Abstract
Curcumin, a natural phenolic compound derived from the plant Curcuma longa, was physically entrapped and stabilized in silk hydrogel films and its influence on human bone marrow-derived mesenchymal stem cells (hBMSCs) was assessed related to adipogenic differentiation. The presence of curcumin significantly reduced silk gelation time and changed the porous morphology of gel matrix, but did not change the formation of silk beta-sheet structure. Based on spectrofluorimetric analysis, curcumin likely interacted with hydrophobic residues in silk, interacting with the beta-sheet domains formed in the hydrogels. The antioxidant activity of silk film-associated curcumin remained functional over at least one month in both the dry and hydrated state. Negligible curcumin was released from silk hydrogel films over 48 hours incubation in aqueous solution. For hBMSCs cultured on silk films containing more than 0.25 mg/mL curcumin, cell proliferation was inhibited while adipogenesis was significantly promoted based on transcripts as well as oil red O staining. When hBMSCs were cultured in media containing free curcumin, both proliferation and adipogenesis of hBMSCs were inhibited when curcumin concentrations exceeded 5 μM, which is more than 1,000-times higher than the level of curcumin released from the films in aqueous solution. Thus, silk film-associated curcumin exhibited different effects on hBMSC proliferation and differentiation when compared to curcumin in solution.
Keywords: curcumin, silk fibroin, mesenchymal stem cells, differentiation, adipogenesis
1. Introduction
Human mesenchymal stem cells (hMSCs) are multipotent stromal cells capable of differentiation into bone, cartilage, fat, ligament, muscle, and other connective tissue lineages, offering versatility in regenerative medicine and tissue engineering [1]. In addition, they can be readily expanded ex vivo for several passages without losing their self-renewal capacity. These functions provide therapeutic potential for treating conditions where complex tissue regeneration and remodeling are major concerns, such as bone, cartilage, and soft tissue engineering [2]. For these applications, hMSCs are often seeded on biomaterial-based scaffolds and their lineage differentiations are regulated by growth factors embedded in the scaffolds and released in temporally and spatially controllable manners. Other factors, including small molecule compounds that interact with specific signaling pathways (e.g., Wnt and/or TGF-β/BMP pathways) at different levels also have potentials to regulate hMSC differentiations [3].
Curcumin is the yellow pigment of Curcuma longa found in turmeric spice that has been shown to have potent antioxidant, anti-inflammatory, and anti-carcinogenic effects [4]. Curcumin positively affected both peroxisome proliferator-activated receptor-γ (PPAR- γ) [5-9] and p21 signaling pathways [10], which are intimately involved in adipogenesis. For example, curcumin dramatically induced the PPAR-γ expression and activated PPAR-γ in activated rat hepatic stellate cells [8]. Curcumin treatment produced significant increase of PPAR-γ mRNA and protein levels in the liver of septic rats [6]. Moreover, curcumin moderately increased the p21 level in breast (MCF-7 and T47-D) and prostate (LNCap) cancer cell lines [10]. The ability of curcumin to affect these same pathways in hMSCs has yet to be documented, but make curcumin an attractive candidate for enhancing adipogenesis in hMSCs, which is of significance to soft tissue engineering. It has been found recently that the effect of curcumin on cell proliferation and differentiation is highly dose-dependent, and the solubility and stability of curcumin may also change in cell culture media under different conditions, such as the concentration of serum and frequency of medium replenishment [11,12].
In the studies described here, attention was focused on the role of curcumin in the adipogenic differentiation of hMSCs, laying the basis for developing curcumin-functionalized biomaterials for hMSCs-based soft tissue regeneration. Bone-marrow derived MSCs (hBMSCs) were used in the study, and the findings will be instructive for MSCs from other sources. Recent studies have shown that a large portion of curcumin added to media directly bound to cell membranes and further stimulated intracellular signaling pathways either by interacting with molecular receptors on the membrane or by changing membrane physical properties [12,13]. Our hypothesis was that binding curcumin to the surface of the cell culture substrate may stimulate intracellular signaling pathways in hBMSCs in a more efficient manner than free curcumin in the medium, thus promoting hBMSC differentiation. Surface-associated curcumin may interact with the binding sites (possibly receptors) on hBMSC cell membrane in a controlled and sustainable manner, resulting in the activation of related signaling pathways and promoting adipogenesis. Curcumin delivered in solution may transiently bind to cell membrane and lead to a different cell response.
To address the hypothesis, we compared two curcumin delivery methods: free curcumin in solution and surface-associated curcumin. The material we chose for immobilizing curcumin and supporting cell growth was silk fibroin, the structural protein isolated from Bombyx mori silkworm cocoons. Silk fibroin has been shown to be a versatile biomaterial due to its biocompatibility, mechanical properties, cell-dependent degradation profile, and processibility [14-16]. Silk proteins can be readily fabricated into a variety of material formats through all-aqueous processing, such as porous sponge, particles, films and hydrogels. Recent work in our labs has produced mechanical [17] and chemical methods [18,19] to rapidly gel silk protein solutions by inducing β-sheet formation to generate physically crosslinked hydrogels. Small molecules or proteins can be encapsulated in these hydrogels, and the release profiles can be tailored depending on the extent of β-sheet formation. In the present study, we chose to physically encapsulate curcumin in silk hydrogel films rather than use chemical cross-linking, because hydrophobic curcumin molecules bound tightly to the silk hydrogel matrix with limited release in aqueous solution.
2. Materials and Methods
2.1 Materials
Partially degummed silk fibers were purchased from Xiehe Silk Incorporation, Shengzhou, Zhejiang province, China. Curcumin (Cat#C7727, >80% pure), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). mRNA isolation kits were purchased from Qiagen Inc (Valencia, CA). Cell medium ingredients and qPCR reagents were purchased from Life Technologies (Grand Island, NY).
2.2 Silk purification
The silk fibers had been pre-treated to remove most of the sericin contaminants. Further degumming was performed in the laboratory at a scale of 10 grams for each batch. The fibers were boiled in 0.02 M sodium carbonate solution for 30 min, rinsed with ultrapure water three times, drained and dried in a fume hood overnight. The dried fibers were dissolved in 9.3 M lithium bromide solution, dialyzed against pure water for 2 days to remove lithium bromide and centrifuged to remove insoluble fibrous debris, as reported in the literature [20]. The silk concentration was about 6% (w/v) after purification. The solution obtained was stored at 4°C and further diluted to 4% (w/v) with water before being used to prepare the hydrogel films.
