Abstract
Obesity is associated with increased liver cancer risks and mortality. We recently showed that apo-10’-lycopenoic acid, a lycopene metabolite generated by beta-carotene-9’,10’-oxygenase (BCO2), inhibited carcinogen-initiated, high-fat diet (HFD)-promoted liver inflammation and hepatic tumorigenesis development. The present investigation examined the outstanding question of whether the lycopene could suppress HFD-promoted hepatocellular carcinoma (HCC) progression, and if BCO2 is important in BCO2-knockout (BCO2-KO) and wild-type male mice. Results showed that lycopene supplementation (100 mg/kg diet) for 24 weeks resulted in comparable accumulation of hepatic lycopene (19.4 vs 18.2 nmol/g) and had similar effects on suppressing HFD-promoted HCC incidence (19% vs 20%) and multiplicity (58% vs 62%) in wild-type and BCO2-KO mice, respectively. Intriguingly, lycopene chemopreventive effects in wild-type mice were associated with reduced hepatic pro-inflammatory signaling (phosphorylation of nuclear factor-κB p65 and signal transducer and activator of transcription 3; interleukin-6 protein) and inflammatory foci. In contrast, the protective effects of lycopene in BCO2-KO but not in wild-type mice were associated with reduced hepatic endoplasmic reticulum stress-mediated unfolded protein response (ERUPR), through decreasing ERUPR-mediated protein kinase RNA-activated like kinase– eukaryotic initiation factor 2α activation, and inositol requiring 1α–X-box binding protein 1 signaling. Lycopene supplementation in BCO2-KO mice suppressed oncogenic signals including Met mRNA, β-catenin protein, and mammalian target of rapamycin (mTOR) complex 1 activation, which was associated with increased hepatic microRNA (miR)-199a/b and miR-214 levels. These results provided novel experimental evidence that dietary lycopene can prevent HFD-promoted HCC incidence and multiplicity in mice, and may elicit different mechanisms depending on BCO2 expression.
Keywords: liver cancer, prevention, lycopene metabolism, inflammation, endoplasmic reticulum stress
Introduction
Primary liver cancer is the third leading cause of cancer deaths worldwide (1, 2), and hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for 70-85% of cases (1, 2). Non-alcoholic fatty liver disease (NAFLD) is a pathology that is observed in 75-100% of overweight and obese adults and children (3), and its rising prevalence parallels closely with HCC's escalating morbidity and mortality trends (3). The prevention of liver cancer progression through dietary means represents an important disease control strategy because HCC has a high mortality rate and a poor prognosis (4, 5).
Previous animal studies demonstrated that high fat diet (HFD) and obesity promoted liver tumorigenesis by inducing chronic inflammation through the interleukin-6/signal transducer and activator of transcription 3 (IL-6/STAT3) pathway (6), with STAT3-activated tumors being more aggressive in humans (6-8). Metabolic surplus from excess calorie consumption can also elevate synthesis of hepatic enzymes, which creates excess demand on the endoplasmic reticulum (ER) for proper protein folding (9-11). This excess demand on the ER leads to the induction of ER stress-mediated unfolded protein response (ERUPR) (9-11), which was associated with liver cancer development (11).
Observational studies have shown beneficial associations between lycopene-rich foods against various cancers (as reviewed in (12-14)), including those of gastrointestinal tract origin (14). Patients with NAFLD have significantly reduced plasma lycopene (15), suggesting a potential interactions between low lycopene status and the development of liver diseases. In the rat model, dietary lycopene has been shown to reduce the liver-specific carcinogen diethylnitrosamine (DEN)-initiation of hepatic preneoplastic foci and macroscopic nodules (16-18), inhibit hepatic glutathione S-transferase placental-form positive foci in rats that develop spontaneous liver tumors (19), and ameliorate DEN-initiated, HFD-promoted precancerous lesions (20). However, the primary outcomes for these rat studies were hepatic preneoplastic lesions that may develop into tumors. There are currently no published in vivo studies to demonstrate whether lycopene can effectively reduce HCC development and progression. Our mechanistic understanding of how lycopene functions against hepatic tumorigenesis is also far from complete (21).
Lycopene can be preferentially metabolized by the enzyme beta-carotene 9’,10’-oxygenase (BCO2), and generate apo-10’-lycopenoids including apo-10’-lycopenal, -lycopenol and -lycopenoic acid (APO10LA) (22, 23). It is important to understand whether lycopene effects on various cellular functions and signaling pathways are the results of intact lycopene or apo-10’-lycopenoids (13). We have recently shown that APO10LA supplementation significantly reduced hepatic inflammation (decreased inflammatory foci, tumor necrosis factor α, IL-6, NF-κB p65 protein expression and STAT3 activation) and tumorigenesis in HFD-fed mice (24). Therefore, lycopene metabolites such as APO10LA, may exhibit protective effects against obesity associated hepatic inflammation and tumorigenesis. The outstanding question is whether BCO2 expression is critical for the potential biological effects of lycopene against HFD-promoted liver tumorigenesis. This information is critically needed because nineteen single-nucleotide polymorphisms (SNPs) of BCO2 have been found in humans (25). These BCO2 SNPs in humans are associated with increased circulatory pro-inflammatory IL-18 expression (25), and with reduced circulatory high density lipoprotein (25), suggesting a gene-diet interaction between the BCO2 enzyme and dietary lycopene on human health outcomes. We hypothesize that lycopene is effective in inhibiting HFD-promoted liver tumorigenesis, and lycopene biological actions could be different in the absences of BCO2 expression.
