Abstract
The goal of the current study was to further investigate the late neurodevelopmental hypothesis of schizophrenia by examining cross-sectional, age-related changes in cognitive function among young adult: 1) siblings of individuals with schizophrenia (N = 66); (2) healthy control participants (N = 77); and (3) the siblings of healthy controls (N = 77). All subjects participated in a battery of tasks in four domains: 1) IQ; 2) working memory; 3) episodic memory; and 4) executive function. We found significant group differences in the relationships between age and performance in working memory and episodic memory, with similar patterns for executive function and verbal IQ. The siblings of individuals with schizophrenia showed impaired performance in working memory, episodic memory, and executive function. In addition, healthy controls and/or their siblings showed age-related improvements in all four cognitive domains, while the siblings of individuals with schizophrenia only showed this for verbal IQ.
For many years, researchers have conceptualized schizophrenia as a neurodevelopmental disorder. Theories of early neurodevelopmental aberration (Murray, Jones, & O’Callaghan, 1991; Rapoport, Addington, Frangou, & Psych, 2005) focus on errors in brain development that occur during the pre- and peri-natal period, which may be due to abnormalities in mechanisms such as neuronal migration (Fatemi & Folsom, 2009). In addition, theories of late neurodevelopmental aberration (Karlsgodt et al., 2008) focus on disruptions in the maturation of neural circuits during the peri-pubertal period, such as cortical synaptic pruning (Feinberg, 1982, 1990) and/or gray/white matter growth (Pantelis et al., 2005). In addition, there are “2-hit” models, which postulate that early neurodevelopmental events sets the stage for, or create a vulnerability for, later irregularities in development (Keshavan, 1999; Keshavan & Hogarty, 1999). The goal of the current study is to address questions primarily related to the late neurodevelopmental theory of schizophrenia by examining the developmental trajectory of cognitive function in the siblings of individuals with schizophrenia, who are at increased risk for developing schizophrenia (Gottesman, 1991).
Theories of early neurodevelopmental abnormalities in schizophrenia predict that cognitive, behavioral and neuroanatomical antecedents of schizophrenia should be present from a very early age in individuals who develop schizophrenia, and then remain static until the onset of the acute syndrome. Such theories are consistent with the large body of data, including the work of Walker and colleagues reporting subtle neuromotor deficits in toddlers who eventually develop schizophrenia (Walker, Savoie, & Davis, 1994). In addition, others have reported cognitive deficits, in particular disproportionate deficits in working memory and selective attention, in children who go to develop schizophrenia (Cornblatt, Obuchowski, Roberts, Pollack, & Erlenmeyer-Kimling, 1999; Niendam et al., 2003).
Late neurodevelopmental theories of schizophrenia are more in keeping with the post-pubertal onset of schizophrenia. Also, the literature on normative cognitive development during puberty provides potential candidate mechanisms for disruptions that could presage the onset of schizophrenia. A wealth of research has pointed to the maturation of a range of cognitive functions across the course of puberty, including working memory, executive control, and specific aspects of attention and episodic memory function (e.g., Davidson, Amso, Anderson, & Diamond, 2006; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Importantly, these are all cognitive domains known to be impaired in schizophrenia (Barch, 2005). Human and non-human animal data have begun to elucidate the neural mechanisms that underlie the development of cognition during puberty. For example, research has shown that gray matter development is characterized by period of growth, followed by gray matter volume reductions driven by selective synaptic pruning (Huttenlocher & Dabholkar, 1997; Rakic, Bourgeois, & Goldman-Rakic, 1994). The timing of gray matter development varies across brain regions (Casey, Giedd, & Thomas, 2000; Lenroot & Giedd, 2006). Importantly, gray matter growth peaks relatively late in the dorsolateral prefrontal cortex (Giedd et al., 1999; Gogtay et al., 2004), a region thought to be critical for executive control, working memory, and many aspects of episodic memory as well. In contrast, white matter growth is characterized by a relatively linear increase from childhood to adulthood (Giedd et al., 1999), with increases in white matter linked to improvements in cognitive function with age (Edin, Macoveanu, Olesen, Tegner, & Klingberg, 2007; Nagy, Westerberg, & Klingberg, 2004; Olesen, Nagy, Westerberg, & Klingberg, 2003). In addition, a number of studies have shown that functional activation in dorsolateral prefrontal regions in response to working memory and cognitive control demands increases with age (Brahmbhatt, McAuley, & Barch, 2008; Casey et al., 1995; Ciesielski, Lesnik, Savoy, Grant, & Ahlfors, 2006; Klingberg, 2006; Klingberg, Forssberg, & Westerberg, 2002; Schweinsburg, Nagel, & Tapert, 2005), such that activity is greater in adults than children, though a few studies have shown greater activation in children then adults (Klingberg, Forssberg, & Westerberg, 2002; Schweinsburg, Nagel, & Tapert, 2005; Tsujimoto, Yamamoto, Kawaguchi, Koizumi, & Sawaguchi, 2004).
Given this data on normative developmental mechanisms, late neurodevelopmental hypotheses of schizophrenia have postulated the occurrence of abnormalities of synaptic pruning (enhanced) (Feinberg, 1982), abnormal white matter growth (e.g., impaired myelination) (Bartzokis, 2002), and accompanying abnormalities in age related improvements in cognitive function (Karlsgodt et al., 2008). Logically, the critical test of late neurodevelopmental hypotheses of schizophrenia is to examine abnormalities in the longitudinal course of development. Giedd has recently argued elegantly for the need to examine such neurodevelopmental trajectories as indicators of risk for neuropsychiatric disorders (Giedd et al., 2008). However, few studies have examined trajectories of cognitive development in individuals at risk for schizophrenia. Cornblatt et al., found that attention deficits were present very early in children who went on to develop schizophrenia, and that the severity of these deficits were stable across the measurement period (Cornblatt et al., 1999). Further, at least one other study found that IQ deficits present in high-risk offspring actually diminished with development, rather than increasing across the course of development (Goodman, 1987).
