Abstract
Objective
To evaluate the long-term prognostic impact of resting heart rate (HR) at index myocardial infarction (MI) and during the first year post MI among one-year survivors.
Patients and Methods
The community-based cohort consisted of 1571 patients hospitalized with an incident MI from January 1, 1983 through December 31, 2007 in Olmsted County, Minnesota, who were in sinus rhythm at index MI and had HR measurements on an ECG at index and during the first year after MI. Outcomes were all-cause and cardiovascular deaths.
Results
During a median follow-up of 7.0 years, 627 deaths and 311 cardiovascular deaths occurred. Using patients with HR ≤ 60 beats/minute (bpm) as the referent, long-term all-cause mortality risk increased progressively with increasing HR at index (hazard ratio 1.62; 95% CI 1.25–2.09) and even more with HR during the first year after MI (hazard ratio 2.16; 95% CI 1.64–2.84) for patients with HR > 90 bpm, adjusting for clinical characteristics and beta blocker use. Similar results were observed for cardiovascular mortality [adjusted hazard ratios (95% CI) 1.66 (1.14–2.42) and 1.93 (1.27–2.94) for HR at index and within one year after MI, respectively].
Conclusion
These data from a large MI community cohort indicate that HR is a strong predictor of long-term all-cause and cardiovascular mortality, not only at initial presentation of MI but also during the first year of follow-up.
Keywords: heart rate, mortality, myocardial infarction
INTRODUCTION
Resting heart rate (HR) measured at hospital admission predicts mortality after acute myocardial infarction (MI)1–11 However, during this initial phase, sinus tachycardia can accompany chest pain and/or stress and subside when adrenergic triggers are relieved. Thus, HR measured upon admission is quite dependent on the sympathetic activity and this risk indicator has not gained widespread clinical acceptance, possibly because of the circumstances under which it is measured. A similar risk of death is associated with elevated HR during hospital stay, but there is no study that ascertained HR after hospital dismissal during the first year of MI follow-up. Moreover, no published data evaluating the role of HR measurement in MI patients reflect the experience of a community study with longitudinal follow-up, and the long-term predictive value of HR at the time of the index MI and during the first year of follow-up is unknown. This is important as demonstrating a persistently high risk of death associated with elevated HR after MI during the first year post MI could help clinical risk stratification.
Thus, we sought to evaluate the prognostic impact of resting HR evaluated at the time of the MI and during the first year of follow-up in first year survivors using the Rochester Epidemiology Project, which provides complete ascertainment of all health care events, including out-patient encounters, in disease-based community cohorts. This constitutes a robust platform from which to address these aforementioned gaps in knowledge.
METHODS
Study Setting
In Olmsted County, Minnesota, few providers (chiefly Mayo Clinic and Olmsted Medical Center) deliver nearly all medical care to county residents. With the exception of a higher proportion employed in health care, the characteristics of this population are similar to those of US whites.12 Each provider uses a medical record which captures information for all encounters and can be retrieved because the Mayo Clinic maintains indices based on all diagnoses and procedures as described previously.13–15 Since 1966, similar indices have been implemented for non-Mayo providers through the Rochester Epidemiology Project, resulting in the linkage of medical records from all sources of care. This provides a unique infrastructure to analyze disease occurrence and outcomes at the population level, with comprehensive access to the records of all hospitalizations and outpatient clinic visits. This study received approval from the Mayo Clinic and Olmsted Medical Center institutional review boards along with a waiver of informed consent as it was a chart review study only. We excluded all patients who did not provide authorization for their medical records to be used for research.
Myocardial Infarction Ascertainment
All patients aged 18 years or older hospitalized in Olmsted County for an incident MI between 1983 and 2007 were eligible for the study. MI was defined according to validated criteria including cardiac pain, biomarkers, and Minnesota coding of the electrocardiograms.16,17 The procedures used to assemble the MI incidence cohort and their reliability have been previously described.18,19 Patients not in sinus rhythm at the time of the MI were excluded from the study. To evaluate the prognostic impact of HR in first-year survivors, we excluded patients who died during the first year after MI or who had less than 1 year of follow-up. Patient residency in Olmsted County and incident status of MI were confirmed by complete review of the community medical records.
Patient characteristics that were recorded included demographic data, cardiovascular risk factors and clinical data. Beta blocker use at index MI and at hospital dismissal and reperfusion/revascularization procedures used during the initial MI hospitalization were also recorded.
