Abstract
The fungus Candida albicans is a benign member of the mucosal microbiota, but can cause mucosal infections and life-threatening disseminated invasive infections in susceptible individuals. The ability to switch between yeast, pseudohyphal, and hyphal growth forms (polymorphism) is one of the most investigated virulence attributes of C. albicans. Recent studies suggest that hyphal development in C. albicans requires two temporally linked regulations for initiation and maintenance of the hyphal transcriptional program. Hyphal initiation requires a rapid but temporary disappearance of the Nrg1 transcriptional repressor of hyphal morphogenesis. Hyphal maintenance requires active sensing of the surrounding environment, leading to exclusion of Nrg1 binding to promoters of hypha-specific genes or reduced NRG1 expression. We discuss recent advances in understanding the complex transcriptional regulation of hyphal gene expression. These provide molecular mechanisms underpinning phenotypic plasticity of C. albicans polymorphism.
Keywords: Candida albicans, hyphal initiation, hyphal elongation, removing Nrg1 repression
Yeast and hyphal forms of Candida albicans
Candida albicans is a common opportunistic fungal pathogen of humans. It asymptomatically colonizes the skin and mucosal surfaces of most healthy individuals [1, 2]. However, alterations in host immunity, physiology, and/or microbiota can lead to the inability to control C. albicans colonization on mucosal surfaces and the development of disease [3]. Disseminated invasive candidiasis has an estimated mortality rate of 40%, even with the use of antifungal drugs[4, 5]. With the limited types of antifungal drugs available and rising populations of susceptible patients, there is a pressing need for understanding mechanisms of Candida pathogenesis in order to develop new approaches for treating invasive candidiasis.
A defining feature of C. albicans is its ability to grow either as a unicellular budding yeast or in filamentous forms [2]. Unlike dimorphic fungal pathogens of humans (e.g. Histoplasma capsulatum, Paracoccidioides brasiliensis and Penicillium marneffei) that normally grow in filamentous forms outside the human body but convert to yeast form in human tissues [6], C. albicans is able to switch reversibly between yeast, pseudohyphae, and hyphal growth forms, and is found in both yeast and filamentous forms in the host [7]. The morphological plasticity of C. albicans is a critical virulence determinant. The hyphal form plays key roles in the infection process, and can promote tissue penetration and escape from immune cells [8, 9]. Hyphal morphogenesis is coupled with virulence, as genes that control hyphal morphology are co-regulated with genes encoding virulence factors. Hypha-specific genes UME6 and HGC1 are regulators of hyphal transcription and morphogenesis. Levels of the transcription factor Ume6 control the levels and duration of hypha-specific transcription [10–12]. Dosage studies of Ume6 suggest that pseudohyphae are an intermediate state between yeast cells and hyphae, rather than a distinct fate [12]. Hyphal G1-type cyclin1 (Hgc1)-Cdc28 is responsible for olarized growth at the hyphal tips and cell chain formation [13–20]. How polarized growth is initiated and maintained during C. albicans hyphal development is comprehensively reviewed [21]. Hypha-specific genes HWP1, ALS3, and RBT5 encode cell wall proteins that are important for adhesion to host cells and iron acquisition from the host [22–25].
The yeast-to-hypha transition is triggered by many nutritional and environmental cues, including serum [26], N-acetylglucosamine (GlcNAc) [27], neutral pH [28], high temperature, nutrient starvation [29], hypoxia, CO2 [30], and adherence [31]. Many of the strong hyphal inducing signals are sensed and integrated at the adenylate cyclase Cyr1, which is essential for hyphal development under all hyphal induction conditions [32–35]. The target of Cyr1 is cAMP-dependent protein kinase A (PKA). The cAMP-PKA pathway and additional signaling pathways that operate to promote the yeast-to-hypha transition, and the transcription factors that are targeted by these pathways have been thoroughly reviewed [35–39]. This review concentrates on recent findings that provide molecular mechanisms for phenotypic plasticity and signal integration in the transcriptional regulation of hyphal development in C. albicans. We emphasize the finding that hyphal development involves two temporally linked regulations: initiation and maintenance. Key signaling pathways and transcription factors important in hyphal initiation and maintenance will also be discussed.
Hyphal initiation and maintenance: two phases of removing Nrg1 inhibition
Hypha-specific gene expression is negatively regulated by a complex consisting of the general transcriptional corepressor Tup1 in association with the transcriptional repressor Nrg1 [40–43]. Cells lacking either of the two repressors constitutively grow as long pseudohyphae, and the expression of hypha-specific genes is derepressed. Ectopic expression of NRG1 inhibits hyphal filamentation in all in vitro growth conditions, and also during invasive infection, leading to attenuated virulence in a systemic infection model [44, 45]. The significance of Nrg1 as the key transcriptional repressor of the hyphal transcriptional program is underscored by phenotypic profiling of 143 transcriptional regulator knockout mutants, where only nrg1 and tup1 mutants are filamentous under all conditions examined [46]. Therefore, removing the transcriptional repression by Nrg1 should lead to the yeast-to-hypha transition in C. albicans. Indeed, Nrg1 is at the promoters of hypha-specific genes to repress their expression during yeast growth. Upon hyphal induction, Nrg1 dissociates rapidly from the promoters and remains at low levels during hyphal elongation [29]. Nrg1 protein levels decrease sharply during the first 30 min upon hyphal induction at 37°C, coinciding with germ tube formation and disappearance of the Nrg1 protein from the promoter of hypha-specific genes [29]. Interestingly, the Nrg1 protein level recovers after 1 h of hyphal induction, but the level of Nrg1 protein at the promoters of hypha-specific genes remain low during hyphal development [29]. The temporary disappearance of Nrg1 is essential for hyphal induction, as ectopically expressing Nrg1 blocks germ tube formation even under robust hyphal induction conditions [29]. A shift in temperature to 37°C and inoculation of a small amount of cells from a saturated culture into fresh medium is sufficient for the rapid clearance of Nrg1. Other hyphal induction conditions, such as serum and starvation, are not essential for the disappearance of Nrg1 during hyphal initiation. Instead, they are critical for excluding Nrg1 from promoters when Nrg1 protein levels recover during hyphal elongation [29]. Therefore, hyphal development involves two phases of removing Nrg1 repression: (i) for initiation and (ii) for maintenance. Initiation requires a transient downregulation of the Nrg1 protein level, whereas maintenance requires a regulation that prevents Nrg1 from binding at the promoters of hypha-specific genes.
