Abstract
Objectives
The aim of this study was to determine if the benefit of implantable cardioverter-defibrillators (ICDs) is modulated by medical comorbidity.
Background
Primary prevention ICDs improve survival in patients at risk for sudden cardiac death. Their benefit in patients with significant comorbid illness has not been demonstrated.
Methods
Original, patient-level datasets from MADIT I (Multicenter Automatic Defibrillator Implantation Trial I), MADIT II, DEFINITE (Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation), and SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) were combined. Patients in the combined population (N = 3,348) were assessed with respect to the following comorbidities: smoking, pulmonary disease, diabetes, peripheral vascular disease, atrial fibrillation, ischemic heart disease, and chronic kidney disease. The primary outcome was overall mortality, using the hazard ratio (HR) of time to death for patients receiving an ICD versus no ICD by extent of medical comorbidity, and adjusted for age, sex, race, left ventricular ejection fraction, use of antiarrhythmic drugs, beta-blockers, and angiotensin-converting enzyme inhibitors.
Results
Overall, 25% of patients (n = 830) had <2 comorbid conditions versus 75% (n = 2,518) with significant comorbidity (≥2). The unadjusted hazard of death for patients with an ICD versus no ICD was significantly lower, but this effect was less for patients with ≥2 comorbidities (unadjusted HR: 0.71; 95% confidence interval: 0.61 to 0.84) compared with those with <2 comorbidities (unadjusted HR: 0.59; 95% confidence interval: 0.40 to 0.87). After adjustment, the benefit of an ICD decreased with increasing number of comorbidities (p = 0.004).
Conclusions
Patients with extensive comorbid medical illnesses may experience less benefit from primary prevention ICDs than those with less comorbidity; implantation should be carefully considered in sick patients. Further study of ICDs in medically complex patients is warranted.
Keywords: comorbid illness, implantable cardioverter-defibrillator, outcomes, randomized trials
Sudden cardiac death (SCD) is a leading cause of death worldwide, and rates of SCD are substantially higher in patients with pre-existing structural heart disease. The implantable cardioverter-defibrillator (ICD) has been a major advancement in the prevention of SCD. Several randomized controlled trials have shown a significantly reduced rate of all-cause mortality in patients with ICDs (compared with control) across several populations with low left ventricular ejection fraction (LVEF). These include patients with low LVEF attributable to coronary artery disease or nonischemic causes accompanied by symptomatic heart failure as well as patients with clinical sustained or inducible ventricular tachyarrhythmias (1–5).
Subsequent analyses have shown the cost-effectiveness of such devices (6,7), and several analyses have attempted to characterize the benefit of ICDs in subpopulations, although without adequate power (8–11). Additionally, these studies have assessed comorbidities in isolation and, as such, did not address the question of the benefit of ICDs in patients with several comorbidities, which may exert competing risks of mortality from alternative causes. To date, analyses of such medically complex patients with ICDs for the prevention of SCD have been limited and largely observational (12). Furthermore, subgroup analyses of individual randomized trials of ICDs are inadequately powered to assess benefit in these types of patients.
Therefore, we conducted the present study to assess the treatment effect of ICDs in medically complex patients in a large combined analysis of 4 randomized controlled trials. Our aims were: 1) to define the burden of specific comorbidities in randomized controlled trial populations; 2) to determine whether the mortality benefit of ICDs observed in these trial populations extended to sicker patients; and 3) to assess if complications of ICDs were higher in patients with extensive medical comorbidities.
Methods
Only prospective randomized controlled trials of primary prevention ICDs compared with no ICDs were considered for this analysis. Furthermore, because we sought to identify if overall treatment benefit extended to patients with additional comorbidities, trials in which there was no significant overall treatment benefit were excluded, as were trials without comorbidity data. As such, the CABG (Coronary Artery Bypass Graft)-Patch Trial and DINAMIT (Defibrillator in Acute Myocardial Infarction Trial) were excluded because they did not show significant benefit of ICD implantation. The Multicenter Unsustained Tachycardia Trial was excluded because it did not record many comorbidities (13). Subsequently, patient-level data from 4 major prospective randomized trials of ICDs were combined: MADIT I (Multicenter Automatic Defibrillator Implantation Trial I) (1), MADIT II (3), DEFINITE (Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation) (4), and SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) (5). The amiodarone arm of SCD-HeFT was excluded to isolate the treatment effect of ICDs.