In contrast, Worland et al found that verbal IQ showed a decline from age 8 to age 16 in the offspring of individuals with schizophrenia (Wolters & Phaf, 1990). In addition, Cosway found that high-risk individuals whose symptoms increased also showed a decline in IQ (Cosway et al., 2000). Further, Kremen found that IQ decline from age 4–7 predicted adult onset psychosis (Kremen et al., 1998), and MacCabe et al. found that decline in verbal ability from ages 13 to 18 predicted increased risk for psychosis (MacCabe et al., 2013). Interestingly, the Worland study was consistent with a 2-hit model, given that the offspring of individuals with schizophrenia showed early IQ impairment followed by further decline across puberty. Thus, the literature on cognitive development in relationship to schizophrenia risk is mixed as best, with both positive and negative results and with few studies focusing on more than a single cognitive domain. Interestingly, at least the Worland study was consistent with a 2-hit model, given that IQ impairment was present even at age 8 in the offspring of individuals with schizophrenia, but there was also some evidence of greater IQ decline across puberty in these offspring, at least compared to the offspring of healthy controls. Further, recent research has also shown that decline in temporal lobe gray matter and verbal IQ across late childhood into adolescence predicted an increase in psychosis in individuals with 22q11 deletion syndrome (Kates et al., 2011), a syndrome associated with an enhanced risk of developing schizophrenia.
Providing further evidence for the late neurodevelopmental theory, children with childhood onset schizophrenia have been shown to have deviant developmental trajectories, with both decreased white matter growth (Gogtay et al., 2008; Vidal et al., 2006) and increased frontal gray matter loss (Vidal et al., 2006). Similarly, first episode patients with schizophrenia, as well as prodromal individuals, have been shown to have enhanced thinning of the dorsal surfaces of the frontal lobes (Sun et al., 2008). In addition, genetically high-risk subjects demonstrate greater reductions in right frontal lobe volumes over time, though this abnormality did not distinguish between high-risk subjects who did and did not develop schizophrenia (Job, Whalley, Johnstone, & Lawrie, 2005). Finally, Gogtay et al examined cortical brain development in the nonpsychotic siblings of individuals with childhood onset schizophrenia between the ages of 8 and 28. These researchers found evidence of gray matter loss in frontal and superior temporal regions that started at age 8, but disappeared by age 20 in frontal regions, particularly among those with improved function (Gogtay et al., 2007). However, although this sample did contain some younger children, the mean age for the first scan was 16, and only healthy siblings (no psychosis or schizotypal personality disorder) were included, which could have lead to a sample less saturated with risk for schizophrenia.
The goal of the current study is to shed further light on early versus late neurodevelopmental hypotheses of schizophrenia by examining the developmental trajectory of cognitive function in the siblings of individuals with schizophrenia compared to controls and their siblings. In the current study, we examined age-related changes in four cognitive domains (working memory, executive control, episodic memory, and verbal IQ) during puberty and early adulthood in the siblings of individuals with schizophrenia as compared to healthy controls and their siblings. An early neurodevelopmental hypothesis of schizophrenia would predict that we should see cognitive impairments even at our earliest ages among the siblings of individuals with schizophrenia, and would not predict either altered age-related changes in cognitive function or a further enhancement of group differences in cognitive function with increasing age. In contrast, a late neurodevelopmental hypothesis of schizophrenia would predict abnormalities in the normal patterns of age-related improvement in cognitive function across the course of puberty into adult hood and an enhancement of group differences in cognitive function with increasing age. Finally, a 2-hit model would predict both – the presence of cognitive impairments prior to puberty, but also impaired age-related maturation of cognitive function and enhanced group differences in cognition as a function of increasing age.
Methods
The subjects for this study were recruited through the Conte Center for the Neuroscience of Mental Disorders (CCNMD) at Washington University School of Medicine in St. Louis, and included: (1) non-psychotic siblings of individuals with DSM-IV schizophrenia (SIB: N=66); (2) healthy control participants (CON: N=77); and (3) the siblings of healthy controls (SCN: N=77). A subset of these subjects were included in a previous report on cognition and symptoms in the siblings of patients with schizophrenia (Delawalla et al., 2006). Siblings were full siblings, based on self-report. All subjects gave written informed consent for participation following a complete description of the risks and benefits of participating in the study. The probands with schizophrenia (by which we recruited the siblings of individuals with schizophrenia) all had a SCID confirmed diagnosis of schizophrenia or schizoaffective disorder, using the methods described below. Although the probands with schizophrenia completed all of the same cognitive and clinical assessments as the other three groups, there were not included in the current project because their age range did not go sufficiently young to be able to examine developmental changes. We included siblings of healthy control participants as well as healthy controls to address confounds associated with differential recruitment and screening criteria for controls versus the siblings of patients. Our controls were required to have no family history of psychosis and no personal history of any AXIS I disorder. However, we could not impose such a criterion on the siblings of individuals with schizophrenia, as many have past depression or anxiety and to exclude such individuals would result in an unrepresentative sample. Thus, we also recruited the siblings of controls, and allowed them to have the same personal history of non-psychotic AXIS I disorders as the siblings of individuals with schizophrenia. Thus, the two sets of siblings were recruited with the same methods and inclusion/exclusion criteria, other than the diagnosis of their sibling.
All subjects were diagnosed using DSM-IV criteria on the basis of a consensus between a research psychiatrist who conducted a semi-structured interview and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P) (First, Spitzer, Gibbon, & Williams, 2001). Participants were excluded if they: (a) met DSM-IV criteria for substance dependence or severe/moderate abuse during the 6 months preceding assessment; (b) had a clinically unstable or severe medical disorder, or a medical disorder that confounded the assessment of psychiatric diagnosis or rendered research participation dangerous; (c) had a history of head injury with documented neurological sequelae or loss of consciousness; or (d) met DSM-IV criteria for mental retardation (mild or greater in severity).
The individuals with schizophrenia were all outpatients at the time of their assessment, and were stabilized on antipsychotic medication for at least two weeks before participating in the study. CON were recruited using local advertisements in the same community, and were required to have no lifetime history of Axis I psychotic or major mood disorders and no first-degree relatives with a psychotic disorder. Potential SIB or SCN subjects were excluded if they had a lifetime history of any DSM-IV Axis I psychotic disorder, but not other DSM-IV Axis I disorders.
