Abstract
Ribes fasciculatum var. chinense MAX. (R. fasciculatum) has traditionally been used in Korea to treat inflammatory diseases. However, the exact mechanism that accounts for the anti-inflammatory effect of R. fasciculatum is not completely understood. We aimed to ascertain the pharmacological effects of R. fasciculatum on both compound 48/80- or histamine-induced scratching behaviors and 2, 4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis (AD) in mice. Additionally, to find a possible explanation for the anti-inflammatory effects of R. fasciculatum, we evaluated the effects of R. fasciculatum on the production of inflammatory mediators in LPS-stimulated macrophage cells. Treatment of R. fasciculatum significantly reduced compound 48/80- or histamine-induced the pruritus in mice. R. fasciculatum attenuated the AD symptoms such as eczematous, erythema and dryness and serum IgE levels in AD model. Additionally, R. fasciculatum inhibited the production of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). The maximal rates of TNF-α and IL-6 inhibition by R. fasciculatum (1 mg/ml) were approximately 32.12% and 46.24%, respectively. We also showed that R. fasciculatum inhibited the activation of nuclear factor-kappa B in LPS-stimulated macrophages. Collectively, the findings of this study provide us with novel insights into the pharmacological actions of R. fasciculatum as a potential molecule for use in the treatment of allergic inflammatory diseases.
Keywords: Ribes fasciculatum, Inflammatory mediators, Nuclear factor-kappa B, Macrophage, Allergic inflammation
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by eczematous inflammation of the skin (Buske-Kirschbaum et al., 2001). The incidence of this disease has increased steadily in recent years. AD is known to be the result of an immune system dysregulation, ultimately resulting in allergic inflammation (Gold and Kemp, 2005). Activation of macrophages is a hallmark of inflammation, especially in the AD skin where they can mediate chronic inflammation by producing cytokines. It has been previously reported that macrophages can be found in larger numbers in AD lesional skin (McCormick et al., 2000). In response to various stimuli, macrophages generate a variety of cytokines, including interleukin (IL)-6, and tumor necrosis factor (TNF)-α. TNF-α has an important amplifying effect in asthmatic inflammation and stimulates airway epithelial cells to produce cytokines, including IL-6, IL-8 and GM-CSF (Boero et al., 2010). IL-6 is an important co-factor in IL-4 dependent IgE synthesis. The release of these cytokines may be of major importance in the development of a variety of inflammatory skin disorders (Trefzer et al., 2003). Therefore, the inhibition of cytokine secretion can aid in the development of a useful therapeutic strategy for allergic inflammatory diseases such as AD. Nuclear factor-kappa B (NF-κB) plays a crucial role in the regulation of many genes involved in immune and inflammatory responses (Tegeder et al., 2001). In the nucleus, NF-κB activates gene transcription; thus, NF-κB plays a pivotal role in the regulation of immune and inflammatory responses, via control of the transcription of inflammatory cytokine genes (Gadaleta et al., 2011). An increase in NF-κB activity associated with the secretion of high levels of IL-6 and TNF-α has also been noted in the context of allergic inflammatory responses (Mukaida, 2000). The results of these studies demonstrated that NF-κB activation and the subsequent activation of pro-inflammatory cytokine gene expression are critically important in the initiation and perpetuation of allergic inflammation. Traditional medicine has been the subject of increased interest for its potential in the treatment of inflammation. Although traditional herbal medicines have long been used effectively in treating diseases, the pharmacological mechanisms of action of most herbal medicines have not yet been elucidated. Ribes fasciculatum var. chinense MAX. (R. fasciculatum) is used in traditional oriental medicine for various medicinal purposes. It was reported that R. fasciculatum inhibited the nuclear factor of activated T cells (NFAT) transcription factor (Dat et al., 2005). This result suggested that R. fasciculatum may be useful in the treatment of autoimmune diseases. Unfortunately, the exact mechanism that accounts for the anti-allergic and anti-inflammatory effects of the R. fasciculatum is still not understood. In this study, we attempted to ascertain the mechanisms underlying the pharmacological effects of R. fasciculatum on both compound 48/80- or histamine-induced scratching behaviors and 2, 4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis in mice. Additionally, to find a possible explanation for the anti-inflammatory mechanisms of R. fasciculatum, we evaluated the effects of R. fasciculatum on the production of inflammatory cytokines and activation of NF-κB in LPS-stimulated macrophages.