Hyphal initiation requires two independent mechanisms of downregulating Nrg1
Hyphal initiation requires the temperature of 37°C and inoculation of a small amount of cells from saturated cultures into a fresh medium under most in vitro conditions. Under the induction condition of 37°C and inoculation, Nrg1 disappears rapidly through transcriptional downregulation of NRG1 and degradation of Nrg1 (Figure 1). The decrease in NRG1 expression is dependent on the cAMP-PKA pathway because the adenylyl cyclase Cyr1 or Tpk2 (a catalytic subunit of the PKA) is required for reduced NRG1 expression during hyphal initiation [47]. CYR1 in C. albicans is essential for hyphal formation but not yeast-form growth [32, 48]. Cyr1 stimulates cAMP production, which then activates protein kinase A (PKA) [35]. There are two catalytic subunits of PKA, Tpk1 and Tpk2 [49, 50]. Deletion of TPK2 impairs hyphal development in liquid media. The requirement of Cyr1 and Tpk2 for hyphal development in all media conditions is consistent with their necessity for downregulation of NRG1 transcription during hyphal initiation. In fact, the major function of Tpk2 in hyphal development is to downregulate Nrg1, as the tpk2 nrg1 double mutant is constitutively filamentous similar to the nrg1 single mutant [47]. The transcription factors Efg1 and Flo8, believed to function downstream of the cAMP-PKA pathway in hyphal development [51, 52], are required for the downregulation of NRG1 expression [29]. The temperature of 37°C is a requirement of the observed transcriptional downregulation of NRG1 during hyphal initiation. Elevated temperature seems to be sensed by heat shock protein 90 (Hsp90), which inhibits hyphal development, as pharmacological inhibition of Hsp90 by geldanamycin leads to hyphal growth [53]. Hsp90 signaling requires an intact cAMP pathway, as a mutation in any of the cAMP-PKA pathway components blocks the hypha-inducing effects of Hsp90 inhibition. But this data does not exclude the possibility that Hsp90 functions in parallel with the cAMP-PKA pathway [53, 54].
Figure 1.
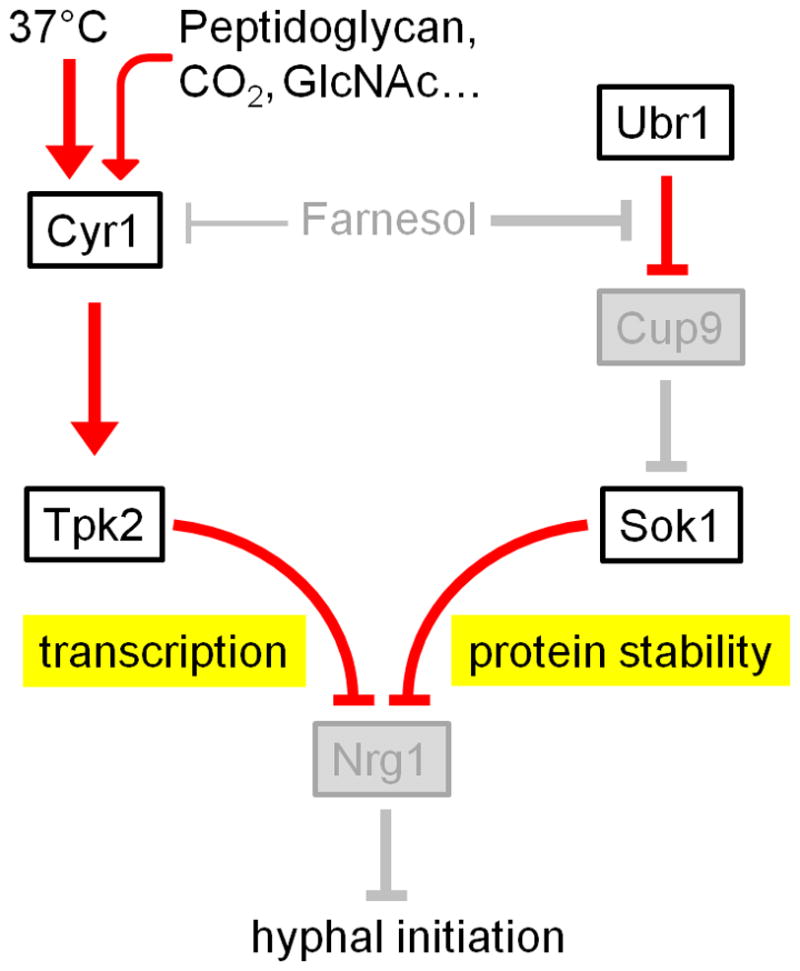
A schematic diagram depicting the two independent pathways involved in downregulation of Nrg1 protein during hyphal initiation. NRG1 transcriptional downregulation requires the activation of the cAMP-PKA pathway, whereas Nrg1 protein degradation requires release from farnesol inhibition. The function of genes indicated by white boxes is activated, and the function of genes in gray boxes is repressed. Red lines represent active regulatory relationships; gray lines represent relationships that are inactive.
