Abstract
Materials with differing surfaces have been developed for clinical implant therapy in dentistry and orthopedics. This study was designed to evaluate bone response to titanium alloy containing Ti-32Nb-5Zr with nanostructure, anodic oxidation, heat treatment, and ibandronate coating. Rats were randomly assigned to two groups for implantation of titanium alloy (untreated) as the control group and titanium alloy group coated with ibandronate as the experimental group. Then, the implants were inserted in both tibiae of the rats for four weeks. After implantation, bone implant interface, trabecular microstructure, mechanical fixation was evaluated by histology, micro-computed tomography (μCT) and the push-out test, respectively. We found that the anodized, heat-treated and ibandronate-coated titanium alloy triggered pronounced bone implant integration and early bone formation. Ibandronate-coated implants showed elevated values for removal torque and a higher level of BV/TV, trabecular thickness and separation upon analysis with μCT and mechanical testing. Similarly, higher bone contact and a larger percentage bone area were observed via histology compared to untreated alloy. Furthermore, well coating of ibandronate with alloy was observed by vitro releasing experiment. Our study provided evidences that the coating of bisphosphonate onto the anodized and heat-treated nanostructure of titanium alloy had a positive effect on implant fixation.
Keywords: Dental implants, Osseointegration, Titanium alloy, Ibandronate, Nanotubes
INTRODUCTION
Biomaterials are defined as any material that has contact with living tissue; their clinical applications in orthopedics and dentistry are widespread. Implantation of biomaterials induces foreign body reactions that significantly affect the performance and biocompatibility of implants. To maximize interaction, implants have been modified, mixed and coated with various elements and drugs. These materials elicit the formation of bone and interact with the surrounding tissue. Titanium and its alloys are extensively used in medical devices (as well as in the dental, orthopedic and cardiovascular fields) due to their relatively low elastic moduli, high corrosion resistance and excellent biocompatibility (Pohler, 2000). Titanium and its alloys are bioinert materials. Instead of chemically bonding to the bone, titanium implants are simply incorporated into bone contact (Yan et al., 1997).
To provide the metal with bonding ability and thus improve the osseointegration of dental implants, researchers are investigating alternative methods, such as using titanium alloys to coat metallic implants with bisphosphonates (BPs) along with heat treatment. The recent formation of titanium implant surfaces with nanostructures could significantly reinforce osseointegration. An oxide layer of nanotube titania with an ordered array, a larger surface area and higher level of surface energy can be formed by an anodic oxidation treatment of pure titanium or on titanium surfaces (Popat et al., 2007a; Balasundaram et al., 2008; Brammer et al., 2009). The surface of the nanostructure can induce the migration of osteoblasts (Popat et al., 2007a; Balasundaram et al., 2008; Brammer et al., 2009) and mesenchymal stem cells (Popat et al., 2007b; Brammer et al., 2009; Park et al., 2009) and promote cell differentiation and matrix synthesis, accelerating the osseointegration process. Since materials such as hydroxyapatite and collagen (which form on implant surfaces during osseointegration) have nanosized structures, nanostructured surfaces should be more favorable for osseointegration. Drug immobilization into a nanotube can be applied to various chemicals and biomolecules in sizes ranging from small molecules to proteins, which can reduce inflammation and promote osteoblastic functions, thereby accelerating osseointegration (Bae et al., 2010).
Bisphosphonates (BPs), currently most widely used class of antiresorptive drugs, are analogues of pyrophosphate; ibandronate is one of the five widely used BPs. Bisphosphonates are selectively taken up by osteoclasts in the bone matrix that contain hydroxyapatite, and they have direct effects at the cellular level (Fleisch, 1998). For example, BPs inhibits the recruitment and differentiation of osteoblast precursors (Hughes et al., 1989). as well as the resorptive activity of mature osteoclasts (Carano et al., 1990). BPs induce apoptosis of macrophages and mature osteoclasts (Reszka et al., 1999) Furthermore, BPs have been shown to directly enhance the proliferation, differentiation, and bone-forming activity of osteoblasts (García-Moreno et al., 1998). Because of this ability, several researchers have investigated implants coated with bisphosphonates and their effects on implant fixation (Shanbhag et al., 1997; Meraw and Reeve, 1999; Bhandari et al., 2005; Hilding and Aspenberg, 2006). The general findings are increased osseointegration and reduced implant migration due to satisfactory attachment at the microcellular level. Given the good performance of the alloy on the bone/implant interface and also considering the surgical technique of bone compaction on the peri-implant bone, we chose to investigate whether implant fixation can be further improved by the application of ibandronate and heat treatments.
We previously investigated the effect of biphosphonate on the osseointegration of anodized and heat treated (AH) titanium surface (Lee et al., 2011). Now, in this study we compared the quantity of new bone that formed around the newly developed implant, which is a mixture of titanium Ti-32Nb-5Zr with a nanostructure treated with anodic oxidation and heat and then further coated with ibandronate. We hypothesized that the alloy and ibandronate produce a double action to enhance biocompatibility in vivo. We analyzed integration through various mechanisms, such as histology, mechanical testing and microstructure parameters by micro computed tomography (μCT) analysis. Furthermore, we carried out in vitro release tests for ibandronate over two weeks and found an adequate coating of ibandronate on the implant surface; ibandronate was detected up to 15 days following the implant. Therefore, our data showed that nanostructure titanium alloy (Ti-32Nb-5Zr) that has been anodized and heat- and bisphosphonate-treated had the potential to reinforce osseointegration.