2.3 Silk-curcumin hydrogel film preparation
A stock solution was prepared by dissolving curcumin in absolute ethanol to obtain a concentration of 5 mg/mL. The solution was further diluted with ethanol to obtain working solutions at various curcumin concentrations. To prepare a large hydrogel film for material characterization, 2 mL of curcumin working solution was mixed with 8 mL of 4% silk solution and the mixed solution was poured into a 100 mm plastic Petri dish. The dish was covered and placed in a fume hood overnight until the solution formed a hydrogel that did not flow when inverted. The cover was then removed to allow the gel to dry in the air, forming a transparent yellow thin film at the bottom of dish. For curcumin release and stability testing, the film was peeled off and cut into small pieces weighing approximately 5 mg each. Ethanol alone was used to prepare the control sample of plain silk film with the same ethanol to silk ratio as used above. The film pieces were placed in Eppendorf tubes for storage or testing. To prepare small hydrogel films for cell culture, curcumin was mixed with silk in the same way, and 200 μL aliquots of the mixture were added to 24-well plates. The silk and curcumin solution were filtered through 0.45 μm sterile membranes (Millipore, Billerica, Massachusetts) before mixing, and the mixing step as well as the subsequent film drying were performed in a laminar flow hood. After drying, the plates were wrapped with aluminum foil and stored at room temperature before use. Curcumin concentrations in silk hydrogels are listed in Table 1. The concentrations in mg/mL were used to distinguish them from free curcumin in the cell culture and release medium in molar concentrations (μM).
Table 1. Curcumin concentrations used and effects on hBMSCs.
| Concentration in hydrogel (mg/mL) | Concentration in medium (μM) | Effect on hBMSC proliferation | Effect on hBMSC adipogenesis | |
|---|---|---|---|---|
| Free curcumin in medium | N.A. | 0 | No effect | No effect |
| N.A. | 0.1 | No effect | No effect | |
| N.A. | 1 | No effect | No effect | |
| N.A. | 5 | Inhibition | N.A. | |
| N.A. | 10 | Inhibition | Inhibition | |
| Film-associated curcumin | 0 | 0 | No effect | No effect |
| 0.025 | <0.001 | No effect | No effect | |
| 0.05 | <0.005 | No effect | No effect | |
| 0.125 | <0.005 | No effect | Promotion | |
| 0.25 | <0.005 | Inhibition | Promotion |
2.4 Fluorescence spectroscopy
Curcumin/DMSO stock solution (2.71 mM) was diluted in an aqueous solution containing silk. The final concentration of curcumin was kept constant at 25 μM, while the concentration of silk varied from 0 μM (pure water) to 12.5, 25, 50 μM. After mixing, 1 mL of the mixture was added into a quartz cuvette, which was loaded into a spectrofluorimeter (FluoroMax-4, HORIBA Jobin Yvon Inc, Edison, NJ) for fluorescence measurements. The excitation wavelength for curcumin was set at 420 nm and the fluorescence emission was scanned in the wavelength range of 450-700 nm. For the fluorescence kinetic studies, silk solution with a concentration of 12.5 μM was used. The curcumin emission wavelength was set at 580 nm and 530 nm for the curcumin alone and curcumin-silk mixture, respectively. The fluorescence intensity was recorded over 96 hours.
2.5 Laser scanning confocal microscopy
The distribution of curcumin in silk films was investigated by confocal microscopy. A piece of film (approximately 5×5 mm) with initial curcumin concentration of 0.25 mg/ml was imaged on an Olympus microscope (FV 1000, Japan) at an excitation wavelength of 488 nm. Single xy scans were collected with an optical slice of 1 μm along the z-direction. Four squares (approximately 200 μm×200 μm each) were randomly chosen from every image and the intensities were measured by Image J (National Institutes of Health).
2.6 Scanning electron microscopy (SEM)
Curcumin-silk hydrogels were prepared as described above. The final concentration of curcumin in the mixture was 0, 0.1, 0.25 or 0.5 mg/mL. After preparation the hydrogels were cut into small pieces, which were frozen at -20 °C overnight and loaded in a lyophilizer (CHRIST Alpha 2-4 LSCplus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). After 3 days, the dried samples were mounted on sample stubs, with the cross sections facing to the top. To determine the thickness of silk-curcumin films, the dry films were cut into small pieces and mounted on sample stubs directly. The samples were sputter-coated with Au, and images were taken using a Hitachi Scanning Electron Microscope (S-4800, Tokyo, Japan) at 3.0 kV.
2.7 Fourier transform infrared spectroscopy (FTIR)
The freeze-dried curcumin-silk hydrogel pieces as described above were ground into fine powders, mixed with potassium bromide (KBr) powder in a weight ratio of 1:100, pressed into a solid pellet using a hand-operated press, and loaded into a FTIR spectrometer (Nicolet 5700, ThermoFisher Scientific Inc, Waltham, MA) for measurement under transmission mode. The curves that had absorption bands in the frequency range of 1620 - 1630 cm-1 and 1695 – 1700 cm-1 represented enriched β-sheet structure in silk II form [21].
2.8 Determination of curcumin stability using DPPH
Curcumin-silk hydrogel films with different concentrations of curcumin were cut into small pieces weighing approximately 5 mg each and placed into 2-mL Eppendorf tubes. The tubes were filled with 1 mL PBS buffer, pH 7.4, or remained empty. All the tubes were tightly sealed and stored at 37°C and 60°C for up to 28 days. At desired time points, four tubes from each testing condition were removed. For the tubes filled with PBS buffer, the buffer was completely removed and 1 mL of DPPH substrate solution was added to the tubes. For the empty tubes, 1 mL of DPPH substrate solution was added to the tubes to start anti-oxidation tests. DPPH substrate solution was prepared by dissolving 3.95 mg DPPH powder in 100 mL methanol. The reaction time was 60 min. After the reaction, the films were removed and the solution was subjected to absorbance measurements at 517 nm [22]. The absorbance value was compared with the DPPH solution in the absence of curcumin to obtain the percentage scavenging value.
2.9 Determination of curcumin release from silk hydrogel films
For a typical release experiment, 2 mL of release medium, either PBS buffer, pH 7.4, or PBS buffer supplemented with 0.5% Tween80 and 3% methanol, was added to a tube containing a piece of silk hydrogel film [22]. The tubes were sealed and incubated on a shaker at a speed of 80 rpm and placed in a 37 °C incubator. At desired time points (1, 2, 3, 96, 144 and 148h), the release medium was carefully moved to an empty tube and the film was resuspended in 2 mL fresh medium. Curcumin concentration in the released medium was determined by measuring absorbance at 424 nm and comparing with a standard curve. The percentage of release was obtained by comparing these values with the initial loading of curcumin in the film. Each test group contains four repeat samples.