Resting Heart Rate Ascertainment
Heart rate measurements at index MI and during the first year of follow-up were obtained from the 12-lead electrocardiographic (ECG) recordings retrieved from an electronic ECG database. Heart rate measurement at index MI was obtained from the first available ECG after MI onset with a maximum time frame of 24 hours after MI onset. Heart rate measurement during the first year after index MI was obtained from an ECG at a follow-up visit for clinical evaluation. The ECG closest to 6 months post MI was used, with an allowable range of 3–9 months.
Ascertainment of Death
The primary study endpoint was all-cause death with a secondary endpoint of cardiovascular death. Death was ascertained from several sources. In addition to the deaths noted during clinical care, all death certificates for Olmsted County residents are obtained annually from the county office. The Mayo Clinic registration office records the obituaries and notices of death in the local newspapers. Finally, data on all Minnesota deaths are obtained from the State of Minnesota annually. The American Heart Association categories were used to define cardiovascular deaths.20
Statistical Analysis
Patient characteristics are presented as frequency, mean ± standard deviation (SD) or median (percentiles) as appropriate. Heart rate was divided into the following categories: ≤ 60 beats per minute (bpm), 61–70 bpm, 71–80 bmp, 81–90 bpm, and > 90 bpm. Trends in patient characteristics with HR level at index MI were analyzed with the Mantel-Haenszel chi-square test for categorical variables and linear regression for continuous variables with a 5-level categorical variable representing HR levels. Proportional hazards regression was used to examine the association between outcome (long-term all-cause death and cardiovascular death) and HR categories, unadjusted and adjusted for confounding factors. Heart failure post MI was modeled as a time-dependent covariate. In the models examining the association between HR during the first year of follow-up and long-term mortality, adjustment was also made for participation in cardiac rehabilitation. A propensity score21 for participation was estimated and inverse probability of treatment weights assigned to each subject 22,23(1/P and 1/(1-P) for those who participated and did not participate in cardiac rehabilitation, respectively, where P is the propensity score for participation). Analyses were conducted using weighted proportional hazards regression models. The proportional hazards assumption was tested using scaled Schoenfeld residuals24 and found to be valid. Missing values did not exceed 1% for any variable used in the regression analyses except for HR measurements. In addition to a complete-case analysis, in sensitivity analyses we used multiple imputation analysis25 to account for missing HR values. Ten datasets were created with missing HR values replaced by imputed values. The model used to impute HR values included demographic and clinical variables along with an indicator for death and the cumulative baseline hazard of death approximated by the Nelson-Aalen estimator.26 The results from analyzing the individual imputed datasets were combined using Rubin’s rules.25 All p-values were from two-tailed significance tests with 0.05 selected as the threshold of statistical significance. Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Clinical Characteristics at Index MI
Among the 3227 Olmsted County residents with an incident MI between January 1, 1983 and December 31, 2007, 205 were not in sinus rhythm at index MI and 650 died within the first year post MI or had less than 1 year of follow-up, resulting in 2372 patients eligible for the study. Of these, 205 patients had missing HR during the first 24 hours and during the first year because the ECG was not retained in the record, and 596 patients had missing HR during the first year. Thus, the study included 1571 patients with HR measurements available at index MI and during the first year of follow-up after index MI.
The mean (SD) age at index MI was 65 (14) years and 62% of the patients were men. The mean (SD) HR at the time of the MI admission was 78 (19) bpm. Baseline clinical and demographic characteristics of the 1571 patients included did not differ from those of the 801 patients with missing HR except for Killip class (29% vs. 21% with Killip class > 1, respectively; p<0.001) and reperfusion/revascularization during the MI hospitalization (68% vs. 60%, respectively; p<0.001). Baseline characteristics of the 1571 patients according to HR categories at index MI are summarized in Table 1. Patients with higher HR were older, more likely to be women, and had more hypertension, heart failure and diabetes mellitus. They also had higher systolic blood pressure, higher Killip class and lower estimated glomerular filtration rate. They were less likely to be prescribed beta blockers and were less likely to be reperfused/revascularized during the hospitalization.
Table 1.