MATERIALS AND METHODS
Biomaterials and coatings
Ti-32Nb-5Zr alloy was prepared and cut to 20×10×1.2 mm in size. Five specimens were polished sequentially using #220 to #1000 SiC paper, ultrasonically washed with acetone and alcohol, and then rinsed with deionized water. Ten implant specimens with dimensions of 1.2×1.2×10 mm were prepared and divided into two groups. One group was left untreated, and the other group was coated with ibandronate after an anodizing treatment. All samples were stored in a dryer at 50°C for more than 24 hours before the anodic oxidation treatment.
Before oxidation treatment, all samples were acid-etched by immersion in a HNO3: HF:H2O (12:7:81) solution for 10 seconds, washed by shaking in deionized water 5 times, and then dried. Electrolyte solutions were prepared by mixing glycerol with 20 wt% H2O and 1 wt% NH4F. To form the nanotubular TiO2 layer, a specimen and platinum plate were connected to the cathode and anode of a direct-current power generator, respectively, and a pulse power of 20 V and 20 mA/cm2 in 0.01 ms cycles was applied for 60 minutes. After the anodic oxidation treatment, the samples were rinsed ultrasonically for one minute in deionized water and dried at 16°C. Then, to achieve structural stability of the nanotubular TiO2 layer and the removal of impurities, the samples were heat-treated at 500°C for two hours. Five untreated and drug-coated mini-implants of Ti-32Nb-5Zr alloy with dimensions of 1.2×1.2×5 mm were prepared. For the drug coating treatment, these implants were immersed in 1 mg/ml of ibandronate at 37°C in an incubator for 24 hours to allow absorption of the drug.
Animals
Experimental rats were purchased from Orient-Bio, Korea. They were housed four per cage under a 12-hour light/dark cycle at a temperature of 26 ± 2°C and a humidity of 40–60%. The rats were provided with commercial food and tap water. The experiments were approved by the Chonbuk National University Animal Care Committee.
Surgical procedure
Surgical procedures were performed under general anesthesia. Two groups of male Wistar rats weighing between 200–225 g each were divided into the control and drug-treated groups; there were four rats in each group. General anesthesia was performed using ketamine/xylazine (80 to 100 mg/kg and 10 to 20 mg/kg, respectively). Additionally, a 2% lidocaine solution containing epinephrine (1:100,000) was also administered for local anesthesia, and then the surgical site was shaved and disinfected with a Betadine scrub. This process was followed by the elevation of full-thickness mucoperiosteal flaps. The implants were placed in the distal regions of bilateral tibia diaphyses using a stepwise drilling procedure and irrigated with sterile saline during placement. The implants were placed in the prepared sites using a self-tapping process and then sutured with resorbable sutures. Postoperatively, amoxicillin (1 ml/kg) antibiotics and ketoprofen (5 mg/kg) analgesics were administered intramuscularly. Four weeks later, the rats were sacrificed with an overdose of thiopental then all tibiae and muscle around the implants were collected for further analysis.
Histological examination
For histological examinations, we collected tibiae from sacrificed rats and stored them in 10% formalin (10% formalin-0.1 M phosphate buffer, pH 7.4) for one day, then decalcified them with 10% EDTA (pH 7.4) that was changed every two days for a period of two weeks. Specimens were then dehydrated using an ethanol series, embedded in paraffin and sectioned. The sections of tibiae (5–10 μm thickness) were stained with hematoxylin and eosin. Stained sections to determine bone area and bone contact were viewed at 25× and 50× magnification, and images of these sections were captured using a color digital video camera (ZEISS, Germany). The bone contact is the length ratio of the direct bone implant interface to the total implant surface, and the bone area is the ratio of newly formed bone area to the whole area within a length of 0.1 mm width. The values for bone contact and total bone area were calculated using software (Soft Imaging System, Analysis). The values for bone contact and total bone area were obtained by averaging all 50 readings [10 location×5 different slides] for each group.
μ-CT analysis
Bone implant interfaces and trabecular microstructures at the knee were analyzed at one and four week after implantation by a μCT 80 scanner (Skyscan1076, Skyscan, Aartselaar, Belgium). The bone and the implant were positioned in a costom to be scanned through a μCT system. The obtained images were evaluated using the software. The value of interest (VOI) was defined as the 100 slices from 3.0 mm below the growth plate and limited in a ring from the implant axis with a diameter of 2.0 mm. The following morphometric indices were directly analyzed from the binarized VOI: bone volume ratio (BV/TV), trabecular thickness (Tb/Th), trabecular separation (Tb/Sp) and trabecular no (Tb/N). The percentage of VOI was calculated to assess the bone-implant interface detail defined as the ratio of bone voxels to total voxels in direct contact with the implant.