MATERIALS AND METHODS
Reagents
Compound 48/80, LPS, avidin peroxidase (AP), 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid (ABTS), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and DNCB were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco’s Modified Eagles Medium (DMEM) was purchased from Gibco BRL (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from JR Scientific, Inc. (Woodland, CA, USA). Anti-mouse TNF-α/IL-6, recombinant TNF-α/IL-6, biotinylated TNF-α/IL-6, anti-mouse IgE, recombinant IgE and biotinylated IgE were purchased from Pharmingen (San Diego, CA, USA). NF-κB, and histone antibodies (Abs) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Animals
The original stock of male ICR mice (5 weeks, 25–30 g), BALB/c mice (5 weeks, 19–20 g) and SD rats (7 weeks, 250–300 g) were purchased from Orient Co.,Ltd, a branch of Charles River Labortories (Seoul, Korea). Animals were housed 10 per cage, allowed access to water and food ad libitum, and maintained at a constant temperature (24 ± 1°C) and humidity (60 ± 10%) under a 12-h light/dark cycle (light on 08:00.20:00 h). Animal experimental procedures were approved by the ethics committee of Daegu Haany University, Korea.
Preparation of R. fasciculatum
The dried of R. fasciculatum were purchased from the Human herb (Gyeongbuk, Korea). The roots (100 g) were chopped using a blender with 1 L of 70% ethanol solution under room temperature for 24 h and then concentrated under a vacuum. Then the extract solution obtained was filtered, concentrated on a water bath under vacuo, frozen and lyophilized to yield ethanol extracts (yield: 5.83%). Dilutions were made in saline and filtered through 0.22-μm syringe filter.
Compound 48/80-induced systemic anaphylactic reaction
Mice were given an intraperitoneal injection of the mast cell degranulator compound 48/80 (8 mg/kg). R. fasciculatum dissolved in saline was administered orally 1 h before the injection of compound 48/80. Mortality was monitored for 23 min after induction of an anaphylactic reaction.
Scratching behavioral experiment
Before the experiment, the ICR mice (n=6) were put into acrylic cages (22×22×24 cm) for about 30 min for acclimation. The behavioral experiments were performed according to the method of Sugimoto et al. (1998). The rostral part of the skin on the back of mice was clipped, and compound 48/80 (50 mg/kg) or histamine (100 mg/kg) for each mouse was intradermally injected. The scratching agents were dissolved in tween 80 and then used. Control mice received a tween 80 injection in the place of the scratching agent. Immediately after the intradermal injection, the mice (one animal/cage) were put back into the same cage; and for the observation of scratching. Scratching of the injected site by the hind paws was counted and compared with that of other sites, such as the ears. Each mouse was used for only one experiment. The mice generally showed several scratches for 1 s, and a series of these behaviors was counted as one incident of scratching for 30 min. R. fasciculatum (200 mg/kg) was orally administered 1 h before the scratching agents.
DNCB-induced atopic dermatitis
Experiments were conducted in accordance with a previously described protocol (Gao et al., 2005). The dorsal skin of the BALB/c mice (n=6) was shaved and treated with a depilatory prior to the experiment. The mice were sensitized with 100 ml of 0.15% DNCB in acetone-olive oil (3:1) applied to the dorsal skin twice per week for 5 weeks. Control mice received vehicle (acetone/olive oil=3:1). After 3 weeks, R. fasciculatum (200 mg/kg) was orally administered 2 weeks until the end of the experiment.
Cell culture
Raw 264.7 cells, macrophage cell line, were grown in DMEM medium supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated FBS at 37°C in 5% CO2.
MTT assay
To test the cell viability by each concentration of R. fasciculatum, the MTT colorimetric assay was performed. Briefly, cells (3×105 cells/well) were incubated with R. fasciculatum (0.01–1 mg/ml) for 12 h. After the addition of MTT solution, the cells were incubated at 37°C for 4 h. The crystallized MTT (formazan) was dissolved in dimethyl sulfoxide and measured the absorbance at 540 nm.
Assay of cytokines
Cytokine (TNF-α and IL-6) assay was performed by a modified enzyme-linked immunosorbent assay (ELISA). In this method, the wells of 96-well plates were coated with mouse monoclonal Abs specific for TNF-α and IL-6. The coated plates were washed with PBS containing 0.05% Tween 20 prior to subsequent steps in the assay. All reagents used in this assay were incubated for 2 h at 37°C. Recombinant TNF-α and IL-6 were diluted and used as standards. The assay plates were sequentially exposed to biotinylated mouse TNF-α, IL-6, AP and ABTS substrate solution containing 30% H2O2. The absorbance values of the plates were recorded at 405 nm.