Baseline characteristics of first year survivors after myocardial infarction according to heart rate at index MI
| Total N=1571 |
Heart rate (beats/min)
|
||||||
|---|---|---|---|---|---|---|---|
| ≤60 N=304 |
61–70 N=313 |
71–80 N=318 |
81–90 N=257 |
>90 N=379 |
Ptrend | ||
| Age (years), mean±SD | 65±14 | 65±13 | 64±13 | 64±14 | 64±14 | 67±15 | 0.009 |
| Men | 980 (62) | 216 (71) | 213 (68) | 186 (59) | 163 (63) | 202 (53) | <0.001 |
| BMI (kg/m2), mean±SD | 28±8 | 28±12 | 28±5 | 29±6 | 29±7 | 28±6 | 0.26 |
| Former or current smoker | 1010 (64) | 194 (64) | 201 (64) | 205 (65) | 179 (70) | 231 (61) | 0.76 |
| Hyperlipidemia | 726 (46) | 145 (48) | 147 (47) | 141 (45) | 117 (46) | 176 (46) | 0.70 |
| Hypertension | 906 (58) | 161 (53) | 166 (53) | 176 (56) | 161 (63) | 242 (64) | <0.001 |
| Heart failure | 109 (7) | 14 (5) | 16 (5) | 13 (4) | 19 (7) | 47 (12) | <0.001 |
| Diabetes mellitus | 317 (20) | 37 (12) | 44 (14) | 69 (22) | 67 (26) | 100 (26) | <0.001 |
| Systolic blood pressure (mmHg), mean±SD | 148±33 | 137±34 | 146±31 | 152±28 | 151±33 | 153±36 | <0.001 |
| Killip class > 1 | 446 (29) | 72 (24) | 70 (22) | 70 (22) | 79 (31) | 155 (41) | <0.001 |
| Estimated glomerular filtration rate (ml/min per 1.73 m2), mean±SD | 62±21 | 64±18 | 64±20 | 64±21 | 60±24 | 59±22 | <0.001 |
| Beta blocker use at admission | 326 (21) | 74 (24) | 78 (25) | 61 (19) | 47 (18) | 66 (18) | 0.005 |
| Beta blocker use at discharge | 1118 (71) | 222 (73) | 234 (75) | 241 (77) | 178 (70) | 243 (64) | 0.002 |
| Reperfusion/revascularization during hospitalization | 1069 (68) | 232 (76) | 247 (79) | 215 (68) | 168 (65) | 207 (55) | <0.001 |
SD = standard deviation; BMI = body mass index; MI = myocardial infarction.
Results presented as n (%) unless otherwise specified. Missing values were less than 1%.
Heart Rate at Index and Long-term Mortality after MI
Patients had a median (25th–75th percentile) follow-up of 7.0 (3.6–13.0) years. After the first year post MI, 627 deaths occurred. Long-term all-cause mortality increased with increasing HR level at index MI (Figure 1A). Using patients with HR ≤ 60 bpm as the referent, patients with HR > 90 bpm had a 62% increase in mortality risk (hazard ratio 1.62; 95% CI 1.25–2.09) (Table 2), after adjustment for age, sex, cardiovascular risk factors, reperfusion/revascularization procedures and beta blocker use at admission. Further adjustment for heart failure prior to the MI produced similar results. Cause of death was not available for 14 patients. Most deaths were from cardiovascular causes [n=311 (51%)]. The associations between HR levels and cardiovascular mortality were similar to those reported for all-cause mortality (Table 2).
Figure 1.
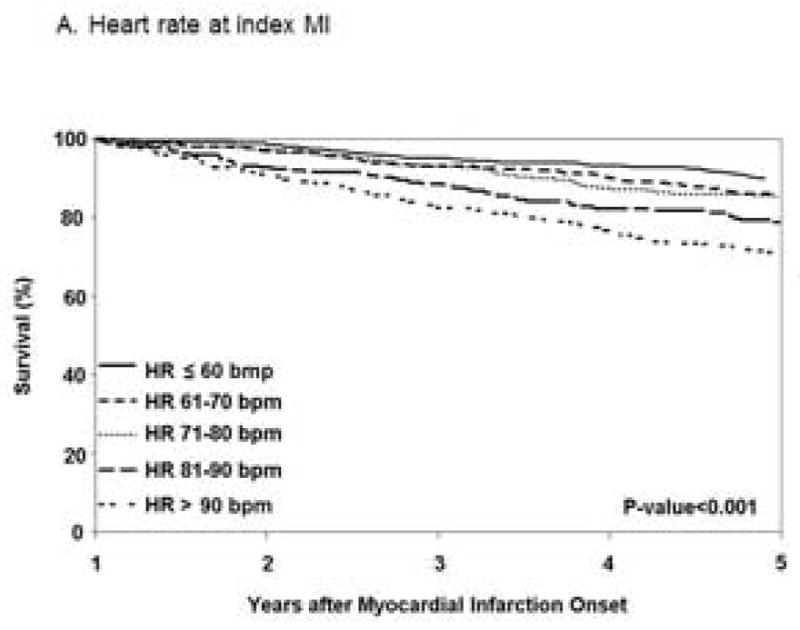
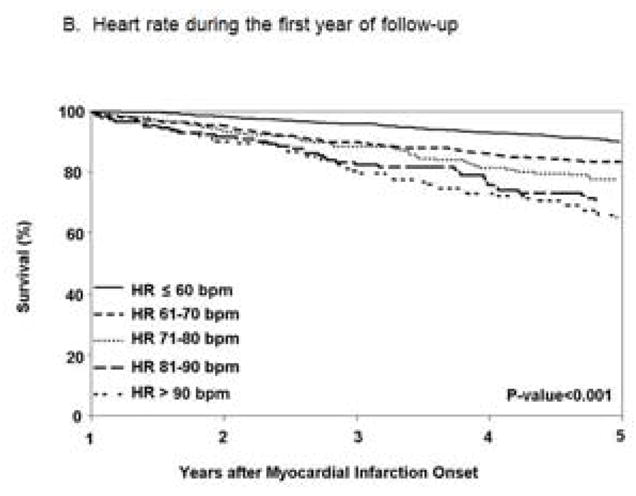
Figure 1A. Kaplan–Meier survival curves for long-term all-cause mortality among first year survivors after myocardial infarction according to heart rate at index MI and during the first year of follow-up (n=1571).