Clinical and Cognitive Assessments
Psychopathology and cognitive function were assessed as previously described (Delawalla et al., 2006; Harms et al., 2007). Briefly, psychopathology was assessed using the Scale for the Assessment of Negative Symptoms (SANS), the Scale for the Assessment of Positive Symptoms (SAPS)(Andreasen, 1983), the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 1999), and the Chapman Psychosis Proneness Scales (Chapman, Chapman, & Kwapil, 1995). The raw scores from the clinical measures were first standardized by z-scores using the means and standard deviations computed across all subjects who have participated in research studies at the CCNMD at Washington University, and the z-scores from specific measures were then averaged to yield three clinical domains. The negative symptom domain consisted of the global scores from the SANS, the negative symptoms scores from the SIPS, and the Chapman social and physical anhedonia scales. The positive symptoms scores consisted of the global hallucinations and delusions SAPS scores, the positive symptom scores from the SIPS and the Chapman Perceptual Aberration and Magical Ideation scales. The disorganization symptom domain included the global scores for formal thought disorder and bizarre behavior from the SAPS and the disorganization symptoms from the SIPS.
Neurocognition was assessed using a battery of neuropsychological tests. The raw scores from the individual neuropsychological tests were first standardized by z-scores using the means and standard deviations computed on this sample and the z-scores from specific tests were then averaged to yield four cognitive domains – verbal IQ (only the WAIS/WASI Vocabulary measure), working memory, episodic memory, and executive function. The working memory domain (α=0.75) consisted of subtests from the Wechsler Memory Scale----Third Edition (raw scores on letter-number sequencing, digit span, and spatial span) (Wechsler, 1997), percentage correct on the 2-back version of the N-back task (Braver et al., 1997), and the 4-item d′ score from the Continuous Performance Task (CPT). The episodic memory domain (α=0.48) consisted of raw scores on immediate recall on family pictures and logical memory (also subtests of Wechsler Memory Scale----Third Edition), and the free recall score for trials 1–5 on the California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 2000). The executive function domain (α=0.66) included time to completion on Trails B (Reitan, 1958), number of novel words generated on the category and verbal fluency tasks (Benton, 1968), raw score on the matrix reasoning subtest from the WAIS-III and the score for perseverative errors (reversed in sign) from the Wisconsin Card Sort (Berg, 1948).
Results
As shown in Table 1, the three groups did not differ in age, F(1, 219) = 2.26, p=.11, ε2 = .02, personal education, F(1,219) = 0.43, p>.6, ε2 = .004, parental education, F(1,219) = 0.04, p>.9, ε2 = .0001, gender, X2(2) = 4.67, p=.10, ϕ = .15, or race, X2(2) = 3.6, p=.46, ϕ = .13.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Healthy Controls (CON) | Siblings of Controls (SCN) | Siblings of Schizophrenia (SIB) | ||||
|
| ||||||
| Measure | Mean | SD | Mean | SD | Mean | SD |
| Age | 20.79 | 3.72 | 20.16 | 3.94 | 21.51 | 3.63 |
| Gender (% male) | 51% | 35% | 47% | |||
| Race Distribution | ||||||
| % Caucasian | 69% | 70% | 59% | |||
| % African-American | 30% | 29% | 41% | |||
| % Asian | 1% | 1% | 0% | |||
| Education | 12.81 | 2.72 | 12.42 | 3.01 | 12.45 | 2.84 |
| Parental Education | 14.74 | 2.03 | 14.64 | 2.04 | 14.70 | 2.80 |
| Negative Symptoms*1 | −.39 | .28 | −0.30 | .40 | −0.10 | .51 |
| Positive Symptoms*b | −.41 | .36 | −0.31 | .36 | −0.21 | .36 |
| Disorganization Symptoms*c | −.33 | .25 | −0.29 | .29 | −0.14 | .42 |
Symptom scores are reported in Z scores relative to the mean of a sample that included the probands with schizophrenia. See Methods for details.
SIB < CON, SCN at p<.01, CON=SCN
SIB < CON, SCN at p<.05, CON=SCN
SIB < CON at p<.01, CON=SCN
Cognitive Measures
We began by examining overall group differences in cognition across the three groups, using a MANOVA with the four cognitive domain scores as the dependent variables. The omnibus Wilk’s lambda was significant, F(8, 424) = 2.57, p<.01, ε2 = 0.046. As shown in Table 2, follow-up univariate ANOVAs indicated that the groups differed in working memory, episodic memory and executive function, all ps<.01, 0.048 > ε2 <0.058, but not verbal IQ, F(2,215) = 1.72, p=.18, ε2 = .016. As shown in Table 2, post-hoc contrasts using Tukey’s HSD indicated that the siblings of individuals with schizophrenia demonstrated significantly impaired performance on working memory, episodic memory, and executive function compared to both the healthy controls and the siblings of healthy controls. There were no significant differences between healthy controls and their siblings on any measure (all ps>0.65).
Table 2.
Group Differences in the Four Cognitive Domains
| Group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Healthy Controls (CON) | Siblings of Controls (SCN) | Siblings of Schizophrenia (SIB) | ||||
|
| ||||||
| Measure | Mean | SE | Mean | SE | Mean | SE |
| IQ | .14 | .11 | .01 | .11 | −.17 | .12 |
| Working Memorya,b,c | .13 | .07 | .04 | .07 | −.21 | .08 |
| Episodic Memorya,c | .11 | .08 | .11 | .08 | −.25 | .08 |
| Executive Functiona,c | .13 | .07 | .07 | .07 | −.21 | .08 |
SIB < CON at p<.01
SIB < SCN at p=.05
CON=SCN
We next examined the relationship between age and cognition across the groups using hierarchical regressions, with one regression for each cognitive domain. In step one, we entered age and group status (siblings of individuals with schizophrenia versus healthy controls and their siblings) to predict the cognitive domain score. In Step 2, we entered an interaction term between age and group status, to determine if there were group differences in the relationship between age and cognition. Step 1 was significant for all four domain scores, all ps<.001. Age predicted cognitive function for working memory, age β = 0.28, p<.001, executive function, age β = 0.24, p<.001, and verbal IQ, age β = 0.43, p<.001, but not for episodic memory, age β = 0.64, p=.33. Consistent with the MANOVA results presented above, group status predicted cognitive function for working memory, group β = −.24, p<.001, episodic memory, group β = −.25, p<.001, and executive function, group β = −.26, p<.001. However, in the regression, group also predicted verbal IQ, group β = −.16, p<.01, though the effect size was smaller than for the other cognitive domains. In addition, Step 2 was significant for both working memory, Fchange(1,216) = 3.84, p=.05, and episodic memory, Fchange (1,216) = 3.9, p<.05, indicating a significant diagnostic group difference in the relationship between age and performance for working memory (β = −0.74, p=.05, and episodic memory, β = −0.77, p<.05. Step 2 was not significant for executive function, Fchange(1,216) = 1.54, p>.2, or verbal IQ, Fchange(1,216) = 2.55, p>.1.