Mechanical testing
Bond strength was measured four weeks after placing the implants in the tibia. Rats were sacrificed with an overdose of thiopental. The implant sites in the rat tibiae were surgically exposed via sharp dissection to the bone and were clinically examined after careful removal of the overgrowing bone and soft tissues. We conducted a push-shear bond test at a cross-head speed of 0.5 mm/min. Mean shear bond strengths were calculated for each group.
In vitro release test of ibandronate
The amount of drug released from the samples was examined using a pure titanium sample and a TiO2 nanotube titanium alloy sample after anodic oxidation and a thermal treatment; both sample types were 1 cm×2 cm in size. Each sample was exposed to ibandronate (1 mg/ml) solution and dried in a cell culture hood for one day. On a daily basis over 12 days, each sample was immersed in a mobile phase for 24 hours. The amount of drug released was examined by high-performance liquid chromatography (Xie et al., 2006). A mixture of 18 mM of an n-amylamine solution (pH 7.0, titrated with acetic acid) and acetonitrile (95:5, v/v) was used as the mobile phase. Ibandronate was separated on a C18 column at 25°C with a flow rate of 1 ml/min and detected at 220 nm.
Osteogenesis-associated mRNA analysis by RT-PCR
Tissue around the implant was collected in a sterile condition and further processed for RT-PCR analysis. All tissues were kept in frozen condition using liquid nitrogen until homogenization for mRNA extraction. Total RNAs were isolated from tissues using TRIzol (Invitrogen) and cDNA synthesis was performed using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Primer sequences and PCR condition used in this study are listed in Table 1. After an initial denaturation step at 95°C for 1 min, PCR was performed for various cycles (30 sec at 94°C, 1 min at the primer-pair specific annealing temperature, and 2 min at 72°C) using Taq polymerase (Promega, Madison, WI, USA). Reaction products (10 ml) were separated on a 0.8% agarose gel stained with ethidium bromide, and analyzed densitometrically. Band intensity was analyzed by densitometry using a phosphoimager and Quantity One software (Version 4.3.1) (Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequences and conditions for RT-PCR
| Target genes (Accession number) | Primers Forward/Reverse | PCR condition | ||
|---|---|---|---|---|
|
| ||||
| Tm(°C) | Cycles | Size(bp) | ||
| Collagen 1 | 5′-ttctcctggtaaagatggtgc-3′ | 50 | 35 | 254 |
| (NM_007742) | 5′-tgttaaaggtgatgctggtcc-3′ | |||
| OCN | 5′-cagcttggtgcacacctagc-3′ | 58 | 30 | 242 |
| (NM_001032298) | 5′-ggagcagtgtcacgttaacct-3′ | |||
| β-actin | 5′- ttctacaatgagctgcgtgt-3′ | 60 | 25 | 456 |
| (NM_007393) | 5′- ctcatagctcttctccaggg-3′ | |||
Statistical analysis
All experimental data are expressed as means ± S.E.M., and all experiments were repeated at least three times unless otherwise indicated. Statistical analyses were performed by Dunnett’s multiple comparison test using the Statistical Package for the Social Sciences (SPSS) ver. 12.0 software, and p-values less than 0.05 were considered statistically significant. Other methods not specifically mentioned in this manuscript were as same as described elsewhere (Nepal et al., 2013).
RESULTS
Histological evaluation of bone
To analyze the effect of ibandronate-treated alloy on bone, we evaluated new bone formation around tibial implants after four weeks. The rats were implanted with either an untreated alloy, which was used as a positive control, or ibandronate and a heat-treated alloy placed between the tibia and the femur of the male rats for four weeks, which was our experimental condition (Fig. 1A). Previous studies have established that BP has the ability to promote osteoblast formation in bone and can also enhance osseointegration. As expected, four weeks after implantation, circumferential bone tissue formation was detectable on both the implants and the bone that was in close contact with the implant on both the untreated as well as the ibandronate-coated implants. Furthermore, healing and hard tissue integration of the implants was achieved over a four-week period, and no signs of inflammation were detected in any of the specimens.
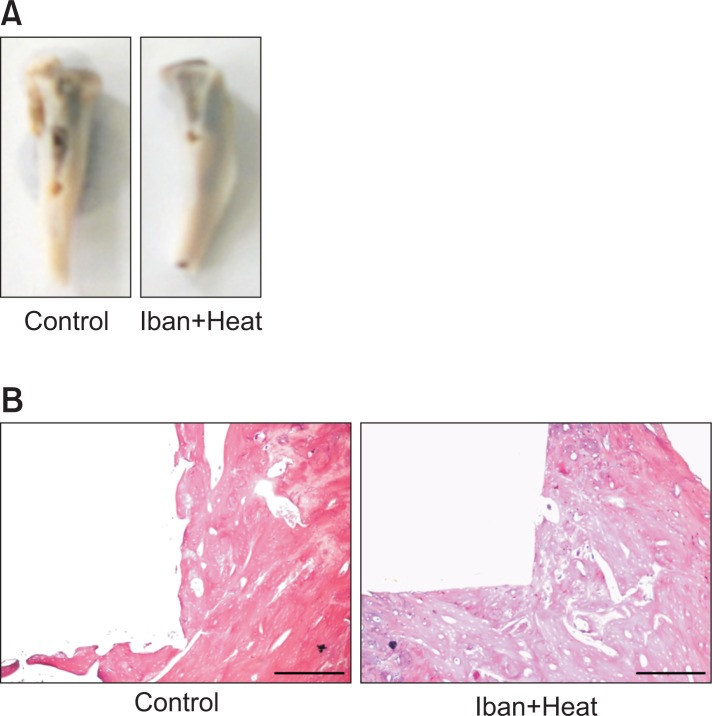
Fig. 1.