Each of the included studies was a prospective randomized controlled trial of ICDs versus control, including optimal medical therapy. Additionally, MADIT I required that patients exhibit prior, nonsustained ventricular tachycardia or have inducible arrhythmias during electrophysiological testing (1). No data on shocks during follow-up, whether appropriate or inappropriate, were available. Trial details, strength of evidence, and patient population characteristics of each of the trials are shown in the Online Appendix.
Patient inclusion criteria were LVEF ≤35%, either no prior myocardial infarction or time from myocardial infarction to randomization >40 days, and availability of data on important covariates. Patients with New York Heart Association functional class IV heart failure were excluded. The derivation of the study population is shown in Figure 1 and resulted in a final cohort of 3,348 patients from 4 clinical trials: 179 from MADIT I, 1,089 from MADIT II, 458 from DEFINITE, and 1,622 from SCD-HeFT.
Figure 1. Derivation of Study Population With Exclusions by Trial.
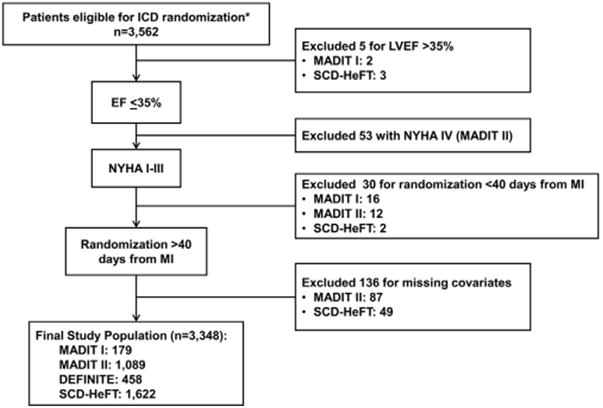
The subgroups do not add up because some patients were excluded for >1 reason. *Excluding patients randomized to drug treatment. DEFINITE = Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation; EF = ejection fraction; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; MADIT I = Multicenter Automatic Defibrillator Implantation Trial I; MADIT II = Multicenter Automatic Defibrillator Implantation Trial II; MI = myocardial infarction; NYHA = New York Heart Association; SCD-HeFT = Sudden Cardiac Death in Heart Failure Trial.
Seven comorbidities were selected for assessment on the basis that they are common, contribute substantially to morbidity and mortality, can be objectively measured, and have readily available data in clinical trial populations: smoking, ischemic heart disease, chronic kidney disease, diabetes, pulmonary disease, atrial fibrillation (AF), and peripheral vascular disease. All patients had left ventricular systolic dysfunction; however, many did not have concomitant ischemic heart disease, which poses a major risk of adverse events. Therefore, it was included as a medical comorbid condition (i.e., coronary artery disease). Comorbidities were categorized on the basis of criteria from the primary trial, with the exception of chronic kidney disease. For consistency with previous studies, chronic kidney disease was defined as an estimated glomerular filtration rate of <60 ml/min/1.73 m2, as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (14). All conditions were assessed at baseline only.
The primary endpoint was all-cause mortality at last follow-up. Secondary endpoints included all-cause rehospitalization and cause-specific mortality. Cause of hospitalization was not available.
