ketotifen inhibited the invasion of MDA-MB-231 and HT-1080 cells. (A) Effect of ketotifen on MDA-MB-231 cell invasion. MDAMB-231 cells (5×104/well) were treated with vehicle or increasing concentration (1, 5, 10, 25 μM) of ketotifen for 24 h. Cells invaded through the membrane after 24 h of incubation. (B) Photographs of invaded MDA-MB-231 cells. (C) Effect of ketotifen on HT-1080 cell invasion. HT-1080 cells (5×104/well) were treated with vehicle or increasing concentrations (1, 5, 10, 25 μM) of ketotifen and treated with TPA (20 nM) for 24 h. Cells invaded through the membrane after 24 h of incubation were stained and photographed. (D) Photographs of invaded HT-1080 cells. For the invasion assay, the upper-chamber transwells were coated with Matrigel (500 μg/ml). After incubation, cells invading the lower surface of the membrane were fixed with methanol, stained with hematoxylin and eosin (H&E), and subsequently counted under four randomly selected high-power (400×) fields. The lower chambers contained 10% FBS except NT (not treated with 10% FBS). *p<0.05, **p<0.01.
Effect of ketotifen on expressions of CDC42, Rac, and Rho of cancer cells
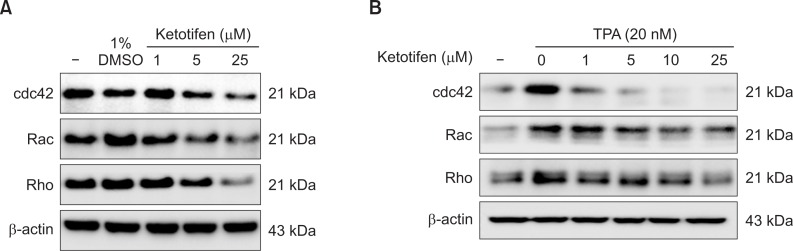
In the first step of cancer cell migration, lamelipodium extension is regulated by CDC42 and Rac expression, and cell-body contraction is regulated by Rho protein and Rac. Therefore, we examined the effects of ketotifen on the expression of CDC42, Rac, and Rho in MDA-MB-231 and HT-1080 cells, especially since ketotifen inhibited those cells’ migration. In MDA-MB-231 cells, ketotifen treatment suppressed CDC42, Rac and Rho expression (Fig. 4A). The most significant suppression was shown at 25 μM (Fig. 4A). In HT-1080 cells, ketotifen strongly suppressed the expressions of CDC42, Rac and Rho, even at low concentrations (Fig. 4B).
Fig. 4.
Ketotifen reduced the expression of CDC42, Rac and Rho of MDA-MB-231 and HT-1080 cells. (A) Effect of ketotifen on expression of CDC42, Rac and Rho in MDA-MB-231 cells. MDA-MB-231 cells were treated with ketotifen (1, 5, 25 μM) for 24 h. (B) Effect of ketotifen on expression of CDC42, Rac and Rho in TPA-treated HT-1080 cells. HT-1080 cells were treated with ketotifen (1, 5, 10, 25 μM) and with TPA (20 nM) for 24 h. Whole-cell lysates (10 μg) were prepared, the protein level was subjected to 10% SDS-PAGE, and the expressions of several proteins were determined by western blotting.