Photographs of bone after removing an implant. The bone was fixed according to the shape of the implant (A). Histological appearance of bone after staining with hematoxylin and eosin (B). The picture depicts the ongoing fixation of the bone to the implant. Photographs captured at 50X, and the scale bar is 50 μM.
More precise integration was observed in rats that received implants treated with BP than was seen in rats receiving implants alone (Fig. 1B). Based on this integration, the bone-to-implant contact (BIC) was calculated using an image-analysis program. The bone contact was defined as the length ratio of the direct bone-implant interface to the total implant surface, and the bone area was defined as the ratio of newly formed bone area to the whole area within a length of 0.1 mm width.
The bone responses to control and ibandronate-coated implants were significantly different. Four weeks following implantation, 324.205 μm2 of bone area (BA) and 29.330 μm of bone contact (BC) surface were observed in the control group, whereas these same measurements were 479.721 μm2 and 53.51 μm, respectively, in the ibandronate and heat-treated implants (Fig. 2A, B) The implants treated with ibandronate showed a significantly higher bone contact ratio (p<0.05) compared to the control implants. Additionally, the relative bone area was also found to be significantly higher than in the controls. These significant increases in both bone contact and bone area suggest that ibandronate coating is a promising method for enhancing the biocompatibility of implant materials.
Fig. 2.
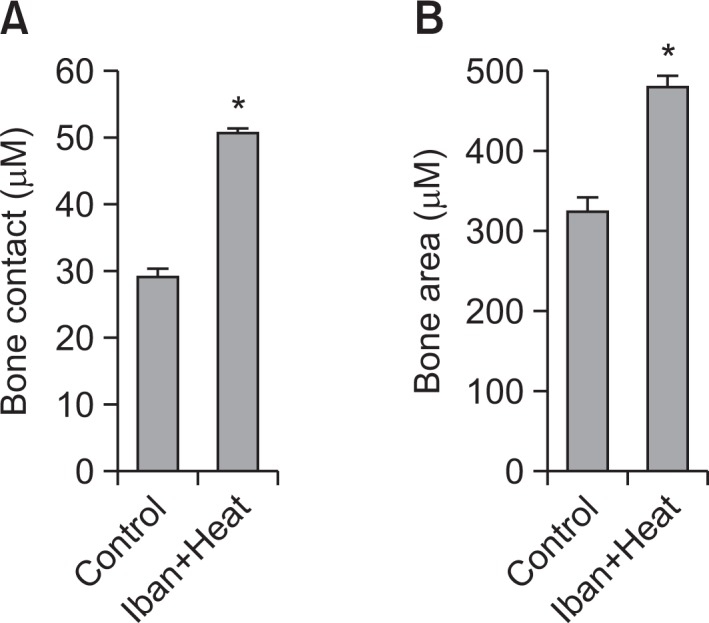
Histomorphometric analysis of the bone area and percentage bone contact. The means of 50 individual readings (10×5 slides) were calculated. The bone contact (A) is defined as the direct bone implant interface to the total implant surface, and the bone area (B) is defined as the ratio of newly formed bone area to the whole area within the figure. Data were expressed as mean ± S.E.M. *p<0.05 vs. the control group.
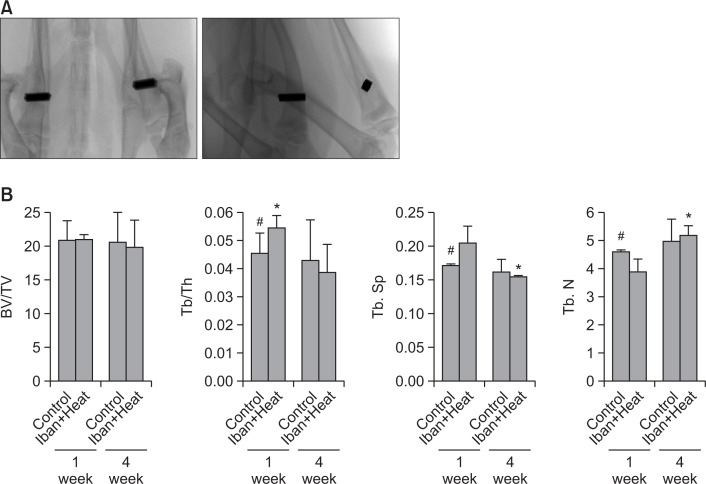
μCT evaluation
μCT analysis of the sample revealed effects in various microstructure parameters, such as BV/TV, Tb/Th, Tb/Sp, and Tb/N, over periods of one and four weeks after implantation (Fig. 3A). Our quantitative evaluation provided more detailed information on osseointegration and trabecular parameters around the screws. Compared with controls, the treatment combination that included ibandronate significantly improved the trabecular bone/trabecular thickness (Tb/Th) in 1 week and trabecular number (Tb N) in 4 weeks, while a significant decrease in trabecular separation (Tb.Sp) in 4 weeks was observed (Fig. 3B). Most changes in microstructure parameters were statistically significant when compared with the control alloy, which was treated with heat alone. This demonstrates that bisphosphonate coating and heat treatment of implants improves microstructure parameters, which provides evidence of ongoing osseointegration.
