Abstract
Background
Elevated CSF tau is considered a biomarker of neuronal injury in newly developed Alzheimer’s disease (AD) and mild cognitive impairment (MCI) criteria. However, previous studies have failed to detect alterations of tau species in other primary tauopathies. We assessed CSF tau protein abnormalities in AD, a tauopathy with prominent Aβ pathology, and progressive supranuclear palsy (PSP), a primary tauopathy characterized by deposition of four microtubule binding repeat (4R) tau with minimal Aβ pathology.
Methods
26 normal control (NC), 37 AD, and 24 PSP patients participated in the study. AD and PSP were matched for severity using the clinical dementia rating sum of boxes (CDR-sb) scores. The INNO BIA AlzBio3 multiplex immunoassay was used to measure CSF Aβ, total tau, and ptau181. Additional, novel ELISAs targeting different N-terminal and central tau epitopes were developed to examine CSF tau components and to investigate interactions between diagnostic group, demographics, and genetic variables.
Results
PSP had lower CSF N-terminal and C-terminal tau concentrations than NC and AD measured with both the novel tau ELISAs and the standard AlzBio3 tau and ptau assays. AD had higher total tau and ptau levels than NC and PSP. There was a gender by diagnosis interaction in both AD and PSP for most tau species, with lower concentrations for male compared to female patients.
Conclusions
CSF tau fragment concentrations are different in PSP compared with AD despite the presence of severe tau pathology and neuronal injury in both disorders. CSF tau concentration likely reflects multiple factors in addition to the degree of neuronal injury.
Keywords: Alzheimer’s disease, Progressive supranuclear palsy, CSF, Tau
INTRODUCTION
Alzheimer’s disease (AD) and progressive supranuclear palsy (PSP) are two common neurodegenerative tauopathies. In both, the severity and neuroanatomic distribution of tau pathology at autopsy is correlated with disease phenomenology prior to death.1–3 AD displays an equal ratio of three to four microtubule binding repeats (3R = 4R) and is also characterized by the accumulation of amyloid plaques in addition to tau. In contrast, PSP is a relatively “pure” tauopathy and is characterized by deposition of 4R tau. The prevailing hypothesis is that amyloid accumulates prior to alterations in CSF concentrations of tau, which increase in parallel with neuronal damage in AD.4 Elevated concentrations of tau can be measured in AD CSF using assays that quantify central tau epitopes. Because PSP is a pure tauopathy, using tau as a biomarker may have better potential to demonstrate efficacy of tau-directed therapies than studies of more pathologically heterogeneous disorders such as AD.
PSP is less common than AD, but is genetically and biochemically more strongly linked to tau. 3,5 However, CSF tau concentrations in PSP are comparable to or lower than age-matched controls.6–9 Similar results have been reported for human tau mutation carriers, 10 and transgenic mice that express such mutations display decreasing tau concentrations in the brain interstitial fluid in parallel with deposition of insoluble tau and onset of neuronal dysfunction. 11 Decreases in CSF tau in PSP and other primary tauopathies might reflect a different propensity of 4R tau to aggregate, be released from cells,12 or undergo proteolytic cleavage13 as compared to equal mixtures of 3R and 4R tau found in AD. 14 Alternatively, standard CSF tau assays might not detect elevations of tau species that are elevated in primary tauopathies. To examine this possibility, we used novel ELISAs targeting N-terminal and mid-domain epitopes15 to compare CSF tau concentrations in severity matched AD and PSP patients.
METHODS
Study Population
24 PSP patients, 37 AD patients, and 26 normal controls (NC) were evaluated at the University of California, San Francisco Memory and Aging Center. PSP patients were diagnosed with possible or probable PSP according to the National Institute of Neurological Disorders and Stroke-Society for Progressive Supranuclear Palsy (NINDS-SPSP) criteria.16 A second set of AD patients (N = 13) that were matched in age, education, and gender, which were purchased from Precision Med (Solana Beach, CA), were also analyzed due to unexpectedly high Abeta levels in the initial AD group. AD patients met the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable Alzheimer's disease17. NC reported no cognitive symptoms and had normal neurological and neuropsychological examinations. All aspects of the study were approved by the UCSF Institutional Review Board, and written informed consent was obtained from all participants.