Figure 1B. Heart rate during the first year of follow-up.
Table 2.
Resting heart rate and risk of death in first year survivors after myocardial infarction. Results are presented as hazard rations (95% confidence intervals).
| HR at index MI | 61–70bpm | 71–80bpm | 81–90bpm | >90bpm | ptrend |
|---|---|---|---|---|---|
| All-cause death | |||||
| Unadjusted | 1.26 (0.96–1.65) | 1.26 (0.96–1.65) | 1.75 (1.33–2.31) | 2.42 (1.90–3.10) | <0.001 |
| Adjusteda | 1.28 (0.97–1.68) | 1.27 (0.96–1.67) | 1.90 (1.44–2.50) | 2.23 (1.74–2.85) | <0.001 |
| Adjustedb | 1.35 (1.03–1.78) | 1.24 (0.94–1.64) | 1.52 (1.15–2.02) | 1.62 (1.25–2.09) | <0.001 |
| Adjustedc | 1.36 (1.03–1.78) | 1.29 (0.98–1.71) | 1.59 (1.20–2.11) | 1.61 (1.24–2.08) | <0.001 |
| Cardiovascular death | |||||
| Unadjusted | 1.57 (1.06–2.32) | 1.34 (0.89–2.01) | 2.00 (1.34–2.99) | 2.69 (1.87–3.86) | <0.001 |
| Adjusteda | 1.60 (1.08–2.36) | 1.36 (0.90–2.04) | 2.17 (1.45–3.24) | 2.45 (1.70–3.53) | <0.001 |
| Adjustedb | 1.67 (1.13–2.47) | 1.33 (0.88–2.01) | 1.64 (1.09–2.48) | 1.66 (1.14–2.42) | 0.03 |
| Adjustedc | 1.67 (1.13–2.48) | 1.38 (0.92–2.09) | 1.73 (1.14–2.61) | 1.65 (1.13–2.40) | 0.03 |
| HR during the first year of follow-up | 61–70bpm | 71–80bpm | 81–90bpm | >90bpm | |
| All-cause death | |||||
| Unadjusted | 1.71 (1.37–2.14) | 2.70 (2.13–3.41) | 3.15 (2.41–4.13) | 4.10 (3.17–5.30) | <0.001 |
| Adjusteda | 1.63(1.30–2.05) | 2.19 (1.72–2.78) | 2.90 (2.21–3.81) | 3.32 (2.56–4.30) | <0.001 |
| Adjustedd | 1.57 (1.25–1.98) | 1.58 (1.23–2.03) | 2.00 (1.51–2.66) | 2.16 (1.64–2.84) | <0.001 |
| Adjustede | 1.56 (1.24–1.96) | 1.61 (1.25–2.07) | 1.99 (1.50–2.64) | 2.21 (1.68–2.92) | <0.001 |
| Cardiovascular death | |||||
| Unadjusted | 2.05 (1.48–2.84) | 3.21 (2.28–4.51) | 4.23 (2.92–6.15) | 3.87 (2.61–5.75) | <0.001 |
| Adjusteda | 1.95 (1.41–2.72) | 2.56 (1.81–3.62) | 3.85 (2.64–5.61) | 3.11 (2.08–4.63) | <0.001 |
| Adjustedd | 1.86 (1.33–2.60) | 1.85 (1.29–2.66) | 2.64 (1.78–3.91) | 1.93 (1.27–2.94) | <0.001 |
| Adjustede | 1.84 (1.32–2.58) | 1.88 (1.31–2.70) | 2.61 (1.76–3.87) | 1.99 (1.31–3.03) | <0.001 |
Calculation of hazard ratio used HR ≤ 60 bpm as the reference.