Fig. 3.
Microstructure parameters measured by μCT. Appearance of the implants by μCT scanner (A). Bone volume/tissue volume, trabecular bone/trabecular thickness, trabecular separation and a trabecular number for one and four weeks, respectively (B). Data are expressed as mean± S.E.M. *p<0.05 vs. control (#).
Biomechanical analysis
To determine the integration between the implant and the bone, we evaluated mechanical force using a push-out assay. Implants placed in the bone exerted noticeable force between the bone and the implant. The ibandronate-coated and heat-treated alloys showed the maximal push-out force and ultimate shear strength. The maximum mean force for the control alloy was about 0.4 Mpa, while for heat and ibandronate-coated implant, the mean force was about 4 Mpa, 10-fold greater than the control implants (Fig. 4). This force indicates strong osseointegration between ibandronate-treated implants and bone. From this result, we concluded that heat treatment along with biphosphonate coating not only improved implant integration to the bone but also promoted early bone formation from one week to four weeks postoperatively, which can ultimately treat implant loosening.
Fig. 4.
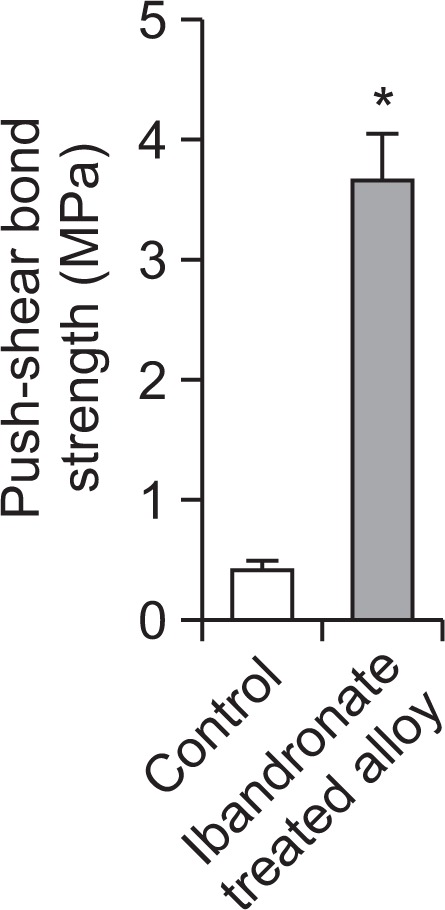
Interfacial shear strength between control and titanium alloy. Four weeks after implantation, the mechanical fixation of control and nanotubular titanium alloy coated with ibandronate was measured via the push-out test, respectively. Data are expressed as mean ± S.E.M. *p<0.05 vs. the control.
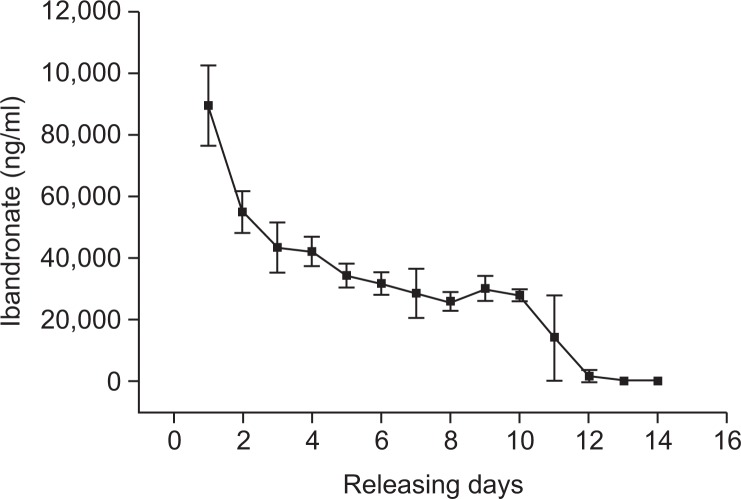
In vitro release experiment
We performed in vitro release tests of ibandronate to test the coating of the BP with alloy. Heat-treated implants were exposed to 1 mg/ml of ibandronate for 24 hours. Immediately following exposure, the water was collected to assess the concentration of ibandronate by high-performance liquid chromatography. This process was continued for two weeks. In the initial phase of testing that took place during the first 2 days, ibandronate was released sharply; from 3 days onward, it was released gradually up to 12 days (Fig. 5). No further ibandronate was detectable after 14 days. In comparison to previous studies, BP was released sharply in the initial four days of our study then released gradually for the remaining days. In our experiment, ibandronate was released gradually, which likely resulted from extensive contact with the nanotubular surface of the implant.