Statistical Methods
The total number of observed comorbidities for each patient was assessed, and the study population was subsequently stratified into low or high comorbidity groups on the basis of the median comorbidity count for descriptive purposes. Baseline characteristics and the distribution of each comorbid disease were compared between the 2 groups and across treatment assignment (ICD vs. control). Unadjusted all-cause mortality event rates were summarized with Kaplan-Meier survival curves, and differences in survival between ICD recipients and nonrecipients were assessed with log-rank tests for each comorbidity group. The use of the observed count of comorbidities is limited in that it can implicitly penalize subjects in trials that recorded more comorbid conditions. To mitigate this limitation, our models for inferential analysis allowed for imputation of missing comorbid condition values using empirical frequencies from trials with similar patient populations. Patients in trials that included nonischemic cardiomyopathy (DEFINITE, the nonischemic subgroup of SCD-HeFT) were used to impute data for each other; in contrast, the remaining trials (MADIT I, MADIT II, and the ischemic subgroup of SCD-HeFT) enrolled similar patients and were used to impute missing data on comorbidities for each other. To combine trial data, we used Bayesian-Weibull survival regression modeling. Assuming random effects for trial-specific treatment effects and for parameters defining trial-specific baseline hazard functions, a model including the main effects of the ICD and the imputed comorbidity count as well as the multiplicative interaction was fitted. Moreover, our model adjusted for age, sex, race, LVEF, and use of antiarrhythmic drugs, beta-blockers, and angiotensin-converting enzyme inhibitors. Statistical significance for the interaction between ICD and comorbidity was determined when the 2-sided posterior probability of null interaction was <0.05. Similarly, we examined the secondary endpoint of rehospitalization with Bayesian logistic regression modeling. The DEFINITE study was excluded from the latter analyses because it did not record data for hospitalizations. All statistical analyses of the de-identified data were performed using R version 3.0.1 and WinBUGS version 1.4.3 (15). Detailed descriptions of the statistical and imputation methods are provided in the Online Appendix.
Results
Study Population
The study population included 3,348 patients: 1,771 treated with an ICD and 1,577 controls. The median follow-up time was 2.6 years. The median number of observed comorbidities was 2, and for descriptive analyses the population was subsequently dichotomized into 2 groups: patients with <2 observed comorbidities (low comorbidity) and those with ≥2 observed comorbidities (high comorbidity). Overall, 830 patients had <2 observed comorbidities (442 treated with an ICD) and 2,518 had ≥2 comorbidities (1,329 treated with an ICD). Rates and distribution of the selected comorbidities in the present analysis are shown in Table 1. Smoking was the most common comorbidity, followed by ischemic heart disease. Rates of AF and peripheral vascular disease were low overall in this population. Patient-level baseline characteristics, stratified by extent of comorbidity, are shown in Online Tables 1 to 4.
Table 1. Comorbidities Included in the Analysis.
| <2 Comorbidities (n = 830) |
≥2 Comorbidities (n = 2,518) |
|||
|---|---|---|---|---|
|
|
|
|||
| Control (n = 388) |
ICD (n = 442) |
Control (n = 1,189) |
ICD (n = 1,329) |
|
| Smoking | 221 (58) | 211 (48) | 1,047 (89) | 1,165 (88) |
|
| ||||
| Ischemic heart disease | 55 (14) | 77 (17) | 877 (74) | 1,041 (79) |
|
| ||||
| Chronic kidney disease | 15 () | 17 (6) | 484 (46) | 524 (43) |
|
| ||||
| Diabetes | 20 (5) | 33 (8) | 462 (39) | 476 (36) |
|
| ||||
| Pulmonary disease | 2 (1) | 12 (3) | 180 (26) | 183 (28) |
|
| ||||
| Atrial fibrillation | 6 (2) | 8 (2) | 97 (14) | 103 (16) |
|
| ||||
| Peripheral vascular disease | 0 (0) | 0 (0) | 20 (10) | 18(10) |
Values are n (%).
ICD = implantable cardioverter-defibrillator.
Outcomes
Without adjusting for additional confounders, use of an ICD resulted in significant improvement in survival in patients with low comorbidity (unadjusted hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.40 to 0.87) and to a lesser extent in patients with extensive comorbid illness (unadjusted HR: 0.71; 95% CI: 0.61 to 0.84). The proportion of deaths due to arrhythmia were higher for patients in the control group (40% and 37% of deaths with <2 and ≥2 comorbidities, respectively) compared with patients in the ICD group (12% and 22% of deaths with <2 and ≥2 comorbidities, respectively). However, hospitalization rates were lowest in patients with <2 comorbidities who did not receive an ICD (54%) and highest for patients with ≥2 comorbidities who received an ICD (74%). Similarly, adverse event rates were lowest in patients with low comorbidity not receiving an ICD (0%) and highest in patients with high comorbidity receiving an ICD (21%). Complete unadjusted outcomes are shown in Online Tables 1 to 4.