Clinical Evaluations
All individuals from UCSF received a neuropsychological assessment that included the Mini-Mental State Exam (MMSE)18; the second set of AD patients were also evaluated with the MMSE. Visuospatial abilities were examined using the Benson Figure copy (Rey). 19 Immediate and working memory was assessed using the forward (FDS) and backward (BDS) digit span. 20 Episodic memory was measured by the short form of the California Verbal Learning Test-2 (CVLT-2, including 30 second and 10 minute delays)21 and 10 minute delayed-recall of the Benson figure (Rey10m). Executive function was tested using a modified Trail-making test (lines per second).22 Phonemic fluency (D words per minute) 23, category fluency (animals per minute) 23, and a 15-item Boston naming task (BNT) 24 examined language capacities. Clinical dementia rating (CDR)25 and sum of boxes (CDR-sb)12 scores assessed disease burden and functional abilities. The Unified Parkinson’s Disease Rating Scale (UPDRS) motor quantified parkinsonism in all groups. 26
Lumbar Punctures (LP)
LPs were performed fasting and sitting up, in accordance with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) recommended procedure (http://www.adni-info.org/ADNIStudyProcedures/LumbarPunctures.aspx) and occurred within a maximum of 5 months of the clinical assessment. Samples were collected in the morning after a 12 hour fast into sterile 13 ml polypropylene tubes. The first 2 ml were discarded. CSF was then transferred into 1 ml aliquots and put on dry ice for an hour before storing in a −80° C freezer.
CSF ELISAs
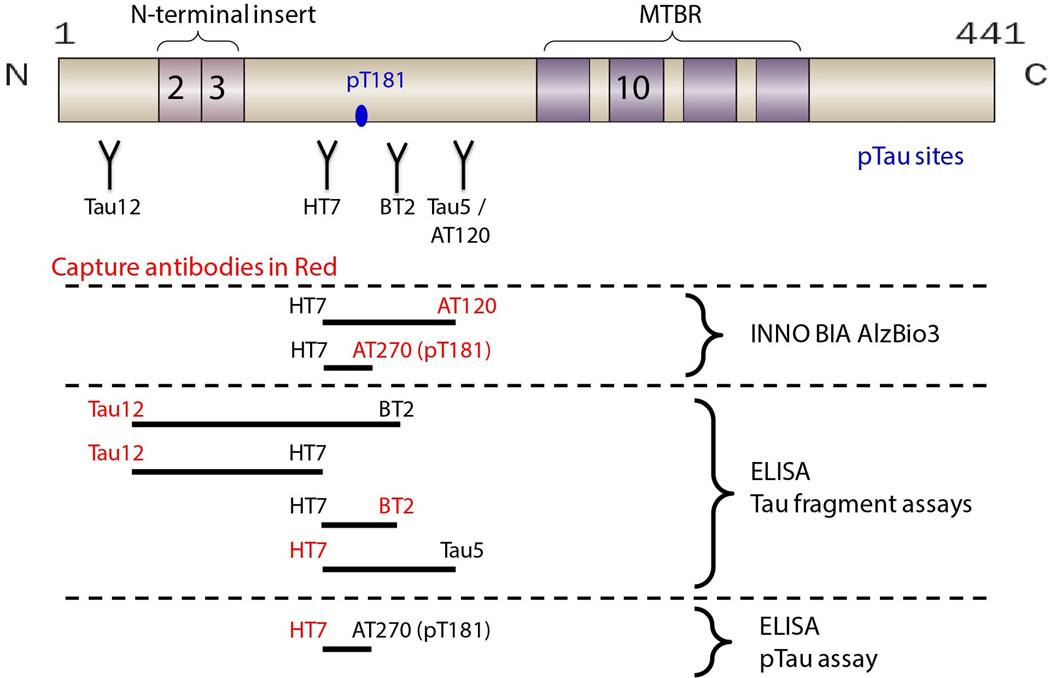
INNO BIA AlzBio3 (Fujirebio/Innogenetics), a multiplex assay for Aβ1–42, tau, and ptau, was performed using standard methods, including a set of three quality control (QC) standards. The novel ELISAs were performed using previously described methods. 15 Different combinations of standard monoclonal antibodies to N-terminal and central epitopes were developed to detect different overlapping regions of the tau protein. The following capture and readout antibodies combinations were used: Tau12-HT7, Tau12-BT2, BT2-HT7, HT7-Tau5, and HT7-PT181 (Figure 1). Standards and samples were run in triplicate; replicate variability was <20% (CV). All samples were included in the same assay run; inter-plate QC variability was <10% (CV). The assays demonstrated ~100-fold dynamic range of quantitation with LLQs ranging from 2 pg/ml to 10 pg/ml. 15 CSF signal specificity was confirmed using a combination of immunodepletion, spike recovery, and peptide competition. 15 One PSP patient was an outlier on all analyses (> 2.5 standard deviations from the group mean) and was excluded from the analyses.
Figure 1.
