Abstract
Cartilage tissue lacks an intrinsic capacity for self-regeneration due to slow matrix turnover, a limited supply of mature chondrocytes and insufficient vasculature. Although cartilage tissue engineering has achieved some success using agarose as a scaffolding material, major challenges of agarose-based cartilage repair, including non-degradability, poor tissue–scaffold integration and limited processing capability, have prompted the search for an alternative biomaterial. In this study, silk fiber–hydrogel composites (SF–silk hydrogels) made from silk microfibers and silk hydrogels were investigated for their potential use as a support material for engineered cartilage. We demonstrated the use of 100% silk-based fiber–hydrogel composite scaffolds for the development of cartilage constructs with properties comparable to those made with agarose. Cartilage constructs with an equilibrium modulus in the native tissue range were fabricated by mimicking the collagen fiber and proteoglycan composite architecture of native cartilage using biocompatible, biodegradable silk fibroin from Bombyx mori. Excellent chondrocyte response was observed on SF–silk hydrogels, and fiber reinforcement resulted in the development of more mechanically robust constructs after 42 days in culture compared to silk hydrogels alone. Thus, we demonstrate the versatility of silk fibroin as a composite scaffolding material for use in cartilage tissue repair to create functional cartilage constructs that overcome the limitations of agarose biomaterials, and provide a much-needed alternative to the agarose standard.
Keywords: Cartilage, Hydrogel, Tissue engineering, Chondrocyte, Silk, Silk fiber, Agarose
1. Introduction
Epidemiological studies have revealed that more than 70% of adults between the ages of 55 and 78 experience disability due to osteoarthritis [1]. Coupled with high disease prevalence, the limited capacity of adult cartilage to undergo self-regeneration has prompted the urgent development of functional cartilage tissue replacement therapy. Poor vascularization, slow matrix turnover, a limited number of progenitor cells and a scarcity of mature, non-dividing chondrocytes all contribute to the inability of cartilage lesions to heal, particular in the elderly [2]. While autografting, allografting and microfracturing used currently to surgically repair lesions have provided some relief, these procedures are not linked with high success rates [3].
Cartilage tissue engineering (TE) has emerged as a promising therapeutic for joint repair. Since native cartilage demonstrates compressive moduli of the order of 300–800 kPa [4], mechanical strength and structural resilience is one of the key requirements for cartilage scaffold fabrication [5]. At a structural level, native cartilage is a multilayer connective tissue composed of chondrocytes dispersed in a dense extracellular matrix (ECM). The hydrophilic environment of the ECM, due to negatively charged glycosaminoglycans (GAGs) that attract water, enables cartilage tissue to experience a swelling pressure, which is countered by the tensile strength generated from the interspersed collagen network [6]. Despite modest success in cartilage TE, the non-homogeneous, depth-dependent composition of articular cartilage, coupled with differences in layer thickness, cellular morphology and ECM composition, makes this tissue difficult to mimic structurally [7–9].
Unlike other scaffold formats, hydrogels provide swelling kinetics and a hydrated environment similar to native tissue [5]. The injectability of hydrogels into a damaged site, the shape-conforming capacity of the gel within the defect, and the ability to homogeneously suspend cells within the hydrogel network and preserve chondrogenic phenotype in vitro makes hydrogels a highly attractive platform for cartilage repair [10, 11]. The development of functional cartilage tissue has evolved through the use of various synthetic and natural hydrogel materials. While synthetic polymers, such as poly(ethylene glycol) (PEG) or polyglycolic acid (PGA), provide well-controlled systems for determining the effects of isolated material properties on scaffold design (e.g. degree of crosslinking, mechanics), these materials are relatively inert and offer limited support for chondrogenesis and cartilage matrix production [12, 13]. Instead, natural materials, such as collagen, hyaluronic acid, agarose, alginate, fibrin and elastin, are favored due to their abundance, environmentally friendly processing and inherent prochondrogenic properties [14].
Agarose hydrogels in particular have been studied extensively to determine the effects of factors such as mechanical loading and cell-seeding density on cartilage tissue formation [15]. Due to its superior support for chondrogenesis and higher deposition of GAG compared to other natural hydrogels made from fibrin, collagen, alginate and PGA, agarose has been labeled as the “gold standard” biomaterial for in vitro cartilage tissue formation [16]. However, despite its promising performance in vitro, agarose has undergone limited study in animal models due to its poor biocompatibility and inability to degrade in vivo, which prevents graft integration with the host tissue [17]. Furthermore, cartilage tissue formation within an agarose hydrogel is complicated by an inability to customize agarose scaffold structure and composition [18, 19]. Thus, limitations of agarose-based joint therapy have prompted the search for an alternative natural biomaterial for cartilage tissue engineering.
Silk fibroin from Bombyx mori silkworms is a promising substitute for agarose in cartilage repair due to its robust mechanical properties, superior biocompatibility, degradability, ease of fabrication and tunable processing parameters [20]. Extensive in vivo study on the implantation of silk scaffolds has proven that silk elicits little to no immune response, degrades in a controlled manner via ubiquitous protease-mediated digestion, and can be conjugated with functional groups or RGD peptide modification to promote cell adhesion [21–23]. In addition, silk fibroin can easily be assembled into a versatile array of material formats (e.g. films, sponges, microspheres, electrospun fibers, hydrogels) using aqueous-based processing [24]. The formation of silk hydrogels in particular is accomplished by a variety of mechanisms marked by a change in silk conformation from amorphous random coil to organized crystalline β-sheet structures. The silk sol–gel process that leads to physical alignment of the protein chains relies on a balance between protein concentration, temperature, pH and salt/ion concentration during the phase transition [25]. Sonication-mediated gelation utilizes shear force from ultrasound waves to initiate gelation, and affords fine control over gelation kinetics by tuning silk fibroin concentration or sonication parameters, such as duration time and energy output. Thus, this time delay allows for encapsulation of cells or biomolecules within the silk prior to gelation [26].
Previously, we demonstrated that one type of silk hydrogel, prepared by sonication-induced gelation of a silk solution, could support chondrocyte viability and yield cartilaginous constructs with biochemical properties mimicking those of native cartilage tissue [27]. While these silk hydrogels provided an excellent scaffold for chondrocyte attachment and cartilage matrix deposition, further improvement in the mechanical properties of these hydrogels is necessary to construct optimal load-bearing cartilage tissue constructs. Efforts in cartilage TE over the past few decades have improved hydrogel mechanics using methods such as chemical crosslinking [28, 29], double-network hydrogels [30, 31] and hydrogel interpenetrating scaffolds [32, 33]. While fiber reinforcement has also successfully enhanced the mechanical performance of hydrogel systems, little is known about the effects of fiber–gel composite systems on long-term cell viability and tissue development [34] [35].