Adjusted for age and sex.
Adjusted for age, sex, body mass index, former or current smoker, hyperlipidemia, hypertension, heart failure, diabetes mellitus, reperfusion/revascularization procedures and beta blockers at index MI.
Adjusted for age, sex, body mass index, former or current smoker, hyperlipidemia, hypertension, heart failure, diabetes mellitus, reperfusion/revascularization procedures, beta blockers at index MI and prior HF.
Adjusted for age, sex, body mass index, former or current smoker, hyperlipidemia, hypertension, heart failure post MI, diabetes mellitus, reperfusion/revascularization procedures and beta blockers at discharge.
Adjusted for age, sex, body mass index, former or current smoker, hyperlipidemia, hypertension, heart failure post MI, diabetes mellitus, reperfusion/revascularization procedures, beta blockers at discharge and prior HF.
Heart Rate During the First Year of Follow-up and Long-term Mortality after MI
Heart rate during the first year of follow-up was measured at a mean (SD) of 5.9 (3.0) months. The mean (SD) HR during the first year of follow-up was 68 (16) bpm; 594 (38%) patients had HR ≤ 60 bpm, 425 (27%) patients had HR between 61 and 70 bpm, 254 (16%) patients had HR between 71 and 80 bpm, 145 (9%) patients had HR between 81 and 90 bpm and 153 (10%) patients had HR > 90 bpm. Long-term all-cause mortality increased with increasing HR (Figure 1B). Patients with HR > 90 bpm had more than a 2-fold increase in mortality risk compared to patients with HR ≤ 60 bpm (hazard ratio 2.16; 95% CI 1.64–2.84) (Table 2), after adjustment for age, sex, cardiovascular risk factors, reperfusion/revascularization procedures and beta blocker use at discharge. Further adjustment for heart failure prior to the MI and participation in cardiac rehabilitation produced similar results. Similar associations were seen between HR levels and cardiovascular mortality.
Influence of Beta Blocker Therapy
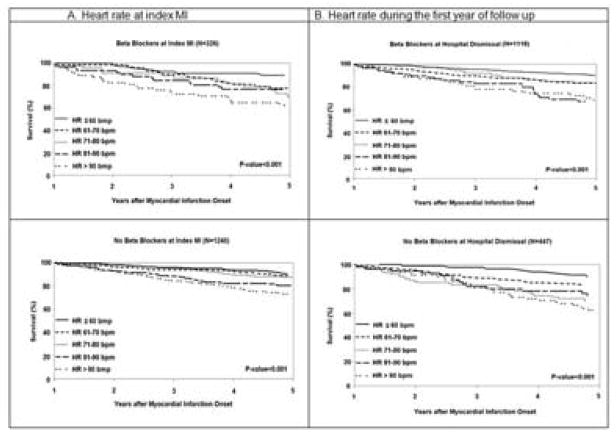
The mean (SD) HR at index MI for patients prescribed and not prescribed beta blockers prior to index MI were 75 (18) bpm and 79 (19) bpm, respectively (p=0.003). Similar trends in long-term all-cause mortality by HR at index MI were observed for patients prescribed beta blockers prior to index MI vs. those who were not (Figure 2). The association between HR at index MI and long-term all-cause mortality did not differ by beta blocker use prior to the MI (pinteraction = 0.53), adjusting for age, sex, cardiovascular risk factors, and reperfusion/revascularization.
Figure 2.
Kaplan–Meier all-cause survival curves for first year survivors from myocardial infarction according to heart rate at index MI and HR during the first year of follow-up after MI and beta blocker use.
The mean (SD) HR during the first year of follow-up were 65 (15) bpm and 75 (17) bpm in patients prescribed and not prescribed beta blockers at hospital dismissal, respectively (p<0.001). Similar trends in long-term all-cause mortality by HR during the first year of follow-up were observed for patients prescribed beta blockers at hospital dismissal (Figure 2). The association between HR during the first year of follow-up and long-term all-cause mortality did not differ by beta blocker use after the MI (pinteraction = 0.82), adjusting for age, sex, cardiovascular risk factors, and reperfusion/revascularization.