2.10 hBMSC Isolation and Expansion
Bone marrow aspirates were obtained from Lonza Walkersville Inc. (Walkersville, MD), and plated in tissue culture plates. hBMSCs were isolated from the aspirate by their ability to adhere to tissue culture plates. These passage 0 (P0) cells were grown in high glucose Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic, 1% non-essential amino acids, and 2 ng/mL basic fibroblast growth factor (human recombinant, Invitrogen) in a humidified incubator at 37 °C. At 90% confluency the P0 cells were detached and frozen in liquid nitrogen. Before use, the frozen cells were thawed, suspended in growth medium and plated in tissue culture treated T75 flasks at a density of 500,000 cells/flask. The cells were expanded in a humidified incubator at 37 °C. Cells were grown to 80% confluency, then trypsinized and re-plated in tissue culture treated 24-well plates at a density of 3,000 cells/well for proliferation studies and 15,000 cells/well for differentiation studies. Cells from one single donor were used in all experiments.
2.11 hBMSC proliferation and differentiation
For the free curcumin experiment, curcumin was dissolved in DMSO at various concentrations, which were further diluted into growth medium to obtain the desired final concentrations (Table 1). The DMSO final concentration was kept at 0.1% in the medium, lower than the concentration causing cytotoxicity [23]. During cell culture, the medium was changed every 2-3 days, and curcumin was added to the medium every time before use. For the film-associated curcumin experiments, plain growth medium was added directly to the 24-well plates containing films. At designated time points, three wells of hBMSCs from each curcumin concentration group were washed with PBS buffer. Using the PicoGreen assay (Invitrogen), cells were lysed and the relative dsDNA content was determined following the manufacturer's instructions. The cell morphology change was monitored using a phase contrast light microscope (Carl Zeiss, Jena, Germany) equipped with a Sony Exwave HAD 3CCD color video camera.
For adipogenic differentiation, hBMSCs were cultured in growth medium until confluency Adipogenic medium containing high glucose DMEM supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic, 1% non-essential amino acids, 1 μM dexamethasone, 50 μM indomethacin, 0.5 mM isobutylxanthine, and 5 μg/mL insulin (human recombinant, Sigma) was added to the wells to induce adipogenic differentiation [24]. For the free curcumin experiment, curcumin was added to the differentiation medium in the same way as mentioned above. The final curcumin concentrations in the media are shown in Table 1. For the film-associated curcumin experiments, plain differentiation medium was added directly to the wells. Medium was changed every 2-3 days until the end of differentiation.
2.12 Gene expression analysis using real-time PCR
At designated time points, three wells from each condition were lysed in 0.35 mL Buffer RLT (Qiagen) containing 10% mercaptoethanol, followed by shredding in a QIAshedder (Qiagen). mRNA was isolated from the cells using an RNeasy Mini Kit (Qiagen). From this mRNA, cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Invitrogen #4368814) and Stratagene Mx3000 qPCR system. The cDNA samples were analyzed for gene expression relative to the GAPDH housekeeping gene (ABI #Hs99999905_m1) using TaqMan® Universal PCR Master Mix (Invitrogen #4304437). Cells cultured in adipogenic medium were analyzed for peroxisome proliferator-activated receptor gamma (PPAR-γ, #Hs00234592_m1), fatty acid-binding protein-4 (FABp4, #Hs00609791_m1), glucose transporter-4 (Glut4, #Hs00168966_m1), and lipoprotein lipase (LPL, #Hs00173425_m1). Expression of these genes was compared to control samples cultured on TCP and plain silk films. For each sample, the Ct value was defined as the cycle number at which the amplification of each target gene was in the linear range of the reaction. Relative expression levels of each gene were calculated by normalizing to the Ct value of the housekeeping gene GAPDH (2ΔCt, Perkin Elmer User Bulletin #2). Data from three separate cultures of each type were averaged.
2.13 Oil Red O Staining
Lipid droplets in cells exposed to adipogenic medium were stained with 1-([4-(xylylazo)xylyl]azo)-2-naphthol (Oil Red O, Sigma). Cells were fixed in 4% neutral buffered formalin overnight at 4 °C, and then washed with 60% isopropanol (500 μL) on a shaker for 30 minutes. Oil Red O was dissolved in isopropanol (175 mg / 50 mL), diluted into PBS to make a 60% working solution, and then filtered through a 0.45 μM syringe filter. The isopropanol wash was aspirated from the wells, and the Oil Red O solution was added (200 μL) and placed on a shaker for 30 minutes. The dye solution was then aspirated and the wells were washed with PBS (500 μL) four times over the course of one hour. Finally, the cells were counterstained with hematoxylin and imaged using a light microscope.
2.14 Statistical Analysis
All measurements for DNA content, real time RT-PCR, and oil red O staining were collected with N = 3 independent determinations per data point and expressed as means ± standard deviations. Data for these measurements were analyzed in Instat (version 3.36) with one-way analysis of variance (ANOVA) and Student–Newman–Keuls Multiple Comparisons Test. Differences were considered significant when p≤0.05, very significant when p≤0.005.
3. Results and Discussion
3.1 Interaction between curcumin and silk
Silk hydrogel formation can be induced by either adding chemicals to the silk solution, such as acids, salt, and alcohols, or applying energy, such as heating and sonication [25]. Since curcumin has a relatively high solubility in ethanol, we chose ethanol as a solvent to dissolve curcumin as well as induce silk gelation. Silk gelation time was dependent on curcumin concentration in the mixture. Figure 1A shows a typical experiment in which silk gelation time decreased from approximately 5 hours at 0 mg/mL curcumin (pure ethanol) to a few minutes at 2.71 mM curcumin. Silk gelation is mainly caused by the intra- and inter-molecular interaction between hydrophobic beta-sheet forming domains [26]. Since curcumin is hydrophobic, it is likely that curcumin bound to these hydrophobic domains on the silk chains in solution, inducing a transition in secondary structure from random coil to beta-sheet, which crosslinked into the hydrogel network. The interaction between curcumin and solution-state silk was further studied using spectrofluorimetry The fluorescence emission maximum of curcumin in aqueous solution was about 580 nm (Figure 2), and after mixing with silk the emission maximum blue-shifted to 530 nm. In addition, fluorescence intensity increased as the concentration of silk increased. At the highest silk to curcumin molar ratio (2:1), the maximum curcumin fluorescence intensity increased more than 5-fold when compared to the control sample of curcumin alone (Figure 2). This result indicated that the microenvironment in silk solution where curcumin was bound was more hydrophobic than that in the absence of silk. Since silk fibroin mainly consists of recurrent amino acid sequences of GAGAGS, it is likely that curcumin bound to these hydrophobic regions. Binding to silk molecules increased the solution stability of curcumin. The fluorescence intensity of curcumin at 580 nm decreased from 660 to 200 over 48 hours, while the fluorescence intensity in silk solution remained stable for more than 96 hours (Figure 2, inset). Curcumin was unstable in aqueous solution but can be stabilized after binding to plasma proteins, such as albumin, or being entrapped in carrier materials, such as silk nanoparticles and porous scaffolds [22,27,28]. The data presented in this study is consistent with these previous reports.
Figure 1. Influence of curcumin on silk gelation.