HPLC analysis
The analysis was carried out using a model 1100 series LC system (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was performed on Hypersil GOLD column (4.6×250 mm, 5 μm, Thermo Scientific). The mobile phase consisted of acetonitrile and acetic acid. The total running time was 60 min, and the flow rate was 1.0 ml/min. The UV detection wavelength was set at 360 nm.
RESULTS
Effect of R. fasciculatum on compound 48/80-induced systemic anaphylaxis
To assess the contribution of R. fasciculatum in anaphylactic reactions, the in vivo model of systemic anaphylaxis was used initially. As a non-immunologic stimulator, compound 48/80 (8 mg/kg) was used. After the injection of the compound 48/80, the mice were monitored for 23 min, after which the mortality rate was determined. As shown in Table 1, an oral administration of saline as a control induced a fatal reaction in 100% of each group. When the R. fasciculatum was orally administered before the compound 48/80 injections, the mortality was reduced (Table 1).
Table 1.
Effect of R. fasciculatum on compound 48/80-induced systemic anaphylactic reaction in mice
The groups of mice (n=18) were orally pretreated with saline or R. fasciculatum was given at various doses 1 h before the compound 48/80 injection.
The compound 48/80 solution was intraperitoneally given to the groups of mice.
Mortality (%) is presented as the ‘Number of dead mice×100/Total number of experimental mice. The results were examined using Fisher’s exact test.
p<0.01 as compared with compound 48/80-treated group.
Effect of R. fasciculatum on histamine-induced scratching behavior in mice
In addition, we investigate the contribution of R. fasciculatum in histamine-induced scratching behavior. As shown in Fig. 1, orally administered R. fasciculatum inhibited the scratching behaviors by 46.11%. Terfenadine was used as a positive control in this study.
Fig. 1.
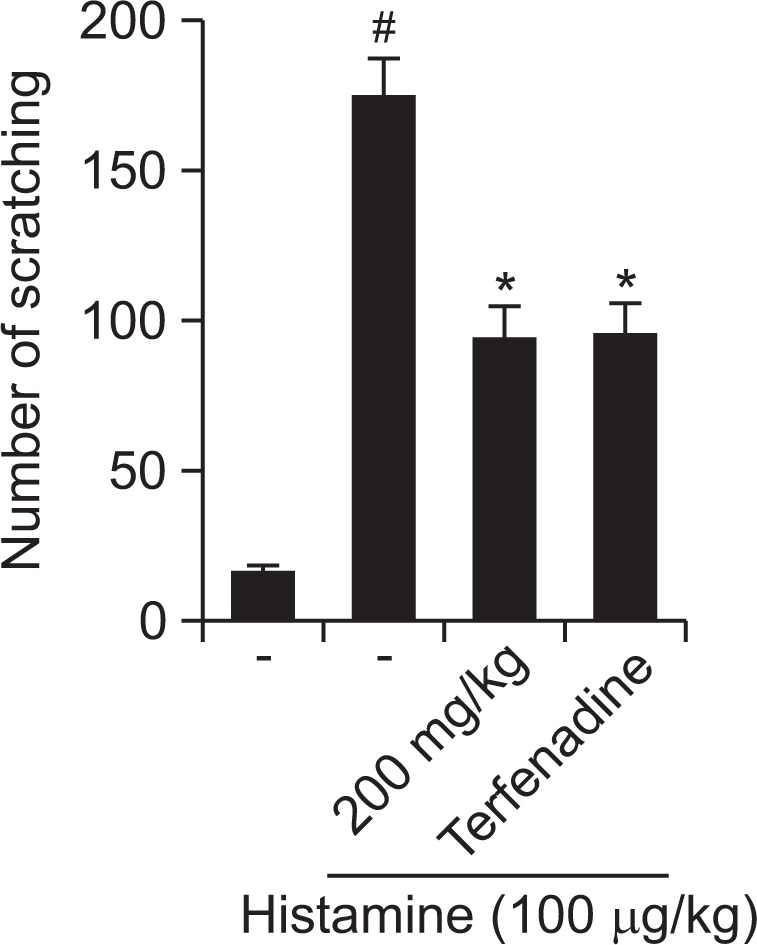
Effect of R. fasciculatum on scratching behavior in ICR mice. R. fasciculatum (200 mg/kg) was orally administered 1 h before compound 48/80 (50 μg/kg) intradermally injection. Scratching behaviors was counted as one incident of scratching for 30 min. Statistical evaluation of the results was performed by ANOVA with a Tukey post hoc test. Each datum represents the means ± S.D. of experiments (#p<0.05 vs. control group, *p<0.05 vs. compound 48/80 or histamine-treated group).