Genotyping
Genotyping of the ApoE allele (rs429358 and rs7412) and MAPT (rs1560310) H1 or H2 haplotype defining variants was carried out using a TaqMan Allelic Discrimination Assay on an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions and using the same methodology as previously described.27
Statistical analyses
Data were analyzed using SPSS (Version 21, SPSS Inc., Chicago) and R (www.r-project.org). Normality for individual variables was determined by the Shapiro-Wilk test. Depending on normality, mean values for demographic, neuropsychological and CSF analyte levels between each group were compared using a univariate ANOVA with False Discovery Rate (FDR) corrected post-hoc analysis, or a Kruskal-Wallis test with a FDR corrected Mann-Whitney U-test for post-hoc analysis. In addition, a linear regression model was used to examine differences in CSF biomarker values across diagnostic groups and to investigate interactions between diagnostic group and gender, age, ApoE genotype, and tau haplotype. Although there were group differences in education levels, education did not explain a significant amount of the variance in the mean CSF concentrations (all p values > 0.443) and was therefore not included in the model. Because the distribution of CSF biomarkers was skewed, inferences were performed using a resampling-based technique that does not require (normal) distributional assumptions. Specifically, accuracy of parameter estimates was examined using a non-parametric bootstrap technique with 2,500 iterations. Bootstrap (or a general linear model) post-hoc testing within each diagnostic group was used to describe differences in CSF biomarkers between groups. Statistical significance was examined based on p-values generated by bootstrap analysis, accompanied by F-statistic values and degrees of freedom from ANOVA and FDR corrected for multiple comparisons. Lastly, a receiver operating characteristic (ROC) curve analysis utilizing a nonparametric approach was performed to explore diagnostic accuracy of the CSF tau and ptau assays that demonstrated lower levels in PSP patients compared to the other groups.
RESULTS
Demographics and neuropsychological performance
There were no differences in gender, age, and ApoE4 and Tau Haplotype status between groups. CDR and CDR-sb scores were higher in AD and PSP patients compared to NC (p < 0.001) but not different between patient groups. Disease duration (defined as the interval from disease onset to the time of CSF collection) was shorter in AD than PSP (p = 0.016). PSP and AD patients had fewer years of education than NC (p < 0.01). PSP patients were impaired relative to NC on the MMSE, CVLT 30 seconds, Rey, Rey10m, FDS, BDS, and phonemic and category fluency (p < 0.026) and had higher UPDRS scores (p < 0.001) (Table 1). PSP patients never performed worse on any neuropsychological test than AD patients. AD patients performed worse than NC on MT correct, CVLT 10 minute delay, MMSE, Rey10m, and BDS (p < 0.001). AD also had worse performance than PSP patients on the MMSE, CVLT 30 seconds and 10 minute delay, Rey10m, and BDS (p < 0.007) but had less Parkinsonism on the UPDRS (p < 0.001). AD patients performed worse than both NC and PSP patients on the BNT (p < 0.001).
Table 1.
