Abstract
A new procedure is reported for high-yield isolation of guard cell protoplasts from Vicia faba L. Delayed light emission and P700 content plus absorption and fluorescence emission spectra of these protoplast extracts are reported. It is concluded that both photosystems are present. The presence of photosystem II and the absence of the reductive-step enzyme of the Calvin-Benson Cycle (Outlaw WH Jr, J Manchester, CH DiCamelli, DD Randall, B Rapp, GM Veith 1979 Proc Natl Acad Sci USA 76: 6371-6375) in a cell has no precedent in the literature. It is speculated that noncyclic photosynthetic electron flow is an environmental sensor which causes stomata to remain open in light.
Full text
PDF
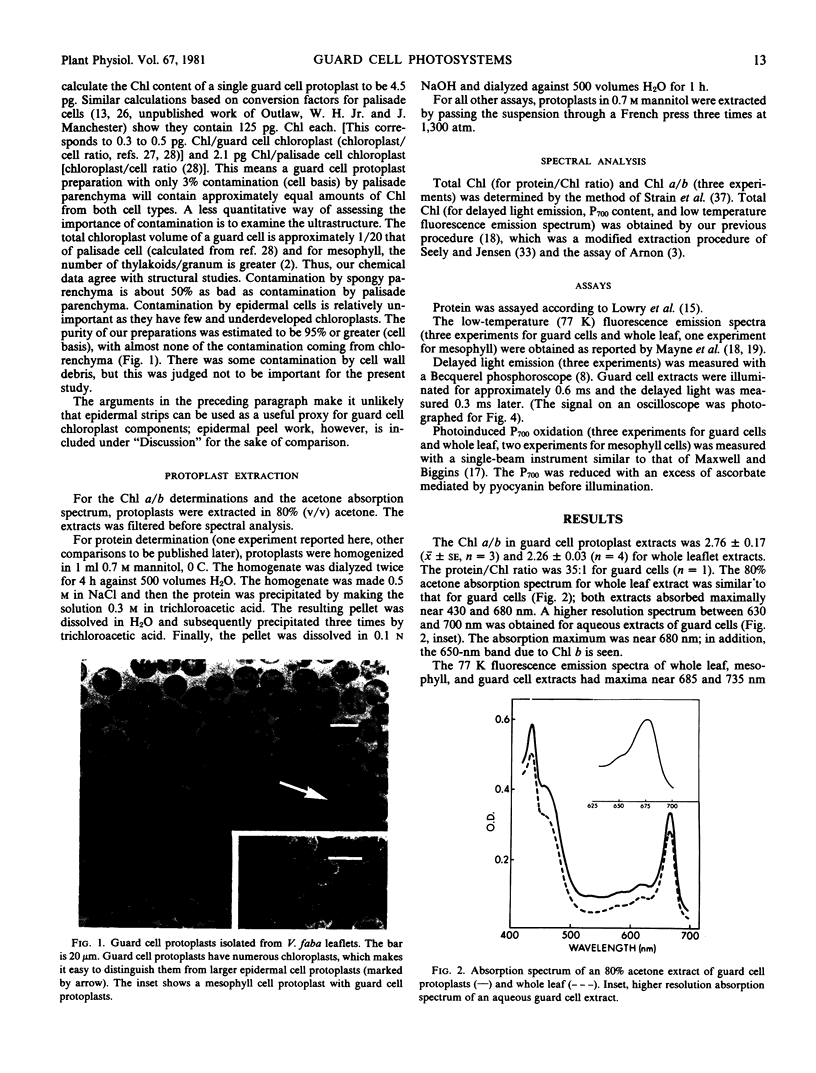
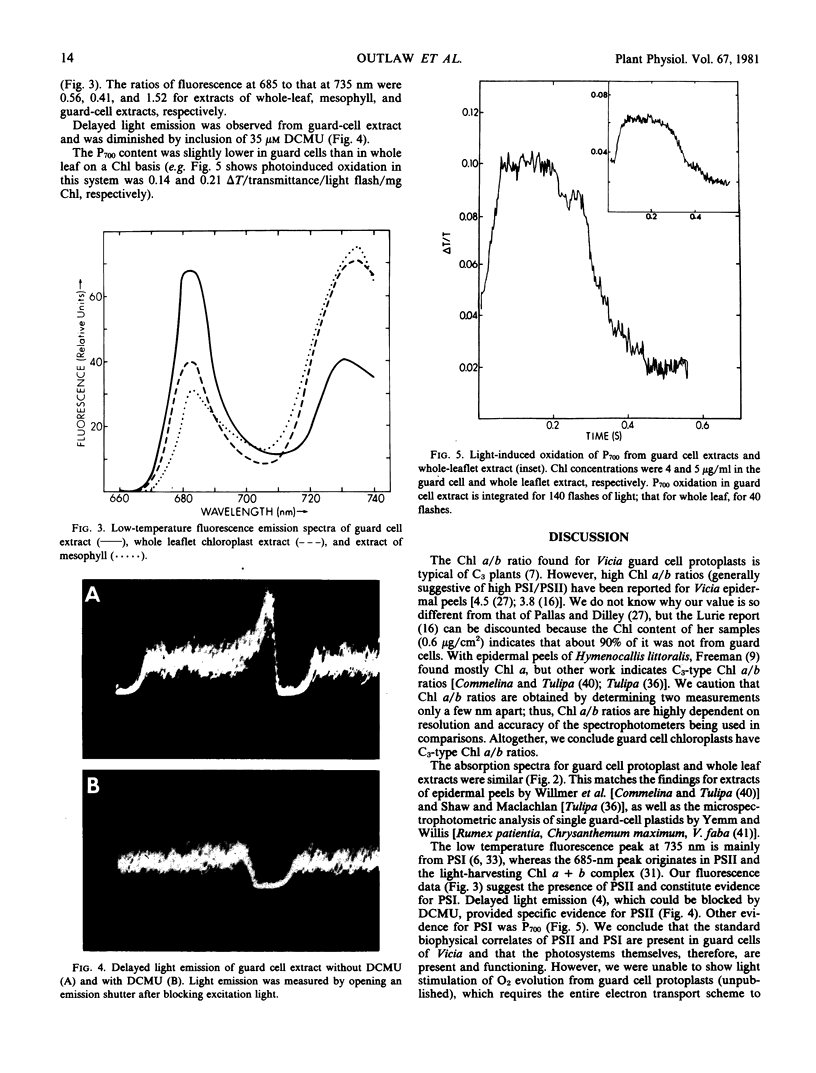


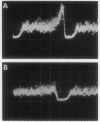
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W., Anderson J. M. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Aug;56(2):586–593. doi: 10.1073/pnas.56.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. CHARACTERISTICS OF FLUORESCENCE AND DELAYED LIGHT EMISSION FROM GREEN PHOTOSYNTHETIC BACTERIA AND ALGAE. J Gen Physiol. 1965 Mar;48:633–646. doi: 10.1085/jgp.48.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREELAND R. O. The green pigment and physiology of guard cells. Science. 1951 Jul 27;114(2952):94–95. doi: 10.1126/science.114.2952.94. [DOI] [PubMed] [Google Scholar]
- Humble G. D., Hsiao T. C. Light-dependent Influx and Efflux of Potassium of Guard Cells during Stomatal Opening and Closing. Plant Physiol. 1970 Sep;46(3):483–487. doi: 10.1104/pp.46.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble G. D., Hsiao T. C. Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiol. 1969 Feb;44(2):230–234. doi: 10.1104/pp.44.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper P. J. Dependence upon Wavelength of Stomatal Movement in Epidermal Tissue of Senecio odoris. Plant Physiol. 1964 Nov;39(6):952–955. doi: 10.1104/pp.39.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maxwell P. C., Biggins J. Role of cyclic electron transport in photosynthesis as measured by the photoinduced turnover of P700 in vivo. Biochemistry. 1976 Sep 7;15(18):3975–3981. doi: 10.1021/bi00663a011. [DOI] [PubMed] [Google Scholar]