Effect of R. fasciculatum on compound 48/80-induced scratching behavior in mice
The anti-pruritic effects of R. fasciculatum were investigated on the compound 48/80-induced scratching behavior animal model. When the R. fasciculatum was orally administered 1 h before compound 48/80 injections, the scratching behaviors was reduced. The inhibition rate of R. fasciculatum (200 mg/ kg) was approximately 45.81% (Fig. 2).
Fig. 2.
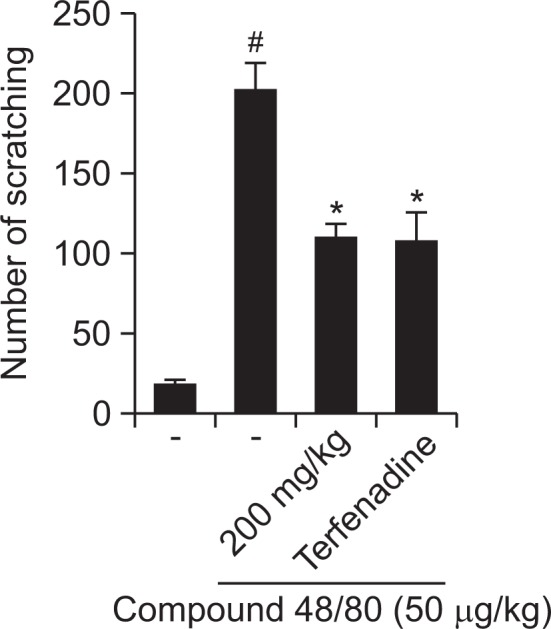
Effect of R. fasciculatum on scratching behavior in ICR mice. R. fasciculatum (200 mg/kg) was orally administered 1 h before histamine (100 μg/kg) intradermally injection. Scratching behaviors was counted as one incident of scratching for 30 min. Statistical evaluation of the results was performed by ANOVA with a Tukey post hoc test. Each datum represents the means ± S.D. of experiments (#p<0.05 vs. control group, *p<0.05 vs. compound 48/80 or histamine-treated group).
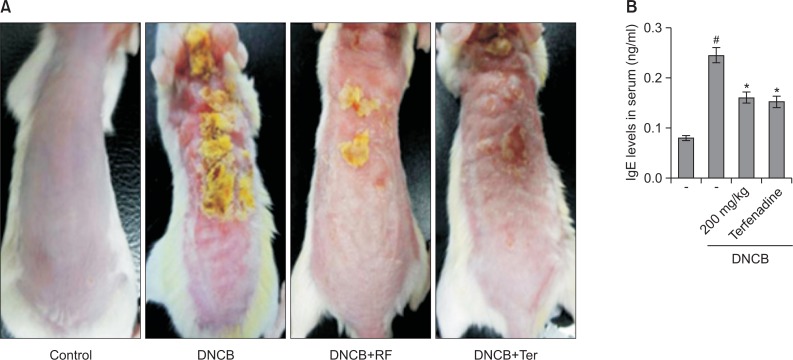
Effect of R. fasciculatum on DNCB-induced atopic dermatitis and IgE levels in serum
In order to evaluate the regulatory effects of R. fasciculatum in an atopic dermatitis in vivo model, DNCB was administered to BALB/c mice. As shown in Fig. 3A, when mice were treated for 2 week with R. fasciculatum, the atopic dermatitis was recovered to a significant extent. To evaluate the effects of R. fasciculatum on IgE levels in serum, blood samples were collected. The levels of IgE were measured via ELISA. The results showed that IgE levels were increased as the result of DNCB exposure, but this phenomenon was significantly reduced in the R. fasciculatum group (Fig. 3B).
Fig. 3.
Effect of R. fasciculatum on the DNCB-induced dermatitis and serum IgE level. (A) The BALB/c mice (n=6) were sensitized with 100 μl of 0.1% DNCB in acetone-olive oil (3:1) or vehicle (acetone/olive oil=3:1) applied to the dorsal skin twice each week for a total period of 5 weeks. After 3 weeks, R. fasciculatum (200 mg/kg) was orally administered 2 week prior to the end of the experiment. (B) Blood samples were collected and then levels of serum IgE in the indicated groups were measured using ELISA method. Statistical evaluation of the results was performed by independent t-test. Each datum represents the means ± S.D. of three independent experiments (#p<0.05 vs. control group, *p<0.05 vs. DNCB -treated group).