Utilizing the BCO2 knockout (BCO2-KO) and wild-type mice in the present study, we investigated the potential inhibitory effects of lycopene against HFD-promoted hepatic tumorigenesis, and elucidated the underlying mechanisms by which lycopene exhibited these chemopreventive effects.
Materials and Methods
Study Design
The in vivo experimental protocol was adapted from previous publications that studied hepatic tumorigenesis (6, 24, 26). All study protocols were approved by the Institutional Animal Care and Use Committee at the Jean Mayer-USDA Human Nutrition Research Center on Aging at Tufts University. The generation of BCO2-KO mice with BCO2 ablation at the protein level was described in previous study (27). The respective wild-type control mice with a 129SvJ/SvEvTac F1 generation mixed genetic background were established by conventional cross breeding in our animal facility. The rationale for the selected wild-type background was based upon the embryonic stem (ES) cell mouse strain utilized to generate the BCO2-KO mice (27). Therefore, utilizing mice that share the same genetic background as these ES cells would sufficiently represent the biological effects of BCO2 enzyme expression. The schematic for the study design is shown in Figure 1A. Study mice were fed the standard laboratory chow (Harlan Laboratories, MA, USA), maintained on a 12-hour light/dark cycle in a controlled temperature and humidity room, and given water ad libitum. Two-week-old male wild-type and BCO2-KO mice were injected intraperitoneally with a liver-specific carcinogen, diethylnitrosamine (DEN; Sigma-Aldrich, USA) at a dosage of 25 mg/kg body weight as previously described (6, 24). At 6 weeks of age, wild-type and BCO2-KO mice were randomized to either an obesogenic HFD (F6635; Bio-Serv, NJ, USA; WT, wild-type on HFD; KO, BCO2-KO on HFD), in which 45% of energy was fat derived, or the same HFD supplemented with lycopene (WT+Ly or KO+Ly; 100 mg/kg diet) for 24 weeks (Figure 1A). All study mice were weighed weekly, given fresh diets every 2-3 days, and maintained on their respective diets until the experiment was completed. Mice were euthanized at 30 weeks of age by exsanguination under deep anesthesia without being food deprived.
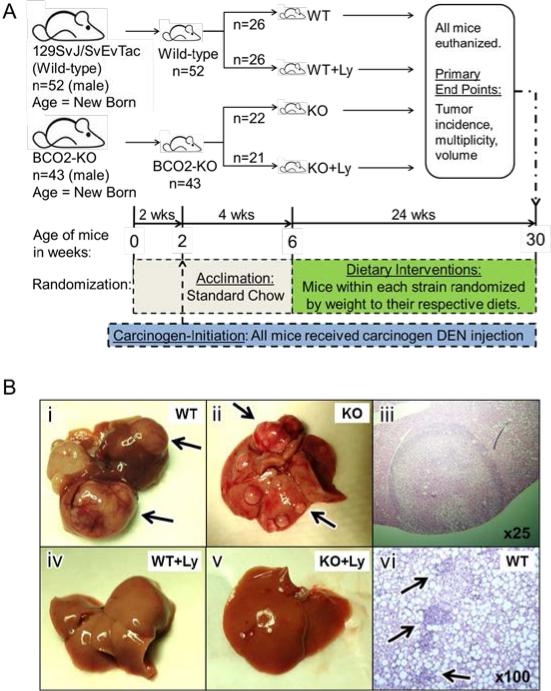
Figure 1. Study design, gross and histopathology of liver tumor and inflammation.
Panel A. Schematic for study design. Panel B: Representative picture or light micrograph of livers from WT, WT+Ly, KO or KO+Ly: B-i, liver from WT with tumors (arrows). B-ii, liver from KO with tumors (arrows). B-iii, H&E stained liver tumor at 25X. B-iv, liver from WT+Ly. B-v, liver from KO+Ly. B-vi, H&E stained liver from WT with inflammatory cell infiltration (arrows) at 100X. BCO2, beta-carotene-9’,10’-oxygenase; BCO2-KO, BCO2 knockout; DEN, diethylnitrosamine; HFD, high fat diet; KO, knockout on HFD; Ly, lycopene; WT, wild-type on HFD.
Lycopene treatment
Both lycopene in the form of a 10% lycopene beadlet or placebo beadlet without lycopene (BASF, Ludwigshafen, Germany) was incorporated directly into the HFD to achieve a homogenous diet mixture. Only lycopene but not lycopene metabolites was detectable in the lycopene-supplemented diets using our HPLC analysis. Both diets were made every 2-4 weeks, and were kept at 4°C (< 1 week) or −20°C (up to 4 weeks) inside opaque boxes. The rationale for selecting this lycopene dose was based on the assumption that BCO2-KO and wild-type mice have similar carotenoid absorption as other strains of mice on a carotenoid-supplemented semi-purified diet (~1/10 of human absorption) (28-30). An established equation was used to calculate the dosage equivalence for human consumption (31, 32), which indicated that the lycopene supplemented dose of 100 mg/kg diet is equivalent to approximately 8.1 mg lycopene/day in a 60 kg adult man. The average human dietary lycopene is approximately 8 mg/day (12, 33), and lycopene doses used in dietary supplements are between 15-30 mg/day.
Liver tumors quantification and liver tissue processing
Whole livers were removed from study mice after euthanization and processed as previously described (24). Briefly, two investigators unaware of treatment groups counted the surface liver tumors (tumor multiplicity). Livers were weighed and washed with saline for further processing. Surface liver tumors were removed, snap-frozen in liquid nitrogen and stored at −80°C. The left lobe of mouse liver was fixed in 10% buffered formalin solution (Thermo Fisher Scientific, USA), processed and embedded in paraffin for serial sectioning as described in previous study (24). The remaining sections of liver were divided into smaller portions, snap-frozen in liquid nitrogen and stored at −80°C.