Nutrients and other conditions affect the robustness of hyphal initiation and Nrg1 downregulation [29]. For example, the timing of hyphal initiation and Nrg1 disappearance is slower in medium with mannitol than that with glucose, consistent with the activation of the cAMP pathway by glucose. In addition, Cyr1 is known to integrate signals that induce hyphal development, such as N-acetylglucosamine, CO2, or the bacterial peptidoglycan found in serum [30, 55, 56]. Although these signals are not essential for the downregulation of Nrg1 during hyphal initiation induced by 37°C and inoculation, they can increase the robustness of hyphal initiation and may even bypass the need for inoculation or temperature upshift. Molecular mechanisms for the downregulation of NRG1 transcription are not known and likely complex. A recent publication suggests the involvement of an antisense NRG1 transcript in the downregulation of NRG1 transcript levels during hyphal development [57]. Further experiments are needed to elucidate how the cAMP-PKA pathway and its downstream transcriptional regulators control the downregulation of NRG1 transcription during hyphal initiation. It is also necessary to determine if and how other signals, such as temperature and pH, control hyphal initiation by downregulating NRG1 transcript levels.
In addition to 37°C growth temperature or nutrient signals, the inoculation procedure is another requirement for hyphal initiation in vitro. It releases cells from inhibition by farnesol, a quorum-sensing molecule in C. albicans that can inhibit germ-tube formation [58]. Farnesol is thought to block hyphal initiation by inhibiting the Ras1-Cyr1 pathway [39, 59]. However, the expression level of NRG1 is still dramatically reduced in the presence of farnesol [47]. The major function of farnesol is to inhibit Nrg1 degradation and this regulation is independent of the cAMP-PKA pathway. During inoculation, when cells are released from farnesol inhibition, the Cup9 transcriptional repressor is degraded [47]. Cup9 is a homeodomain-containing transcriptional repressor, and is degraded by the N-end rule E3 ligase Ubr1 [47, 60]. It is not clear how Ubr1 senses farnesol to regulate Cup9 degradation. In Saccharomyces cerevisiae, Cup9 degradation is regulated by a conformational change of Ubr1, triggered by binding with dipeptides [61]. It is possible that farnesol adopts a similar mechanism to inhibit the binding of Ubr1 to Cup9 in C. albicans. Additional experiments are needed to uncover the molecular mechanism of the Ubr1-mediated Cup9 degradation by farnesol. The rapid degradation of Cup9 transiently derepresses the expression of Sok1 to promote Nrg1 protein degradation. Deletion of SOK1 inhibits hyphal initiation and Nrg1 degradation upon inoculation, and overexpression of SOK1 can overcome farnesol-mediated inhibition of germ-tube formation. Therefore, release from farnesol inhibition triggers Nrg1 degradation through transient expression of SOK1 [47]. The major function of Sok1 is to downregulate Nrg1, as the sok1 nrg1 double mutant is similar in phenotype to the nrg1 single mutant [47]. In addition to farnesol, other quorum-sensing molecules may also regulate hyphal development of C. albicans. For example, the quorum-sensing molecule 3-oxo-C12-homoserine lactone, which is secreted by Pseudomonas aeruginosa, can also inhibit the yeast-to-hyphal transition [58, 62, 63]. Altogether, these results demonstrate that NRG1 transcriptional downregulation requires the cAMP-PKA pathway, whereas release from farnesol inhibition during inoculation triggers Nrg1 degradation. The two pathways are both required for rapid clearing of Nrg1 to initiate hyphal development.
In addition to the Nrg1-controlled hyphal transcriptional program, post-transcriptional regulations during hyphal initiation have been found important for the initiation of polarized growth [16, 20]. Future research should identify additional post-transcriptional regulations that are necessary for hyphal initiation, but are independent of the hyphal transcriptional program.
Hyphal maintenance in air requires Brg1- and Hda1-mediated chromatin remodeling
Unlike hyphal initiation, hyphal maintenance requires active sensing of the surrounding environment. After hyphal initiation, Nrg1 protein levels increase gradually, and return to the levels similar to that in yeast cells. However, Nrg1 is excluded from hyphal promoters to sustain hyphal development (Figure 2A, I). Cells deleted of Hda1, a class II histone deacetylase (HDAC) [64], are unable to maintain hyphal growth [29]. Hda1 is recruited to the hyphal promoters during hyphal elongation in response to environmental signals known to sustain hyphal development. With the exception of serum in YPD, media that favor sustained hyphal development are often nutrient-poor, such as Lee’s medium [65], Spider medium (with mannitol as a carbon source) [66], and mammalian tissue culture media M199. Treatment of C. abicans cells with a sub-lethal level of rapamycin in a rich medium mimics a nutrient-poor medium and induces robust hyphal elongation [29]. Rapamycin inhibits Tor1 kinase, a central regulator of cell growth in response to nitrogen and amino acid availability in yeast [67] and is conserved in C. albicans [68].