Unadjusted and adjusted absolute differences in mortality are shown in Table 2. The Bayesian posterior probability suggested significant interaction between use of an ICD and level of comorbid disease on all-cause mortality (pinteraction = 0.004), but no such effect was detected for hospitalization (pinteraction= 0.23). Kaplan-Meier curves for each comorbidity group, stratified by ICD use, are shown in Figure 2. When measured on a discrete scale, after adjustment, increasing comorbidity was associated with decreasing treatment benefit from an ICD (p = 0.004) (Figure 3).
Table 2. Unadjusted and Adjusted Survival Differences Between ICD and Control Groups at Five Years by Number of Comorbidities.
| No. of Comorbidities | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
|
|
|||
| Median Survival Difference (ICD – Control) |
95% CI | Median Survival Difference (ICD – Control) |
95% CI | |
| 0 | 0.11 | (0.05 to 0.17) | 0.13 | (0.06 to 0.19) |
|
| ||||
| 1 | 0.12 | (0.07 to 0.18) | 0.13 | (0.07 to 0.19) |
|
| ||||
| 2 | 0.13 | (0.08 to 0.18) | 0.13 | (0.08 to 0.18) |
|
| ||||
| 3 | 0.11 | (0.06 to 0.16) | 0.11 | (0.06 to 0.15) |
|
| ||||
| 4 | 0.06 | (-0.01 to 0.14) | 0.06 | (0.00 to 0.14) |
|
| ||||
| 5 | 0.00 | (-0.13 to 0.12) | 0.00 | (-0.10 to 0.12) |
|
| ||||
| 6 | -0.06 | (-0.19 to 0.07) | -0.05 | (-0.18 to 0.09) |
No patient had all 7 comorbidities. Posterior probability of interaction between treatment and comorbidity, p < 0.01 (unadjusted and adjusted).
CI = confidence interval; ICD = implantable cardioverter-defibrillator.
Figure 2. Kaplan-Meier Curves for All-Cause Mortality by Treatment Group (Stratified by Comorbidity Group) and Unadjusted HRs.
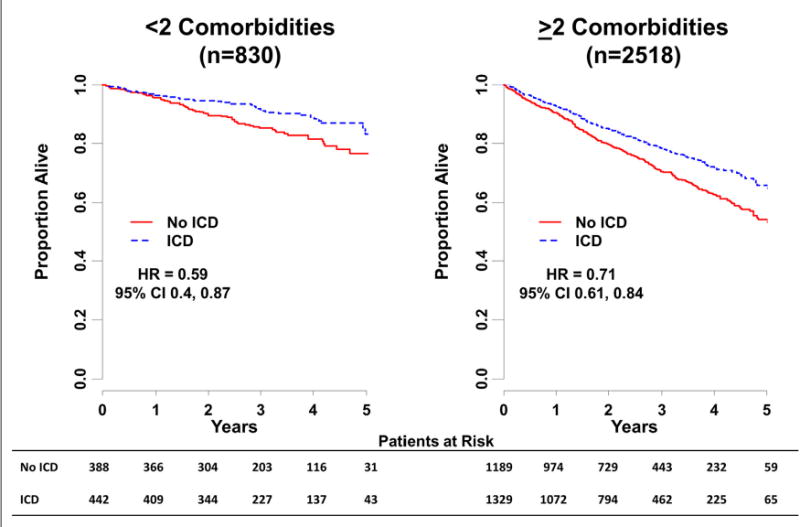
CI = confidence interval; HR = hazard ratio; other abbreviations as in Figure 1.
Figure 3. Results Assessing Benefit of an ICD (vs. Control) in Patients With Increasing Number of Comorbidities.

Models were adjusted for age, sex, race, left ventricular ejection fraction, and use of antiarrhythmic drugs, beta-blockers, and angiotensin-converting enzyme inhibitors. Abbreviation as in Figure 1.
Sensitivity Analyses
To further test the robustness of our findings, sensitivity analyses were performed. These include extreme imputation cases for missing comorbidities (all patients had comorbidity, no patients had comorbidity), which resulted in findings consistent with the primary analysis (Online Tables 5 and 6).