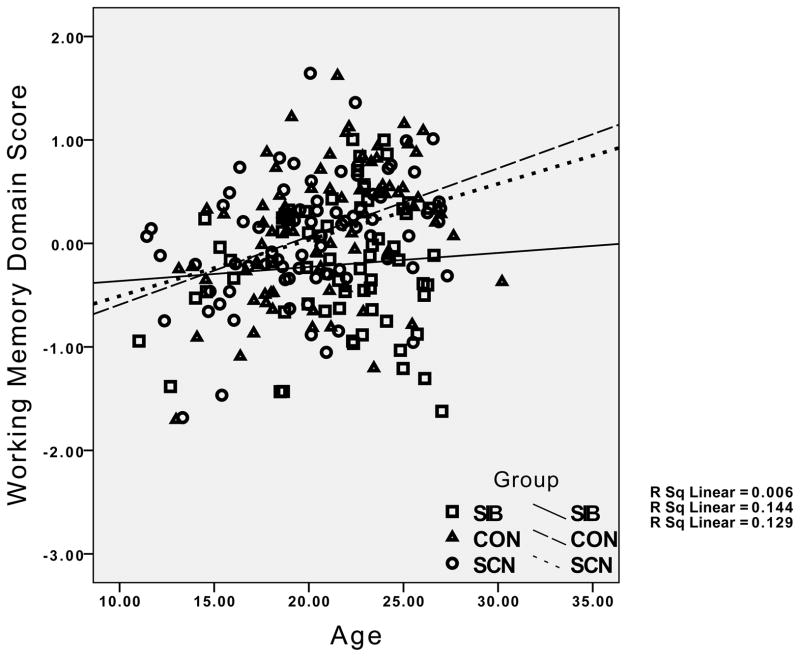
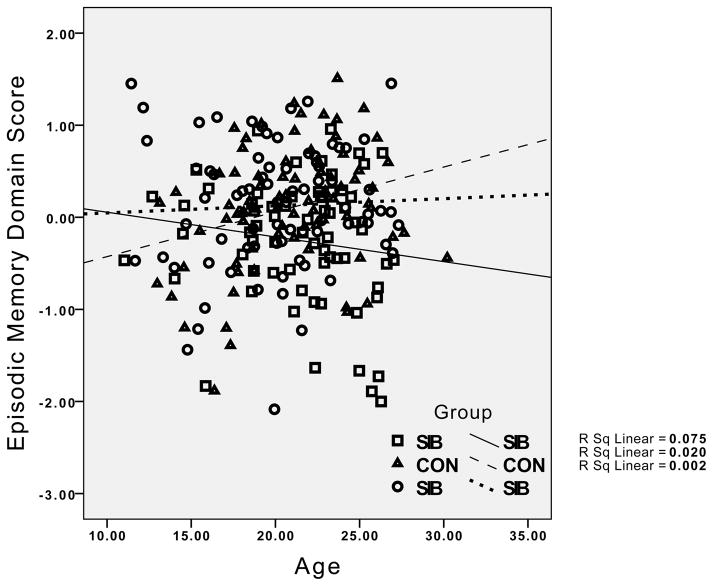
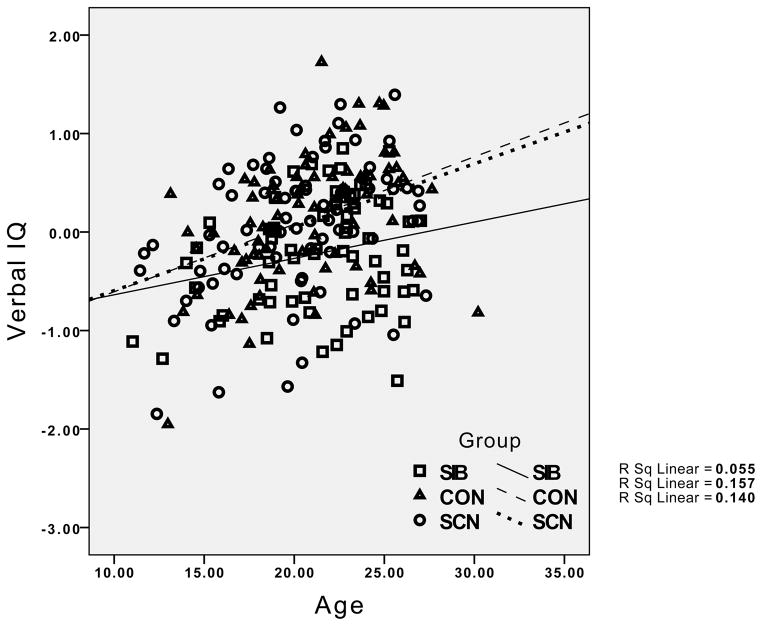
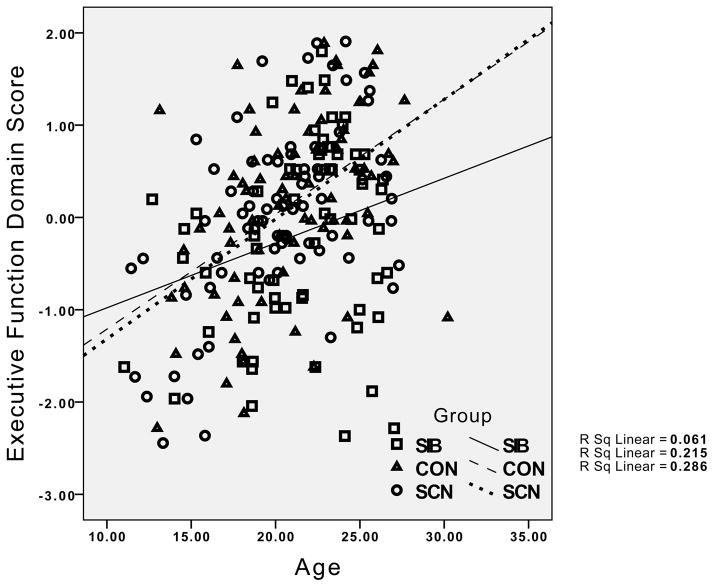
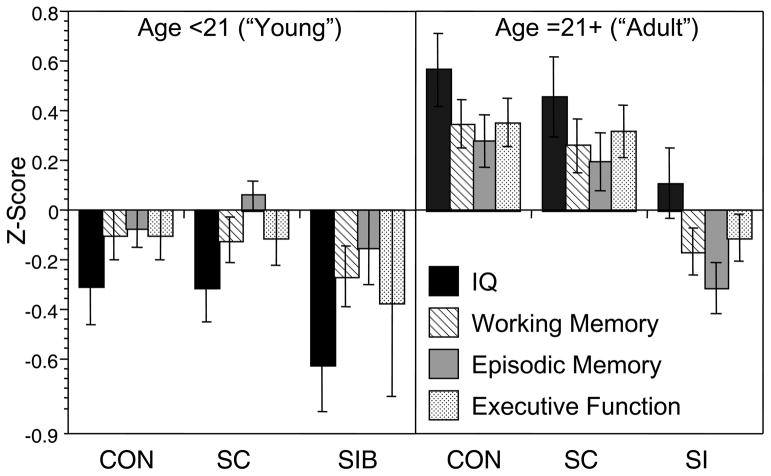
The group differences in the relationship between age and working memory performance reflected the presence of the expected significant positive correlations in both the healthy controls (r = .38, p < .01) and their siblings (r = .36, p < .01), but the absence of a significant correlation between age and working memory in the siblings of the individuals with schizophrenia (r = .08, p > .20). For episodic memory, the healthy controls showed a significant positive relationship between age and episodic memory (r = .27, p < .05), while this correlation was non-significant in the siblings of controls (r = .04, p < .20), and even negative in the siblings of individuals with schizophrenia (r = −.14, p > .10). Although the interaction between age and group was not significant for executive function and verbal IQ, the correlations indicated strong positive correlations between age and executive function and age and IQ for both healthy controls and their siblings (.53 < rs >.37, all ps < .01). In contrast, the correlation between executive function and IQ was not significant for the siblings of patients with schizophrenia (r = .24, p > .10), and the correlation between age and executive function was relatively weak in siblings of individuals with schizophrenia compared to the other groups (r = .25, p < .05). The pattern for all four cognitive domains is graphically illustrated in Figure 1.
Figure 1.
Graphs depicting the relationship between age and performance for IQ, working memory, episodic memory and executive function, with results for healthy controls and their siblings (circles) and the siblings of individuals with schizophrenia (diamonds) plotted separately. CON = Healthy Controls; SCN = siblings of healthy controls; SIB = siblings of individuals with schizophrenia.
Visual examination of the graphs in Figure 1 suggests relatively little difference between the groups in cognitive performance at younger age, but a greater difference at older ages. To examine this statistically, we categorized participants as “young” if they were under 21, and “adult” if they were 21 or older. We then conducted separate MANOVAs for the young and old groups, examining group differences in the four cognitive domains. The Wilk’s Lambda was not significant for group differences in the young group, F(8, 200) = 0.59, p>0.75, ε2 = 0.023, but it was significant in the adult group, F(8, 212) = 3.16, p<.005, ε2 = 0.11, as illustrated in Figure 2. Follow-up univariate ANOVAs for each