Histopathological evaluation of liver tissue
Five μm sections of formalin-fixed, paraffin-embedded liver tissue were stained with hematoxylin and eosin (H&E) for histopathological examination. H&E stained liver slides were examined by two independent investigators blinded to treatment groups under light microscopy (Zeiss, USA). Liver histopathology of non-tumor areas was graded in 20 random fields at 100x magnification, according to the degree of liver inflammation severity as described previously (24, 34). Briefly, inflammatory foci were evaluated by the number of inflammatory-cell clusters, which mainly constitute the infiltration of mononuclear inflammatory cells. Mean foci per field was calculated and reported as inflammatory-cell clusters per cm2. The liver tumor was confirmed as HCC by two independent investigators according to the following criteria: 1) the presence of trabecular pattern with 3+ cell-thick hepatocellular plates/cords; 2) mitotic figure; 3) enlarged convoluted nuclei or high nuclei/cytoplasmic ratio; 4) the presence of tumor giant cells with compact growth pattern; and 5) the presence of endothelial cells lining of sinusoids that surround enlarged hepatocellular plates/cords.
HPLC analysis
Lycopene (all-trans and 5-, 9- and 13-cis-isomers) and lycopene metabolites including apo-10’-lycopenal, apo-10’-lycopenol and apo-10’-lycopenoic acid concentrations in liver tissue and diets were measured by gradient reverse phase HPLC consisted of a Waters 2695 separations module and a Waters 2996 photodiodearray detector (Waters, USA) as previously described (23, 29). Lycopene and metabolites were quantified relative to the internal standard by determining peak areas calibrated against known amounts of standard.
RNA and microRNA (miR) extraction and quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from frozen liver sections with Trizol reagent (Invitrogen, USA), as previously described (24). cDNA was prepared from the RNA samples using M-MLV (for mRNA; Invitrogen, USA) or M-MuLV (for miR; BioLabs, MA, USA) reverse transcriptases and an automated thermal cycler PTC-200 (MJ Research, USA). qRTPCR was performed using FastStart Universal SYBR Green Master (ROX) (Roche, USA). Relative gene expression was determined using the 2-△△CT method. Primer sequences are listed in Supplementary Table S1.
Protein isolation and western blotting
Both tissue protein preparation and Western blotting analysis were as described previously (24). The following antibodies were used for western blotting: mammalian target of rapamycin (mTOR), nuclear factor kappa-B (NF-κB) p65, eukaryotic initiation factor (eIF) 2α, phosphorylated-eIF2α (Ser51), phosphorylated NF-κB p65 (Ser536), (phosphorylated-STAT3 (Tyr705), phosphorylated S6 (Ser235/236), S6, STAT3 (Cell Signaling, MA, USA), IL-6 (R&D, MN, USA), CCAAT/enhancer-binding protein homology protein (CHOP), and cyclin D1 (Santa Cruz, TX, USA). Proteins were detected by a horseradish peroxidase-conjugated secondary antibody (Bio-Rad, CA, USA). The specific bands were visualized by a SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce, IL, USA) according to the manufacturer's instructions. Dilution series and calibration curve were performed for each of the antibodies used to quantify protein. Anti-actin antibody (Sigma-Aldrich) was used to detect β-actin for loading normalization of some proteins. Intensities of protein bands were quantified using GS-710 Calibrated Imaging Densitometer (Bio-Rad).
Statistical analysis
SAS 9.3 software was used to perform the statistical analysis. Two-way ANOVA analysis with Tukey's adjustments for multiple comparisons was used to evaluate the effects of BCO2 protein expression, lycopene supplementation, and the potential interactions between these two factors. Chi-square test was used to examine the effects of mouse strains or dietary lycopene on liver tumor incidence. Student's t-test or Wilcoxon signed-rank test was used to test for the differences between the following comparisons: 1) WT and WT+Ly; 2) KO and KO+Ly. Statistical significance was p < 0.05.
Results
Lycopene supplementation inhibited HCC development in both wild-type and BCO2-KO mice without altering body/liver weights
Food intake by weight was similar among the four groups of mice (Table 1). Lycopene had no significant effect on body or liver weight in either mouse strain (Table 1), although BCO2-KO mice exhibited significantly lower final body and liver weights than wild-type mice (Table 1). DEN-initiation resulted in visible and multiple surface liver tumors in HFD-fed BCO2-KO and wild-type mice (Figure 1B-i through 1B-iii). Liver histopathology of H&E stained, formalin-fixed, and paraffin-embedded liver tumors classified all observed tumors to be either well-, moderate-, or poorly differentiated HCC (Figure 1B-iii). Dietary lycopene supplementation significantly decreased the HCC incidence (17% for wild-type; 20% for BCO2-KO; Table 1) and multiplicity (58% for wild-type; 61% for BCO2-KO; Table 1; Figure 1B-i through 1B-v), irrespective of mouse strain. Hepatic lycopene concentrations were 19.4 and 18.2 nmol/g tissue in WT+Ly and KO+Ly mice respectively, and were not detectable in mice without supplementation (Table 1). Although there was no significant effect of mouse strain on total hepatic lycopene concentrations with lycopene supplementation (Table 1), BCO2-KO mice accumulated a great proportion of hepatic all-trans lycopene (65%), as compared with WT (51% as all-trans lycopene, Table 1). Using our HPLC analysis, we did not detect measurable amounts of lycopene metabolites in hepatic tissue from all groups of mice (data not shown).