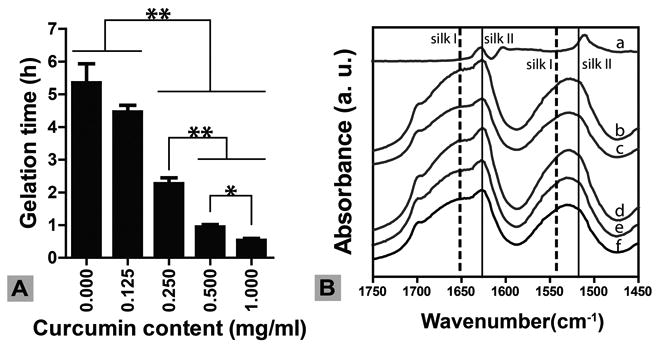
(A) Silk gelation time decreased with increase of curcumin content. N = 5. *indicates significant difference (p < 0.05); ** indicates very significant difference (p < 0.005) (B) Silk secondary structure by FTIR. Characteristic silk I and silk II peaks in silk hydrogels were not influenced by the presence of curcumin. a, free curcumin; b-f, curcumin concentrations of 1, 0.5, 0.25, 0.1, 0 mg/mL in 4 wt% silk hydrogel, respectively. Silk gels were lyophilized prior to FTIR measurement.
Figure 2. Silk-curcumin interactions in solution.
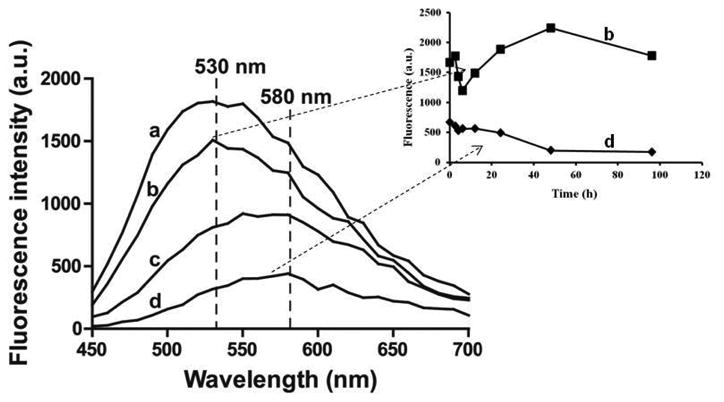
Solution containing 25 μM curcumin and various concentrations of silk fibroin (a-d: 50, 25, 12.5, 0 μM, respectively) was subjected to fluorescence measurement with excitation at 420 nm and emission of 450-700 nm. Inset: fluorescence kinetics for b and d measured at 580 nm.
Silk undergoes a change in secondary structure from random coil to beta-sheet during gelation [26]. Beta-sheets, indicated by characteristic FTIR peaks at 1630 and 1520 cm-1 (silk II), formed in ethanol-induced silk hydrogels (Figure 1B). The presence of curcumin, with concentrations from 0.27 to 2.7 mM, did not significantly change the secondary structure of the silk. All samples showed similar silk II spectral peaks (Figure 1B). The presence of curcumin, however, changed silk hydrogel morphology as determined by SEM (Figure 3). The plain silk hydrogel showed smooth lamellar structures as previously reported (Figure 3A, E) [18], while the curcumin-loaded samples showed interconnected porous structure.
Figure 3. SEM images of lyophilized silk-curcumin hydrogels.
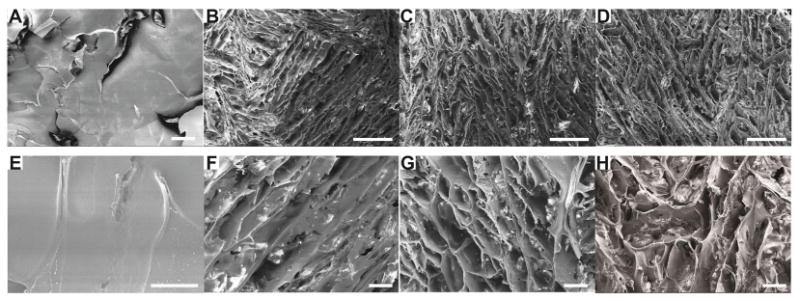
Curcumin concentration in silk hydrogels was 0 mg/mL (A, E); 0.1 mg/mL (B, F); 0.25 mg/mL (C, G) and 0.5 mg/mL (D, H). Scale bars: A, F, G and H, 50 μm; B, C, D, 250 μm; E, 25μm.
3.2. Distribution of curcumin in silk films
The thickness of the dry silk-curcumin films was approximately 20 μm, as determined by a caliper and SEM imaging (Figure 4). Curcumin emitted green fluorescence in silk films. With the scan proceeding from the top to the bottom of the film with 1 μm interval, the green fluorescence changed from weak to high to weak, covering the full film thickness (A-F in the upper images in Figure 4). Images C,D,E in Figure 4 with scanning depth of 15-35 μm and relatively high fluorescence intensities exhibited the distribution of curcumin in the film from the top surface (facing the air) to the bottom (attaching the dish). The curcumin distribution was relatively homogeneous in the silk film, although the concentration was a bit higher in the middle than at the edge of the film. The weak fluorescence determined near the top and bottom of the film was likely due to the surface roughness, leading to uneven distribution of fluorescence on the images (Figure 4). Washing and drying the film did not change curcumin distribution (data not shown).
Figure 4.
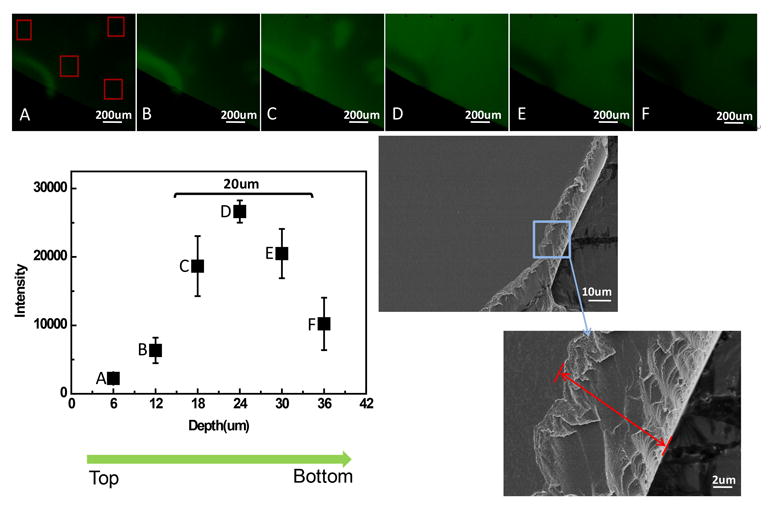
Distribution of curcumin in silk-curcumin hydrogel films by confocal microscopy. (A-F) Selected z-series images showed the distribution of curcmin with increasing film depth. The fluorescent intensity was measured by Image J software. The thickness of silk-curcumin film was measured in the cross-sectioal SEM images of the film.