Fig. 5.
In vitro release of ibandronate from anodized and heat-treated nanotubular implants over a two-week period.
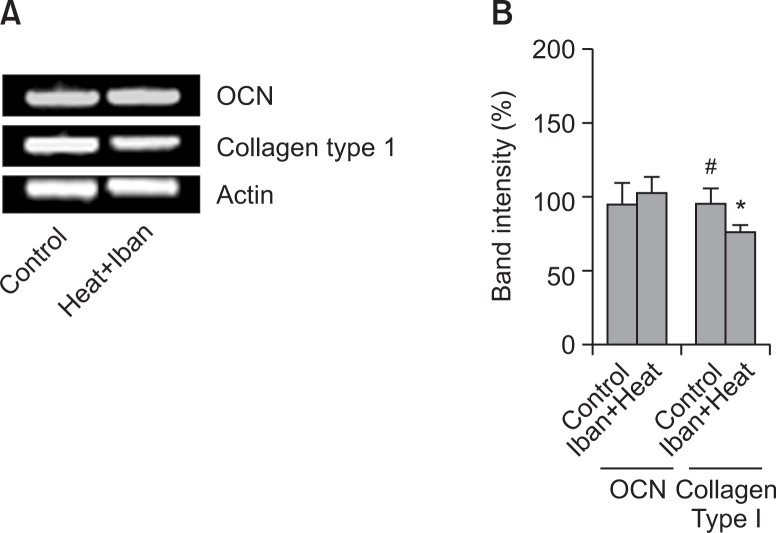
Expression of osteogenesis-associated genes
Several transcription factors and genes play a vital role for bone formation. In our study we tried to see the mRNA expression of Collagen type I and osteocalcin (OCN) in the tissue around the implant. To do so we have extracted the tissue around the implant after four week of implantation. The mRNA expression of Collagen type I was down regulated while the expression of OCN remained unchanged. Expression of these genes was analyzed by RT-PCR in both control and ibandronate coated titanium alloy (Fig. 6).
Fig. 6.
The mRNA expression of marker genes for bone formation. The mRNAs of Collagen type I and OCN from heat-, and ibanronate-treated rats and control, respectively were extracted and measured by RT-PCR (A). The histogram depicts the mRNA of Collagen type I and osteocalcin (OCN) compared to β-actin (B). Asterisk (*) indicates a significant difference (p<0.05) compared with the control (#). Each column represents the mean ± S.E.M. of three or four separate experiments.
DISCUSSION
Several materials have long been used in dental prosthetics, and design, modification and production of alloys has developed over time. Currently, research is focused on advanced materials and alloys. It has been reported that the formation of a titanium implant surface with a nanostructure could significantly reinforce osteointegration. An oxide layer of nanotube titania with an ordered array, a larger surface area and higher level of surface energy can be formed by oxidation treatment of pure titanium or titanium alloy surfaces (Jones, 2001; Giavaresi et al., 2003).
In this study, we determined the bioactivity of heat-treated nanotubular Ti-32Nb-5Zr alloy coated with ibandronate. Titanium (Ti) is considered to be the best material for endosseous implants. In addition to its mechanical, chemical and physical properties, Ti is known to possess excellent biocompatibility (Ratner, 2001). Osteointegration may be improved by modifying implants with different metals. Appropriately modified Ti may therefore be the key factor in achieving fast and stable implant fixation through optimal osseointegration. In this study we used a titanium alloy coated with BP, which was expected to enhance implant osseointegration. We observed solid contact between tissue and implant surfaces. The increased expression of osteogenesis-associated genes indirectly reflects increased bone formation in nanotubular alloys treated with heat and ibandronate. Type I collagen is one of the common collagenous bone proteins that accounts for more than 90% of the bone matrix and reflects early bone formation. Osteocalcin is an osteogenic marker that indicates the functions of osteoblasts in association with minerals contained in the bone matrix. Therefore, the expressions of type I collagen and osteocalcin indicate bone formation activity. Since BPs increase early bone formation around implants, mRNA levels of Type 1 collagen and OCN may return to normal or low in 4 weeks (Meraw et al., 1999; Jakobsen et al., 2007).
Because the osteointegration of Ti takes place through a process that involves the chemical modification of the outer layer of Ti (Textor et al., 2001) and the mixing of other elements, and because coating with BPs enhances osteoblast differentiation and cell proliferation, this process may become the basis for intimate bond formation between alloys and bone. Previous studies have shown that BPs not only promote osteoblast formation but also enhance the biomechanical parameters of implants (Jakobsen et al., 2007). BPs can inhibit bone resorption and normalize the high rate of bone turnover that characterizes osteoporosis. Although there has been some controversy over the relationship between oral BP treatments and jaw osteonecrosis (Motohashi et al., 1999), the beneficial effects of BP alendronate (synthetic treatment) on early bone integration to HA-coated implants or implant fixation have been demonstrated in different studies (Pazianas et al., 2007). Moreover, a previous study clearly showed that OVX-induced osteopenia impairs the osteointegration of HA-coated titanium implants, and daily subcutaneous injections of ibandronate improve implant integration in osteopenic, ovariectomized rats (Jensen et al., 2007). In addition to their beneficial effects when used systemically, local administration of BPs has been proven to be highly efficient to gather BPs at the site of implantation and thus to increase early bone formation around implants and the biomechanical fixation of implants inserted into normal bone (Meraw et al., 1999; Jakobsen et al., 2007).