The aim of this study was to leverage the versatility of silk fibroin to generate a mechanically reinforced silk microfiber silk hydrogel (SF–silk hydrogel) composite for functional cartilage TE. Osteoconductive materials have been successfully developed using silk microfiber-reinforced porous scaffolds; however, silk fiber reinforcement has not yet been applied to a hydrogel system [36]. For the first time, two formats of silk—microfiber and sonicated hydrogel—have been united to develop a mechanically reinforced hydrogel for cartilage tissue engineering. The composite SF-silk hydrogel system was optimized according to diffusivity and mechanical properties, and compared to the agarose hydrogel standard to identify a potential alternative to the current “gold standard” biomaterial. Silk microfibers and primary chondrocytes were encapsulated within the gel during the sonication-induced sol–gel transition to test the hypothesis that SF–silk hydrogels exhibit comparable properties to agarose and can yield cartilage constructs that mimic native cartilage after only 6 weeks of in vitro culture. Through this work, we provide valuable insights into the role of silk microfibers in hydrogel reinforcement, and demonstrate the development of SF–silk hydrogel cartilage constructs with properties approaching those of native cartilage.
2. Materials and methods
2.1 Preparation of silk solution
Silk fibroin was extracted from cocoons of B. mori as previously described [27]. Briefly, silk cocoons were boiled in an aqueous solution of 0.02 M Na2CO3 and washed with deionized (DI) water. The resultant dry silk fibroin was then dissolved in a 9.3 M LiBr solution (25% w/v) at 60°C for 4–6 h, and dialyzed against DI water using 3500 dalton molecular weight cut off dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA). The final concentration of the aqueous silk solution was 6–8% w/v, which was determined by weighing the remaining silk solid after drying a known volume.
2.2 Preparation of silk microfibers
Micron-sized, non-immunogenic silk fibers were fabricated according to protocol by Mandal et al. [36]. Briefly, 0.35 g of dried, degummed silk fibers were incubated in a 17.5 M NaOH solution. To obtain large (>500 μm), medium (400–500 μm) and small (150–200 μm) microfibers, the hydrolysis reaction was carried out for 30, 60 and 180 s, respectively. The reaction was quenched with DI water and the microfibers were washed repeatedly. Dried fibers were subsequently obtained through lyophilization. Microfibers were stored at ambient conditions until further use.
2.3 Chondrocyte isolation
Cartilage was harvested from fresh bovine carpometacarpal joints of 4 month old calves (Green Village Packing Co., NJ). Cartilage flakes were digested for 10 h using collagenase (390 U ml−1, type V; Sigma Aldrich, St Louis, MO) in high-glucose Dulbecco’s Modified Eagle’s Medium (hgDMEM supplemented with 5% FBS). The digesting suspension was filtered through a 70 μm cell strainer, and plated at high density in growth medium (hgDMEM supplemented with 10% FBS).
2.4 Preparation of silk microfiber-reinforced hydrogels
Sterilized silk solution (8% w/v) as mixed with DMEM powder and NaHCO3 and sonicated using a Branson 450 Sonifier (Branson Ultrasonics Co., Danbury, CT) for 15 s with 12% amplitude to initiate gelation. Silk microfibers were soaked with 70% ethanol, air dried overnight and resuspended in phosphate-buffered saline (PBS) prior to mixing with the sonicated silk solution to obtain a final microfiber concentration of 2% w/v in the hydrogel. For SF–silk hydrogel, sterile filtered presonicated silk solution was mixed with an equal volume of silk microfibers and cells to obtain a final concentration of 4% silk hydrogel, 2% fibers and 20 × 106 cells ml−1. SF–agarose hydrogel was prepared by mixing 4% low-melt agarose (type VII, Sigma) in PBS at 37°C with an equal volume of silk microfibers and cells. The resultant agarose mixture consisted of 2% agarose, 2% microfibers and 20 × 106 cells ml−1. The mixture was cast in sterile molds made of two glass plates separated by a 2.5 mm thick glass spacer. Cylindrical disks (4 mm diameter) were cored out using a biopsy punch. Fiber- or cell-free constructs were prepared in a similar manner without the addition of silk fibers or chondrocytes to the hydrogel solution.
2.5 Scanning electron microscopy
Cell-free hydrogels were freeze-dried at −80°C and fractured into sections using a razor blade. The fractures surfaces were sputter-coated with gold/palladium. An Amray 1830 scanning electron microscope was used to visualize surface features at 200× and 500× magnification (MicroVision Labs, Inc., Chelmsford, MA).
2.6 Construct cultivation
Cartilage tissue development was performed in chondrogenic medium (hgDMEM supplemented with 5 mg ml−1 proline, 1% ITS+, 100 nM dexamethasone, 50 μg ml−1 ascorbate and 10 ng ml−1 TGF-β3 for the first 2 weeks) [37]. Constructs from SF–silk hydrogel, SF–agarose hydrogel, silk hydrogel and agarose hydrogel were maintained in culture for 42 days with biweekly medium changes.
2.7 Diffusion measurement by FRAP
Acellular agarose constructs of 2% and 4% were prepared by diluting 4% and 8% low-melt agarose solution in PBS, respectively. Acellular silk constructs of 2% and 4% were prepared by sonicating 2% and 4% silk solutions using the parameters as previously described. Constructs were cast in sterile molds and cored out to obtain cylindrical samples 4 mm in diameter × 2.5 mm high. Acellular agarose and silk constructs were soaked in a saturated (0.5 mg ml−1) 70 kDa fluorescein-conjugated dextran solution (Invitrogen, Carlsbad, CA) for over 24 h. Samples were placed on an Olympus Fluoview FV1000 confocal microscope and diffusion properties were measured via fluorescent recovery after photobleaching (FRAP) of a thin line using a 405 nm laser for 30 s. Images (320 × 320 pixels) were acquired using a 20× objective before, during and after the photobleaching process. FRAP analysis was performed using a custom MATLAB code according to Albro et al. [38], which fits the FRAP images to the Gaussian equation , where I is the fluorescence intensity as a function of position (x) and time (t), M is the total amount of bleached species and w is related to the diffusion coefficient, D, by the equation w2 = 4Dt. A linear regression was applied to a plot of w2 against t (r2 ≈ 0.99) to acquire a value for D.