Discussion
Our analysis highlights the importance of medical comorbidities in patients with cardiovascular disease at high risk for SCD; the burden of concomitant illness in this population of patients is not low. In addition to their severe cardiovascular pathology, many patients have competing risks of morbidity and mortality from diseases of other organ systems. These patients with relatively higher comorbidity may not derive the same benefit from an ICD. Additionally, there is a numeric excess of hospitalization in patients with additional comorbidity.
These data have important implications for the application of clinical trial data to daily practice. Nontrial patients may have a higher burden of illness than the patients with higher comorbidity in the present analysis (16). These differences were highlighted in a recent analysis from the National Cardiovascular Data Registry for ICD implantation, which compared those patients with 2 unrestricted trial populations (17). They showed that older patients with more comorbid illnesses were receiving ICDs in clinical practice as compared with those enrolled in the clinical trials (SCD-HeFT and MADIT II). Although the patients in the registry who were matched to ICD recipients in the trials had equivalent outcomes, it remains unclear if patients with a higher burden of disease in clinical practice derive the same benefit. Our analysis suggests that the benefit of an ICD in such patients may be attenuated.
Prior data have shown the substantial risk of mortality in patients with an ICD who have concomitant noncardiac disease. In an observational study of 2,467 patients undergoing ICD implantation in Ontario, Canada, Lee et al. (18) followed up patients for up to 2 years. They found significantly increased rates of all-cause mortality in patients with congestive heart failure, peripheral vascular disease, pulmonary disease, and/or kidney disease. Similarly, a U.S. population–based study found a 1-year mortality rate of 21% in patients undergoing ICD implantation with 2 or more high-risk features (age older than 80 years, history of AF, creatinine level >1.8 mg/dl, or New York Heart Association functional class III or IV) (19). However, these cohorts did not provide insight into the incremental benefit, if any, of ICD use in these sicker patients.
Such an analysis was performed by Chan et al. (12) in an observational study matching 494 ICD recipients to similar patients who did not receive ICDs. Using multivariable Cox regression techniques, they concluded that ICD implantation should not be withheld from patients with increased comorbidities (counting those similar to the ones in the present analysis). However, this analysis had some limitations, most importantly that ICD use was not randomized and there was likely residual unmeasured confounding. Furthermore, their analysis showed an attenuation of ICD benefit as the number of comorbidities increased (adjusted HR: 0.89; 95% CI: 0.55 to 1.45 for ICD benefit in patients with a comorbidity score ≥3; n = 195). Our study provides stronger data from a population randomly assigned to ICDs, showing that survival benefit is questionable in patients with extensive comorbidity. Additionally, these results are independent of assessment of comorbidity level; in dichotomization (<2 vs. ≥2), treatment effect was attenuated in the high comorbidity group and the HR for benefit of ICD implantation decreased as the number of comorbidities increased (Table 2, Figure 3).
Our data on cause-specific mortality suggest that in patients with high comorbidity, the competing risk of nonsudden death may outweigh the benefit of ICD protection from sudden death. At the very least, the incremental benefit of the ICD is diminished in this population, because these data also suggest higher rates of hospitalization and adverse events in medically complex patients. Although limited in power, the results of these secondary endpoints are consistent with the primary analysis; patients with additional comorbidities are likely at particular risk for adverse outcomes other than sudden death, and additional data on safety and effectiveness are needed to optimally define the benefit (if any) of ICD use in these patients.
Study Limitations
The analysis in this study was performed on the basis of a combined population from several randomized controlled trials. Although this presents advantages, there are some limitations. We did not assign different weights to each comorbid illness. There are several reasons for this. First, there are subtle differences in the definitions among the different trials, complicating comparisons across trials. Second, variability in severity likely exists within each diagnosis, such that assigning different values to different diseases would exacerbate such heterogeneity. For example, prior data have suggested variable benefit from an ICD across the spectrum of chronic kidney disease (11). The use of a single HR overall may not be accurate for all patients with an ICD. However, we believe that weighting such diagnoses would make it difficult to derive meaningful clinical interpretations from such data.