Demographic and neuropsychological variables
| Variable | NC | AD1 | AD2 | PSP |
|---|---|---|---|---|
| Education | 17.8 ± 1.6 | 16.1 ± 4.3c | 15 ± 1.15f | 15.8 ± 2.3e |
| Gender (M/F) | 26/11 | 21/16 | 7/6 | 11/12 |
| Age | 62.9 ± 12.5 | 64.1 ± 9.1 | 68.23 ± 6.23 | 68.0 ± 6.7 |
| ApoE4 Status (non-carrier/carrier) | 15/10 | 21/16 | 16/3 | |
| Tau Haplotype (H1/H2 vs H1/H1) | 6/16 | 12/22 | 2/18 | |
| Disease Duration | NA | 4.5 ± 3.6d | 6.3 ± 2.6 | |
| CDR-total | 0a,b | 0.7 ± 0.7 | 0.4 ± 0.5 | |
| CDR-sb | 0a,b | 4.9 ± 3.0 | 4.4 ± 2.5 | |
| MMSE | 29.2 ± 1.0 | 20.4 ± 5.9c,d | 28.46 ± 2.99g | 26.2 ± 3.6e |
| Rey | 15.5 ± 0.7 | 12.1 ± 4.5c | 13.1 ± 1.9e | |
| Rey 10m | 11.9 ± 3.3 | 4.6 ± 4.1c,d | 9.4 ± 2.8e | |
| FDS | 7.4 ± 0.8 | 4.8 ± 1.5c | 5.6 ± 1.3e | |
| BDS | 6.3 ± 1.0 | 2.9 ± 1.0c,d | 3.9 ± 1.3e | |
| Phonemic Fluency | 15.4 ± 4.5 | 8.2 ± 4.4c | 6.8 ± 4.0e | |
| Category Fluency | 25.0 ± 4.1 | 9.8 ± 5.5c | 10.9 ± 4.2e | |
| BNT | 14.8 ± 0.4 | 10.9 ± 3.8c,d | 14.1 ± 1.0 | |
| Trails Correct | 14.0 ± 0 | 8.8 ± 5.3c | 12.8 ± 2.4 | |
| UPDRS-III | 0.3 ± 0.7a,b | 9.0 ± 12.2d | 32.1 ± 8.2 | |
| CVLT 30 secs | 8.4 ± 1.1 | 3.2 ± 2.0c,d | 6.7 ± 1.6e | |
| CVLT 10 min | 8.3 ± 1.4 | 2.0 ± 2.4c,d | 6.3 ± 2.0e |
NC: normal control, AD1: Alzheimer's disease in the original cohort, AD2: Alzheimer’s disease in the second cohort, PSP: progressive supranuclear palsy, ApoE4 status: ApoE4 non-carrier vs carrier, Disease duration: number of years from the onset of the disease to the date of CSF collection, CDR: Clinical Dementia Rating, CDR- sb: Clinical Dementia Rating sum of boxes, MMSE: Mini-Mental State Examination, Rey and Rey10: Benson Figure copy test immediate recall and 10 minute delayed recall, FDS: Digit Span Forward, BDS: Digit Span Backward, Phonemic Fluency: number of correct words beginning with D in 1 minute, Categorical Fluency: number of animals in 1 minute, BNT: Boston naming task (out of 15), Trails Correct: Modified Trail-making test number of correct items, UPDRS-III: Unified Parkinson’s Disease Rating Scale–part III motor scale, CVLT 30 secs and 10 min: number of correct items remembered on the California verbal learning task after a 30 second or 10 minute delay.
NC < AD1 (p < 0.05)
NC < PSP (p < 0.05)
AD1 < NC (p < 0.05)
AD1 < PSP (p < 0.05)
PSP < NC (p < 0.05)
NC < AD2 (p < 0.05)
PSP < AD2 (p < 0.05)
CSF Analytes
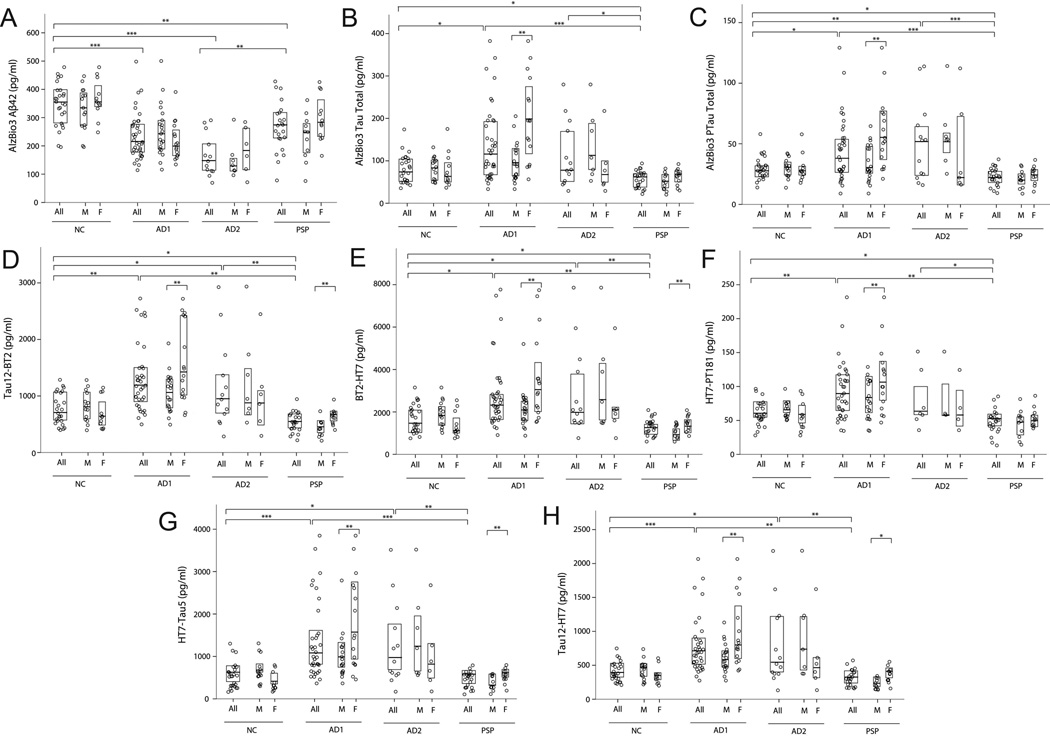
Group differences in mean CSF analyte concentrations from the novel ELISAs, and AlzBio3 ELISAs were examined using linear models. As shown in Figure 2, lower CSF tau levels were identified in PSP as compared to NC with the novel tau ELISAs in several N-terminal and mid-domain epitopes: Tau12-BT2 (p = 0.018), BT2-HT7 (p = 0.019), HT7-PT181 (p = 0.05) and the AlzBio3 assays for total tau (p = 0.034) and ptau (p = 0.028). Lower Aβ levels were seen in PSP (p = 0.002) than NC. Compared to NC, AD patients displayed lower Aβ levels (p < 0.001) and higher CSF tau levels in all the novel ELISAs (p < 0.013) and AlzBio3 total tau (p = 0.012) and ptau (p = 0.01) assays. There were no differences between AD and PSP patients for Aβ. AD patients also had higher CSF tau levels than PSP patients in all the novel ELISAs (p < 0.007), AlzBio3 total tau (p < 0.001) and ptau (p < 0.001) assays (Table 2).