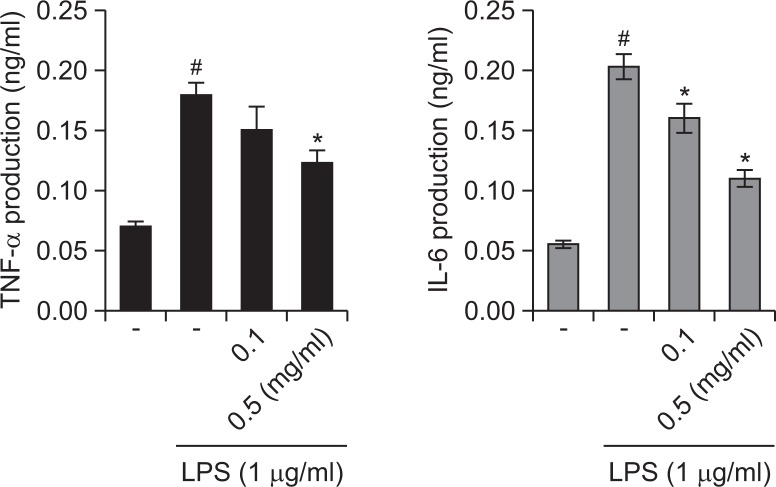
Effect of R. fasciculatum on the TNF-α and IL-6 production in LPS-stimulated macrophage
In an effort to determine the molecular mechanism of R. fasciculatum, the macrophage cell line, Raw 264.7, was employed in this study. We determined whether R. fasciculatum modulates the LPS-induced production of TNF-α and IL-6. The levels of TNF-α and IL-6 in culture supernatants were measured via ELISA. As is shown in Fig. 4, the production of TNF-α and IL-6 in response to LPS was inhibited as the result of pre-treatment with R. fasciculatum in a dose-dependent manner. The maximal rates of TNF-α and IL-6 inhibition by R. fasciculatum (1 mg/ml) were approximately 32.12% and 46.24%, respectively. Additionally, we observed that R. fasciculatum did not affect cell viability (data not shown).
Fig. 4.
Effects of R. fasciculatum on the production of inflammatory cytokines in LPS -stimulated Raw 264.7 cells. Cells were pre-treated with R. fasciculatum (0.1–0.5 mg/ml) for 1 h and then stimulated LPS (1 μg/ml) for 12 h. The levels of inflammatory cytokines (TNF-α and IL-6) were measured from cell supernatant using ELISA. Statistical evaluation of the results was performed by independent t-test. All data were represented in the mean ± S.D. of triplicate determinations from triplicate separate experiments (#p<0.05 vs. control, *p<0.05 vs. LPS alone).
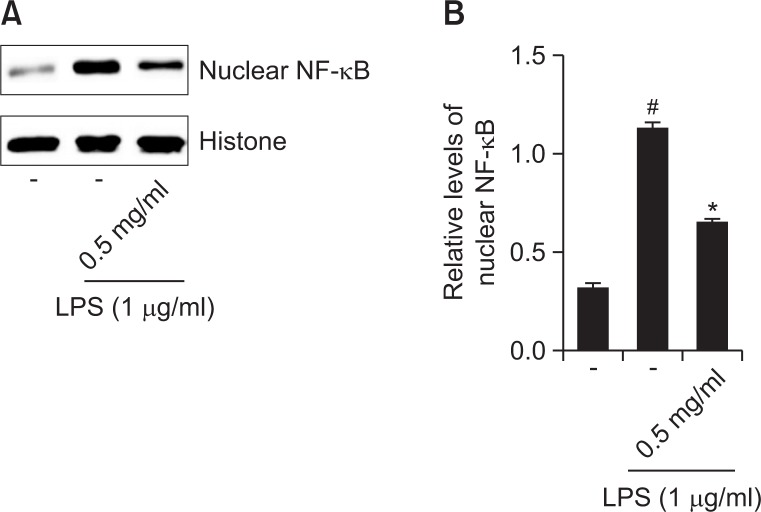
Effect of R. fasciculatum on NF-κB activation in the nuclei of LPS-stimulated RAW 267.4 cells
As the suppression of NF-κB activation has been linked with anti-inflammation, we speculated that the effects of R. fasciculatum might be mediated, at least in part, via the suppression of NF-κB activation. Additionally, because NF-κB activation requires the nuclear translocation of the RelA/p65 subunit of NF-κB, we evaluated the effects of R. fasciculatum on the nuclear pool of RelA/p65 protein via western blot analysis. In LPS-stimulated cells, the levels of Rel/p65 were increased, but R. fasciculatum reduced these enhanced nuclear levels of Rel/p65 (Fig. 5A). The relative levels of NF-κB (in nucleus) were represented in Fig. 5B.