Similar results were obtained for cardiovascular mortality (pinteraction = 0.99 and 0.69 for the interaction between HR at index MI and beta blocker use prior to index MI and for the interaction between HR during the first year of follow-up and beta blocker use after MI, respectively).
Sensitivity Analyses
Analyses including patients who died during the first year of follow-up post MI resulted in similar associations between increasing HR at the index MI and increased risk of all-cause and cardiovascular mortality. Similar trends were also observed for HR during the first year of follow-up.
Additionally, analyses after multiple imputation of HR in the 801 patients missing HR at the index MI and/or during the first year post MI resulted in similar associations between elevated HR at the index MI and during the first year of follow-up, and long-term all-cause mortality and cardiovascular mortality reported herein.
DISCUSSION
Our population-based data pertaining to a large MI incidence cohort indicate that, in first year survivors after MI, there was an important increase in long-term all-cause and cardiovascular mortality with increasing resting HR during the first year of follow-up regardless of other known confounders, including beta blocker use. Patients with HR > 80 bpm had a two-fold increased risk of long-term mortality compared with patients with HR ≤ 60 bpm; similar results were observed for long-term cardiovascular mortality.
To the best of our knowledge, the present study is unique as it reports on the long-term prognostic impact of resting HR at the initial presentation and during the first year of follow-up post MI. It extends previous studies indicating HR is a strong prognostic marker of mortality after acute MI5,6,8,27,28 and in a population free of cardiovascular disease.29 This study demonstrates for the first time that HR is a useful long-term mortality predictor, not only at the initial presentation of MI but even more during the first year of follow-up post MI. Although prior studies on coronary syndromes reported an association between HR and mortality, patients included in these studies had proven or suspected coronary disease defined as angina pectoris without prior MI, hypertensive heart disease, valvular heart disease, or cardiomyopathy.30–32 Moreover, HR assessments during follow-up inform the risk appraisal as, herein, HR at the time of the MI was a strong prognostic marker of all-cause and cardiovascular mortality for HR > 80 bpm whereas the excess risk of all-cause and cardiovascular mortality emerged with HR > 60 bpm during the first year of follow-up.
Pathophysiologic considerations and clinical data substantiate that elevated resting HR is an intrinsic risk factor for poor outcomes after MI rather than merely a marker of other cardiovascular risk factors.1–6,28,33–35 Indeed, HR might theoretically contribute to increased mortality by increasing myocardial oxygen consumption,36 thus favoring ischemia,37 increase in infarct size38 and influencing the atherosclerotic coronary disease progression39–41 and plaque stability.42 Experimental studies suggest that HR is not only important in the development of ischemia, but also influences whether ischemic episodes trigger serious arrhythmias.43,44
Modulation of HR by conventional rate control agents as beta blockers affects cardiovascular mortality.45 These agents have protection effects beyond rate control.46 Herein, we adjusted for the use of beta blockers, which enabled evaluating the independent value of resting HR. As there was no detectable interaction between HR and beta blocker use, the better prognosis of the patients with low HR cannot be explained only by the protective effect of beta blockers because the HR-mortality association did not differ by beta blocker use.
Limitations and Strengths
Potential limitations of the current study need to be considered when interpreting the data. First, not all patients with an incident MI were included in this study. To allow evaluation of the association’s effect size between mortality and HR at the time of the MI, and mortality and HR during the first year after MI, we focused on first year MI survivors with available ECGs for HR measurement at the time of the MI and during the first year post MI. However, analyses after multiple imputation of missing HR data confirmed these associations. Second, echocardiography and radionuclide studies were not systematically performed in these patients, and left ventricular ejection fraction was not routinely measured. Therefore we cannot exclude that elevated HR may have marked depressed left ventricular function that is clinically unrecognized. Third, data on the proportion of patients treated with appropriate, guidelines-recommended doses of beta blockers as well as therapeutic adherence are lacking. Finally, while the racial and ethnic composition of Olmsted County may preclude generalizing findings to groups not adequately represented in this population, epidemiological studies in Olmsted County underscore that the results are generalizable to a large portion of the United States population and that cardiovascular disease trends measured in Olmsted County parallel national trends, further supporting its generalizability.47
Our study has several important strengths. Our population-based design reflects the experience of an entire community and thus is less subject to selection bias.48 This, in turn, optimizes the clinical relevance of our data. The internal validity of the present data are quite robust because our ascertainment identified all consecutive incident MIs in the community validated through rigorous criteria, and follow-up was extensive and comprehensive with few missing data. This allowed evaluating the prognostic impact of HR over an extended period of time. The predictive value of resting HR remained after adjusting for potential confounders in multivariable analyses indicating the robustness of the association with mortality. Moreover, despite the high rates of use of beta blockers in the study population, HR was an important independent risk predictor for all-cause and cardiovascular mortality, even in the small group of patients not prescribed beta blockers at hospital dismissal.