Figure 2.
Table 2.
Mean CSF analyte concentrations.
| Variable | NC | AD1 | AD2 | PSP |
|---|---|---|---|---|
| AlzBio3 Aβ42 (pg/ml) | 348.1 ± 75.7 | 237.5 ± 82.7b | 167.06 ± 73.49 f,g | 269.0 ± 87.0c |
| AlzBio3 total tau (pg/ml) | 79.0 ± 33.1a | 138.4 ± 88.6 | 111.09 ± 78.17 | 55.9 ± 20.1c,d,h |
| AlzBio3 ptau (pg/ml) | 29.1 ± 9.4a,e | 44.7 ± 25.7 | 50.47 ± 33.15 | 22.1 ± 7.4c,d,h |
| Tau12-BT2 (pg/ml) | 773.3 ± 275.8a,e | 1312.4 ± 591.5 | 1180.03 ± 774.95 | 549.3 ± 173.9c,d,h |
| BT2-HT7 (pg/ml) | 1587.2 ± 652.9a,e | 2719.4 ± 1630.1 | 2786.06 ± 2079.67 | 1205.6 ± 390.2c,d,h |
| HT7-Tau5 (pg/ml) | 639.7 ± 300.1a,e | 1442.1 ± 909.6 | 1353.13 ± 989.99 | 546.2 ± 186.5d,h |
| HT7-PTau181 (pg/ml) | 64.0 ± 17.4a,e | 96.4 ± 42.1 | 82.06 ± 42.49 | 51.7 ± 17.5c,d,h |
| Tau12-HT7 (pg/ml) | 409.9 ± 141.9a,e | 788.2 ± 414.0 | 789.08 ± 603.01 | 321.5 ± 117.7d,h |
Group differences in CSF AlzBio3 and CSF tau assays. CSF levels are displayed by means and standard deviations within each group.
NC < AD1 (p < 0.05)
AD1 < NC (p < 0.05)
PSP < NC (p < 0.05)
PSP < AD1 (p < 0.05)
NC < AD2 (p < 0.05)
AD2 < NC (p < 0.05)
AD2 < PSP (p < 0.05)
PSP < AD2 (p < 0.05)
Because Aβ levels were higher than expected in the initial AD group (AD1), an additional analysis in a different group of AD patients (AD2) was pursued. This group demonstrated lower Aβ levels than PSP (p < 0.0014) and NC (p < 0.0001). PSP patients had lower Aβ levels than NC (p < 0.0014). Differences in tau levels were also analyzed. The results with this AD group were similar to the original AD cohort (Table 2).
There was an interaction between gender and CSF tau concentration in the AD1 and PSP groups, but not AD2 or NC (Figure 2). Male PSP patients had lower CSF tau levels than female patients in the N-terminal fragments Tau12-BT2 (p = 0.003) and Tau12-HT7 (p = 0.013), and in the mid-domain fragments BT2-HT7 (p = 0.028) and HT7-Tau5 (p = 0.008) ELISAs. The same pattern was seen in AD patients; males had lower CSF tau levels than females in all the novel ELISAs (p < 0.007), AlzBio3 total tau (p = 0.002) and ptau (p = 0.001) assays. None of the other demographic or genetic variables influenced CSF analyte concentrations.
ROC analyses were used to determine the ability of CSF tau concentration to differentiate PSP from NC and AD (Table 3). Multiple CSF tau assays were able to differentiate PSP from AD (AUC range between 0.794–0.948) with the N-terminal fragment Tau12-BT2 assay (AUC = 0.948, p < 0.001) having the highest AUC value. Most CSF tau assays also differentiated PSP from NC, however AUC values were lower (AUC range between 0.654–0.730), p < 0.02.
Table 3.