2.8 Mechanical assessment
The mechanical properties of the constructs were measured in unconfined compression using a custom-made mechanical testing device [39]. Constructs (n = 6) were placed in a testing chamber containing PBS at 37 °C, equilibrated under a creep tare load (0.5 g for 30 min) and subjected to stress-relaxation test (ramp velocity of 1 μm m s−1 up to 10% strain) to obtain the equilibrium Young’s modulus (EY). The dynamic modulus (E*) was calculated from analyzing the stress–strain response of the construct to the applied load of 1% strain at frequencies of 1, 0.5 and 0.1 Hz for 1 min at each frequency. The frequencies that were selected are based on literature values that are designed to mimic the repetitive physiological stresses experienced by the joint during walking from low to moderate activity.
2.9 Biochemical composition assay
Silk and agarose hydrogel constructs (n = 5) were harvested, weighed and digested with 20 μl ml−1 papain in 1 mg ml−1 of proteinase K (Fisher Scientific, Pittsburgh, PA) containing 1 mM iodoacetamide and 10 mg ml−1 pepstatin-A (Sigma Aldrich). For GAG content, aliquots of digested constructs were analyzed using the 1,9-dimethylmethylene blue dye binding (DMMB) assay [40]. Ortho-hydroxyproline (OHP) content was determined via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde [41] and converted to total collagen content using the 1:7.64 ratio of OHP to collagen [42]. Total DNA content was quantified using PicoGreen assay (Invitrogen) following the manufacturer’s protocol. Biochemical content were reported as μg per μg of construct wet weight (% wwt).
2.10 Histology and immunohistochemistry
Constructs were rinsed with PBS, fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin. Sections were stained with Alcian Blue to detect GAG. For collagen immunohistochemistry staining, tissue sections were hydrated, and antigen retrieval was performed using heated 0.01 M citrate buffer with pH 6.0 for 15 min. The sections were incubated with blocking serum and incubated with a 1:1000 dilution of type II or type I collagen monoclonal antibody (Millipore, Temecula, CA). Biotinylated secondary antibody was applied to the sections followed by signal detection using a 3,3′-diaminobenzidine (DAB) peroxidase substrate kit (Vectastain ABC, Burlingame, CA).
2.11 Statistical analysis
Statistics were performed with Statistica software (Statsoft, Tulsa, OK). Data were expressed as the average ± SD of n = 4–6 samples per group at each time point. The differences in construct properties between the groups were evaluated using two-way ANOVA, followed by Turkey’s honest significant difference test. A P-value <0.05 was considered statistically significant.
3. Results
3.1 Diffusivity of solute molecules in silk and agarose hydrogels
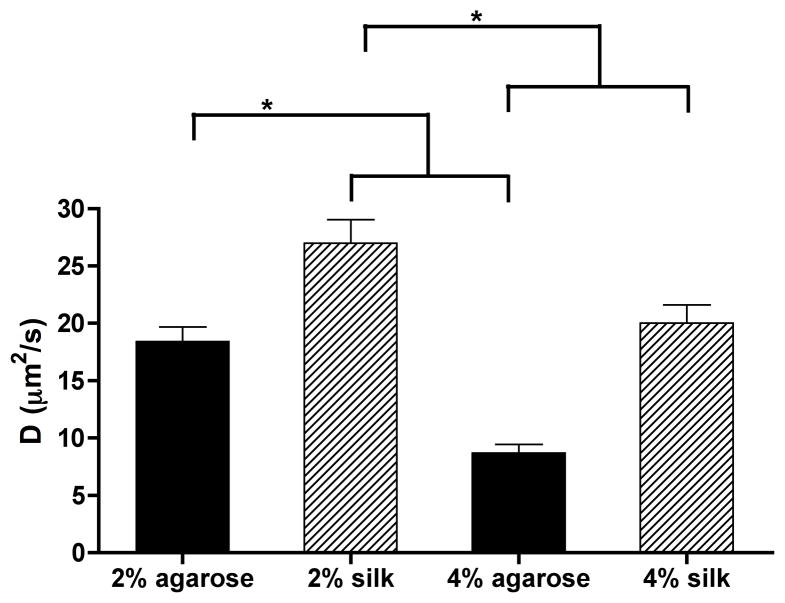
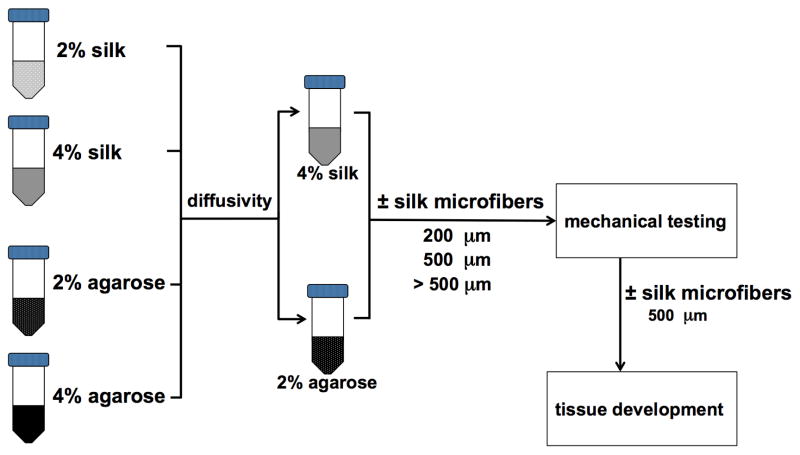
We first assessed the diffusivity of the silk and agarose hydrogels. FRAP results indicated that the diffusivity of agarose and silk hydrogels was significantly lower at 4% compared to 2% for the same material (P < 0.01). Interestingly, 2% agarose hydrogels did not show significant differences in diffusivity compared to 4% silk hydrogels (P > 0.05). Based on the similarity in diffusivity for these two groups, 2% agarose and 4% silk hydrogels were chosen for incorporation of silk microfibers and further evaluation through mechanical testing and in vitro cartilage development.