3.3 Bioactive curcumin entrapped in silk hydrogel films
Anti-oxidation activity of curcumin entrapped in silk hydrogel films was examined using a DPPH assay. After incubation at 37 °C and 60 °C the films were pre-wet to allow DPPH substrate penetration into the film matrix to react with the entrapped curcumin. Pilot experiments showed no significant difference in DPPH activity for sample films containing curcumin concentrations above 0.5 mg/mL, even at 60 °C. Thus, subsequent stability and activity studies were performed at low curcumin concentrations (0.025 and 0.05 mg/mL). Figure 5A shows the samples stored in PBS buffer at two different temperatures. At 37 °C, DPPH activity remained stable for both samples, while at 60 °C, activity decreased more than two-fold within 28 days. In contrast, the DPPH activity of the samples stored dry remained stable at both temperatures (Figure 5B). Curcumin decomposes quickly under certain conditions, such as change in pH and exposure to UV light [28,29]. The data from the present study indicate that the curcumin encapsulated in silk maintained anti-oxidation activity in aqueous solution at 37 °C, which is significant for cell culture studies, such as hBMSC proliferation and differentiation.
Figure 5. Curcumin stability in silk hydrogel films.
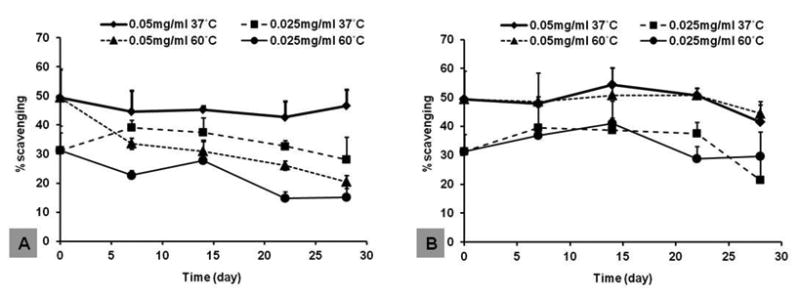
Curcumin-loaded silk films were incubated at different temperatures for 28 days, and anti-oxidant activity of curcumin was determined by DPPH. A, silk films incubated in PBS buffer, pH 7.4. B, silk films incubated in empty tubes (dry). N = 4.
3.4 Curcumin release from silk hydrogel films
When silk hydrogel films containing 0.1, 0.25, 0.5, 1 mg/mL curcumin were immersed in PBS buffer, a burst release of curcumin was recorded in the first 24 hours. For the 0.1 mg/mL sample, the highest curcumin concentration was 0.00096 ± 0.00033 μM, while it was 0.002-0.005 μM for higher concentration samples (0.25-1 mg/mL). After the burst release, curcumin concentrations dropped more than 10-fold within 48 hours for all samples (Figure 6A). The cumulative percentage release was 3-15% over 168 hours for all samples, where the main contribution was from the burst release (Figure 6B). Thus, for the curcumin-loaded samples immersed in aqueous solution, any release mainly occurred in the first 24 hours and subsequent levels released were negligible. In contrast, when the film samples were immersed in medium composed of PBS, Tween-80 and methanol, curcumin release was accelerated. The highest curcumin concentration was 0.00487 ± 0.00130 μM for the 0.1 mg/mL sample, and 0.015-0.035 μM for the other three samples with concentrations of 0.25-1 mg/mL (Figure 6C). The decline in curcumin concentration after the initial burst release was more gradual than that in PBS. For all samples, cumulative percentage release was 50-60% within a time frame of 168 hours, significantly higher than that in PBS (Figure 6D). The data confirmed the conclusion that curcumin was tightly bound to the silk hydrogel matrix and sustained release at low levels in aqueous solution.
Figure 6. Curcumin release from silk hydrogel films.
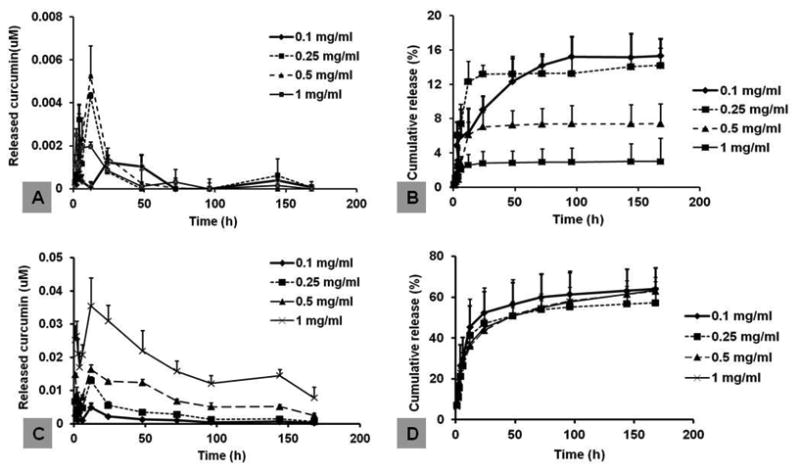
Curcumin-loaded silk hydrogel films were immersed in either PBS buffer, pH 7.4 (A, B), or PBS buffer supplemented with Tween 80 and methanol (C,D) for release study. The concentration of released curcumin in solution was determined by absorbance at 424 nm. A,C, curcumin concentrations (μM) determined in the released medium. B,D, cumulative release calculated based on original curcumin loaded to the films. N=4.