Receiver operating characteristic (ROC) curve analysis
| ROC analysis | AUC | Asymp. Significance, P |
|---|---|---|
| PSP vs NC | ||
| AlzBio3 total tau | 0.702 | 0.015 |
| AlzBio3 ptau | 0.716 | 0.010 |
| Tau12-BT2 | 0.730 | 0.006 |
| BT2-HT7 | 0.654 | 0.065 |
| HT7-PTau181 | 0.694 | 0.020 |
| PSP vs AD1 | ||
| AlzBio3 total tau | 0.846 | < 0.001 |
| AlzBio3 ptau | 0.794 | < 0.001 |
| Tau12-BT2 | 0.948 | < 0.001 |
| BT2-HT7 | 0.904 | < 0.001 |
| HT7-PTau181 | 0.861 | < 0.001 |
| PSP vs NC and AD1 | ||
| AlzBio3 total tau | 0.787 | < 0.001 |
| AlzBio3 ptau | 0.762 | < 0.001 |
| Tau12-BT2 | 0.859 | < 0.001 |
| BT2-HT7 | 0.801 | < 0.001 |
| HT7-PTau181 | 0.790 | < 0.001 |
AUC: area under the curve, Asymp = asymptotic.
DISCUSSION
We identified divergent patterns of CSF tau abnormalities in two common tauopathies, AD and PSP, using both the standard AlzBio3 CSF tau assays used in most AD research and a series of novel ELISAs designed to detect other CSF tau fragments 15. In AD and PSP patients matched for disease severity, AD patients displayed higher CSF tau and ptau levels than NC, whereas PSP patients had lower levels than NC. In PSP patients and one of the AD cohorts there was an interaction with gender, such that males had lower CSF tau levels than females in most assays. There were no effects of other demographic factors or the disease-associated genotypes ApoE4 and tau H1 haplotype. Although the current literature, which is mainly based on studies of AD, suggests that elevated CSF tau concentrations reflect neuronal injury and cell death in neurodegenerative disease,28,4 our data demonstrate that this model does not hold true for the primary four repeat tauopathy, PSP. Further investigations into the mechanisms of CSF tau alterations may reveal differences in the pathophysiology of tau-related neurodegeneration in AD and PSP11,29.
The standard AlzBio3 tau assays may detect only a subset of potential CSF tau fragments.15, 30, 31 Therefore we used novel CSF tau ELISAs designed to measure tau fragments containing N-terminal and mid-domain sequences. The absolute concentrations of tau fragments measured in the novel ELISAs were higher than those measured with AlzBio3. This likely resulted from technical differences in the assays due to differences in antibodies, matrix effects or standards. Without a more quantitative measurement of tau fragment levels, such as mass spectrometry, it is impossible to directly compare these values. Nonetheless, using these assays, we found lower tau fragment levels in PSP patients compared to controls and AD.
Unexpectedly, in the first AD cohort (AD1), CSF Aβ42 levels in AD and PSP patients were comparable, and lower than NC as in one previous study,8 but different from other reports in PSP that found higher Aβ42 levels.7, 8, 32 33 One possible explanation for low CSF Aβ42 in some PSP patients would be the presence of concurrent AD pathology.34 To further explore the potential confounding effects of concurrent AD pathology we compared PSP cases with low Aβ42 (below the mean) with those with higher Aβ42 (mean or higher) and found that PSP patients with low CSF Aβ42 had even lower total tau levels and tau fragment levels than PSP patients with high CSF Aβ42 levels (p < 0.034). This suggests that even in combined PSP and AD, CSF tau levels are lower than normal controls, however further analyses in autopsy-confirmed cases will be necessary to confirm this hypothesis.
We also found lower CSF total tau and ptau levels using the standard AlzBio3 assays in PSP compared to AD and controls. Although several previous studies have found PSP CSF total tau concentrations comparable to controls,7, 8, 32 the lack of observed differences in those studies may have been confounded by tau levels being near the limit of detection; a more recent study6 demonstrated lower ptau in PSP compared to controls, similar to our results.
There was an effect of gender on CSF tau levels measured in many of the tau assays in AD1 and PSP, but not in NC. Both AD1 and PSP male patients had lower CSF tau than their female counterparts (Figure 2). This suggests that our ability to detect CSF tau concentration differences between PSP and NC could also reflect differences in the gender composition of both groups as compared to previous studies. A previous study demonstrated that normal elder female ApoE4 carriers have increased CSF tau levels relative to non-carriers, similar to our results in AD.35 Another study found AD patients who are tau H1/H1 haplotype carriers have lower CSF tau levels as compared to H1/H2 carriers.36 We did not observe either effect but differences in sample size, age, and gender distribution may have influenced our findings since the gender effect was not present in the AD2 group.