Fig. 5.
Effect of R. fasciculatum on the NF-κB activation in the nuclei of LPS-stimulated Raw 264.7 cells. Cells were pre-treated with R. fasciculatum (0.5 mg/ml) for 1 h and then stimulated with LPS for 2 h. (A) Nuclear extracts were prepared as described in the Materials and Methods section and evaluated for RelA/p65 via Western blot analysis. (B) The relative levels of NF-κB were represented. Statistical evaluation of the results was performed by independent t-test. All data were represented in the mean ± S.D. of triplicate determinations from triplicate separate experiments (#p<0.05 vs. control, *p<0.05 vs. LPS alone).
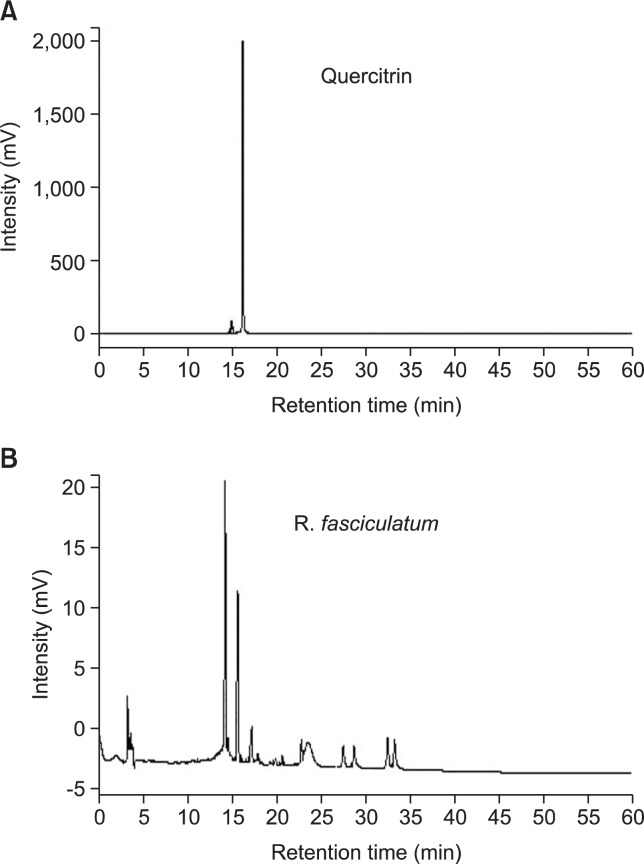
Qualitative analysis of R. fasciculatum by HPLC
To confirm the constituents of R. fasciculatum, an HPLC analysis was performed. HPLC measurement of R. fasciculatum demonstrated various chromatographic peaks. Comparing the chromatographic peaks of R. fasciculatum with reference chromatographic peaks, quercitrin was identified (Fig. 6).
Fig. 6.
HPLC chromatogram of R. fasciculatum. The retention time of quercitrin was specified on the chromatogram for the comparison. The mobile phase was 0.1% aqueous acetic acid (A) and acetonitrile (B) with a gradient program as follows: 0–5 min; isocratic 95% A; 5–60 min, linear gradient elution, 95-0% A; at a flow rate of 1 mL/min. The sample injection volume was 20 μL and the peak was monitored at a wavelength of 360 nm.
DISCUSSION
In this study, we demonstrated the molecular mechanisms of action of R. fasciculatum on allergic inflammation in vivo and in vitro. The findings of this study show that R. fasciculatum attenuated the compound 48/80- or histamine-induced scratching behaviors and inhibited DNCB-induced atopic dermatitis under in vivo conditions. Additionally, R. fasciculatum inhibited the production of TNF-α and IL-6 and activation of NF-κB in LPS-simulated macrophages.