Figure 2.
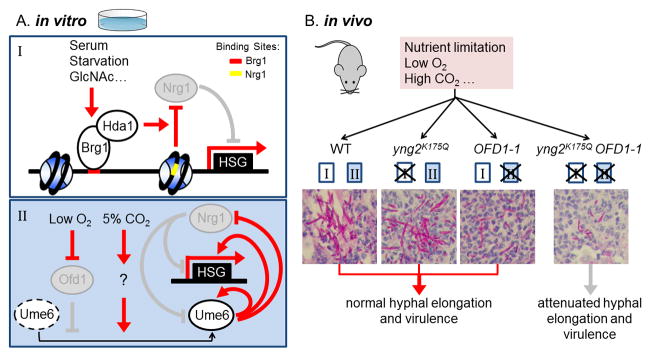
Two parallel pathways control hyphal elongation and virulence during invasive infection. (A) Hyphal maintenance in vitro is controlled by two pathways: (I) Brg1-mediated chromatin remodeling and (II) Ume6 stabilization in hypoxia plus 5% CO2. Red lines represent active regulatory relationships; gray lines represent relationships that are inactive. Dashed circles represent degraded proteins. (B) Synergy between two hyphal elongation pathways for C. albicans pathogenesis. Virulence is attenuated only when both hyphal elongation pathways are blocked. Kidney tissues from the mice infected with the indicated strains were fixed, sectioned, and stained to visualize fungal cells. The rectangles in (B) represent the two hyphal elongation pathways of corresponding colors in (A). The images from the mouse kidneys in (B) are from [88]. Abbreviations: HSG, hypha-specific genes; WT, wildtype.
The major function of Hda1 in hyphal development is to deacetylate Yng2. Yng2 is a subunit of NuA4 histone acetyltransferase (HAT) module [69]. Hda1 deacetylates Yng2 at K175, leading to Yng2 degradation. This regulation of Yng2 is critical for blocking Nrg1 binding to the promoters and sustaining hyphal elongation in vitro. Substituting K175 with glutamine (K175Q, a mutation mimicking constitutive acetylation) results in defective hyphal maintenance under all media known to support prolonged hyphal development [29]. Conversely, the yng2K175R mutant (a mutant mimicking the constitutive deacetylation state of Yng2) completely bypasses the requirement of Hda1 in hyphal elongation [29]. In addition to Hda1, the Set3/Hos2 histone deacetylase complex has been shown to inhibit the yeast-to-filament transition and modulate transient expression changes of key transcription factors that influence morphogenesis [70, 71]. Therefore, not just DNA-binding transcription factors, but also chromatin-modifying enzymes, play critical roles in the regulation of hyphal transcriptional program in C. albicans.
Brg1, a GATA family transcription factor, is required for both biofilm formation and hyphal elongation in C. albicans [57, 72–74]. Through a forward genetic screen, Brg1 was identified as the transcription factor that recruits Hda1 to promoters of hypha-specific genes for chromatin remodeling, leading to occlusion of Nrg1 binding during hyphal elongation [72]. BRG1 expression requires both the removal of Nrg1 and a sub-growth inhibitory level of rapamycin; therefore, it is a sensitive readout of Tor1 signaling [72, 75]. Overexpression of Brg1 sustains hyphal development at 37°C in the absence of environmental signals for hyphal elongation [72], indicating that hyphal development is maintained through activation of Brg1 expression. Brg1 expression is activated by several hypha-inducing conditions, including rapamycin [72]. Reduced Tor1 signaling lowers the basal activity of the HOG (high osmolarity) mitogen-activated protein kinase (MAPK) to activate BRG1 expression. Hog1 is activated by osmotic stress, oxidative stress, and heavy metal stress, and is required for the survival of C. albicans cells when they encounter these stresses [76–79]. In contrast to stress-induced rapid Hog1 activation, rapamycin treatment leads to a downregulation of Hog1 basal activity for a prolonged period of time through the functions of the two Hog1 tyrosine phosphatases, Ptp2 and Ptp3, leading to the activation of BRG1 expression [75]. In addition, the Set3/Hos2 complex also modulates the transcription kinetics of BRG1 during hyphal development [71]. Brg1 sustains hyphal elongation by prolonging Ume6 expression. UME6 expression is dependent on Brg1 and Hda1. Ectopically expressing Ume6 rescues the hyphal growth defect of the brg1 and hda1 mutants [72]. Therefore, hyphal elongation in response to nutrient limitation is maintained through the activation of BRG1 expression, which in turn activates UME6 expression. Transcriptional regulation of BRG1 or UME6 expression is critical for sustained hyphal development. Their regulations are likely complex considering that both genes have long upstream sequences. Future research should determine if and how different signaling pathways and transcriptional regulators, such as Eed1 [80, 81], Sfl2 [82, 83], Cph2 [84], and Rim101 [85, 86], converge to regulate their expression. In addition to transcriptional regulation, both BRG1 and UME6 transcripts contain a long 5′ untranslated region (UTR). The 5′ UTR of UME6 has recently been found to regulate Ume6 translational efficiency [87]. Considering that transcripts of many hyphal regulators have a long 5′ UTR, translational regulation may be another level of regulation important for polymorphism that awaits further investigation.