3.2 Mechanical reinforcement of silk hydrogels using silk microfibers
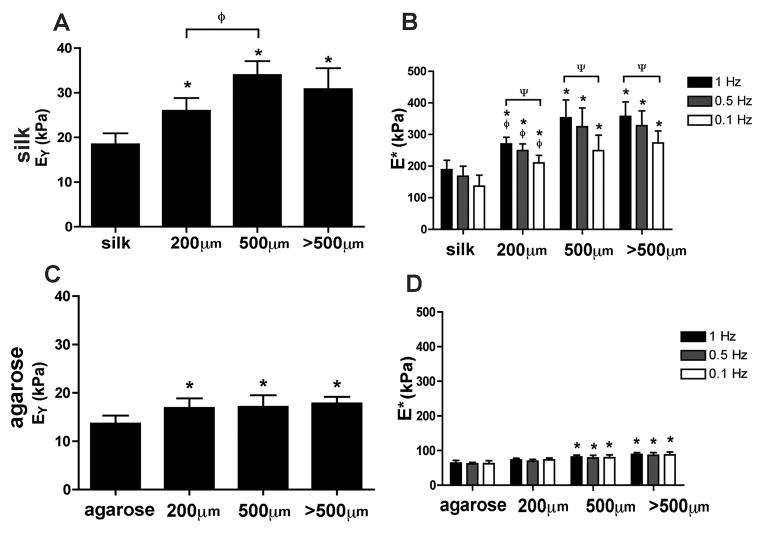
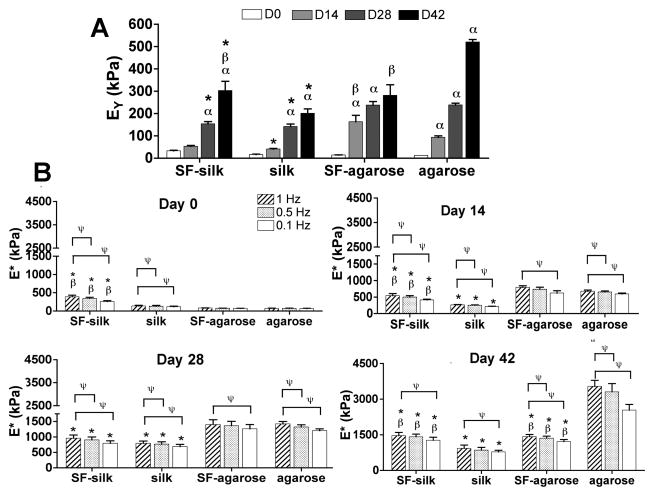
Based on findings from the FRAP diffusivity testing (Fig. 2), 2% agarose and 4% silk hydrogels were chosen for mechanical evaluation with and without reinforcement using silk microfibers. Acellular hydrogel constructs containing silk microfibers were tested in unconfined compression to determine equilibrium (EY) and dynamic moduli (E*). Silk microfiber incorporation and length affected both the dynamic and equilibrium moduli of silk hydrogels with silk microfibers (SF–silk hydrogels) (Fig. 3). The highest Young’s modulus (EY) was observed in SF–silk hydrogels reinforced with 500 μm fibers (34.0 ± 3.1 kPa) (Fig. 3A). All SF–silk hydrogel groups displayed frequency-dependent dynamic moduli, indicating a stiffening response to dynamic loading (Fig. 3B). The silk hydrogel with 500 μm silk microfibers exhibited the highest dynamic modulus at 1 Hz (357.2 ± 45.7 kPa). For the agarose hydrogels, incorporation of silk fibers increased the Young’s moduli in all groups. However, microfiber length did not affect the Young’s moduli of the SF-agarose hydrogels (Fig. 3C). When 500 μm or >500 μm fibers were incorporated into agarose hydrogel, the dynamic moduli of SF–agarose significantly increased compared to the no-fiber agarose control (Fig. 3D). Interestingly, none of the SF–agarose hydrogel groups demonstrated a frequency-dependent change in dynamic modulus as was observed in the silk hydrogel groups.
Figure 2.
Diffusivity of FITC–dextran in agarose and silk hydrogels. The diffusivity of FITC–dextran (MW = 70 kDa) in silk hydrogels made from varying silk protein (n = 5 for all groups). 70 kDa FITC–dextran was selected as its size is similar to the major cartilage growth factor TGF-β.
Figure 3.
Mechanical properties of hydrogels reinforced with silk microfibers. The equilibrium and dynamic moduli of hydrogel scaffolds made of silk (A, B) and agarose (C, D) with and without incorporation of silk microfibers of different lengths (200, 500 and >500 μm) were determined in unconfined compression testing. *P < 0.05 indicates statistical significance compared with the no-fiber control group. ΨP < 0.05 indicates statistical significance compared with the 1 Hz frequency testing within the same hydrogel group, ϕP < 0.05 indicates statistical significance compared with the 500 μm hydrogel group (n = 20 for all groups).
3.3 Organization of silk microfibers in hydrogels
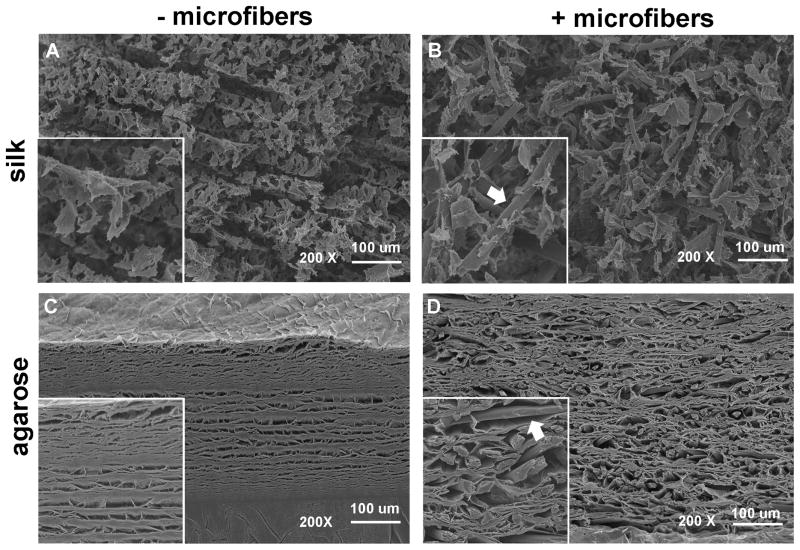
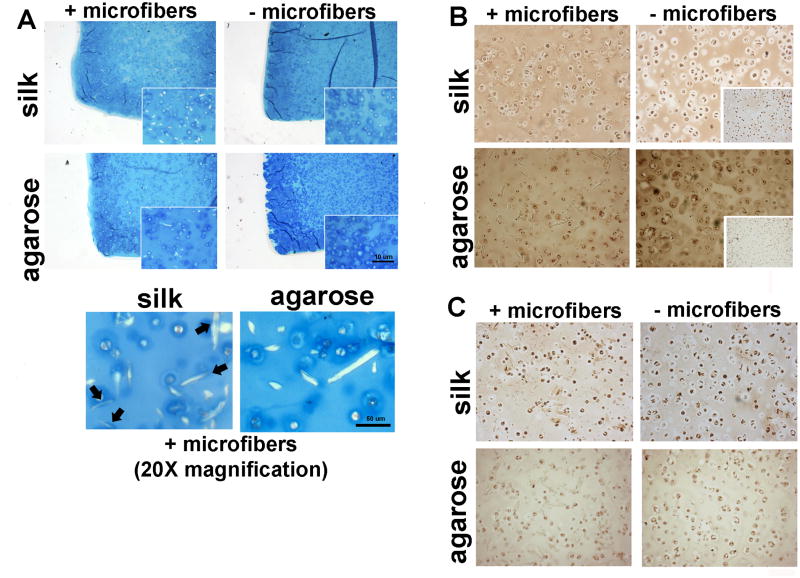
The microarchitecture of silk and agarose hydrogels with and without 500 μm fibers was visualized by scanning electron microscopy (SEM). Organized, loose sheet-like structures were observed in SF–silk and silk control hydrogels (Fig. 4A). Introduction of silk microfibers resulted in disruption of this lamellar-type organization and disorientation of the silk hydrogel sheets. Physical adherence of the microfibers to the silk sheets was observed in the SF–silk hydrogels, with noticeable fusion of the silk microfibers with the surrounding silk hydrogel matrix (Fig. 4B). In contrast, agarose hydrogels formed a single intact sheet with internal porosity (Fig. 4C), while SF–agarose hydrogels displayed larger pore sizes of ~50 – 100 μm (Fig. 4D). A clear separation was observed between the agarose hydrogel sheets and the silk microfibers, indicating that the agarose was unable to bind the fibers, resulting in a smooth appearance along the surface of the fiber (Fig. 4D).