AD is a chronic inflammatory skin disease and is characterized by erythema, edema, and scaling (Leung and Bieber, 2003). Generally, steroid therapy is very important in the treatment of AD, but it cannot be administered over a long-term, owing to its deleterious side-effects. Therefore, several researchers have attempted to find a new drug, which is effective in the treatment of AD (Shiohara et al., 2004). AD is characterized by a potent skin inflammation associated with an elevated level of IgE against many types of allergens (Allam and Novak, 2006; Brenninkmeijer et al., 2008). On the basis of the results of these studies, we aimed to evaluate the effects of R. fasciculatum on DNCB-induced allergic reactions in vivo. The findings of this study revealed that R. fasciculatum significantly reduced DNCB-induced atopic dermatitis. Additionally, R. fasciculatum caused a reduction in serum IgE levels induced by DNCB. These results demonstrate the potential effect of R. fasciculatum on anti-allergic responses via the regulation of IgE levels.
In pathological skin conditions, histamine is involved in the induction of itching and edema (Minami and Kamei, 2004). This study focused on the manner in which R. fasciculatum regulates histamine-induced scratching behaviors in mice. We showed that R. fasciculatum inhibited the histamine-induced scratching behaviors in mice. Compound 48/80 is a well-known histamine releaser (Kim et al., 2003). Antihistamines decreased the release of inflammatory mediators from inflammatory cells and the activation of eosinophils in an in vitro study (Assanasen and Naclerio, 2002). Thus, compound 48/80 has been used as a direct and convenient reagent to study the mechanism of anaphylactic reaction. The results demonstrated that R. fasciculatum attenuated compound 48/80-induced scratching behavior and anaphylactic reaction in mice. These results indicate that R. fasciculatum regulates the allergic response in vivo.
In inflammatory processes, inflammatory cytokines recruit activated immune and inflammatory cells to the site of lesions, thereby amplifying and perpetuating the inflammatory state. Macrophages are important effecter cells in allergic inflammatory diseases, such as asthma and AD. Inflammatory cytokines derived from macrophages play an important role in the development of inflammatory reactions. To gain further insights into the mechanisms underlying R. fasciculatum-mediated inhibition of LPS-induced inflammatory mediators (TNF-α and IL-6), we examined the regulatory effect of R. fasciculatum on intracellular signaling molecules involved in the LPS signaling pathways in macrophages. In this research, we demonstrated that R. fasciculatum inhibited the secretion of TNF-α and IL-6 in LPS-simulated macrophages. The maximal inhibition rates of TNF-α and IL-6 production by R. fasciculatum were approximately 32.12% and 46.24%, respectively. These results demonstrate that R. fasciculatum exerts an anti-inflammatory effect via the regulation of inflammatory cytokine production.
Cytokine production is associated with increased activation of the gene transcriptional regulator, NF-κB (Gilmore and Garbati, 2011). After a variety of stimuli, the IκB proteins are phosphorylated, and degraded, allowing NF-κB to translocate into the nucleus where it can bind to specific DNA sequences located in the promoter regions of target genes and activate gene transcription, thereby indicating its pivotal role in the regulation of inflammatory responses, via control of the transcription of inflammatory cytokine genes. Based on this, inhibition of NF-κB activation has been suggested as an anti-inflammatory treatment strategy in AD. Therefore, we attempted to determine whether the anti-inflammatory effect of R. fasciculatum is via the regulation of NF-κB activation. The results demonstrated that R. fasciculatum inhibited the NF-κB translocation into nucleus in the LPS-stimulated RAW 264.7 cells. Therefore, we hypothesized that R. fasciculatum might exert anti-inflammatory effects via NF-κB activation. Although R. fasciculatum attenuated the activation of NF-κB, the effect of R. fasciculatum on the pathways involving NF-κB (phosphorylation of IκB-α and IKK activation) was not determined. Therefore, further studies will be necessary in order to clarify more precisely the role of R. fasciculatum in the NF-κB pathway.
The chemical constituents of R. fasciculatum are cathecin, sarmentosin, quercitrin and so on (Dat et al., 2005). It was reported that these compounds regulated the allergic inflammation. For example, quercitrin attenuated the degranulation of mast cell, histamine release and allergic anaphylaxis reaction in mice (Cruz et al., 2008). Shimamura et al., also showed that catechin may be useful in the treatment of atopic dermatitis via suppression of TNF-α production (Hisano et al., 2003). Although we confirmed that quercitrin is one of compounds from R. fasciculatum in this study, the pharmacological effects of these compounds were not investigated. Therefore, further studies will be necessary to determine the effect and mechanism of these compounds on allergic reaction.