- Mayne B. C. Spectral, Physical, and Electron Transport Activities in the Photosynthetic Apparatus of Mesophyll Cells and Bundle Sheath Cells of Digitaria sanguinalis (L.) Scop. Plant Physiol. 1971 May;47(5):600–605. doi: 10.1104/pp.47.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Schmuck C. L., Tolbert N. E. Photosynthetic Carbon Metabolism in the Palisade Parenchyma and Spongy Parenchyma of Vicia faba L. Plant Physiol. 1976 Aug;58(2):186–189. doi: 10.1104/pp.58.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E. Photophosphorylation Can Provide Sufficient Adenosine 5'-Triphosphate to Drive K Movements during Stomatal Opening. Plant Physiol. 1972 Apr;49(4):649–650. doi: 10.1104/pp.49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins H. B., Harper J. R., Higinbotham N. Membrane potentials of vallisneria leaf cells and their relation to photosynthesis. Plant Physiol. 1980 Jan;65(1):1–5. doi: 10.1104/pp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Butler W. L. Low temperature spectral properties of subchloroplast fractions purified from spinach. Plant Physiol. 1978 Mar;61(3):373–379. doi: 10.1104/pp.61.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Zelitch I. Some Effects of Metabolic Inhibitors, Temperature, & Anaerobic Conditions on Stomatal Movement. Plant Physiol. 1963 Jul;38(4):390–396. doi: 10.1104/pp.38.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. Chlorophyll and photosynthesis in stomatal guard cells. Nature. 1954 Apr 17;173(4407):726–726. doi: 10.1038/173726a0. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Hepler P. K. Production of guard cell protoplasts from onion and tobacco. Plant Physiol. 1976 Oct;58(4):492–498. doi: 10.1104/pp.58.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]