Effect of ketotifen on MMP-9 expression and activity of cancer cells
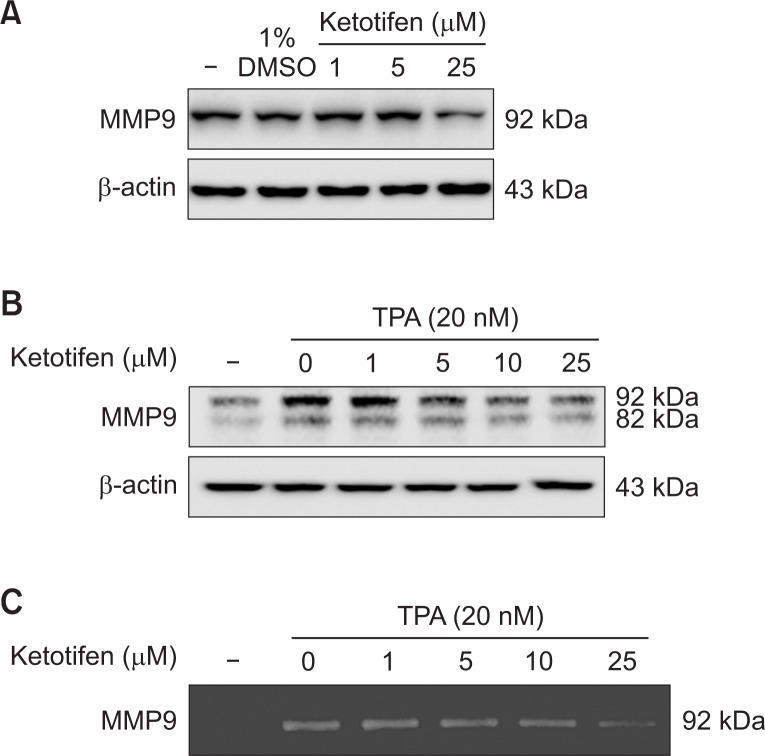
Next, since ketotifen suppressed the invasion of metastatic MDA-MB-231 and HT-1080 cells, we determined whether it also suppresses MMP-9 expression in those cells (Fig. 3). In the results, ketotifen dose-dependently suppressed the expression of MMP-9 in the MDA-MB-231 and HT-1080 cells (Fig. 5). Ketotifen’s suppressive effects were markedly stronger on the HT-1080 cells than on the MDA-MB-231 cells (Fig. 5A, 5B). And, because MMP-9 is secreted to media, we confirmed the effects of ketotifen on HT-1080 cells’ MMP-9 activities by zymography. The data showed that ketotifen suppressed those activities (Fig. 5C).
Fig. 5.
Ketotifen reduced MMP-9 expression of MDA-MB-231 and HT-1080 cells. (A) Effect of ketotifen on expression of MMP-9 in MDA-MB-231 cells. MDA-MB-231 cells were treated with ketotifen (1, 5, 25 μM) for 24 h. (B) Effect of ketotifen on expression of MMP-9 in TPA-treated HT-1080 cells. HT-1080 cells were treated with ketotifen (1, 5, 10, 25 μM) and with TPA (20 nM) for 24 h. Whole-cell lysates (10 μg) were prepared, the protein level was subjected to 10% SDS-PAGE, and the expressions of several proteins were determined by western blotting. (C) Effect of ketotifen on MMP-9 activity in HT-1080 cells. To test the activity of MMP-9, HT-1080 cells were treated with ketotifen (1, 5, 10, 25 μM) and with TPA (20 nM) for 24 h. Conditioned media were collected, and gelatin zymography was performed as described in Materials and Methods.
DISCUSSION
Only one of every 5000–10,000 prospective antcancer agents receives FDA approval and only 5% of oncology drugs entering Phase I clinical trials are ultimately approved (Zamboni et al., 2012; Gupta et al., 2013). Drug repositioning is one way to overcome the high costs and attrition rates.
Ketotifen is a second-generation non-competitive H1-anti-histamine and mast-cell stabilizer (Grahnen et al., 1992). In its ophthalmic form, it is used to treat allergic conjunctivitis, or the itchy red eyes caused by allergies (Pacharn and Vichyanond, 2013). In its oral form, it is used to prevent asthma attacks (Schwarzer et al., 2004). Some reports have suggested that ketotifen might be effectively utilized as an anticancer drug (Zhang and Berger, 2003).
HT-1080 and MDA-MB-231 cell lines were chosen since HT-1080 and MDA-MB-231 cell lines are well-known to migrate and invade well (Park et al., 2013).
As Fig. 2, 3 illustrate, ketotifen inhibited the migration and invasion of MDA-MB-231 and HT-1080 cancer cells. Given that ketotifen is an antihistamine drug, it has been suggested that histamine might be involved in migration and invasion of MDA-MB-231 and HT-1080 cancer cells. It was reported that in experimental mammary carcinomas, histamine becomes an autocrine growth factor capable of regulating cell proliferation via histamin receptor 1 and 2 (H1R, H2R), which is one of the first steps responsible for the onset of malignant transformation (Medina and Rivera, 2010). Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression (Kobayashi et al., 2000). The histamine receptor 4 (H4R) agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis (Meng et al., 2011). The clinical trials carried out with H2R antagonists in cancer patients thus far, unfortunately, have returned controversial results for breast cancer (Medina and Rivera, 2010). Histamine increased MDA-MB-231 cell proliferation and also migration via H3R, whereas H4R suppressed MDA-MB-231 cell proliferation (Medina et al., 2008). The role of histamine in fibrosarcoma has been less thoroughly studied, though one investigation reported that histamine-containing zinc chelator suppressed the invasion of HT-1080 fibrosarcoma cells (Ferry et al., 1998).