Hyphal elongation in hypoxia and high CO2 is maintained by stabilizing Ume6
Hda1-mediated deacetylation of Yng2 at K175 is essential for hyphal extension in vitro. However, the yng2K175Q mutant is not defective in virulence and hyphal elongation during disseminated infection in mice [88]. Thus, conditions to which C. albicans is exposed within the host must activate a signaling pathway that is independent of the Hda1-mediated hyphal elongation pathway. In the foci of infection, fungal cells are exposed to both hypoxia and hypercarbia relative to standard in vitro growth conditions. Indeed, hypoxia together with 5% CO2, but neither condition alone, maintains hyphal development. This condition bypasses the brg1 or yng2K175Q, but not ume6 mutant for hyphal elongation [88]. Ume6 is continuously degraded in air, partially stabilized in either low oxygen or high CO2, but is completely stable under low oxygen combined with 5% CO2 [88]. Similar to Ume6, Hgc1 is also stabilized only in both hypoxia and 5% CO2, suggesting that C. albicans uses a common pathway to stabilize hyphal regulators in hypoxia plus high CO2. Stable Ume6 can activate its own expression and repress NRG1, thus bypassing the requirement for Brg1 and Hda1 in hyphal maintenance (Figure 2A,II).
Ofd1, a prolyl 4-hydroxylase-like 2-oxoglutarate-Fe(II) dioxygenase, is an oxygen sensor [88, 89]. Deletion of Ofd1 in C. albicans results in stabilization of Ume6 in 5% CO2, but not in air [88]. This indicates that Ofd1 senses oxygen concentration to regulate Ume6 stability; but in parallel to Ofd1, an additional regulator(s) that senses high CO2 may exist and further stabilize Ume6. CO2 has been shown to regulate hyphal morphogenesis through the activation of the adenylyl cyclase Cyr1, resulting in activation of the cAMP-PKA pathway [30]. Stabilization of Ume6 by CO2 is likely mediated through a Cyr1-independent pathway, as CO2 and hypoxia promote hyphal elongation, not initiation. Ofd1 has two functionally distinct domains: the N-terminal dioxygenase domain is required for oxygen sensing, and inhibits the activity of the C-terminal degradation domain in an O2-dependent manner. Removal of the N-terminal dioxygenase domain creates a constitutively active Ofd1 (designated OFD1–1) that is no longer inhibited by hypoxia. Ectopically expressing OFD1–1 leads to Ume6 degradation and impaired hyphal elongation even in hypoxia with 5% CO2. However, OFD1–1 has no effect in hyphal elongation in rapamycin-containing media in air. Conversely, yng2K175Q mutants are defective in hyphal elongation in air, but not in hypoxia plus 5% CO2 [88]. Therefore, C. albicans employs two different strategies to maintain hyphal elongation in air versus hypoxia. Disrupting one pathway blocks hyphal elongation only in response to its corresponding inducing conditions.
Two parallel pathways control hyphal elongation and virulence during disseminated infection
Ofd1-mediated regulation also functions in parallel to the Brg1-Hda1 pathway in controlling hyphal elongation and virulence in vivo (Figure 2B). The OFD1–1 single mutant does not show a defect in hyphal maintenance and virulence compared to wild-type OFD1 during disseminated infection; but the yng2K175QOFD1–1 double mutant displays a profound defect in hyphal elongation and attenuated virulence in comparison to the yng2K175Q single mutant [88]. Therefore, hyphal elongation in vivo is regulated by two parallel pathways that share overlapping functions in hyphal elongation and pathogenesis. Virulence and hyphal elongation in vivo are attenuated only when both pathways are blocked. This synergy between two pathways of hyphal elongation for virulence indicates that nutrient limitation, as well as hypoxia and high CO2, must all exist at the same time during the disseminated infection. The multitude of host signals and the redundancy for hyphal regulation may explain why some C. albicans mutants have profound defects in hyphal formation and elongation in vitro, yet have normal virulence in mice [90]. These findings suggest that C. albicans can sense multiple host conditions through parallel pathways to promote hyphal elongation and pathogenicity during systemic infections.
Temporal connection between hyphal initiation and maintenance
Temporal regulation of cell fate by different signaling pathways is common in development of organisms. For hyphal development in C. albicans, the two phases of regulation for initiation and maintenance are temporally linked. Nrg1 removal during hyphal initiation is a prerequisite for the subsequent Brg1-Hda1 mediated hyphal maintenance. Moreover, adding serum or rapamycin after 2 h of hyphal induction showed no effect on hyphal elongation [29]. Therefore, the time period of reduced Nrg1 during hyphal initiation can be viewed as a window of opportunity for establishing the sustained hyphal transcription program (Figure 3). The dynamic change of nucleosome positions during yeast-to-hypha transition determines promoter accessibility to Nrg1 and Brg1 in yeast and hyphal states, which establishes the temporal connection between hyphal initiation and maintenance. In yeast cells, the Nrg1 binding site is located in the nucleosome free region in the middle of the UAS region on the HWP1 promoter, whereas the Brg1 binding site is occupied by a nucleosome [72]. Removal of Nrg1 during hyphal initiation leads to rapid nucleosome disassembly and repositioning so both Brg1 and Nrg1 binding sites are accessible. Nrg1 also represses BRG1 expression. Removal of Nrg1 during initiation allows the activation of BRG1 expression in response to environmental signals that promote hyphal elongation. Therefore, during this time window, accumulated Brg1 can recruit Hda1 to promoters of hypha-specific genes to reposition nucleosomes, leading to obstruction of Nrg1 binding sites and sustained hyphal development. The removal of Nrg1 repression during hyphal initiation also allows transient expression of hypha-specific genes, including UME6. If cells are under hypoxia and high CO2 condition during this time window, Ume6 is then stabilized and further activates its own transcription and represses NRG1 expression. These positive feedback loops sustain cells in the hyphal form.