cognitive domain in the adult group indicated significant group differences in working memory, F(2,109) = 8.08, p=.001, episodic memory, F(2,109) = 9.30, p<.001, executive function, F(2,109) = 7.22, p=.001, and a trend for verbal IQ, F(2,109) = 2.76, p=.05. Posthoc contrasts indicated that the siblings of the schizophrenia patients performed worse than healthy controls and the siblings of controls on working memory, episodic memory and executive function, all ps<.05.
Figure 2.
Graph illustrated cognitive performance for IQ, working memory, episodic memory and executive function as a function of diagnostic group separately for young and adult age group. CON = Healthy Controls; SCN = siblings of healthy controls; SIB = siblings of individuals with schizophrenia.
Psychopathological Measures
We examined overall group differences in symptoms across the three groups, using a MANOVA with the three symptom domain scores as the dependent variables. The omnibus Wilk’s lambda was significant, F(6, 430) = 6.00, p<.001, ε2 = 0.07). As shown in Table 1, follow-up univariate ANOVAs indicated that the groups differed in all three symptom domains (all ps<.005, 0.052 > ε2 <0.11), and post-hoc contrasts using Tukey’s HSD indicated that the siblings of individuals with schizophrenia demonstrated greater negative and disorganization symptoms than both healthy controls and the siblings of healthy controls (all ps<.05,), but only demonstrated greater positive symptoms than healthy controls (p<.01) and not their siblings (p=.17). Next, we conducted hierarchical regressions for the three symptom domains analogous to those conducted for the cognitive domains. Step 1 was significant for all three symptom domains (all p<.005). However, only group and not age predicted positive, age β = −0.10, p>.15; group β = 0.21, p<.005, negative, age β = −0.09, p>.15; group β = 0.35, p<.005, and disorganization, age β = −0.05, p>.49; group β = 0.25, p<.005, symptoms. In addition, Step 2 was significant for negative symptoms, Fchange(1,216) = 4.5, p<.05, R2change=.018, indicating a significant diagnostic group difference in the relationship between age and performance for negative symptoms, β = −0.81, p=.05. However, follow-up within group correlations indicated that this interaction was due to a positive but non-significant correlation between age and negative symptoms in the SCN group, r = .14, p = .21, but negative and non-significant correlations in the CON and SIB groups, r = −.23, p=.06 and r = −.20, p = .09 respectively. Step 2 was not significant for either positive symptoms, Fchange(1,216) = 0.13, p>.7, R2change=.001, or disorganization symptoms, Fchange(1,216) = 0.01 p>.9, R2change=.0001.