In Fig. 2, 3, TPA was used to induce migration and invasion of HT-1080 cancer cells since TPA acts as protein kinase C activator and enhanced MMP-9 expression in HT-1080 cancer cell lines leading to increased invasion (Moore et al., 1997). Therefore TPA treatment increased invasion of HT-1080 cells.
It is not clear how ketotifen inhibited the migration and invasion of MDA-MB-231 and HT-1080 cells in the present study. In fact, the effects of ketotifen on CDC42, Rac, and Rho expression had not been studied previously. We showed that reduced expressions of CDC42, Rac, and Rho were involved in the anti-migratory and anti-invasive effects of ketotifen (Fig. 4). CDC42, Rac, and Rho proteins show largely enhanced expressions in several tumor samples including breast cancer (Fritz et al., 1999; Fritz et al., 2002; Ellenbroek and Collard, 2007; Bray et al., 2013). It is indeed interesting that ketotifen suppressed the expressions of CDC42, Rac, and Rho (Fig. 4); however, we still do not understand the mechanisms involved. Ketotifen suppressed the calcium entry into mast cells (Franzius et al., 1994). So calcium entry antagonizing effects of ketotifen might be related with the expression and activation of small GTPases such as rho, CDC42, and rac (Aspenstrom, 2004).
MMPs are a major group of enzymes, zinc- and calcium-dependent endopeptidases, that regulate the ECM composition (Kim et al., 2014). MMP-9 in particular, considered to be one of the critical MMPs involved in cancer invasion, has been found to be directly associated with breast cancer invasion, metastasis, and poor prognosis (Brinckerhoff and Matrisian, 2002). Histamine, meanwhile, is involved in MMP-9 expression in keratinocytes and PANC-1 cells (Cricco et al., 2006; Gschwandtner et al., 2008; Medina and Rivera, 2010). And because ketotifen suppressed MMP-9 expression in the present study, it might be suggested that H1R is involved in MDAMB-231 cells’ MMP-9 expression (Fig. 5).
In summation, we found that ketotifen suppressed the migration and invasion of MDA-MB-231 and HT-1080 cancer cells via inhibition of CDC42, Rac, Rho, and MMP-9 expression. These results suggest that ketotifen might be effectively employed as an anti-migratory and anti-invasive drug for metastatic cancer and, moreover, be considered a candidate for drug reposition.
Acknowledgments
This study was supported by grants from the GRRC program of Gyeonggi Province (GRRC Dongguk2013-B01).
REFERENCES
- Aspenstrom P. Integration of signalling pathways regulated by small GTPases and calcium. Biochim. Biophys. Acta. 2004;1742:51–58. doi: 10.1016/j.bbamcr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K, Gillette M, Young J, Loughran E, Hwang M, Sears JC, Vargo-Gogola T. Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res. 2013;15:R91. doi: 10.1186/bcr3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. NatRev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Cailleau R, Young R, Olive M, Reeves WJ., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cricco G, Nunez M, Medina V, Garbarino G, Mohamad N, Gutierrez A, Cocca C, Bergoc R, Rivera E, Martin G. Histamine modulates cellular events involved in tumour invasiveness in pancreatic carcinoma cells. Inflamm.Res. 2006;55(Suppl 1):S83–84. doi: 10.1007/s00011-005-0054-9. [DOI] [PubMed] [Google Scholar]
- Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin. Exp. Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- Elliott RL. Four lessons from global health drug discovery: medicine for an ailing industry? ACS Med Chem Lett. 2012;3:688–690. doi: 10.1021/ml3002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry G, Boutin JA, Hennig P, Genton A, Desmet C, Fauchere JL, Atassi G, Tucker GC. A zinc chelator inhibiting gelatinases exerts potent in vitro anti-invasive effects. Eur J Pharmacol. 1998;351:225–233. doi: 10.1016/s0014-2999(98)00304-5. [DOI] [PubMed] [Google Scholar]
- Franzius D, Hoth M, Penner R. Non-specific effects of calcium entry antagonists in mast cells. Pflugers Arch. 1994;428:433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br.J. Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Geiser AG, Anderson MJ, Stanbridge EJ. Suppression of tumorigenicity in human cell hybrids derived from cell lines expressing different activated ras oncogenes. Cancer Res. 1989;49:1572–1577. [PubMed] [Google Scholar]
- Gommerman JL, Berger SA. Protection from apoptosis by steel factor but not interleukin-3 is reversed through blockade of calcium influx. Blood. 1998;91:1891–1900. [PubMed] [Google Scholar]