Figure 4.
Scanning electron micrographs of hydrogels reinforced with silk microfibers. The morphology and microarchitecture of freeze-dried, acellular (A) 4% silk hydrogel, (B) 4% SF–silk hydrogel, (C) 2% agarose hydrogel and (D) 2% SF–agarose hydrogel were visualized by SEM at 200× magnification. Silk microfibers are indicated by arrows. Scale bars are 100 μm. Insert boxes show magnified images of hydrogels. Silk microfibers are indicated by arrows.
3.4 Cartilage tissue development in silk microfiber-reinforced hydrogels
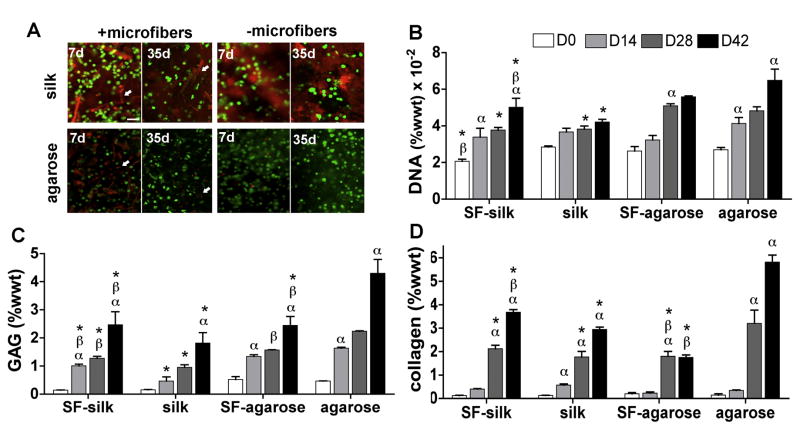
Acellular 4% w/v silk hydrogel constructs reinforced with 500 μm long silk microfibers displayed the highest dynamic and equilibrium moduli, and were selected for in vitro cartilage tissue development (Fig. 3). A homogeneous distribution of live chondrocytes was observed in all hydrogels at 7 and 35 days post-seeding (Fig. 5A). DNA content significantly increased in all hydrogel groups with time. At day 42, the no-fiber agarose control group displayed significantly higher DNA content than the SF–silk gels or the no-fiber silk controls (P < 0.01) (Fig. 5B). SF–silk hydrogel displayed higher DNA content than the no-fiber silk hydrogel control (P < 0.05). No significant difference was observed in total DNA content between the agarose hydrogel groups at day 42 of culture.
Figure 5. Chondrocyte viability and biological components of constructs.
Primary bovine chondrocytes were encapsulated in silk or agarose hydrogels with or without 500 μm microfibers and cultured for 42 days.(A) Live/dead assay revealed a homogeneous distribution of viable chondrocytes in all hydrogel groups at 7 and 35 days post-seeding. Silk microfibers and silk hydrogels exhibited red autofluorescence in the background. Scale bars are 200 μm. Biochemical evaluation was performed on silk and agarose hydrogels with and without 500 μm fiber reinforcement to determine total (B) DNA, (C) GAG and (D) collagen content. αP < 0.05 indicates statistical significance compared to a previous time point within the same group; βP < 0.05 indicates statistical significance compared to the same hydrogel group; *P < 0.05 indicates statistical significance compared to agarose hydrogels at the same time point (n = 5 for all groups).
An increase in proteoglycan and collagen production was observed in all groups with time (Fig. 5C and D). However, the no-fiber agarose control constructs displayed GAG and collagen content that was significantly higher than all other groups at day 42. For silk hydrogels, incorporation of silk microfibers significantly increased GAG and collagen production (P < 0.01).
3.5 Mechanical and immunohistological analysis of silk microfiber-reinforced cartilage constructs
Corresponding with biochemical content, the mechanical properties of all groups improved with time. At the end of the 42 day culture period, the no-fiber agarose control constructs yielded the highest Young’s modulus (521 ± 11 kPa) (Fig. 6A). Silk microfiber incorporation in silk hydrogels (SF–silk) significantly improved the dynamic modulus of the silk hydrogels under 1 Hz dynamic loading, while incorporation of silk microfibers into agarose hydrogels resulted in an adverse affect on dynamic modulus of the agarose construct. Interestingly, the equilibrium and dynamic moduli of agarose hydrogels with and without fiber reinforcement were comparable at days 0, 14 and 28. After 42 days in culture, SF–agarose hydrogels displayed inferior mechanical properties to the no-fiber agarose control hydrogels (Fig. 6A,B).
Figure 6.
Mechanical properties of engineered cartilage constructs. Constructs (n = 5 in all groups) were mechanically tested under confined compression to determine (A) the Young’s modulus (EY) and (B) the dynamic modulus (E*) every 2 weeks over the course of a 6 week culture period. αP<0.05 indicates statistical significance compared to the previous time point within the same group; βP < 0.05 indicates statistical significance compared to the same hydrogel group, * p < 0.05 indicates statistical significance compared to agarose hydrogels at the same time point; ΨP < 0.05 indicates statistical significance compared to 1 Hz frequency dynamic testing within the same group (n = 5 for all groups).
By day 42 in culture, SF–silk constructs exhibited uniform cartilage-like tissue organization and GAG production similar to the no-fiber agarose control constructs (Fig. 7A). Light GAG staining was observed in engineered cartilage constructs made from SF–agarose hydrogels. Higher magnification revealed that the highest GAG content in the SF–silk hydrogels was clustered around the embedded silk microfibers as indicated by arrows. This phenomenon was not observed in SF–agarose hydrogels. Collagen type II was found in higher abundance in the no-fiber agarose control constructs compared to all other groups (Fig. 7B). Among the silk hydrogel groups, SF–silk constructs displayed stronger collagen type II staining than the no-fiber silk control constructs. Immunostaining was unable to detect collagen type I in any hydrogel group (Fig. 7C).