Table 1.
Body weights, liver weights, food consumption, hepatic lycopene concentration, inflammatory foci and tumor outcomes of DEN-induced wild-type or BCO2-KO mice with or without lycopene supplementation for 24 weeks.1
| Study Group: | Wild-type | BCO2-KO | p-values for Two-way ANOVA or Wilcoxon signed-rank test | |||||
|---|---|---|---|---|---|---|---|---|
| WT | WT | KO | KO | Overall | Diet Effect | Strain Effect | Diet1 Strain Effect | |
| Animal (n) | 26 | 26 | 22 | 21 | ||||
| Food Consumption, g/d | 2.6±0.1 | 3.1±0.5 | 3.0±0.1 | 2.9±0.1 | 0.35 | 0.59 | 0.54 | 0.11 |
| Final Body Weights, g | 52.2±1.1 | 49.1±1.3 | 43.6±1.9 | 41.6±1.7 | <0.01 | 0.06 | <0.01 | 0.71 |
| Liver Weight, g | 2.4±0.2 | 2.3±0.1 | 1.5±0.1 | 1.3±0.1 | <0.01 | 0.14 | <0.01 | 0.98 |
| Liver/Body Weight, % | 4.6±0.2 | 4.6±0.2 | 3.4±0.1 | 3.2±0.1 | <0.01 | 0.64 | <0.01 | 0.55 |
| Liver Tumor Outcomes: | ||||||||
| Incidence, % | 88 | 71 | 100 | 80 (p=0.10) | NA | 0.03 | 0.48 | NA |
| Multiplicity, n | 17.8±4.5 | 7.4±1.81 | 10.3±2.2 | 4.0±0.71 | 0.05 | 0.01 | 0.52 | 0.98 |
| Hepatic Lycopene, nmol/g tissue | ND | 19.4±4.11 | ND | 18.2±3.21 | 0.01 | <0.01 | 0.82 | 0.90 |
| All-trans:cis-isomers, % | ND | 51:49 | ND | 65:35 | NA | NA | <0.01 | NA |
| Inflammatory Foci, number/cm2 | 0.95±0.6 | 0.55±0.1 (p=0.06) | 0.0±0.0 | 0.1±0.1 | NA | 0.29 | 0.04 | NA |
Values are means ± SEMs or n (%). Two-way ANOVA was used to examine the overall, diet, strain, and diet*strain effects. Student's t-test, Chi-square test or Wilcoxon signed-rank test was used to compare between WT and WT+Ly or KO and KO+Ly.
Different from WT or KO, P < 0.05. NA, not applicable; ND, not detected.
Lycopene-mediated suppression in hepatic tumorigenesis was associated with reduced hepatic inflammation in wild-type mice
Numerous animal studies suggested that HFD-promoted liver tumorigenesis was associated with an elevated pro-inflammatory response, by inducing the NF-κB, and the IL-6/STAT3 signaling pathway (6, 7, 35). In the present study, H&E staining of liver tissues showed the infiltration of inflammatory cells in wild-type and BCO2-KO mice (Figure 1B-vi). Lycopene supplementation reduced the number of hepatic inflammatory foci (58%; p=0.06; Table 1) in wild-type mice. Lycopene supplementation in wild-type mice significantly reduced hepatic pro-inflammatory biomarkers including IL-6 (58%; Figure 2A) protein expression, phosphorylation of NF-κB p65 (Ser536; 42%; Figure 2B) and STAT3 (Tyr705; 43%; Figure 2C), as compared to non-supplemented mice. None of these lycopene-mediated modulations were observed in BCO2-KO mice.
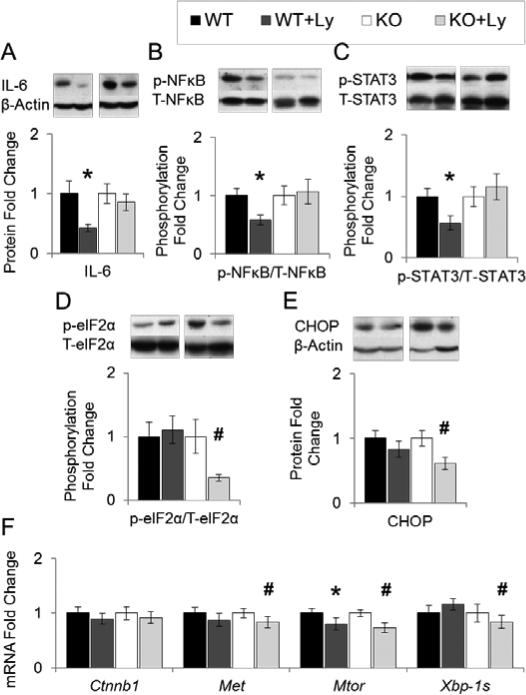
Figure 2. Effects of lycopene supplementation on hepatic pro-inflammatory and ER-stress biomarkers.