In this study, ibandronate treatment was able to increase the quantity and quality of new bone formation around implants. Heat treatment and ibandronate coating also enhanced biomechanical parameters, which demonstrated a positive correlation between amount of tissue-to-bone contact and biomechanical implant fixation. Therefore, increased force is required to push out an ibandronate-coated implant.
The empty space within a TiO2 nanotube can be used as a drug delivery carrier to reduce inflammation and accelerate osseointegration by promoting osteoblast functions and suppressing osteoclast activities. According to Yao and Webster, when penicillin was loaded into the anodized titanium with a nanotube structure using a co-precipitation method in vitro it was released over a three-week period via first-order kinetics. Compared to untreated titanium or anodized titanium, the level of bone cell adhesion was increased in participants receiving penicillin (Yao and Webster, 2009). In addition, according to Bae et al., after loading immobilized rhBMP-2 in vitro, the drugs were released gradually from anodized titanium using a nanotube structure over a three-week period. The activity of osteoblasts was higher than observed in non-anodized titanium samples. The results of the present study suggest that the controlled release of BPs might have continuously promoted osseointegration of the nanotube alloy during the healing period, as demonstrated by the higher mean removal of torque value in BP-coated groups.
An earlier study also noted that the positive effect of BPs on implant fixation is partially attributed to their action on osteoblasts; they can up-regulate key osteoblastic genes and exert a direct anabolic effect on osteoblast activity (von Knoch et al., 2005). Another striking finding is that the application of immobilized BPs to prevent implant loosening seems more efficient in osteoporotic bones than in normal bones. Steel screws with fibronectin/PAM/ibandronate coatings exhibited an increase of 28% in pull-out force compared to a control group (Tengvall et al., 2004). In the present study, the increase of the maximum push-out force was greater in ibandronate-coated implants than in controls. The mechanism of this phenomenon is not yet known, but it could be related to activate osteoclasts, which are a putative molecular target of BPs action. As previously noted, the elevated osteoclast activity in osteoporosis likely precipitates the dissolution of bone mineral and impairs the tight coupling between bone formation and resorption, while incomplete filling of resorptive cavities leads to net bone loss and implant loosening (Akesson, 2003). Compared to normal bone, osteoporotic bone provides a larger region to be exposed to drugs; local resorption and osteoclastic activities at the site of implantation are also much stronger. In this condition, BPs not only reduce the aseptic loosening associated with particulate debris but also inhibit the osteoporosis-related osteolysis around the implants to shift the dynamic equilibrium of bone metabolism in favor of stronger bone (Cohen, 2003). Therefore, the increase of mechanical fixation induced by immobilized BPs was not surprising.
In conclusion, heat-treated and ibandronate-coated titanium alloy Ti-32Nb-5Zr with nanotubes significantly improved bone fixation and osseointegration in comparison with controls. The alloy showed potentially clinically relevant prevention of implant loosening, and also improved microstructure parameters in bone. Therefore, Ti-32Nb-5Zr alloy may be useful as a material for dental and surgical prosthetics.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. R01-2008-000-20556-0) and research funds from Chonbuk National University (2012).
REFERENCES
- Akesson K. New approaches to pharmacological treatment of osteoporosis. Bull World Health Organ. 2003;81:657–664. [PMC free article] [PubMed] [Google Scholar]
- Bae IH, Yun KD, Kim HS, Jeong BC, Lim HP, Park SW, Lee KM, Lim YC, Lee KK, Yang Y, Koh JT. Anodic oxidized nanotubular titanium implants enhance bone morphogenic protein-2 delivery. J Biomed Mater Res B Appl Biomater. 2010;93:484–491. doi: 10.1002/jbm.b.31606. [DOI] [PubMed] [Google Scholar]
- Balasundaram G, Yao C, Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increase osteoblast adhesion. J. Biomed. Mater. Res. A. 2008;84:447–453. doi: 10.1002/jbm.a.31388. [DOI] [PubMed] [Google Scholar]
- Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schünemann H, Einhorn TA. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A. meta-analysis. J Bone Joint Surg Am. 2005;87:293–301. doi: 10.2106/JBJS.D.01772. [DOI] [PubMed] [Google Scholar]
- Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone forming functuality on diameter-controlled TiO2 nanotube furface. Acta Biometer. 2009;5:3215–3223. doi: 10.1016/j.actbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Carano A, Teitlebaum SL, Konsek JK, Schlesinger PH, Blair HC. Bisphosphonates directly inhibit the resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990;85:456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DP. Anti-osteoporotic medications: traditional and non-traditional. Clin ObstetGynecol. 2003;46:341–348. doi: 10.1097/00003081-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- García-Moreno C, Serrano S, Nacher M, Farré M, Díez A, Mariñoso ML, Carbonell J, Mellibovsky L, Nogués X, Ballester J, Aubía J. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998;22:233–239. doi: 10.1016/s8756-3282(97)00270-6. [DOI] [PubMed] [Google Scholar]