Figure 3.
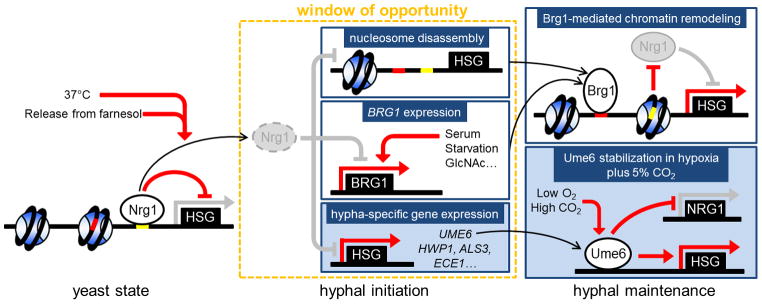
Temporal connection between hyphal initiation and maintenance. The transient disappearance of Nrg1 during hyphal initiation provides a time window to establish the hyphal maintenance program. The function of proteins in white circles is activated, and the function of proteins in gray circles is repressed. Dashed circles represent degraded proteins. Red lines represent active regulatory relationships; gray lines represent relationships that are inactive. Black arrows represent the connection between each state of hyphal development. Abbreviations: HSG, hypha-specific genes.
The temporal link between hyphal initiation and elongation provides underlying mechanisms for the plasticity of polymorphism observed in C. albicans, and how cells can simultaneously grow in both yeast and hyphal forms in the same culture or at the same site in the host. In order to initiate hyphal transcription, Nrg1 must be temporarily removed. Under in vitro conditions, the timing, duration, and extent of Nrg1 downregulation correlates with the timing and efficiency of hyphal initiation, and are sensitive to multiple factors, including the growth state and inoculum size, media, and temperature. The combination of temperature of 37°C and releasing from farnesol inhibition is sufficient to induce robust and synchronous hyphal initiation and temporary disappearance of Nrg1. Because the sustained hyphal transcriptional program can only be established during the absence of Nrg1, duration of the low Nrg1 period in some cells may not be long enough to accumulate enough Brg1 for the Hda1-mediated chromatin remodeling, or accumulate enough Ume6 under hypoxia and high CO2 condition. These cells will grow as yeast. Other cells that have a window of opportunity sufficient to establish the hyphal transcription program will develop into hyphae. Therefore, the different length of window of opportunity in each cell can lead to cell-to-cell variation in hyphal development in a given culture, and quality of the initial hyphal induction can affect the fate of hyphal development. Furthermore, duration of hyphal development is determined by growth environments. Hyphal cells continue to grow as hyphae under hypoxia and high CO2 or nutrient starvation, but convert to yeast when nutrients are replete. These regulations provide underlying mechanisms for the plasticity of polymorphism.
Concluding remarks and future directions
The yeast-to-hyphal transition of C. albicans is linked to a number of properties important for its interactions with the host: adhesion to epithelial and endothelial cells; primary and intercellular invasion via induced endocytosis and active penetration; and escape from phagocytes and immune evasion. The capacity of C. albicans to reversibly switch between yeast, pseudophal and hyphal morphologies is widely believed to be essential for pathogenicity at both superficial and systemic levels. Recent findings reviewed here provide molecular mechanisms for plasticity of polymorphism in C. albicans. Despite the recent advances in our understanding of C. albicans polymorphism in vitro, little is known about temporal-dynamic regulation of C. albicans polymorphism in the host. Future studies are needed to determine morphologies of C. albicans during colonization and infection, and identify host signals that control hyphal initiation and elongation in different host niches (Box 1). Future experiments should also address how these host signals are sensed by C. albicans. Additional studies are also needed to integrate the new and known signaling pathways into the recently identified pathways that repress Nrg1 for hyphal initiation and elongation. In addition, C. albicans may also employ Nrg1-independent regulations to control polymorphism, and this remains to be addressed. Besides studying the regulation of polymorphism and understanding how host environment influences C. albicans growth forms, it is also important to learn more about the roles of the yeast, pseudohyphal and hyphal growth forms in pathogenesis and commensal colonization. We predict that studies along these lines will provide insights on mechanisms that control the yeast-to-hypha transition in the host. Targeted inhibition of this morphological switch should provide an alternative approach to current antifungals for controlling C. albicans infections.
Box 1. Outstanding questions.
What are the morphologies of C. albicans and the signaling pathways that control the morphologies during colonization and infection?
What signals control hyphal initiation and elongation in different host niches?
How are host signals sensed by C. albicans?
How are the signaling pathways integrated to downregulate or repress Nrg1 for hyphal initiation and elongation?
Are there Nrg1-independent regulations that control hyphal development?
What are the roles of the yeast and hyphal growth forms in pathogenesis and commensal colonization?
Highlights.