Study design as described in Figure 1. Protein or mRNA expression in liver lysates (WT, WT+Ly, KO, KO+Ly n=16-20) were analyzed by western blotting or qRT-PCT, and β-Actin was used as loading control unless specified otherwise. Graphical representation of fold changes in: A, IL-6. B, NF-κB p65 (Ser536) phosphorylation (NF-κB p65 as loading control). C, STAT3 (Tyr705) phosphorylation (STAT3 as loading control). D, eIF2α (Ser51) phosphorylation (eIF2α as loading control). E, CHOP. F, Ctnnb1, Met, Mtor, and Xbp-1s mRNA. Representative western blots with 1 sample per group are shown. Fold changes normalized to WT or KO. Values are means ± SEMs. * Different from WT, and # different from KO, P < 0.05. CHOP, CCAAT/enhancer-binding protein homology protein; Ctnnb1, β-catenin mRNA; eIF2α, eukaryotic initiation factor 2α; IL, interleukin; KO, knockout on HFD; Ly, lycopene; mTOR, mammalian target of rapamycin; XBP1, X-box binding protein; NF-κB, nuclear factor-κB; p-, phosphorylated; STAT3, signal transducer and activator of transcription 3; T-, total; WT, wild-type on HFD.
Reduced HCC development by dietary lycopene was associated with attenuated expression of hepatic ER stress markers in BCO2-KO but not wild-type mice
Dietary lycopene significantly suppressed markers of ERUPR-mediated protein kinase RNA-activated like kinase (PERK)-eIF2α signaling in BCO2-KO mice, but not wild-type mice, including the activation of eIF2α by phosphorylation (65%; Figure 2D), and the expression of CHOP protein (39%; Figure 2E). Similarly, lycopene supplementation also significantly reduced ERUPR-mediated activation of inositol requiring (IRE) 1α - X-box binding protein 1 (XBP1) system in BCO2-KO but not wild-type mice, as measured by the splicing of XBP1 mRNA (Xbp-1s; 16%; Figure 2F).
Lycopene supplementation was associated with decreased mTOR activation and protooncogene Met expression in BCO2-KO but not wild-type mice
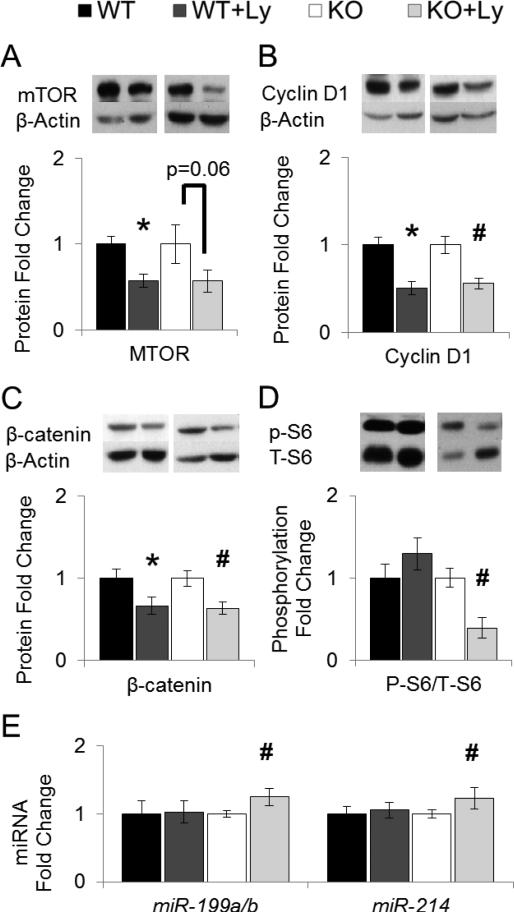
Chronic mTORC1 activation promoted HCC development in mice, through inducing mTOR protein expression and the activation of S6 ribosomal protein by phosphorylation (36). Elevation in proto-oncogenes Met and β-catenin are both positively associated with increased hepatocarcinogenesis, partially through promoting cell proliferation (37, 38). Lycopene chemopreventive effects in BCO2-KO mice was associated with reduced Mtor mRNA (27%; Figure 2F), mTOR protein (43%; p=0.06; Figure 3A), Met mRNA (17%; Figure 2F), cell proliferation marker cyclin D1 protein (44%; Figure 3B), β-catenin protein (33%; Figure 3C), but not β-catenin mRNA (Ctnnb1; Figure 2F). In wild-type mice, dietary lycopene also lessened Mtor mRNA (20%; Figure 2F), mTOR protein (42%; Figure 3A), cyclin D1 protein (44%; Figure 3B) and β-catenin protein (37%; Figure 3C) expression. However, lycopene reduced mTOR signaling as measured by S6 ribosomal protein phosphorylation was only observed in BCO2-KO mice (61%, Figure 3D), but not in wild-type mice. miR-199a/b directly targeted Mtor and Met mRNA, leading to the subsequent down-regulation of their protein products (37, 39). miR-214 induction was shown to reduce β-catenin protein without altering its mRNA (Ctnnb1) expression (38, 40). Decrement in miR-199a/b and miR214 have been associated with HCC development (37, 38, 40-42) , and linked to ERUPR (41). We observed that the lycopene-mediated reduced ERUPR in BCO2-KO mice coincided with the significant elevation in hepatic miR-199a/b (25%; Figure 3E) and miR-214 expression (23%; Figure 3E), but not in wild-type mice.
Figure 3. Effects of lycopene supplementation on hepatic tumorigenic biomarkers and miRNA expression.
Study design as described in Figure 1. Protein or miRNA expression in liver lysates (WT, WT+Ly, KO, KO+Ly n=16-20) were analyzed by western blotting and β-Actin was used as loading control unless specified otherwise. Graphical representation of fold changes in: A, mTOR. B, Cyclin D1. C, β-catenin. D, S6 (Ser235/236) phosphorylation (S6 as loading control). E, miR-199a/b and miR-214 (5S as loading control). Representative western blots with 1 sample per group are shown. Fold changes normalized to WT or KO. Values are means ± SEMs. * Different from WT, and # different from KO, P < 0.05. KO, knockout on HFD; Ly, lycopene; miR, microRNA; mTOR, mammalian target of rapamycin; p-, phosphorylated; T-, total; WT, wild-type on HFD.