Figure 7.
Histological evaluation of silk and agarose hydrogel cartilage constructs. (A) Alcian blue staining was used to visualize glycosaminoglycan (GAG) content within hydrogel constructs. Arrows indicate co-localization of GAGs around silk microfibers in SF–silk hydrogels, a phenomenon not observed in SF–agarose hydrogels. Immunohistochemistry of (B) collagen type II and (C) collagen type I. Collagen type II was found in a higher abundance in the no-fiber agarose control constructs compared to all other groups. Insert boxes represent negative staining.
4. Discussion
In this study, we demonstrated the use of natural silk fibroin from B. mori as a fiber–hydrogel composite cartilage replacement material. Unlike agarose, which is considered the “gold standard” biomaterial for in vitro cartilage development, silk is biodegradable, elicits no immune response and can be fabricated using aqueous-based environmentally friendly protocols into various formats [21, 22]. In addition, silk has been shown to demonstrate exceptional mechanical strength (i.e. ~300 MPa tensile strength for raw silk fiber [43] and 50–400 kPa equilibrium modulus for the sonicated silk hydrogel depending on protein concentration [26]). Initial attempts at using silk scaffolds for cartilage repair by Chao et al. compared cartilage tissue development in two silk scaffold formats (hydrogel and porous scaffold) [27]. Their results demonstrated excellent chondrocyte viability in the silk hydrogel, which, after 6 weeks of culture, developed into a cartilage construct containing newly synthesized extracellular matrix deposits. However, the constructs fell short of the desired mechanical properties of native cartilage [5, 44]. Thus, to address the challenges of constructing a mechanically competent engineered cartilage construct, we developed a novel SF–silk hydrogel composite to generate biocompatible and biodegradable cartilage constructs mimicking both the structure and function of native tissue.
Silk microfiber-reinforced silk hydrogels were tested in parallel with agarose hydrogels with and without fiber reinforcement to determine the effects of the silk microfibers on cartilage tissue development in comparison to agarose. Diffusivity testing was first conducted to select the appropriate concentrations for agarose and silk hydrogels (Fig. 2). As the results showed, 2% agarose and 4% silk hydrogels without fiber reinforcement displayed similar diffusivity values (~18 μm2 s−1) and all the following experiments were conducted using these concentrations.
Both silk and agarose hydrogels were reinforced with silk microfibers of various lengths (200, 500 and >500 μm) to assess the effect of fiber length on mechanical properties. Like most hydrogels, silk gels are brittle and experience cracking under conditions of low stress due to a lack of energy dissipation mechanisms [45]. Hydrogel–fiber composites are devised with the premise that the incorporation of a ductile fibrous network within a brittle hydrogel matrix increases the stiffness and fracture toughness of the hydrogel, enhancing energy absorption and preventing crack propagation [46, 47]. Fiber-reinforced hydrogels have been investigated in tissue engineering by incorporating a variety of polymeric fibers within hydrogel matrices [48, 49]. Structurally, fiber-reinforced hydrogel provides a discontinuous fibrous structure and proteoglycan matrix in native cartilage. Based on the critical fiber length required for maximal stress transfer between the brittle hydrogel and tough fibers, we expected a dependence of composite mechanical properties on silk microfiber length. Critical fiber length is essential in the development of a fiber-reinforced hydrogel scaffold to ensure that sufficient energy transfer occurs from the soft hydrogel matrix to the stiff fibers under mechanical loading [50].
In our studies, the critical fiber length for silk microfibers was determined to be 500 μm. SF–silk hydrogel constructs reinforced with this fiber length displayed superior mechanical properties compared to other SF–silk hydrogel constructs (Fig. 3). Although all silk microfiber lengths tested produced stronger composite silk scaffolds compared to silk hydrogel alone, the 200 μm silk microfibers appeared to fall below the critical fiber length, preventing adequate stress transfer along the fibers. It is interesting to note that while the mean equilibrium and dynamic moduli for 500 μm SF–silk hydrogels were higher than those made with >500 μm long fibers, there was no significant difference between these two groups, suggesting that a fiber length of 500 μm or greater provided sufficient fiber overlap to increase hydrogel stiffness (Fig. 3).
While the addition of microfibers significantly increased the moduli of silk hydrogels, fiber reinforcement contributed a lesser extent to agarose hydrogel mechanics. This suggested weaker interfacial bonding between the silk microfibers and agarose hydrogels, leading to poor fiber–gel stress transfer during loading (Fig. 3D and C). One plausible explanation for the poor agarose–silk binding is due to a difference in non-covalent, intermolecular interactions between the two materials. Agarose monosaccharide units contain many hydroxyl groups, making agarose highly hydrophilic [51]. In contrast, the silk structure is governed by repeated hydrophobic regions of alanine and glycine residues [26]. In an effort to elucidate the physical interaction of the silk microfibers and hydrogel at the fiber–gel interface, the morphology of the freeze-dried, acellular scaffolds was observed by SEM. The silk microfibers physically adhered to the silk hydrogel, but were clearly separated from the agarose hydrogel (Fig. 4). This provided support for the trends observed in equilibrium and dynamic moduli of the agarose hydrogels in Fig. 3. Specifically, the discontinuity of the silk microfiber–agarose hydrogel composite resulted in poor physical stress transfer to the fiber component. While SEM imaging provided information about physical bonding of the fiber to the hydrogel, further studies are required to determine the underlying microfiber–hydrogel interactions.
Based on mechanical testing, SF–silk and SF–agarose hydrogels reinforced with 500 μm silk microfibers were chosen for in vitro cartilage development using primary bovine chondrocyte encapsulation within the hydrogel matrix. A homogeneous distribution of viable chondrocytes was observed, indicating sufficient mass transport throughout silk and agarose hydrogels both with and without fiber reinforcement (Fig. 5A). The DNA content increased significantly with time in all hydrogel groups regardless of microfiber reinforcement. The increase in DNA content observed in SF–silk hydrogels was significantly higher than in the no-fiber silk controls, suggesting that silk microfibers enhanced chondrocyte proliferation in the silk hydrogels. It is possible that the increase in hydrogel stiffness imparted by the microfibers positively affected chondrocyte proliferation in the SF–silk hydrogels. Previous work using chick dorsal root ganglion cells showed that changes in stiffness resulted in significant differences in neurite density, extension and outgrowth within the silk hydrogel [52]. Thus, the stiff, fibrous matrix produced by the dispersion of microfibers within the silk hydrogels may improve chondrocyte adhesion and resulted in the higher cell numbers observed on SF–silk hydrogels. More notably, SF–silk hydrogels matched the increase in DNA content in agarose hydrogel groups, providing further support for the SF–silk hydrogel as a potential alternative to agarose.