- Grahnen A, Lonnebo A, Beck O, Eckernas SA, Dahlstrom B, Lindstrom B. Pharmacokinetics of ketotifen after oral administration to healthy male subjects. Biopharm Drug Dispos. 1992;13:255–262. doi: 10.1002/bdd.2510130404. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Purwar R, Wittmann M, Baumer W, Kietzmann M, Werfel T, Gutzmer R. Histamine upregulates keratinocyte MMP-9 production via the histamine H1 receptor. J Invest Dermatol. 2008;128:2783–2791. doi: 10.1038/jid.2008.153. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Jung KH, Park BH, Hong SS. Progress in cancer therapy targeting c-Met signaling pathway. Arch Pharm Res. 2012;35:595–604. doi: 10.1007/s12272-012-0402-6. [DOI] [PubMed] [Google Scholar]
- Kim HR, Kim JM, Kim MS, Hwang JK, Park YJ, Yang SH, Kim HJ, Ryu DG, Lee DS, Oh H, Kim YC, Rhee YJ, Moon BS, Yun JM, Kwon KB, Lee YR. Saussurea lappa extract suppresses TPA-induced cell invasion via inhibition of NF-kappaB-dependent MMP-9 expression in MCF-7 breast cancer cells. BMC Complement Altern Med. 2014;14:170. doi: 10.1186/1472-6882-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–3984. [PubMed] [Google Scholar]
- Mazzocca A, Carloni V. The metastatic process: methodological advances and pharmacological challenges. Curr Med Chem. 2009;16:1704–1717. doi: 10.2174/092986709788186192. [DOI] [PubMed] [Google Scholar]
- Medina V, Croci M, Crescenti E, Mohamad N, Sanchez-Jimenez F, Massari N, Nunez M, Cricco G, Martin G, Bergoc R, Rivera E. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M, Khanna C. Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol. 2009;41:1452–1462. doi: 10.1016/j.biocel.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Han Y, Staloch D, Francis T, Stokes A, Francis H. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology. 2011;54:1718–1728. doi: 10.1002/hep.24573. [DOI] [PubMed] [Google Scholar]
- Moore DH, Allison B, Look KY, Sutton GP, Bigsby RM. Collagenase expression in ovarian cancer cell lines. Gynecol Oncol. 1997;65:78–82. doi: 10.1006/gyno.1997.4628. [DOI] [PubMed] [Google Scholar]
- Pacharn P, Vichyanond P. Immunomodulators for conjunctivitis. Curr Opin Allergy Clin Immunol. 2013;13:550–557. doi: 10.1097/ACI.0b013e328364d86a. [DOI] [PubMed] [Google Scholar]
- Park MK, Jo SH, Lee HJ, Kang JH, Kim YR, Kim HJ, Lee EJ, Koh JY, Ahn KO, Jung KC, Oh SH, Kim SY, Lee CH. Novel suppressive effects of cardamonin on the activity and expression of transglutaminase-2 lead to blocking the migration and invasion of cancer cells. Life Sci. 2013;92:154–160. doi: 10.1016/j.lfs.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Park MK, Lee CH. Alpinia katsumadai suppresses migration and 12-O-tetradecanoylphorbol-13-acetate-induced invasion of HT-1080 cells through suppression of transglutaminase-2, matrix metalloproteinase-2, and matrix metalloproteinase-9 expression. Cancer Prev Res. 2011;16:326–332. [Google Scholar]
- Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, Bassler D, Mitra A, Ducharme FM, Forster J. Ketotifen alone or as additional medication for long-term control of asthma and wheeze in children. Cochrane Database Syst. Rev. 2004:CD001384. doi: 10.1002/14651858.CD001384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Berger SA. Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. J Biol Chem. 2002;277:13812–13820. doi: 10.1074/jbc.M112129200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Zhang Y, Minden M, Berger SA. Sensitivity of myeloid leukemia cells to calcium influx blockade: application to bone marrow purging. Exp Hematol. 2002;30:1219–1226. doi: 10.1016/s0301-472x(02)00893-7. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, McKee CM, Cao Y, Ding Y, Kessler BM, Muschel RJ. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res. 2010;70:6988–6998. doi: 10.1158/0008-5472.CAN-10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, Lee R, Nel A, Panaro NJ, Grodzinski P. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Berger SA. Ketotifen reverses MDR1-mediated multidrug resistance in human breast cancer cells in vitro and alleviates cardiotoxicity induced by doxorubicin in vivo. Cancer Chemother Pharmacol. 2003;51:407–414. doi: 10.1007/s00280-003-0600-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crump M, Berge SA. Purging of contaminating breast cancer cells from hematopoietic progenitor cell preparations using activation enhanced cell death. Breast Cancer Res Treat. 2002;72:265–278. doi: 10.1023/a:1014965726663. [DOI] [PubMed] [Google Scholar]