Discussion
To the best of our knowledge, the present study provides the first experimental evidence that lycopene supplementation is effective in inhibiting DEN-initiated HCC incidence and multiplicity in two different strains of mice, the BCO2-KO strain and its respective wild-type. The final body weight difference between wild-type and BCO2-KO mice in the present study did not impede the beneficial effects of dietary lycopene against HCC development. This result underscores the potential chemopreventive effects of dietary lycopene against HFD-promoted tumorigenesis in mice, regardless of the amount of body weight gain. Moreover, the hepatic lycopene concentrations in lycopene-supplemented mice (18.2-19.4 nmol/g tissue) were within ranges for humans (0.1-20.7 nmol/g tissue) (43). Therefore, we believe that the lycopene supplemented dosage used in the present study was physiologically relevant to lycopene biological effects in human conditions. It should be pointed out that the in vivo study design for the present study was selected to investigate how lycopene can inhibit HFD-promoted hepatic tumorigenesis after the carcinogen initiation (e.g., i.p injection of DEN to the animals at two weeks of age). It was not our intention to evaluate lycopene effects on the initiation stage of hepatocarcinogenesis in the current study.
The present study suggests that the molecular mechanisms for lycopene chemopreventive effects may be mouse-strain specific. The lycopene-mediated chemoprevention in wild-type mice was associated with reduced hepatic inflammatory foci, lowered hepatic IL-6 protein, as well as with decreased activation of NF-κB p65 (by phosphorylation) and the oncogenic transcription factor STAT3. These lycopene-mediated mechanistic modulations were similar to the chemopreventive effects of the lycopene metabolite APO10LA in C57Bl/6J wild-type mice (24). Intriguingly, our study also revealed that dietary lycopene exhibits chemopreventive effects in the absence of BCO2 expression. In contrast to wild-type mice, lycopene-mediated chemopreventive effects in BCO2-KO mice were associated with reduced ERUPR (IRE1α-XBP1, PERK-eIF2α) and mTORC1 activation, as well as with suppressed oncogenic Met gene and β-catenin protein expression.
Elevated ERUPR is associated with liver cancer development (11). ERUPR consists of three distinct pathways regulated by ER membrane-bound proteins: IRE1α-XBP1 system, PERK-eIF2α signaling, and activating transcription factor (ATF) 6α (9-11). Elevated IRE1α and ATF6α signaling activation in HCC tissue were correlated with increased severity of HCC histological grading (44), and can induce PERK-eIF2α signaling (44). Induced proto-oncogene Met expression, β-catenin protein and chronic mTORC1 activation through S6 phosphorylation promoted HCC development in mice (36-38). These oncogenic signals can be stimulated by ERUPR through suppressing miR-199a/b and miR-214 expression (45-48). The miRNA profile or the “miRNome” identified in human liver tumors found that miR-199a/b and miR-214 are decreased in human HCC (37, 39, 41, 42). Interestingly, we observed in BCO2-KO mice that lycopene-mediated ERUPR inhibition coincided with increased miR-199a/b and miR-214 expression. miR-199a/b up-regulation inhibited proliferation and invasiveness of HCC cell lines (42). miR-199a/b can directly degrade proto-oncogene Met and Mtor mRNA and reduced their encoded protein products (37, 39), whereas transfection of miR-199a/b into HCC-derived cell lines inhibited phosphorylation of S6 (37). The results observed in this present study suggested that lycopene chemopreventive effects in BCO2-KO mice were associated with reduced mTORC1 activation, potentially through ameliorating ERUPR.
Lycopene supplementation in wild-type mice also reduced mTOR mRNA and protein, but had no effects on S6 phosphorylation. The mTORC1 pathway integrates inputs from at least five cellular and extracellular signaling, and mTORC1 kinase activity can be modulated by modifying mTOR-associated proteins within the mTORC1 complex (49). It is plausible that lycopene supplementation in wild-type mice induced upstream signaling(s) that increased mTORC1 activity and counteracted the suppressive effect on S6 phosphorylation from mTOR protein reduction.
Down-regulation of hepatic miR-214 was associated with cell growth, cell invasion, stem-like traits and early recurrence of HCC (38, 40). miR-214 overexpression inhibited proliferation of HCC cells in vitro (41), reduced HCC tumorigenicity and β-catenin protein in vivo (38). We observed in BCO2-KO mice that lycopene-induced hepatic miR-214 expression was associated with decreased cell proliferation marker cyclin D1 protein, β-catenin protein but not mRNA. These results from the present study were consistent with previous findings, where miR-214 reduced β-catenin protein via inhibiting β-catenin mRNA translation (38, 40). Future investigation is needed to elucidate how lycopene ameliorated β-catenin and cyclin D1 protein expression in wild-type mice, without altering miR-214 expression. It should be noted that lycopene chemopreventive effects may be mediated through multiple mechanisms in addition to those examined in this study. Lycopene is a natural dietary agent that may inhibit tumorigenesis. It may not deliver comparable potent effects as other pharmacological drugs against tumor progression, as shown by its modest effects on miR199a/b and miR214 induction. During our manuscript preparation, Tan et al (50) showed that lycopene-mediated hepatic gene regulation in mice could be dependent or independent of BCO2 status. The beta-carotene-15,15’-oxygenase (BCO1) is responsible for the central cleavage of carotenoids at the 15,15’ double bond (51-54). It remains controversial as to whether lycopene is a potential substrate for BCO1, as numerous studies found no detectable activity of BCO1 towards lycopene (21, 52, 54). Nevertheless, the central cleavage product apo-15-lycopenal (acyclo-retinal) was recently detected when lycopene was incubated in purified recombinant human BCO1 (55), and previously in recombinant murine BCO1 (56). Since we did not see a difference on hepatic lycopene levels between WT and BCO2 KO mice, it is possible that lycopene can be cleaved by both BCO1 and BCO2. Apo-15-lycopenoic acid (acyclo-retinoic acid), an oxidative products of apo-15-lycopenal, is structurally similar to acyclic retinoic acid (21). Treatment with acyclic retinoids was shown to inhibit secondary primary tumors in patients with hepatocellular carcinoma (57). Further investigations with BCO1/BCO2 double KO mice are currently ongoing in our laboratory to examine if lycopene metabolites generated by BCO1-mediated cleavage inhibit hepatic tumorigenesis.