3.5 Effect of curcumin on hBMSC proliferation
To determine the effect of free curcumin on hBMSC proliferation, stock solutions of curcumin in DMSO were diluted with growth medium to obtain final concentrations of 0.1, 1, 5, 10 μM (Table 1). Medium containing 0.1% DMSO served as control (0 μM). Cell growth was not affected when the curcumin concentrations were lower than 1 μM (Figure 7, left panel), while concentrations above 5 μM significantly inhibited cell proliferation. Thus, the effect of free curcumin on hBMSCs proliferation was dose dependent, as reported in the literature [30]. Cell viability assay was conducted to determine curcumin cytotoxicity. Curcumin treatment caused a dose-dependent decrease in hBMSC cell viability. After 48-hour curcumin treatment, cell viability was not affected at curcumin concentrations ≤10 μM (Figure S1). Therefore, the cytotoxicity of curcumin should not contribute to the inhibitory effect of curcumin on cell growth in the curcumin concentration range used in this study. To determine the effect of the film-associated curcumin on hBMSC proliferation, curcumin at concentrations of 0.1, 0.25, 0.5 mg/mL were incorporated into silk hydrogel films onto which the hBMSCs were cultured. Since silk does not have specific cell attachment motifs, such as RGD, growth of hBMSCs on silk hydrogel films was slower when compared to growth on tissue culture plastic (TCP), as reported previously (Figure 7, right panel) [31]. When curcumin concentration in the hydrogel film was lower than 0.1 mg/mL, no significant difference between silk and the curcumin-silk groups was observed. A significant decrease in proliferation (p<0.05) occurred when more than 0.25 mg/mL curcumin loaded in the films (Figure 7, right panel). Cells attached to the films showed elongated cell morphology. By day 14, cells formed cell patches on plain silk film, while lower amount of cells were observed on curcumin-loaded silk hydrogel films, indicating inhibited proliferation. Based on the curcumin release experiment shown in Figure 5A, the concentration of curcumin released to the medium should be less than 0.005 μM for the 0.25 mg/mL and 0.5 mg/mL sample, which is 1,000-times lower than the 5 μM free curcumin needed to inhibit proliferation (Figure 7, left panel). Thus, inhibition of hBMSC proliferation in this case would have been caused by the curcumin concentrated at the film surface rather than the curcumin released into the medium. Although curcumin is credited with its antioxidant effect that restrains free radical-mediated lipid peroxidation and DNA damage, an increasing number of reports show that curcumin may also act as a pro-oxidant by inducing the generation of reactive oxygen species (ROS) [32]. As a result, curcumin can induce cell cycle arrest and apoptosis, leading to the inhibition of cell proliferation. It has also been found that the exposure period and dose used in the treatments determine the functions of the curcumin [33]. In future studies it would be useful to know if curcumin utilizes the same mechanisms when associated to silk versus in free solution.
Figure 7. Dose-dependent influence of curumin on hBMSC proliferation.
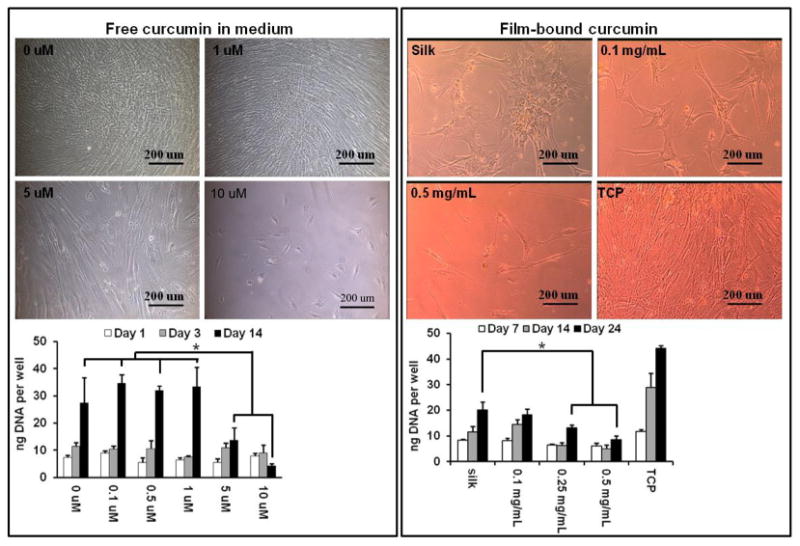
Left panel, hBMSC cultured in a medium supplemented with various amounts of curcumin. Right panel, hBMSC cultured on curcumin-loaded silk films. N = 3. *indicates significant difference (p < 0.05) between groups.
3.6 Inhibition of hBMSC adipogenesis by free curcumin
The effect of free curcumin on adipogenesis of hBMSCs was evaluated initially with transcription profiling using quantitative real-time PCR (qPCR). The four adipogenic marker genes examined were: a) the transcription factor peroxisome proliferator receptor-γ (PPAR-γ) which is critical for driving terminal adipogenic differentiation and promoting adipocyte maturation, b) the enzyme lipoprotein lipase (LPL) that catalyzes hydrolysis of lipids and lipoproteins into fatty acids in mature adipocytes, c) fatty-acid binding protein-4 (FABp4) that is involved in fatty acid uptake, transport, and metabolism, and d) the insulin-regulated glucose transporter-4 (Glut4) found in mature adipocytes [34]. Relative expression levels were obtained by comparing with a housekeeping gene, GAPDH. After 14 days of culture, the expression of three marker genes (FABp4, Glut4, LPL) was significantly (p<0.05) or very significantly (p<0.005) down-regulated for the samples treated with 10 μM free curcumin, when compared to the TCP control (Figure 8). PPAR-γ, however, was not significantly downregulated at the same curcumin concentration. The results confirmed literature reports that free curcumin within a concentration range of 5-10 μM in the medium inhibited hBMSCs adipogenesis [35]. Accumulation of lipid droplets due to adipocyte maturation was then examined by oil red O staining. The number of lipid-containing cells (stained in red color) in the 10 μM group was significantly lower than in the other groups (Figure 10, upper panel), confirming the conclusions from transcript analysis.
Figure 8. hBMSC adipogenesis in the presence of free curcumin.
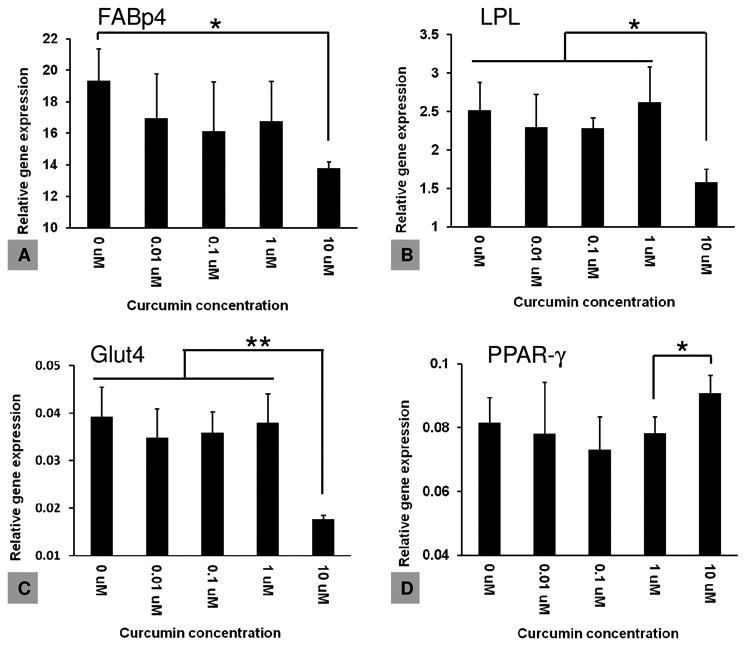
hBMSCs were cultured in an adipogenic differentiation medium supplemented with various concentrations of curcumin, and adipogenesis was evaluated by transcripts using adipogenic markers. N = 3. *indicates significant difference (p < 0.05); ** indicates very significant difference (p < 0.005)
Figure 10. hBMSC adipogenic differentiation by oil red O staining.