GAG and collagen deposition increased in all hydrogel groups with time (Fig. 5C and D). Interestingly, microfiber reinforcement of silk hydrogels enhanced collagen and GAG production after 42 days of culture, while it reduced matrix accumulation in agarose hydrogels, suggesting that microfibers hindered the chondrocytes’ ability to deposit matrix in agarose (Fig. 5F and G). One plausible explanation for this observation relates to pore size. Previous studies have suggested that pore size is negatively correlated with collagen deposition [53]. In their studies, more chondrocytes attached to regions of small pores and resulted in higher collagen production. Since SEM imaging of the SF–agarose gels revealed larger pore sizes compared to the no-fiber agarose controls, this difference in porosity may have affected collagen matrix deposition (Fig. 4). The agarose controls without microfibers may have displayed higher cell density and stimulated more matrix synthesis (Fig. 7B). Further investigation is required to elucidate this potential mechanism and understand the effect of fiber reinforcement on collagen development.
Engineered cartilage constructs made with 500 μm SF–silk hydrogels exhibited a 50% improvement in equilibrium modulus (EY) and a 60% increase in dynamic moduli (E* at 1 Hz) at day 42 compared with constructs generated from silk hydrogels without fibers (Fig. 6A). Interestingly, silk microfibers did not improve the dynamic modulus of SF–agarose constructs. In fact, significantly lower equilibrium and dynamic moduli were exhibited by the SF–agarose constructs compared to the no-fiber agarose controls at day 42 (Fig. 6). In SF–silk hydrogels, histological evaluation revealed higher GAG staining around the fibers, suggesting that newly synthesized proteoglycan was localized to silk microfibers. In contrast, GAG staining in SF–agarose hydrogel was not localized to silk microfibers. These results again suggested that silk microfibers failed to form a physical bond with the agarose matrix, resulting in poor stress transfer. Moreover, silk microfibers may have hindered GAG and collagen deposition in agarose hydrogels.
This study demonstrated the use of silk microfiber-reinforced silk hydrogels to mimic the structure and function of native cartilage for the development of engineered cartilage constructs. SF–silk hydrogels not only demonstrated comparable mechanical properties to agarose hydrogels, but also displayed excellent chondrocyte compatibility and support of GAG and collagen matrix deposition, making silk a feasible alternative to agarose for cartilage tissue engineering. Leveraging the principles of fiber–gel composite engineering, SF–silk hydrogels were fabricated to improve sonicated silk hydrogel mechanics. The fiber–gel composite silk scaffolds exhibited excellent microfiber–hydrogel continuity, allowing deposition and localization of an ECM rich in GAG and collagen around the fibers. In this study, SF–silk hydrogels seeded with chondrocytes resulted in the production of cartilage constructs that matched the range of equilibrium modulus of native cartilage tissue (300–800 kPa) [4].
After 42 weeks of culture, silk microfiber-reinforced silk hydrogel cartilage constructs also fell within the range of equilibrium moduli observed for other engineered cartilage constructs (10–1,000 kPa depending on the choice of hydrogel material) [15, 54–56]. Thus, SF–silk hydrogels provide a platform to effectively mimic both the biomechanical properties and biochemical composition of native cartilage tissue, and exhibit comparable properties to other hydrogel formats currently used in cartilage TE, including agarose. Moreover, since extensive in vivo testing of silk fibroin has demonstrated the material’s excellent biocompatibility, controllable degradation and integration with native host tissue, SF–silk hydrogels hold promise for the next step of testing in an animal cartilage defect model [57–59].
This study reports one of the first composite cartilage tissue engineering scaffolds with both the matrix and reinforcing component made entirely of the same material. The data presented in this work suggest that silk hydrogels reinforced with silk microfibers are well suited for cartilage tissue engineering, providing mature chondrocytes with a structural and mechanical microenvironment that is highly supportive of cartilage matrix deposition. Furthermore, the potential to surface modify the silk fibroin for enhanced cell attachment, or the opportunity to employ silk as a drug depot for local, controlled release of vascular or growth factors to the defect site significantly increases the utility of silk composite materials for cartilage tissue engineering [60–64]. SF-silk hydrogel scaffolds could be further optimized using controlled microfiber alignment in hydrogels that mimics the collagen fiber orientation in superficial and subchondral zones of articular cartilage to sustain multidirectional loading in a joint model.
Figure 1.
Experimental design. Hydrogels made of silk and agarose (2% w/v and 4% w/v, respectively) were evaluated for diffusional properties using FRAP. The 4% silk and 2% agarose hydrogels exhibited similar diffusivity and were selected for further testing and reinforcement with silk microfibers. Silk microfibers of three lengths (200, 500 and >500 μm) were tested, with 500 μm microfibers producing the greatest increase in Young’s modulus in both the silk and agarose hydrogels. Cartilage tissue development was then investigated in silk and agarose hydrogels with and without the addition of the 500 μm silk microfibers.