Hyphal development requires two phases of regulation to remove Nrg1 inhibition.
Nrg1 removal upon activation of cAMP and release from farnesol initiate hyphal growth.
Chromatin regulation and Ume6 stability act in parallel for hyphal elongation in vivo.
Hyphal initiation and maintenance are temporally linked.
Acknowledgments
Research in the authors’ laboratory is supported by the National Institutes of Health grants R01GM/AI55155 and R01AI099190 to H.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 2.Odds FC. Candida and candidosis. Bailliere Tindall; 1988. [Google Scholar]
- 3.Kirkpatrick CH. Chronic mucocutaneous candidiasis. J Am Acad Dermatol. 1994;31:S14–17. doi: 10.1016/s0190-9622(08)81260-1. [DOI] [PubMed] [Google Scholar]
- 4.Kullberg BJ, Filler SG. Candidemia. In: Calderone RA, editor. Candida and Candidiasis. ASM Press; 2002. pp. 327–340. [Google Scholar]
- 5.Filler SG, Kullberg BJ. Deep-seated candidal infections. In: Calderone RA, editor. Candida and candidiasis. ASM Press; 2002. pp. 341–348. [Google Scholar]
- 6.Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol. 2007;10:314–319. doi: 10.1016/j.mib.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romani L, et al. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr Opin Microbiol. 2003;6:338–343. doi: 10.1016/s1369-5274(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 8.Dalle F, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MC, et al. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee M, et al. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell. 2008;19:1354–1365. doi: 10.1091/mbc.E07-11-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeidler U, et al. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res. 2009;9:126–142. doi: 10.1111/j.1567-1364.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 12.Carlisle PL, et al. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci U S A. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng X, et al. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. Embo J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng XD, et al. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 2007;26:3760–3769. doi: 10.1038/sj.emboj.7601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang A, et al. Hyphal chain formation in Candida albicans: Cdc28-Hgc1 phosphorylation of Efg1 represses cell separation genes. Mol Cell Biol. 2009;29:4406–4416. doi: 10.1128/MCB.01502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop A, et al. Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. EMBO J. 2010;29:2930–2942. doi: 10.1038/emboj.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Escribano P, et al. CDK-dependent phosphorylation of Mob2 is essential for hyphal development in Candida albicans. Mol Biol Cell. 2011;22:2458–2469. doi: 10.1091/mbc.E11-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Novo A, et al. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol Biol Cell. 2008;19:1509–1518. doi: 10.1091/mbc.E07-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caballero-Lima D, Sudbery PE. In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol Biol Cell. 2014;25:1097–1110. doi: 10.1091/mbc.E13-11-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha I, et al. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell. 2007;13:421–432. doi: 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Arkowitz RA, Bassilana M. Polarized growth in fungi: symmetry breaking and hyphal formation. Seminars in cell & developmental biology. 2011;22:806–815. doi: 10.1016/j.semcdb.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Staab JF, et al. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 23.Almeida RS, et al. the hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008;4:e1000217. doi: 10.1371/journal.ppat.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan QT, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman Z, Kornitzer D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol. 2004;53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- 26.Taschdjian CL, et al. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. AMA journal of diseases of children. 1960;99:212–215. doi: 10.1001/archpedi.1960.02070030214011. [DOI] [PubMed] [Google Scholar]
- 27.Simonetti N, et al. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974;250:344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- 28.Buffo J, et al. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, et al. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol. 2011;9:e1001105. doi: 10.1371/journal.pbio.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klengel T, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DH, Jr, et al. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol. 1999;34:651–662. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- 32.Rocha CR, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahn YS, Sundstrom P. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J Bacteriol. 2001;183:3211–3223. doi: 10.1128/JB.183.10.3211-3223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou H, et al. Candida albicans Cyr1, Cap1 and G-actin form a sensor/effector apparatus for activating cAMP synthesis in hyphal growth. Mol Microbiol. 2009;75:579–591. doi: 10.1111/j.1365-2958.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- 35.Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future microbiology. 2009;4:1263–1270. doi: 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- 36.Sudbery PE. Growth of Candida albicans hyphae. Nature reviews Microbiology. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 37.Huang G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence. 2012;3:251–261. doi: 10.4161/viru.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas S, et al. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiology and molecular biology reviews : MMBR. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan DA, Muhlschlegel FA. Candida albicans developmental regulation: adenylyl cyclase as a coincidence detector of parallel signals. Curr Opin Microbiol. 2011;14:682–686. doi: 10.1016/j.mib.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 41.Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell. 2005;16:2903–2912. doi: 10.1091/mbc.E05-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun BR, et al. NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–4761. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murad AM, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. Embo J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YN, Morschhauser J. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot Cell. 2005;4:1328–1342. doi: 10.1128/EC.4.8.1328-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saville SP, et al. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrobial agents and chemotherapy. 2006;50:3312–3316. doi: 10.1128/AAC.00628-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homann OR, et al. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009;5:e1000783. doi: 10.1371/journal.pgen.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, et al. Quorum sensing controls hyphal initiation in Candida albicans through Ubr1-mediated protein degradation. Proc Natl Acad Sci U S A. 2014;111:1975–1980. doi: 10.1073/pnas.1318690111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harcus D, et al. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bockmuhl DP, et al. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol. 2001;42:1243–1257. doi: 10.1046/j.1365-2958.2001.02688.x. [DOI] [PubMed] [Google Scholar]