Further, MANOVAs conducted separately for each age group indicated significant main effects of diagnostic group in both the young, F(6,178) = 6.0, p<.001, ε2 = 0.15, and adult, F(6,232) = 2.58, p<.05, ε2 = 0.07, groups. Follow-up univariate analyses indicated significant group differences across all three symptom domains in the adult group (all p<.05, all ε2s > 0.058). However, in the young group, there was a significant group difference for negative symptoms, F(2,105) = 19.4, p<.001, ε2 = 0.27, a trend for positive symptoms, F(2,105) = 2.6, p=.08, ε2 = 0.046, and no significant difference for disorganization symptoms, F(2,105) = 2.0, p>.10, ε2 = 0.036. Thus, unlike the cognitive domains, there was no evidence of age related changes in any of the symptom domains, or any evidence of age related changes in the magnitude of group differences in any of the symptom domains.
The difference in the results for the influence of age as a function of risk-status for cognition versus symptoms led us to ask whether the relationship between symptoms and cognitive varied as a function of age. To do so, we computed correlations between the three symptom domains and the four cognitive domains in the young (<21) and older (21+) participants, controlling for group status, and compared them using Fisher’s r to z transformations. As shown in Supplemental Table 1, there were significant negative correlations between negative symptoms and verbal IQ, working memory and executive function in both the younger and older participants, and no significant age differences in the magnitude of these correlations. In addition, there were significant negative correlations between positive symptoms and verbal IQ in both age groups, and a negative correlation with working memory in the younger participants, but a negative correlation with episodic memory in the older group. Further, disorganization symptoms were negatively correlated with verbal IQ, working memory and executive function in both groups, and negatively correlated with episodic memory in the older group. Again, however, none of these correlations differed significantly as a function of age.
Discussion
The goal of the current study was to shed further light on the early and late neurodevelopmental hypothesis of schizophrenia by examining age related changes in cognitive function among the siblings of individuals with schizophrenia, healthy controls and the siblings of healthy controls. We found significantly impaired age-related development of cognitive function among the siblings of individuals with schizophrenia in both working memory and episodic memory, with similar patterns for executive function and verbal IQ. More specifically, both healthy controls and their siblings showed improvements in performance in each of these four domains as a function of increasing age (with the exception of episodic memory for the siblings of controls). However, the siblings of individuals with schizophrenia did not show evidence of improvements as a function of age in working memory, episodic memory or executive function, though they did show some evidence of improvement as a function of age for verbal IQ. Further, we found that the siblings of individuals with schizophrenia showed impaired cognitive function in working memory, episodic memory, and executive function compared to healthy controls and their siblings in an “adult” age group consisting of individuals 21 and older, but not in the younger age group of individuals under 21.
These results are consistent with a late neurodevelopmental hypothesis of schizophrenia, in which normative neurobiological processes driving cognitive development through puberty are disrupted in those at risk for the development of schizophrenia. Further, these results are consistent with prior studies showing altered development of white matter and gray matter (particularly in frontal cortex) in individuals with childhood onset schizophrenia (Vidal et al., 2006) and prodromal patients who develop psychosis (Sun et al., 2009). However, these results are not consistent with work by Cornblatt and colleagues, who did not see evidence for enhanced cognitive impairment as a function of age in high-risk children who went on to develop schizophrenia. It is not clear why the results of the current study differ from that of the New-York High Risk project, although the most obvious difference is the selection of siblings versus children as high-risk subjects. Also, the current study used a more extensive battery of cognitive tasks than used in the Cornblatt study, which might have allowed us to detect more subtle effects or a wider range of cognitive functions that could change as a function of development.
In contrast, our results provide little evidence consistent with either an early neurodevelopmental hypothesis or a 2-hit hypothesis, at least in terms of cognitive function. Specifically, we did not find that the younger siblings of individuals with schizophrenia demonstrated significantly impaired performance in any cognitive domain compared to healthy controls. This result is not consistent with prior research showing impairments in at least some cognitive domains (i.e., IQ and working memory) among children at risk for schizophrenia (de la Serna et al., 2010; Goldstein et al., 2000; Niendam et al., 2003; Sorensen, Mortensen, Parnas, & Mednick, 2006; Worland, Weeks, Weiner, & Schechtman, 1982). Of note, however, the two domains with the largest effect size for a diagnostic group differences in our younger group were verbal IQ and executive function, which is at least somewhat consistent with the work of Worland and colleagues and other researchers suggesting that IQ impairments are important predictors of risk for psychosis (Ott et al., 1998). Importantly, many of the prior studies examining cognitive function in young high-risk individuals have examined offspring rather than siblings. As such, it is possible that there are enhanced risk factors present in offspring (e.g., pre or peri-natal care issues) that might lead to enhanced evidence of cognitive impairment compared to samples such as ours that consist solely of siblings.
As noted above, research has replicated extensively a link between the severity of cognitive impairments and the severity of clinical symptoms such as negative and disorganization symptoms in both individuals with schizophrenia and their siblings (Barch, Carter, & Cohen, 2003; Barch, Csernansky, Conturo, Snyder, & Ollinger, 2002; Delawalla et al., 2006; Nieuwenstein, Aleman, & de Haan, 2001; Perlstein, Dixit, Carter, Noll, & Cohen, 2003). We replicated these findings in the current sample, showing consistent negative correlations between cognition and both negative and disorganization symptoms, as well as some relationships with positive symptoms. Thus, we also examined whether the severity of subclinical symptoms varied as a function of age in the siblings of individuals with schizophrenia. Interestingly, we did not find age-related differences in any symptom domain, and both the younger and older siblings of individuals with schizophrenia showed elevated subclinical schizotypal symptoms compared to healthy controls and their siblings, with increased negative symptoms being most consistent across the age groups. Further, we did not find any age differences in the magnitude of the relationship between clinical symptoms and cognition. These results suggest one of two possibilities. One possibility is that subclinical schizotypal symptoms and cognitive impairment may be independent expressions of risk for psychosis, as some other researchers have found (Asarnow et al., 2002). However, this interpretation would not be consistent with studies reporting a link between the severity of symptoms and the severity of cognitive impairments in individual with schizophrenia and their relatives (Delawalla et al., 2006), as well as our data showing a significant relationship between clinical symptoms and cognitive function. Alternatively, while cognition and symptomatology may be linked, the emergence of subclinical symptoms may precede the emergence of cognitive impairments or may be a more sensitive indicator of risk. This interpretation would be consistent with the literature on the emergence of social difficulties – one aspect of negative symptoms – in children at risk for the development of schizophrenia (Cannon, Mednick, & Parnas, 1990; MacCrimmon, Cleghorn, Asarnow, & Steffy, 1980; Olin & Mednick, 1996; Sohlberg & Yaniv, 1985).