Clinical Implications
HR is easily measured and can be modified by treatment and/or exercise training. It is a valuable tool to identify high risk subjects. Debate should center now on how to integrate this evidence into clinical practice. A formal evaluation in pragmatic clinical trials seems necessary to assess the optimal clinical strategy in selected MI patients during their early follow-up visits based on their treatment compliance, risks and benefits.
Conclusion
These data from a large MI community cohort indicate that elevated resting HR at the time of the MI and during the first year of follow-up after MI identifies patients at increased risk of all-cause and cardiovascular mortality. Simple HR measurements during the first year of follow-up after MI may help stratify long-term prognosis.
Acknowledgments
Financial Support
This work was supported in part by grants from the Public Health Service and the National Institutes of Health [RO1 HL 59205]; and the National Institute on Aging [AG034676]. Dr. Jabre is supported by INSERM, U970, Paris Descartes University, France; and the French Emergency Physician Society [SFMU].
This study was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigators: Walter A. Rocca, MD and Barbara P. Yawn, MD, MSc).
Abbreviations
- HR
Heart Rate
- MI
Myocardial Infarction
- ECG
Electrocardiographic
- SD
Standard Deviation
- bpm
beats per minute
Footnotes
Disclosure:
No Conflicts of Interest declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parodi G, Bellandi B, Valenti R, et al. Heart rate as an independent prognostic risk factor in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Atherosclerosis. 2010;211(1):255–259. doi: 10.1016/j.atherosclerosis.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD, Morrow DA, Frederick PD, et al. Performance of the thrombolysis in myocardial infarction risk index in the National Registry of Myocardial Infarction-3 and -4: a simple index that predicts mortality in ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44(4):783–789. doi: 10.1016/j.jacc.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Kovar D, Cannon CP, Bentley JH, Charlesworth A, Rogers WJ. Does initial and delayed heart rate predict mortality in patients with acute coronary syndromes? Clin Cardiol. 2004;27(2):80–86. doi: 10.1002/clc.4960270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuanetti G, Mantini L, Hernandez-Bernal F, et al. Relevance of heart rate as a prognostic factor in patients with acute myocardial infarction: insights from the GISSI-2 study. Eur Heart J. 1998;19(Suppl):FF19–26. [PubMed] [Google Scholar]
- 5.Hjalmarson A, Gilpin EA, Kjekshus J, et al. Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol. 1990;65(9):547–553. doi: 10.1016/0002-9149(90)91029-6. [DOI] [PubMed] [Google Scholar]
- 6.Disegni E, Goldbourt U, Reicher-Reiss H, et al. The predictive value of admission heart rate on mortality in patients with acute myocardial infarction. J Clin Epidemiol. 1995;48(10):1197–1205. doi: 10.1016/0895-4356(95)00022-v. [DOI] [PubMed] [Google Scholar]
- 7.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91(6):1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 8.Berton GS, Cordiano R, Palmieri R, Gheno G, Mormino P, Palatini P. Heart rate during myocardial infarction: relationship with one-year global mortality in men and women. Can J Cardiol. 2002;18(5):495–502. [PubMed] [Google Scholar]
- 9.Saraiva F, Antonio N, Lourenco C, et al. Heart rate and prognosis in acute coronary syndromes. Rev Port Cardiol. 2010;29(7–8):1101–1119. [PubMed] [Google Scholar]
- 10.Timoteo AT, Toste A, Ramos R, Oliveira JA, Ferreira ML, Ferreira RC. Admission heart rate as a predictor of mortality in patients with acute coronary syndromes. Acute Card Care. 2011;13(4):205–210. doi: 10.3109/17482941.2011.628028. [DOI] [PubMed] [Google Scholar]
- 11.Facila L, Morillas P, Quiles J, et al. Prognostic significance of heart rate in hospitalized patients presenting with myocardial infarction. World J Cardiol. 2012;4(1):15–19. doi: 10.4330/wjc.v4.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2000 Census. 2000 http://factfinder.census.gov.