- Giavaresi G, Giardino R, Ambrosio L, Battiston G, Gerbasi R, Fini M, Rimondini L, Torricelli P. In vitro biocompatibility of titanium oxide for prosthetic devices nanostructured by low pressure metal-organic chemical vapor deposition. Int. J. Artif. Organs. 2003;26:774–780. doi: 10.1177/039139880302600811. [DOI] [PubMed] [Google Scholar]
- Hilding M, Aspenberg P. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop. 2006;77:912–916. doi: 10.1080/17453670610013213. [DOI] [PubMed] [Google Scholar]
- Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in longterm cultures of human bone marrow. J Clin Invest. 1989;83:1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2007;25:432–441. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- Jensen TB, Bechtold JE, Chen X, Søballe K. Systemic alendronate treatment improves fixation of press-fit implants: a canine study using nonloaded implants. J Orthop Res. 2007;25:772–778. doi: 10.1002/jor.20272. [DOI] [PubMed] [Google Scholar]
- Jones FH. Teeth and bones: applications of surface science to dental materials and related biomaterials. Surf Sci Rep. 2001;42:75–205. [Google Scholar]
- Lee SJ, Oh TJ, Bae TS, Lee MH, Soh Y, Kim BI, Kim HS. Effect of bisphosphonates on anodized and heat-treated titanium surfaces: an animal experimental study. J Periodontol. 2011;82:1035–1042. doi: 10.1902/jop.2010.100608. [DOI] [PubMed] [Google Scholar]
- Meraw SJ, Reeve CM. Qualitative analysis of peripheral peri-implant bone and influence of alendronate sodium on early bone regeneration. J Periodontol. 1999;70:1228–1233. doi: 10.1902/jop.1999.70.10.1228. [DOI] [PubMed] [Google Scholar]
- Meraw SJ, Reeve CM, Wollan PC. Use of alendronate in peri-implant defect regeneration. J Periodontol. 1999;70:151–158. doi: 10.1902/jop.1999.70.2.151. [DOI] [PubMed] [Google Scholar]
- Motohashi M, Shirota T, Tokugawa Y, Ohno K, Michi K, Yamaguchi A. Bone reactions around hydroxyapatite-coated implants in ovariectomized rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:145–152. doi: 10.1016/s1079-2104(99)70264-7. [DOI] [PubMed] [Google Scholar]
- Nepal M, Li L, Cho HK, Park JK, Soh Y. Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/ BMP-2 signaling pathway. Food Chem Toxicol. 2013;62:238–245. doi: 10.1016/j.fct.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Park J, bauer S, Schlegel KA, Neukam EW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15nm-an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5:666–671. doi: 10.1002/smll.200801476. [DOI] [PubMed] [Google Scholar]
- Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala PA. A reivew of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther. 2007;29:1548–1558. doi: 10.1016/j.clinthera.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Pohler OE. Unalloyed titanium for implants in bone surgery. Injury. 2000;31:7–13. doi: 10.1016/s0020-1383(00)80016-9. [DOI] [PubMed] [Google Scholar]
- Popat KC, Eltgroth M, Latempa TJ, Grimes CA, Desai TA. Decreased staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007a;28:4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surface on bone cells. Biomaterials. 2007b;28:3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Ratner BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J Dent Educ. 2001;65:1340–1347. [PubMed] [Google Scholar]
- Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA. Bisphosphonates act directly on the osteoclast to induce caspase cleavage of mst 1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J Biol Chem. 1999;274:34967–34973. doi: 10.1074/jbc.274.49.34967. [DOI] [PubMed] [Google Scholar]
- Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. [PubMed] [Google Scholar]
- Tengvall P, Skoglund B, Askendal A, Aspenberg P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials. 2004;25:2133–2138. doi: 10.1016/j.biomaterials.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Textor M, Sitting C, Frauchiger V, Tosatti S, Brunette D. Properties and biological significance of natural oxide films on titanium and its alloys. In: Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in Medicine. Springer-Verlag; Berlin: 2001. pp. 171–230. [Google Scholar]
- von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–6949. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Xie Z, Jiang Y, Zhang DQ. Simple analysis of four bisphosphonates simultaneously by reverse phase liquid chromatography using n-amylamine as volatile ion-pairing agent. J. Chromatogr. A. 2006;1104:173–178. doi: 10.1016/j.chroma.2005.11.113. [DOI] [PubMed] [Google Scholar]
- Yan WQ, Nakamura T, Kobayashi M, Kim HM, Miyaji F, Kokubo T. Bonding of chemically treated titanium implants to bone. J Biomed Mater Res. 1997;37:267–275. doi: 10.1002/(sici)1097-4636(199711)37:2<267::aid-jbm17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Yao C, Webster TJ. Prolonged antibiotic delivery from anozided nanotubular titanium using a co-precipitation drugs loading method. J Biomed Mater Res B Appl Biomater. 2009;91:87–595. doi: 10.1002/jbm.b.31433. [DOI] [PubMed] [Google Scholar]