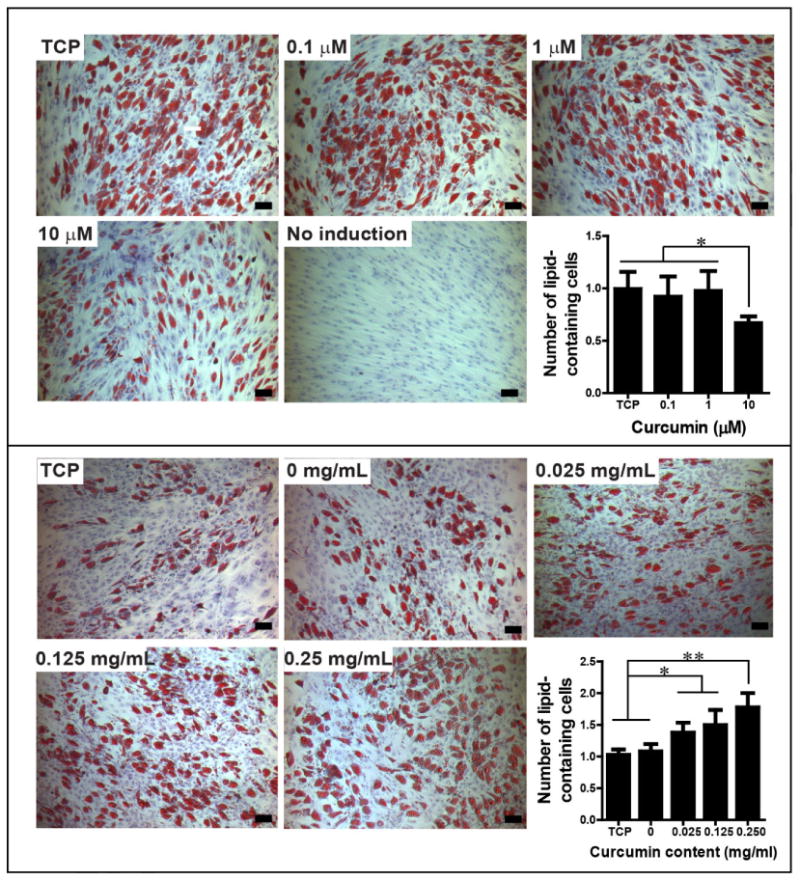
Upper panel, hBMSCs cultured in free curcumin-containing medium, day 14; Lower panel, hBMSCs cultured on curcumin-loaded silk hydrogel films, day 14. The number of lipid-containing cells was normalized to TCP control, N =4-7. *indicates significant difference (p < 0.05); ** indicates very significant difference (p < 0.005). Scale bar, 100 μm. The experiment was repeated twice with similar results.
3.7 Promotion of hBMSC adipogenesis by silk film-associated curcumin
The effect of silk encapsulated curcumin on hBMSC adipogenesis was determined using the same four adipogenic transcripts. At day 7, the expression of all the four genes was significantly (p<0.05) or very significantly (p<0.005) upregulated in the 0.125 mg/mL curcumin group when compared to the TCP, plain silk film, and 0.025 mg/mL curcumin group (Figure 9). Based on the curcumin release study in Figure 6, the amount of curcumin released to the medium in the 0.125 mg/mL group should be less than 0.001 μM, which is more than 10,000-fold lower than the concentration of 10 μM of free curcumin causing inhibition of adipogenesis (Figure 8). Curcumin distribution experiment indicated that a large portion of curcumin remained on the film surface after washing (Figure 4). Thus, the pro-adipogenesis effects observed should be due to the curcumin on the silk film surfaces. It is likely that the surface-associated curcumin molecules in the 0.125 mg/mL group was optimal to stimulate the highest cellular response. At day 14, the expression of the four adipogenic genes was still significantly upregulated in the 0.125 mg/mL curcumin group when compared to the plain silk group (p<0.05 for LPL, Glut4, PPAR-γ and p<0.005 for FABp4, Figure 9). The expression levels in the TCP and 0.25 mg/mL curcumin groups were also upregulated and the levels were significantly higher than those in the plain silk group (p<0.05, Figure 9). The lower curcumin concentration groups (0.025, 0.05 mg/mL) showed significant upregulation of FABp4 and Glut4 transcripts (p<0.05) while no significant upregulation of LPL and PPAR-γ transcripts were found, when compared to the plain silk film group at day 14 (Figure 9). The difference in hBMSC adipogenesis among the various groups was confirmed by oil red O staining of lipid droplets formed at day 14. It is noted that the degree of adipogenic differentiation varied in different experiments, as indicated by the difference in the number of lipid-containing cells in TCP samples in the two independent adipogenic assays (Figure 10). Therefore, the number of lipid-containing cells in each experiment group was normalized to TCP samples. When compared to the plain silk film group, the number of lipid-containing cells (stained in red color) on curcumin-loaded silk films was significantly higher (Figure 10, lower panel). Thus, film-associated curcumin accelerated hBMSC adipogenesis. Curcumin may integrate into the lipid bilayer of cell membranes, change membrane properties or interact with cell membrane proteins, further triggering intracellular signaling pathways, such as MAPK and Wnt/β-catenin pathways that are important for the modulation of osteogenic and adipogenic differentiation of MSCs [35].
Figure 9. hBMSC adipogenesis in the presence film-associated curcumin.