A limitation of this study is the difficulty in matching AD and PSP patients for disease severity given their disparate clinical presentations. We matched patient groups using the CDR-sb which is commonly used to match AD with non-AD neurodegenerative syndromes.33,37 While it is possible the relative disease severity differed between patient groups, the different directions of CSF tau alteration relative to NC argue that such differences would not influence our results, and there were no correlations between disease severity and CSF tau levels in either patient group. A second potential limitation of this work is that we relied on clinical and not neuropathological diagnoses that could quantify the exact degree of underlying tau pathology. Given the disparate clinical presentations and excellent clinic-pathological diagnostic accuracy of the PSP criteria we used16, it is unlikely that misdiagnosis confounded these results; however, it is possible that our results might be influenced by the presence of concurrent AD pathology, which sometimes occurs in PSP.34 A third potential limitation of this study is that the novel ELISAs could not distinguish between 3R and 4R tau isoforms. A previous study 38 found lower 4R tau in CSF from both AD and PSP patients, suggesting that the difference we observed could potentially be explained by a selective alteration in 3R tau levels. Future studies will examine this possibility.
Our findings suggest that the relationship between CSF tau and neurodegenerative disease is a complex process potentially reflecting multiple factors related to the underlying cause of the tauopathy. Therefore, viewing tau only as an indicator of neuronal damage may not be accurate in generating models of tau-related pathogenesis. Possible explanations for the different effects of disease on CSF tau concentrations measured using ELISA based techniques in different tauopathies include: 1) Altered CSF tau fragment conformations may lead to differential affinity for the same monoclonal antibody. 2) Differences in CSF tau fragments produced in each disease may lead to different observed patterns in CSF.13,40 We found the greatest differences between PSP and NC using the N-terminal fragment ELISAs Tau12, BT2, and HT7; selective alterations in N-terminal tau fragments in PSP13, 39 might therefore partially explain these results. 3) Tau isoform ratios differ in PSP and AD, with predominantly 4R tau in PSP, and equal 3R to 4R ratios in AD. A recent study found that 4R tau is not released from cells as readily as 3R tau.12 Since 4R tau accumulates in PSP, more tau may be sequestered in dying cells than in AD, leading to lower extracellular concentrations reflected in CSF measurements.
A variety of new tau-directed therapies are entering human clinical trials for AD and other tauopathies. 14, 40 These drugs were developed in transgenic mouse models that overexpress mutant forms of 4R tau, which produces human clinical disease similar to frontotemporal degeneration or PSP, but not AD. The different associations between disease and CSF tau concentration observed in this study suggest that alterations in CSF tau in clinical trials of tau directed therapies may require different interpretations in AD and PSP, and that results in PSP may be more analogous to treatment effects observed in preclinical models.
Acknowledgments
Funding This study was supported by Bristol Myers Squibb, Tau Research Consortium, and National Institutes of Health grants R01AG038791 and P01 AG19724
SS, MA, HS, JM, VG, and TH are employees of Bristol-Myers Squibb (BMS). MA and HS are BMS shareholders. FB is an employee of Kyowa Hakko Kirin Pharma. JHK receives royalties from Pearson for the publication of commercially available neuropsychological tests. He receives research support from the NIH (2R01AG022983, 1R01AG032289, HHSN271200623661C). GDR receives research support from NIH/NIA (K23-AG031861), Alzheimer’s Association, Tau Consortium, Hellman Family Foundation, John Douglas French Alzheimer’s Foundation, and Avid Radiopharmaceuticals/Eli Lilly; he has served as a consultant for Eli Lily and received a speaking honorarium from GE Healthcare. BLM serves on a scientific advisory board for the Alzheimer's Disease Clinical Study; serves as an Editor for Neurocase and as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Elan Corporation, and Allon Therapeutics, Inc.; serves on speakers' bureaus for Novartis and Pfizer Inc.; and receives research support from Novartis and the NIH (NIA P50 AG23501 [PI] and NIA P01 AG19724[PI]) and the State of California Alzheimer's Center. ALB received personal compensation from Acetylon, Archer, Ipierian and Neurophage for consulting and received research support from Genentech, BMS, TauRx for conducting clinical trials. He is funded by NIH grants R01AG038791 [PI], R01AG031278 [PI], the Alzheimer's Drug Discovery Foundation, CBD Solutions and the Tau Research Consortium
Footnotes
Contributors All authors have made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; drafting of the article or revising it critically for important intellectual content and provided final approval of the version to be published.
Competing interests DW, IL, CS, HH, MCT, ZM, GC, AK, and LV have no conflict of interest.