- 50.Cloutier M, et al. The two isoforms of the cAMP-dependent protein kinase catalytic subunit are involved in the control of dimorphism in the human fungal pathogen Candida albicans. Fungal genetics and biology : FG & B. 2003;38:133–141. doi: 10.1016/s1087-1845(02)00520-0. [DOI] [PubMed] [Google Scholar]
- 51.Bockmuhl DP, Ernst JF. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics. 2001;157:1523–1530. doi: 10.1093/genetics/157.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao F, et al. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shapiro RS, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shapiro RS, Cowen L. Coupling temperature sensing and development: Hsp90 regulates morphogenetic signalling in Candida albicans. Virulence. 2010;1:45–48. doi: 10.4161/viru.1.1.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu XL, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Huang G, et al. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS pathogens. 2010;6:e1000806. doi: 10.1371/journal.ppat.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cleary IA, et al. BRG1 and NRG1 form a novel feedback circuit regulating Candida albicans hypha formation and virulence. Mol Microbiol. 2012;85:557–573. doi: 10.1111/j.1365-2958.2012.08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hornby JM, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis-Hanna A, et al. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner GC, et al. Peptides accelerate their uptake by activating a ubiquitin-dependent proteolytic pathway. Nature. 2000;405:579–583. doi: 10.1038/35014629. [DOI] [PubMed] [Google Scholar]
- 61.Hu RG, et al. The N-end rule pathway is a sensor of heme. Proc Natl Acad Sci U S A. 2008;105:76–81. doi: 10.1073/pnas.0710568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall RA, et al. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell. 10:1034–1042. doi: 10.1128/EC.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogan DA, et al. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 64.Srikantha T, et al. The histone deacetylase genes HDA1 and RPD3 play distinct roles in regulation of high-frequency phenotypic switching in Candida albicans. J Bacteriol. 2001;183:4614–4625. doi: 10.1128/JB.183.15.4614-4625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KL, et al. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, et al. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 67.Wullschleger S, et al. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Bastidas RJ, et al. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 2009;5:e1000294. doi: 10.1371/journal.ppat.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyon Y, Cote J. The highly conserved and multifunctional NuA4 HAT complex. Current opinion in genetics & development. 2004;14:147–154. doi: 10.1016/j.gde.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Hnisz D, et al. The Set3/Hos2 histone deacetylase complex attenuates cAMP/PKA signaling to regulate morphogenesis and virulence of Candida albicans. PLoS Pathog. 2010;6:e1000889. doi: 10.1371/journal.ppat.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hnisz D, et al. A histone deacetylase adjusts transcription kinetics at coding sequences during Candida albicans morphogenesis. PLoS Genet. 2012;8:e1003118. doi: 10.1371/journal.pgen.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y, et al. A GATA transcription factor recruits Hda1 in response to reduced Tor1 signaling to establish a hyphal chromatin state inCandida albicans. PLoS Pathog. 8:e1002663. doi: 10.1371/journal.ppat.1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du H, et al. Roles of Candida albicans Gat2, a GATA-type zinc finger transcription factor, in biofilm formation, filamentous growth and virulence. PloS one. 2012;7:e29707. doi: 10.1371/journal.pone.0029707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobile CJ, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su C, et al. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol Biol Cell. 2013;24:385–397. doi: 10.1091/mbc.E12-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alonso-Monge R, et al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith DA, et al. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arana DM, et al. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology. 2005;151:1033–1049. doi: 10.1099/mic.0.27723-0. [DOI] [PubMed] [Google Scholar]
- 79.Enjalbert B, et al. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell. 2006;17:1018–1032. doi: 10.1091/mbc.E05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin R, et al. The Candida albicans-specific gene EED1 encodes a key regulator of hyphal extension. PloS one. 2011;6:e18394. doi: 10.1371/journal.pone.0018394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wheeler RT, et al. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spiering MJ, et al. Comparative transcript profiling of Candida albicans and Candida dubliniensis identifies SFL2, a C. albicans gene required for virulence in a reconstituted epithelial infection model. Eukaryot Cell. 2010;9:251–265. doi: 10.1128/EC.00291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song W, et al. Candida albicans Sfl2, a temperature-induced transcriptional regulator, is required for virulence in a murine gastrointestinal infection model. FEMS Yeast Res. 2011;11:209–222. doi: 10.1111/j.1567-1364.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 84.Lane S, et al. The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol Cell Biol. 2001;21:6418–6428. doi: 10.1128/MCB.21.19.6418-6428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis D, et al. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–978. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Barkani A, et al. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol Cell Biol. 2000;20:4635–4647. doi: 10.1128/mcb.20.13.4635-4647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Childers DS, et al. A 5′ UTR-mediated translational efficiency mechanism inhibits the Candida albicans morphological transition. Mol Microbiol. 2014;92:570–585. doi: 10.1111/mmi.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu Y, et al. Synergistic regulation of hyphal elongation by hypoxia, CO(2), and nutrient conditions controls the virulence of Candida albicans. Cell Host Microbe. 2013;14:499–509. doi: 10.1016/j.chom.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hughes BT, Espenshade PJ. Oxygen-regulated degradation of fission yeast SREBP by Ofd1, a prolyl hydroxylase family member. Embo J. 2008;27:1491–1501. doi: 10.1038/emboj.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noble SM, et al. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]