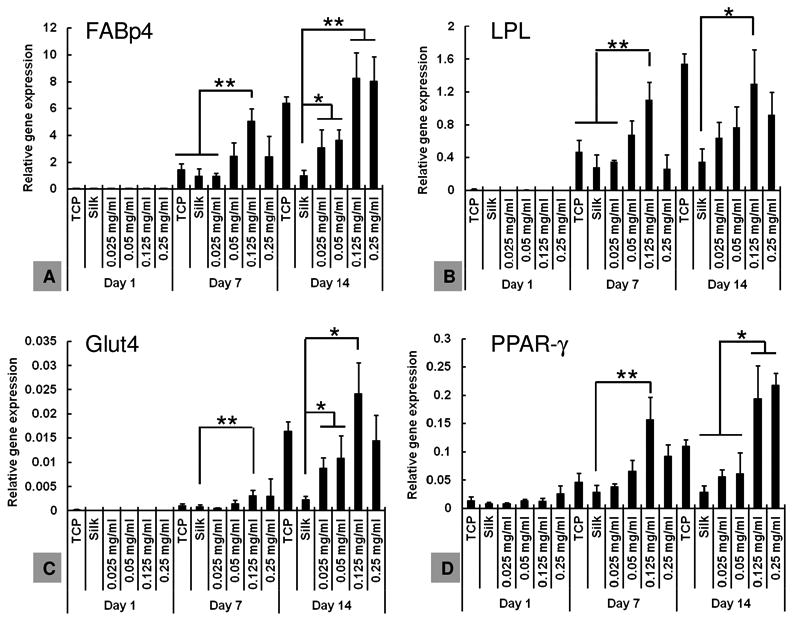
hBMSCs were seeded on curcumin-loaded silk hydrogel films, and adipogenesis was evaluated on transcript level using adipogenic markers. N = 3. *indicates significant difference (p < 0.05); ** indicates very significant difference (p < 0.005)
In the present study, silk hydrogel films were used for 2-D cell culture in order to investigate the role of curcumin in hBMSC proliferation and differentiation. Interestingly, the data obtained suggested that the effects of curcumin are highly dependent on the modes by which it interacts with cells; intracellular (free curcumin in the medium) or interfacial (surface-associated curcumin). The two different modes induced completely different cell responses, resulting in different proliferation and differentiation behavior. Unlike other studies that focused more on the dosing effect of free curcumin, this is the first time that the effect of surface-associated curcumin on hBMSCs lineage differentiation was examined and clarified. The interaction between curcumin and hBMSCs at the silk film interfaces is of great interest in the future studies. The interaction of free curcumin with cell membranes has been investigated recently by Quitschke [36]. The results clearly showed that free curcumin in the culture medium rapidly interacted with the binding sites on cell membranes, with the maximal cellular binding occuring within 0.5 h. Depending on the initial doses and exposure time used, different signal transduction pathways were activated, suggesting the affinities of curcumin for respective binding sites were crucial for the regulation of signaling pathways. Although lack of directly evidence, the author argued that the binding sites were mainly protein components, especially receptors, which competed with the albumin in the medium for curucmin. In the present study, free and silk-associated curcumin might have had different binding kinetics with the receptors on hBMSC membranes, leading to different cellular responses. The interaction of free curcumin with membrane receptors might have taken place transiently due to decomposition, whereas it persisted longer for the silk-assocaited curcumin. It would be interesting to know if curcumin dissociated from silk surface before interacting with membrane receptors, or interacted with the receptors directly without dissociation. In the future studies, functionalization within other material formats, such as 3-D porous silk scaffolds, would be of interest towards novel biomaterial systems utilizing natural anti-oxidants for tissue regeneration and tissue engineering applications.
4. Conclusions
Silk hydrogel films were a useful material format to entrap and stabilize a natural antioxidant, curcumin. The entrapment and stabilization effect was attributed to the strong binding of curcumin to silk, likely to the hydrophobic beta-sheet domains formed in silk hydrogels. Curcumin distributed homogeneously in the dried hydrogel films. Silk film-associated curcumin had almost no release to the aqueous environment and retained high antioxidant activity for at least one month. Silk film-associated curcumin significantly inhibited proliferation while promoting adipogenic differentiation hBMSCs. The effect was dose-dependent, with a critical curcumin concentration range in hydrogel films of 0.125-0.25 mg/mL. Free curcumin in cell culture medium, however, inhibited both hBMSC proliferation and adipogenesis, at a critical concentration range of 5-10 μM, 1,000-times higher than that released into the medium from silk film-associated curcumin. The present study provides evidence that curcumin delivery mode differentially affects the adipogenic differentiation of hBMSCs and also provides a new tool to functionalize biomaterial scaffolds with antioxidants for tissue regeneration.
Acknowledgments
The study was financially supported by the Natural Science Foundation of China grant (project no. 51273138) and US NIH P41 EB002520, AR061988 and AR005593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Marion NW, Mao JJ. Mesenchymal Stem Cells and Tissue Engineering. Methods Enzymol. 2006;420:339–61. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 4.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007;595:127–48. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 5.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Sig. 2008;10:511–45. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 6.Jacob A, Wu R, Zhou M, Wang P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR research. 2007;2007:89369. doi: 10.1155/2007/89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol-Gastrointest Liver Physiol. 2003;285:G20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agr Food Chem. 2005;53:959–63. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda M, Mimaki Y, Nishiyama T, Mae T, Kishida H, Tsukagawa M, et al. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bul. 2005;28:937–9. doi: 10.1248/bpb.28.937. [DOI] [PubMed] [Google Scholar]
- 10.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–68. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 11.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–25. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 12.Quitschke WW. Curcuminoid binding to embryonal carcinoma cells: reductive metabolism, induction of apoptosis, senescence, and inhibition of cell proliferation. PloS one. 2012;7:e39568. doi: 10.1371/journal.pone.0039568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung WC, Chen FY, Lee CC, Sun Y, Lee MT, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–8. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–41. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 15.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 16.Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–64. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–92. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 19.Murphy AR, St John P, Kaplan DL. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials. 2008;29:2829–38. doi: 10.1016/j.biomaterials.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–61. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Kaplan D, Cebe P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules. 2006;39:6161–70. [Google Scholar]
- 22.Kasoju N, Bora U. Fabrication and characterization of curcumin-releasing silk fibroin scaffold. J Biomed Mater Res B. 2012;100:1854–66. doi: 10.1002/jbm.b.32753. [DOI] [PubMed] [Google Scholar]
- 23.Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janderova L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes Res. 2003;11:65–74. doi: 10.1038/oby.2003.11. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto A, Chen J, Collette AL, Kim UJ, Altman GH, Cebe P, et al. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006;110:21630–8. doi: 10.1021/jp056350v. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharmaceut Biomed. 1997;15:1867–76. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 29.Tonnesen HH, Karlsen J, van Henegouwen GB. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Zeitschrift fur Lebensmittel-Untersuchung und -Forschung. 1986;183:116–22. doi: 10.1007/BF01041928. [DOI] [PubMed] [Google Scholar]
- 30.Yagi H, Tan J, Tuan RS. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J Cell Biochem. 2013;114:1163–73. doi: 10.1002/jcb.24459. [DOI] [PubMed] [Google Scholar]
- 31.Morgan AW, Roskov KE, Lin-Gibson S, Kaplan DL, Becker ML, Simon CG., Jr Characterization and optimization of RGD-containing silk blends to support osteoblastic differentiation. Biomaterials. 2008;29:2556–63. doi: 10.1016/j.biomaterials.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005;25:4029–36. [PubMed] [Google Scholar]
- 33.Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol. 2006;44:1362–71. doi: 10.1016/j.fct.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 35.Gu Q, Cai Y, Huang C, Shi Q, Yang H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn Mag. 2012;8:202–8. doi: 10.4103/0973-1296.99285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quitschke WW. Curcuminoid binding to embryonal carcinoma cells: reductive metabolism, induction of apoptosis, senescence, and inhibition of cell proliferation. PLoS ONE. 2012;7:e39568. doi: 10.1371/journal.pone.0039568. [DOI] [PMC free article] [PubMed] [Google Scholar]