REFERENCES
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Schofield EC, Hodges JR, Bak TH, et al. The relationship between clinical and pathological variables in Richardson's syndrome. J Neurol. 2012;259:482–490. doi: 10.1007/s00415-011-6205-8. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boxer AL, Gold M, Huey E, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: the next therapeutic frontier) Alzheimers Dement. 2013;9:189–198. doi: 10.1016/j.jalz.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 7.Urakami K, Wada K, Arai H, et al. Diagnostic significance of tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy. J Neurol Sci. 2001;183:95–98. doi: 10.1016/s0022-510x(00)00480-9. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi M, Yoshita M, Matsumoto Y, et al. Decreased beta-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci. 2005;237:61–65. doi: 10.1016/j.jns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Frank RA, Galasko D, Hampel H, et al. Biological markers for therapeutic trials in Alzheimer's disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer's disease. Neurobiol Aging. 2003;24:521–536. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosso SM, van Herpen E, Pijnenburg YA, et al. Total tau and phosphorylated tau 181 levels in the cerebrospinal fluid of patients with frontotemporal dementia due to P301L and G272V tau mutations. Arch Neurol. 2003;60:1209–1213. doi: 10.1001/archneur.60.9.1209. [DOI] [PubMed] [Google Scholar]
- 11.Yamada K, Cirrito JR, Stewart FR, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karch CM, Jeng AT, Goate AM. Extracellular Tau levels are influenced by variability in Tau that is associated with tauopathies. J Biol Chem. 2012;287:42751–42762. doi: 10.1074/jbc.M112.380642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arai T, Ikeda K, Akiyama H, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–79. doi: 10.1002/ana.10793. [DOI] [PubMed] [Google Scholar]
- 14.Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat Rev Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meredith JE, Jr, Sankaranarayanan S, Guss V, et al. Characterization of Novel CSF Tau and ptau Biomarkers for Alzheimer's Disease. PLoS One. 2013;8:e76523. doi: 10.1371/journal.pone.0076523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Possin KL, Laluz VR, Alcantar OZ, et al. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer's disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49:43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale, Third ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, et al. Ober BA. California Verbal Learning Test, Second Eed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 22.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Delis DC, Kaplan EB, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 24.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 25.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.Fahn S, Elton RL. Committee motUD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health-care Information; 1987. pp. 153–163. [Google Scholar]
- 27.Coppola G, Chinnathambi S, Lee JJ, et al. Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer's diseases. Hum Mol Genet. 2012;21:3500–3512. doi: 10.1093/hmg/dds161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–1280. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 29.Maia LF, Kaeser SA, Reichwald J, et al. Changes in amyloid-beta and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med. 2013;5:194re192. doi: 10.1126/scitranslmed.3006446. [DOI] [PubMed] [Google Scholar]
- 30.Hanisch K, Soininen H, Alafuzoff I, et al. Analysis of human tau in cerebrospinal fluid. J Proteome Res. 2010;9:1476–1482. doi: 10.1021/pr901002t. [DOI] [PubMed] [Google Scholar]
- 31.Ishiguro K, Ohno H, Arai H, et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett. 1999;270:91–94. doi: 10.1016/s0304-3940(99)00476-0. [DOI] [PubMed] [Google Scholar]
- 32.Constantinescu R, Zetterberg H, Holmberg B, et al. Levels of brain related proteins in cerebrospinal fluid: an aid in the differential diagnosis of parkinsonian disorders. Parkinsonism Relat Disord. 2009;15:205–212. doi: 10.1016/j.parkreldis.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Scherling CS, Hall T, Berisha F, et al. CSF neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2013 doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gearing M, Olson DA, Watts RL, et al. Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology. 1994;44:1015–1024. doi: 10.1212/wnl.44.6.1015. [DOI] [PubMed] [Google Scholar]
- 35.Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson A, Zetterberg H, Hakansson A, et al. TAU haplotype and the Saitohin Q7R gene polymorphism do not influence CSF Tau in Alzheimer's disease and are not associated with frontotemporal dementia or Parkinson's disease. Neurodegener Dis. 2005;2:28–35. doi: 10.1159/000086428. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luk C, Compta Y, Magdalinou N, et al. Development and assessment of sensitive immuno-PCR assays for the quantification of cerebrospinal fluid three- and four-repeat tau isoforms in tauopathies. Journal of Neurochemistry. 2012;123:396–405. doi: 10.1111/j.1471-4159.2012.07911.x. [DOI] [PubMed] [Google Scholar]
- 39.Borroni B, Gardoni F, Parnetti L, et al. Pattern of Tau forms in CSF is altered in progressive supranuclear palsy. Neurobiol Aging. 2009;30:34–40. doi: 10.1016/j.neurobiolaging.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]