One of the major limitations of the current study is that it was cross-sectional rather than longitudinal, and therefore cohort effects or sample selection issues could have biased the results. A number of the early offspring high-risk studies did conduct repeated assessments throughout childhood and adolescence, though relatively few tools for non-invasive brain imaging were available for these studies, as they are today. In contrast, many sibling studies have been able to use a range of structural and functional neuroimaging tools, but have not been able to study individuals prior to puberty (Munoz Maniega et al., 2008; Whalley et al., 2006). An additional limitation of the current study is that we studied a familial high-risk population that included a mixture of people who will and will not develop schizophrenia. Thus, it is not yet clear whether alterations in the development of cognitive function is a more general characteristic of familial risk, or a specific predictor of psychosis onset.
Given the limitations of our own study and the extent literature described above, an optimal study to test hypotheses about early, late, or 2-hit neurodevelopmental models would be a longitudinal design in one or more risk populations (e.g., offspring, siblings, 22q11 deletion syndrome) that started at birth and had multiple waves of data collection prior to, during, and after puberty. It would be ideal to use several types of at risk populations so as to determine the generalizability and replicability of any identified predictors. As noted by Giedd (Giedd et al., 2008), at least three assessment points are needed to characterize a trajectory, and ideally we would be able to determine trajectories of development prior to, during and after puberty. Advances in modern imaging have made available many techniques for the non-invasive measurement of brain structure and function across the course of development, even making it feasible to assess such characteristics of brain development in newborn infants (e.g., resting state brain connectivity during sleep) (Smyser et al., 2010). Such a study should include detailed behavioral measures of cognitive, affective and motor function, and as many non-invasive measurements of brain integrity as possible (e.g., gray matter, white matter, resting state functional connectivity, task related activity when age appropriate, perfusion, etc.). By including both more and less expensive, technically demanding, and/or invasive measures, we will be able to determine the relative utility of using more time and cost-consuming methods to clarify and identify predictors of psychosis. It is possible that less expensive/invasive measures may have as much utility (e.g., cognitive or motor function trajectories) and more expensive/invasive ones, and we will only know this by directly comparing them. Further, we should be careful not to think of full blown psychosis as the only relevant outcome in such studies. In addition to full diagnostic outcomes that may not be evident until adulthood, it will be important to look at cognitive, social, or academic function during childhood and adolescence as “outcomes” that could be predicted by earlier measurements. Further, we would need to examine subtle signs and symptoms of psychosis, including indicators of clinical high risk (Cannon et al., 2008; Woods et al., 2009) as intermediate outcome measures that can inform the developmental trajectory of psychosis risk. Given that adolescents and young adults with the “clinical” high risk profile for psychosis already suffer significant distress and impairment in social, educational and occupational function (Fusar-Poli, Yung, McGorry, & van Os, 2013), identifying neurodevelopmental trajectories that predict the onset of clinical high risk symptoms and can allow for targeted early intervention has significant public health benefits in and of itself, even if not all of those individuals will develop psychotic disorders that meet DSM-5 criteria (Fusar-Poli, Bechdolf, et al., 2012; Fusar-Poli, Bonoldi, et al., 2012). Although we fully realize the practical limitations and constraints on the conduct of such a large scale study, it is the only way we will be able to more adequately and definitively characterize and identify abnormalities in cognitive, affective, motor and brain development as a precursor to psychosis.
Supplementary Material
Acknowledgments
The authors would like to thank the participants in this study, who gave generously of their time. We also thank the staff of the Administrative/Assessment and Biostatistical Cores of the CCNMD at Washington University School of Medicine for collection of the clinical and imaging data and data management. The authors would also like to thank Carol Cox for her help in preparing this manuscript. Funding for this study was provided by NIMH grants P50 MH071616 and R01 MH56584.
Contributor Information
Deanna M. Barch, Departments of Psychology, Psychiatry and Radiology, Washington University
Rachel Cohen, Department of Psychology, Washington University.
John Csernansky, Department of Psychiatry, Northwestern University.