In conclusion, R. fasciculatum can regulate the allergic response in vivo, including in compound 48/80- or histamine-induced scratching behaviors and DNCB-induced atopic dermatitis. Additionally, we demonstrated in this study that the anti-inflammatory activities of R. fasciculatum could be attributed, at least in part, to the inhibition of inflammatory cytokine production (TNF-α and IL-6). These effects of R. fasciculatum are caused by the inhibition of LPS-induced activation of NF-κB in LPS-stimulated macrophages. These results provide experimental evidence demonstrating that R. fasciculatum may prove useful in the treatment of allergic inflammatory diseases.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0022703).
REFERENCES
- Allam JP, Novak N. The pathophysiology of atopic eczema. Clin Exp Dermatol. 2006;31:89–93. doi: 10.1111/j.1365-2230.2005.01980.x. [DOI] [PubMed] [Google Scholar]
- Assanasen P, Naclerio RM. Antiallergic anti-inflammatory effects of H1-antihistamines in humans. Clin Allergy Immunol. 2002;17:101–139. [PubMed] [Google Scholar]
- Boero S, Silvestri M, Ullmann N, Rossi GA. Modulation by flunisolide of tumor necrosis factor-alpha-induced stimulation of airway epithelial cell activities related to eosinophil inflammation. J. Asthma. 2010;47:381–387. doi: 10.3109/02770901003759410. [DOI] [PubMed] [Google Scholar]
- Brenninkmeijer EE, Spuls PI, Legierse CM, Lindeboom R, Smitt JH, Bos JD. Clinical differences between atopic and atopiform dermatitis. J Am Acad Dermatol. 2008;58:407–414. doi: 10.1016/j.jaad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom. 2001;70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- Cruz EA, Da-Silva SA, Muzitano MF, Silva PM, Costa SS, Rossi-Bergmann B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int Immunopharmacol. 2008;8:1616–1621. doi: 10.1016/j.intimp.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Dat NT, Cai XF, Shen Q, Lee IS, Kim YH. New inhibitor against nuclear factor of activated T cells transcription from Ribes fasciculatum var. chinense. Chem Pharm Bull. 2005;53:114–117. doi: 10.1248/cpb.53.114. [DOI] [PubMed] [Google Scholar]
- Gadaleta RM, Oldenburg B, Willemsen EC, Spit M, Murzilli S, Salvatore L, Klomp LW, Siersema PD, van Erpecum KJ, van Mil SW. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim. Biophys. Acta. 2011;1812:851–858. doi: 10.1016/j.bbadis.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Gao XK, Fuseda K, Shibata T, Tanaka H, Inagaki N, Nagai H. Kampo medicines for mite antigen-induced allergic dermatitis in NC/Nga mice. Evid Based Complement Alternat Med. 2005;2:191–199. doi: 10.1093/ecam/neh077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD, Garbati MR. Inhibition of NF-κB signaling as a strategy in disease therapy. Curr Top Microbiol Immunol. 2011;349:245–263. doi: 10.1007/82_2010_105. [DOI] [PubMed] [Google Scholar]
- Gold MS, Kemp AS. Atopic disease in childhood. Med J Aust. 2005;182:298–304. doi: 10.5694/j.1326-5377.2005.tb06707.x. [DOI] [PubMed] [Google Scholar]
- Hisano M, Yamaguchi K, Inoue Y, Ikeda Y, Iijima M, Adachi M, Shimamura T. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB) Arch. Dermatol Res. 2003;295:183–189. doi: 10.1007/s00403-003-0411-x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Na HJ, Han SW, Jin JS, Song UY, Lee EJ, Song BK, Hong SH, Kim HM. Forsythia fructus inhibits the mast cell mediated allergic inflammatory reactions. Inflammation. 2003;27:129–135. doi: 10.1023/a:1023865727780. [DOI] [PubMed] [Google Scholar]
- Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- McCormick TS, Stevens SR, Kang K. Macrophages and cutaneous inflammation. Nat Biotechnol. 2000;18:25–26. doi: 10.1038/71879. [DOI] [PubMed] [Google Scholar]
- Minami K, Kamei CA. A chronic model for evaluating the itching associated with allergic conjunctivitis in rats. Int Immunopharmacol. 2004;4:101–108. doi: 10.1016/j.intimp.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–398. [PubMed] [Google Scholar]
- Shiohara T, Hayakawa J, Mizukawa Y. Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci. 2004;36:1–9. doi: 10.1016/j.jdermsci.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effect of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- Trefzer U, Hofmann M, Sterry W, Asadullah K. Cytokine and anti-cytokine therapy in dermatology. Expert Opin Biol Ther. 2003;3:733–743. doi: 10.1517/14712598.3.5.733. [DOI] [PubMed] [Google Scholar]