Acknowledgments
We gratefully acknowledge research funding received from the NIH (grants DE016525 to G.V.N., EB002520 to D.K. and G.V.N., AR061988 to D.K., C.H. and G.V.N.), and the Royal Thai Graduate Fellowship (to S.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Detterline AJ, Goldberg S, Bach BR, Jr, Cole BJ. Treatment options for articular cartilage defects of the knee. Orthop Nurs. 2005;24:361. doi: 10.1097/00006416-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Hirvonen J, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 5.Boschetti F, Pennati G, Gervaso F, Peretti GM, Dubini G. Biomechanical properties of human articular cartilage under compressive loads. Biorheology. 2004;41:159. [PubMed] [Google Scholar]
- 6.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 7.Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- 8.Mansour J. Biomechanics of cartilage. In: Oatis CA, editor. Kinesiology: the mechanics and pathomechanics of human movement. Baltimore, MD: Lippincott Williams & Wilkins; 2003. pp. 1992–6. [Google Scholar]
- 9.Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 10.Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 11.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 12.Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A. 2009;90:456. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein TJ, Rizzi SC, Schrobback K, Reichert JC, Jeon J, Crawford RW, Hutmacher DW. Long-term effects of hydrogel properties on human chondrocyte behavior. Soft Matter. 2010;6:5175. [Google Scholar]
- 14.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32:8771. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 18.De Paepe I, Declercq H, Cornelissen M, Schacht E. Novel hydrogels based on methacrylate- modified agarose. Polym Int. 2002;51:867. [Google Scholar]
- 19.Cao X, Shoichet MS. Photoimmobilization of biomolecules within a 3-dimensional hydrogel matrix. J Biomater Sci Polymer Edn. 2002;13:623. doi: 10.1163/156856202320269120. [DOI] [PubMed] [Google Scholar]
- 20.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen JS, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Ki CS, Park YH, Jung HM, Woo KM, Kim HJ. Electrospun silk fibroin scaffolds with macropores for bone regeneration: an in vitro and in vivo study. Tissue Eng Part A. 2010;16:1271. doi: 10.1089/ten.TEA.2009.0328. [DOI] [PubMed] [Google Scholar]
- 22.Bessa PC, Balmayor ER, Hartinger J, Zanoni G, Dopler D, Meinl A, Banerjee A, Casal M, Redl H, Reis RL, van Griensven M. Silk fibroin microparticles as carriers for delivery of human recombinant bone morphogenetic protein-2: in vitro and in vivo bioactivity. Tissue Eng Part C Methods. 2010;16:937. doi: 10.1089/ten.TEC.2009.0486. [DOI] [PubMed] [Google Scholar]
- 23.Murphy AR, Kaplan DL. Biomedical applications of chemically-modified silk fibroin. J Mater Chem. 2009;19:6443. doi: 10.1039/b905802h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6:1612. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao PH, Yodmuang S, Wang X, Sun L, Kaplan DL, Vunjak-Novakovic G. Silk hydrogel for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:84. doi: 10.1002/jbm.b.31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu F, Cao X, Zeng L, Zhang Q, Chen X. An interpenetrating HA/G/CS biomimic hydrogel via Diels-Alder click chemistry for cartilage tissue engineering. Carbohydr Polym. 2013;97:188. doi: 10.1016/j.carbpol.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, Qing H, Fan HS, Zhang XD. Reinforcement and chemical cross-linking in collagen-based scaffolds in cartilage tissue engineering: a comparive study. Iraninan Polymer Journal. 2013;22:833. [Google Scholar]
- 30.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15:1155. [Google Scholar]
- 31.Ronken S, Wirz D, Daniels AU, Kurokawa T, Gong JP, Arnold MP. Double-network acrylamide hydrogel compositions adapted to achieve cartilage-like dynamic stiffness. Biomech Model Mechanobiol. 2013;12:243. doi: 10.1007/s10237-012-0395-6. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal A, Rahbar N, Calvert PD. Strong fiber-reinforced hydrogel. Acta Biomater. 2013;9:5313. doi: 10.1016/j.actbio.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Cha C, Soman P, Zhu W, Nikkhah M, Camci-Unal G, Chen S, Khademhosseini A. Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds. Biomater Sci. 2014;2:141. doi: 10.1039/C3BM60210A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou C, Wu Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf B Biointerfaces. 2011;84:155. doi: 10.1016/j.colsurfb.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh A, Zahir T, Lapitsky Y, Amsden B, Wan W, Shoichet MS. Hydrogel/electrospun fiber composites influence neural stem/progenitor cell fate. Soft Matter. 2010;6:2227. [Google Scholar]
- 36.Mandal BB, Grinberg A, Gil ES, Panilaitis B, Kaplan DL. High-strength silk protein scaffolds for bone repair. Proc Natl Acad Sci U S A. 2012;109:7699. doi: 10.1073/pnas.1119474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albro MB, Rajan V, Li R, Hung CT, Ateshian GA. Characterization of the concentration-dependence of solute diffusivity and partitioning in a model dextran-agarose transport system. Cell Mol Bioeng. 2009;2:295. doi: 10.1007/s12195-009-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 40.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 41.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 42.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed M, Uskanm MS, Ahsa Q, Islam MM. Silk Fibers and their unidirectional polymer composite. In: Thomas S, Ninan N, Mohan Sneha, Elizabeth Francis, editors. Natural Polymers, Biopolymers, Biomaterials, and Their Composites, Blends, and IPNs. Advances in Materials Science. Ontario: Apple Academic Press; 2013. pp. 80–90. [Google Scholar]
- 44.Vunjak-Novakovic G, Goldstein S. Biomechanical principles of cartilage and bone tissue engineering. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics & Mechanobiology. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 363–365. [Google Scholar]
- 45.Huang T, Xu H, Jiao K, Zhu L, Brown HR, Wang H. A novel hydrogel with high mechanical strength: a macromolecular microsphere composite hydrogel. Adv Mater. 2007;19:1622. [Google Scholar]
- 46.Marshall DB, Cox BN. The mechanics of matrix cracking in brittle-matrix fiber composit. Acta Metallurgica. 1985;33:2013. [Google Scholar]
- 47.Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, Kawano S. High mechanical strength double-network hydrogel with bacterial cellulose. Advanced Functional Materials. 2004;14:1124. [Google Scholar]
- 48.Coburn J, Gibson M, Bandalini PA, Laird C, Mao HQ, Moroni L, Seliktar D, Elisseeff J. Biomimetics of the extracellular matrix: an integrated three-dimensional fiber-hydrogel composite for cartilage tissue engineering. Smart Struct Syst. 2011;7:213. doi: 10.12989/sss.2011.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, Petite H, Sedel L, Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13:87. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 50.Lee SM. Handbook of Composite Reinforcements. Weinheim: VCH Verlag; 1992. p. 91. [Google Scholar]
- 51.Cao Z, Gilbert RJ, He W. Simple agarose-chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules. 2009;10:2954. doi: 10.1021/bm900670n. [DOI] [PubMed] [Google Scholar]
- 52.Hopkins AM, Laporte LD, Tortelli F, Spedden E, Staii C, Atherton TJ, Hubbell JA, Kaplan DL. Silk hydrogels as soft substrates for neural tissue engineering. Advanced Functional Materials. 2013;23:5140. [Google Scholar]
- 53.Woodfield TB, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005;11:1297. doi: 10.1089/ten.2005.11.1297. [DOI] [PubMed] [Google Scholar]
- 54.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 56.Hoemann CD, Sun J, Legare A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13:318. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 59.Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Fang JY, Chen JP, Leu YL, Wang HY. Characterization and evaluation of silk protein hydrogels for drug delivery. Chem Pharm Bull (Tokyo) 2006;54:156. doi: 10.1248/cpb.54.156. [DOI] [PubMed] [Google Scholar]
- 61.Wenk E, Merkle HP, Meinel L. Silk fibroin as a vehicle for drug delivery applications. J Control Release. 2011;150:128. doi: 10.1016/j.jconrel.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Hofmann S, Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111:219. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Meinel L, Kaplan DL. Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 2012;64:1111. doi: 10.1016/j.addr.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67:559. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]