References
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) University of Iowa; 1983. The scale for the assessment of positive symptoms (SAPS) [Google Scholar]
- Asarnow RF, Nuechterlein KH, Asamen J, Fogelson D, Subotnik KL, Zaucha K, Guthrie D. Neurocognitive functioning and schizophrenia spectrum disorders can be independent expressions of familial liability for schizophrenia in community control children: the UCLA family study. Schizophr Res. 2002;54(1–2):111–120. doi: 10.1016/s0920-9964(01)00358-9. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. In: Cannon T, Mineka S, editors. Annual Review of Clinical Psychology. Vol. 1. Washington, D.C: American Psychological Association; 2005. pp. 321–353. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Cohen JD. Context processing deficit in schizophrenia: Diagnostic specificity, 4-week course, and relationships to clinical symptoms. Journal of Abnormal Psychology. 2003;112(132–143):132–143. [PubMed] [Google Scholar]
- Barch DM, Csernansky J, Conturo T, Snyder AZ, Ollinger J. Working and long-term memory deficits in schizophrenia. Is there a common underlying prefrontal mechanism? Journal of Abnormal Psychology. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27(4):672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential effects of frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. The Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt SB, McAuley T, Barch DM. Functional developmental similarities and differences in the neural correlates of verbal and nonverbal working memory tasks. Neuropsychologia. 2008;46(4):1020–1031. doi: 10.1016/j.neuropsychologia.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Mednick SA, Parnas J. Antecedents of predominantly negative- and predominantly positive-symptom schizophrenia in a high-risk population. Archives of General Psychiatry. 1990;47(7):622–632. doi: 10.1001/archpsyc.1990.01810190022003. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor R, Giedd J, Pannier L, Kaysen D, Rapoport JL. Activation of prefrontal cortex in children during a non-spatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine T, Lencz T, Mednick S, editors. Schizotypal personality. New York: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP. Developmental neural networks in children performing a Categorical N-Back Task. Neuroimage. 2006;33(3):980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Developmental Psychopathology. 1999;11(3):487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychological Medicine. 2000;30(5):1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Serna E, Baeza I, Toro J, Andres S, Puig O, Sanchez-Guistau V, Romero S, Bernardo M, Castro-Fornieles J. Relationship between clinical and neuropsychological characteristics in child and adolescent first degree relatives of subjects with schizophrenia. Schizophrenia Research. 2010;116(2–3):159–167. doi: 10.1016/j.schres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, Csernansky JG. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophrenia Bulletin. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. Journal of Cognitive Neuroscience. 2007;19(5):750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia Bulletin. 2009;35(3):528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatric Research. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Cortical pruning and the development of schizophrenia. Schizophrenia Bulletin. 1990;16(4):567–570. doi: 10.1093/schbul/16.4.567. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV-TR Axis I disorders. Washington, D. C: American Psychiatric Press; 2001. [Google Scholar]
- Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, McGuire P. At Risk for Schizophrenic or Affective Psychoses? A Meta-Analysis of DSM/ICD Diagnostic Outcomes in Individuals at High Clinical Risk. Schizophrenia bulletin. 2012 doi: 10.1093/schbul/sbs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of general psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychological medicine. 2013:1–8. doi: 10.1017/S0033291713000184. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Foundation Symposium. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. discussion 112–108, 193–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2007;64(7):772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(41):15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Buka SL, Horton NJ, Donatelli JL, Rieder RO, Tsuang MT. Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychoses. Schizophrenia Bulletin. 2000;26(2):323–334. doi: 10.1093/oxfordjournals.schbul.a033456. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Emory University Project on Children of Disturbed Parents. Schizophrenia Bulletin. 1987;13(3):411–423. doi: 10.1093/schbul/13.3.411. [DOI] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia genesis: The origins of madness. New York: Freeman; 1991. [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. Journal of Neuroscience. 2007;27(50):13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Developmental Psychopathology. 2008;20(4):1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, Botti JA, Kelchner L, McCarthy C. Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biological psychiatry. 2011;69(10):945–952. doi: 10.1016/j.biopsych.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. Journal of Psychiatric Research. 1999;33(6):513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Developmental Psychopathology. 1999;11(3):525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, Tsuang MT. IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. The American Journal of Psychiatry. 1998;155(5):672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, Allebeck P, Dalman C. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70(3):261–270. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- MacCrimmon DJ, Cleghorn JM, Asarnow RF, Steffy RA. Children at risk for schizophrenia. Clinical and attentional characteristics. Archives of General Psychiatry. 1980;37(6):671–674. doi: 10.1001/archpsyc.1980.01780190069008. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symtpom assessment in schizophrenia prodromal states. Psychaitric Quarterly. 1999;70(4):273–288. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S, Lymer GK, Bastin ME, Marjoram D, Job DE, Moorhead TW, Owens DG, Johnstone EC, McIntosh AM, Lawrie SM. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophrenia Research. 2008;106(2–3):132–139. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Murray RM, Jones P, O’Callaghan E. Fetal brain development and later schizophrenia. Ciba Foundation Symposium. 1991;156:155–163. doi: 10.1002/9780470514047.ch10. discussion 163–170. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry. 2003;160(11):2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Aleman A, de Haan EH. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. Wisconsin Card Sorting Test. Continuous Performance Test. Journal of Psychiatric Research. 2001;35(2):119–125. doi: 10.1016/s0022-3956(01)00014-0. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research: Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Olin SC, Mednick SA. Risk factors of psychosis: identifying vulnerable populations premorbidly. Schizophrenia Bulletin. 1996;22(2):223–240. doi: 10.1093/schbul/22.2.223. [DOI] [PubMed] [Google Scholar]
- Ott SL, Spinelli S, Rock D, Roberts S, Amminger GP, Erlenmeyer-Kimling L. The New York High-Risk Project: social and general intelligence in children at risk for schizophrenia. Schizophrenia Research. 1998;31(1):1–11. doi: 10.1016/s0920-9964(98)00010-3. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophrenia Bulletin. 2005;31(3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Progress in Brain Research. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test. Perceptual and Motor Skills. 1958;(8):271–276. [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005;11(5):631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cerebral cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg SC, Yaniv S. Social adjustment and cognitive performance of high-risk children. Schizophrenia Bulletin. 1985;11(1):61–65. doi: 10.1093/schbul/11.1.61. [DOI] [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Parnas J, Mednick SA. Premorbid neurocognitive functioning in schizophrenia spectrum disorder. Schizophrenia Bulletin. 2006;32(3):578–583. doi: 10.1093/schbul/sbj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia Research. 2009 doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, van Erp TG, Thompson PM, Toga AW, Smith DJ, Cannon TD, Pantelis C. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S, Yamamoto T, Kawaguchi H, Koizumi H, Sawaguchi T. Prefrontal cortical activation associated with working memory in adults and preschool children: an event-related optical topography study. Cerebral Cortex. 2004;14(7):703–712. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Rapoport JL, Hayashi KM, Geaga JA, Sui Y, McLemore LE, Alaghband Y, Giedd JN, Gochman P, Blumenthal J, Gogtay N, Nicolson R, Toga AW, Thompson PM. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Archives of General Psychiatry. 2006;63(1):25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophrenia Bulletin. 1994;20(3):441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Whalley H, Simonotto E, Moorhead W, McIntosh AM, Marshall I, Ebmeier KP, Owens DGC, Goddard NH, Johnstone EC, Lawrie SM. Functional imaging as a predictor of schizophrenia. Biological Psychiatry. 2006;60:454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wolters G, Phaf RH. Implicit and explicit memory: Implications for the symbol-manipulation versus connectionism controversy. Psychological Research. 1990;52:137–144. [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophrenia bulletin. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worland J, Weeks DG, Weiner SM, Schechtman J. Longitudinal, prospective evaluations of intelligence in children at risk. Schizophrenia Bulletin. 1982;8(1):135–141. doi: 10.1093/schbul/8.1.135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.