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melton LJ, 3rd, Rocca WA, Roger VL. Development of population research at Mayo Clinic. Mayo Clin Proc. 2014;89(2):e17–20. doi: 10.1016/j.mayocp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):coppr2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 17.Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, Massachusetts: John Wright-PSG, Inc; 1982. [Google Scholar]
- 18.Roger VL, Jacobsen SJ, Weston S, et al. Trends in the Incidence and Survival of Patients with Hospitalized Myocardial Infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002:136341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 22.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc. 1987:82387–394. [Google Scholar]
- 23.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 24.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 26.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copie X, Hnatkova K, Blankoff I, Staunton A, Camm AJ, Malik M. Risk of mortality after myocardial infarction: value of heart rate, its variability and left ventricular ejection fraction. Arch Mal Coeur Vaiss. 1996;89(7):865–871. [PubMed] [Google Scholar]
- 28.Abildstrom SZ, Jensen BT, Agner E, et al. Heart rate versus heart rate variability in risk prediction after myocardial infarction. J Cardiovasc Electrophysiol. 2003;14(2):168–173. doi: 10.1046/j.1540-8167.2003.02367.x. [DOI] [PubMed] [Google Scholar]
- 29.Saxena A, Minton D, Lee DC, et al. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin Proc. 2013;88(12):1420–1426. doi: 10.1016/j.mayocp.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronow WS, Ahn C, Mercando AD, Epstein S. Association of average heart rate on 24-hour ambulatory electrocardiograms with incidence of new coronary events at 48-month follow-up in 1,311 patients (mean age 81 years) with heart disease and sinus rhythm. Am J Cardiol. 1996;78(10):1175–1176. doi: 10.1016/s0002-9149(96)90077-6. [DOI] [PubMed] [Google Scholar]
- 31.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 32.Shaper AG, Wannamethee G, Macfarlane PW, Walker M. Heart rate, ischaemic heart disease, and sudden cardiac death in middle-aged British men. Br Heart J. 1993;70(1):49–55. doi: 10.1136/hrt.70.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56(24):1973–1983. doi: 10.1016/j.jacc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Redwood DR, Smith ER, Epstein SE. Coronary artery occlusion in the conscious dog. Effects of alterations in heart rate and arterial pressure on the degree of myocardial ischemia. Circulation. 1972;46(2):323–332. doi: 10.1161/01.cir.46.2.323. [DOI] [PubMed] [Google Scholar]
- 35.James RG, Arnold JM, Allen JD, Pantridge JF, Shanks RG. The effects of heart rate, myocardial ischemia and vagal stimulation on the threshold for ventricular fibrillation. Circulation. 1977;55(2):311–317. doi: 10.1161/01.cir.55.2.311. [DOI] [PubMed] [Google Scholar]
- 36.Panza JA, Diodati JG, Callahan TS, Epstein SE, Quyyumi AA. Role of increases in heart rate in determining the occurrence and frequency of myocardial ischemia during daily life in patients with stable coronary artery disease. J Am Coll Cardiol. 1992;20(5):1092–1098. doi: 10.1016/0735-1097(92)90363-r. [DOI] [PubMed] [Google Scholar]
- 37.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 38.Maroko PR, Kjekshus JK, Sobel BE, et al. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Perski A, Olsson G, Landou C, de Faire U, Theorell T, Hamsten A. Minimum heart rate and coronary atherosclerosis: independent relations to global severity and rate of progression of angiographic lesions in men with myocardial infarction at a young age. Am Heart J. 1992;123(3):609–616. doi: 10.1016/0002-8703(92)90497-j. [DOI] [PubMed] [Google Scholar]
- 40.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49(25):2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 41.Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol. 2008;126(3):302–312. doi: 10.1016/j.ijcard.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 42.Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104(13):1477–1482. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds RD, Calzadilla SV, Lee RJ. Spontaneous heart rate, propranolol, and ischaemia-induced ventricular fibrillation in the dog. Cardiovasc Res. 1978;12(11):653–658. doi: 10.1093/cvr/12.11.653. [DOI] [PubMed] [Google Scholar]
- 44.Bolli R, Fisher DJ, Entman ML. Factors that determine the occurrence of arrhythmias during acute myocardial ischemia. Am Heart J. 1986;111(2):261–270. doi: 10.1016/0002-8703(86)90138-9. [DOI] [PubMed] [Google Scholar]
- 45.Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. 2007;28(24):3012–3019. doi: 10.1093/eurheartj/ehm489. [DOI] [PubMed] [Google Scholar]
- 46.Sipahi I, Tuzcu EM, Wolski KE, et al. Beta-blockers and progression of coronary atherosclerosis: pooled analysis of 4 intravascular ultrasonography trials. Ann Intern Med. 2007;147(1):10–18. doi: 10.7326/0003-4819-147-1-200707030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113(19):2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 48.Steg PG, Lopez-Sendon J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167(1):68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]