We observed greater hepatic all-trans lycopene (65%) accumulation in BCO2-KO than WT mice (51%). This observation is different from results published by Tan et al (50), where they found that BCO2KO mice accumulated marginally higher % of cis lycopene (68%) than WT mice (63%). The disparities between our results and those published by Tan et al could be due to the difference in lycopene dosage (100 vs 250mg/kg diet), length of lycopene supplementation (24 vs 3 weeks), or the strains of mice selected as the WT (129SvJ/SvEvTac F1 vs C57BL/6 x 129/SvJ F1). Interestingly, certain members of the carotenoid cleavage enzyme family has intrinsic isomerase activity concurrently with carotenoid cleavage (58-60). For example, BCO1-mediated conversion of 9-cis-β-carotene to 9-cis-retinal occurred with a sub-optimal output, indicating that this enzyme can catalyze cis to trans isomerization (59). It is plausible that the BCO1 isomerization capacity in our BCO2-KO study mice in conjunction with a long-term lycopene supplementation yielded the observed hepatic lycopene isomers distribution. Future investigation is also required to determine whether BCO2 can function as an isomerase.
In summary, our results demonstrated that lycopene elicited differential mechanism of chemopreventive effects against hepatic tumorigenesis in mice depending on the present or absence of BCO2. The lycopene-mediated chemopreventive effects were associated with reduced hepatic inflammatory responses in wild-type mice, but were associated with inhibition of ERUPR response in BCO2 knockout mice. Together with our previous report on APO10LA's efficacy against liver cancer, these findings suggest that both lycopene and lycopene metabolites could be effective dietary agents for preventing liver cancer or reducing cancer risk for patients with NAFLD.
Supplementary Material
Acknowledgements
We thank Drs. Alice H. Lichtenstein and Martin S. Obin for their valuable comments. We also thank Ms. Kang-Quan Hu and other members of the Nutrition and Cancer Biology Laboratory, Dr. Donald E. Smith of the Comparative Biology Unit, Mr. John N. Lomartire, as well as Ms. Shahin Sarkarati Smith and Ms. Stephanie Thea Leon Valliere of the Nutrition Evaluation Laboratory (HNRCA at Tufts University) for their assistance and support.
Funding: This work was supported by the NIH grants CA104932 (X.D. Wang), CA176256 (X.D. Wang) and USDA/ARS grant 1950-51000-074S (X.D. Wang). B.C. Ip was supported by the NHLBI/NIH training grants 5T32HL069772-10 and 2T32HL069772-11A1. Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Abbreviations used
- APO10LA
Apo-10'-lycopenoic acid
- ATF
activation of transcription factor 6α
- BCO1
beta-carotene-15,15'-oxygenase
- BCO2
beta-carotene-9',10'-oxygenase
- BCO2-KO
BCO2 knockout
- CHOP
CCAAT/enhancer-binding protein homology protein
- Ctnnb1
β-catenin mRNA
- DEN
diethylnitrosamine
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERUPR
ER-stress mediated unfolded protein response
- ES
embryonic stem
- HFD
high fat diet
- H&E
hematoxylin and eosin
- IL
interleukin
- IRE1α
inositol requiring enzyme 1α
- KO
BCO2-KO mice on HFD
- Ly
lycopene
- miR
microRNA
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- NAFLD
non-alcoholic fatty liver disease
- NF-κB
nuclear factor-κB
- PERK
protein kinase RNA-activated like kinase
- p
phosphorylated
- qRT-PCR
quantitative Real-Time PCR
- SNP
single-nucleotide polymorphisms
- STAT3
signal transducer and activator of transcription 3
- T
total
- XBP1
X-box binding protein
- WT
wild-type mice on HFD
Footnotes
Disclosure of Potential Conflict of Interest: None
Reference
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J Hepatol. 2012;56:1384–91. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Liby KT. Is lycopene an effective agent for preventing prostate cancer? Cancer Prev Res (Phila Pa) 2013;6:384–6. doi: 10.1158/1940-6207.CAPR-13-0026. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–6. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–97. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 10.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–34. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 13.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214S–22S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Haussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with nonalcoholic steatohepatitis (NASH). Eur J Med Res. 2011;16:76–8. doi: 10.1186/2047-783X-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astorg P, Gradelet S, Berges R, Suschetet M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr Cancer. 1997;29:60–8. doi: 10.1080/01635589709514603. [DOI] [PubMed] [Google Scholar]
- 17.Melendez-Martinez AJ, Nascimento AF, Wang Y, Liu C, Mao Y, Wang X-D. Effect of tomato extract supplementation against high-fat diet-induced hepatic lesions. Hepatobiliary Surg Nutr. 2013;2:198. doi: 10.3978/j.issn.2304-3881.2013.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin K, Orhan C, Tuzcu M, Sahin N, Ali S, Bahcecioglu IH, et al. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr Cancer. 2014 doi: 10.1080/01635581.2014.894092. [DOI] [PubMed] [Google Scholar]