Additionally, not all potentially clinically important comorbid conditions were ascertained in each of the trials; therefore, there may be other illnesses conferring competing risk of nonsudden death that we have not captured. Moreover, among the set of comorbid conditions used in our analysis, a number were not available in all trials. In our analysis, we imputed the missing comorbid condition assuming that frequencies are similar between trials with similar patient populations. Similarly, patient-level data on shocks or other tachyarrhythmia therapies were not available. Lastly, these data are derived from randomized trials in a primary prevention setting; as such, this may limit generalizability to clinical practice, and they cannot be extended to patients considered for secondary prevention ICDs (although preliminary data suggest the effect may be consistent) (20).
Conclusions
Many patients eligible for a primary prevention ICD have extensive concomitant morbidities that may attenuate the survival benefit of primary prevention ICDs. The decision to implant an ICD for primary prevention in patients with high comorbid illness should be weighed against the risk of the patient's other diseases. Further data are needed on the real-world outcomes of medically complex patients receiving ICDs.
Supplementary Material
Acknowledgments
This project was supported by grant 5R01HS018505 from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The opinions expressed in this document are those of the authors and do not reflect the official position of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services. Dr. Steinberg was supported by a National Institutes of Health T-32 training grant (5 T32 HL 7101-37; Duke University Medical Center, Durham, North Carolina). Dr. Steinberg has received modest educational support from Medtronic, Inc. Dr. Bardy is founder and inventor of and holds equity and royalties in Cameron Health. Dr. Buxton has received consulting fees/honoraria from Medtronic, Inc., St. Jude Medical, Boston Scientific Corp., and Forest Pharmaceuticals; and has received research support from Biosense Webster and Medtronic, Inc. Dr. Moss has received a research grant from Boston Scientific Corp. Dr. Mark has received research grants from St. Jude Medical and Medtronic, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. William Stevenson, MD, and John R. Teerlink, MD, served as Guest Editors for this paper.
Abbreviations and Acronyms
- AF
atrial fibrillation
- CI
confidence interval
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- SCD
sudden cardiac death
Footnotes
Appendix For the detailed statistical analyses as well as supplemental tables, please see the online version of this paper.
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Mushlin AI, Hall WJ, Zwanziger J, et al. The cost-effectiveness of automatic implantable cardiac defibrillators: results from MADIT. Multicenter Automatic Defibrillator Implantation Trial. Circulation. 1998;97:2129–35. doi: 10.1161/01.cir.97.21.2129. [DOI] [PubMed] [Google Scholar]
- 7.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 8.Albert CM, Quigg R, Saba S, et al. Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am Heart J. 2008;156:367–72. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Huang DT, Sesselberg HW, McNitt S, et al. Improved survival associated with prophylactic implantable defibrillators in elderly patients with prior myocardial infarction and depressed ventricular function: a MADIT-II substudy. J Cardiovasc Electrophysiol. 2007;18:833–8. doi: 10.1111/j.1540-8167.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- 10.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58:409–17. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–90. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Chan PS, Nallamothu BK, Spertus JA, et al. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes. 2009;2:16–24. doi: 10.1161/CIRCOUTCOMES.108.807123. [DOI] [PubMed] [Google Scholar]
- 13.Lee KL, Hafley G, Fisher JD, et al. Effect of implantable defibrillators on arrhythmic events and mortality in the multicenter unsustained tachycardia trial. Circulation. 2002;106:233–8. doi: 10.1161/01.cir.0000021920.73149.c3. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBugs—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–37. [Google Scholar]
- 16.Masoudi FA, Go AS, Magid DJ, et al. Longitudinal study of implantable cardioverter-defibrillators: methods and clinical characteristics of patients receiving implantable cardioverter-defibrillators for primary prevention in contemporary practice. Circ Cardiovasc Qual Outcomes. 2012;5:e78–85. doi: 10.1161/CIRCOUTCOMES.112.965368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khatib SM, Hellkamp A, Bardy GH, et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA. 2013;309:55–62. doi: 10.1001/jama.2012.157182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky MA, McAnulty J, Zipes DP, Baessler C, Hallstrom AP. A history of heart failure predicts arrhythmia treatment efficacy: data from the Antiarrythmics versus Implantable Defibrillators (AVID) study. Am Heart J. 2006;152:724–30. doi: 10.1016/j.ahj.2006.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