- 19.Toledo LP, Ong TP, Pinho AL, Jordao A, Jr., Vanucchi H, Moreno FS. Inhibitory effects of lutein and lycopene on placental glutathione S-transferase-positive preneoplastic lesions and DNA strand breakage induced in Wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr Cancer. 2003;47:62–9. doi: 10.1207/s15327914nc4701_8. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2010;126:1788–96. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip BC, Wang X-D. Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients. 2014;6:124–62. doi: 10.3390/nu6010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 23.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, et al. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila Pa) 2013;6:1304–16. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of beta-carotene 15,15′-monooxygenase 1 (BCMO1) and beta-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–50. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 26.Vesselinovitch SD, Mihailovich N, Rao KV. Morphology and metastatic nature of induced hepatic nodular lesions in C57BL × C3H F1 mice. Cancer Res. 1978;38:2003–10. [PubMed] [Google Scholar]
- 27.Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–59. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C-S, Chuang C-H, Hu M-L. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitam Nutr Res. 2006;76:377–84. doi: 10.1024/0300-9831.76.6.377. [DOI] [PubMed] [Google Scholar]
- 29.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–74. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 30.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr. 2012;142:405–10. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–21. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 33.Riso P, Visioli F, Grande S, Guarnieri S, Gardana C, Simonetti P, et al. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem. 2006;54:2563–6. doi: 10.1021/jf053033c. [DOI] [PubMed] [Google Scholar]
- 34.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 35.Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–88. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5:ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, et al. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–93. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z, et al. MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of beta-catenin. Biochem Biophys Res Commun. 2012;428:525–31. doi: 10.1016/j.bbrc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Lee UJ, Kim MN, Lee E-J, Kim JY, Lee MY, et al. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2). J Biol Chem. 2008;283:18158–66. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 40.Xia H, Ooi LLPJ, Hui KM. MiR-214 Targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS ONE. 2012;7:e44206. doi: 10.1371/journal.pone.0044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan Q, Wang X, Gong W, Ni L, Chen C, He X, et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS ONE. 2012;7:e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–43. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz HH, Poor CL, Wellman R, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613. doi: 10.1093/jn/121.10.1613. [DOI] [PubMed] [Google Scholar]
- 44.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 45.Lerner AG, Upton J-P, Praveen P, Ghosh R, Nakagawa Y, Igbaria A, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–64. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am J Physiol Cell Physiol. 2013;304:C1117–C26. doi: 10.1152/ajpcell.00061.2013. [DOI] [PubMed] [Google Scholar]
- 47.Oikawa D, Tokuda M, Hosoda A, Iwawaki T. Identification of a consensus element recognized and cleaved by IRE1α. Nucleic Acids Res. 2010;38:6265–73. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol. 2003;284:C273–C84. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- 49.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan H-L, Moran NE, Cichon MJ, Riedl KM, Schwartz SJ, Erdman JW, et al. β-carotene-9′, 10′-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor–, stress-, and metabolism-related gene expression in mice. J Nutr. 2014;144:431–9. doi: 10.3945/jn.113.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takitani K, Zhu C-L, Inoue A, Tamai H. Molecular cloning of the rat β-carotene 15, 15′-monooxygenase gene and its regulation by retinoic acid. Eur J Nutr. 2006;45:320–6. doi: 10.1007/s00394-006-0601-3. [DOI] [PubMed] [Google Scholar]
- 52.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving β-carotene to retinal. J Biol Chem. 2000;275:11915–20. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 53.Wyss A, Wirtz G, Woggon W-D, Brugger R, Wyss M, Friedlein A, et al. Cloning and expression of β, β-carotene 15, 15′-dioxygenase. Biochem Biophys Res Commun. 2000;271:334–6. doi: 10.1006/bbrc.2000.2619. [DOI] [PubMed] [Google Scholar]
- 54.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human β-carotene 15, 15′-monooxygenase. J Biol Chem. 2002;277:23942–8. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 55.dela Sena C, Narayanasamy S, Riedl KM, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human β-carotene 15, 15′-oxygenase (BCO1). J Biol Chem. 2013 doi: 10.1074/jbc.M113.507160. jbc. M113. 507160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, et al. Identification, expression, and substrate specificity of a mammalian β-carotene 15, 15′-dioxygenase. J Biol Chem. 2001;276:6560–5. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 57.Takai K, Okuno M, Yasuda I, Matsushima-Nishiwaki R, Uematsu T, Tsurumi H, et al. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Intervirology. 2005;48:39–45. doi: 10.1159/000082093. [DOI] [PubMed] [Google Scholar]
- 58.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308:267–9. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 59.Maeda T, Perusek L, Amengual J, Babino D, Palczewski K, von Lintig J. Dietary 9-cis-β, β-carotene fails to rescue vision in mouse models of leber congenital amaurosis. Mol Pharmacol. 2011;80:943–52. doi: 10.1124/mol.111.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sui X, Kiser PD, Lintig Jv, Palczewski K. Structural basis of carotenoid cleavage: from bacteria to mammals. Arch Biochem Biophys. 2013;539:203–13. doi: 10.1016/j.abb.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.