Abstract
Little is known about trends in depression at antiretroviral therapy (ART) initiation among people living with HIV (PLHIV) in low- and middle-income countries. We used data from an ongoing cohort of treatment-naïve PLHIV in rural Uganda to estimate secular trends in depression among PLHIV at ART initiation. We fitted linear regression models with depression symptom severity as the outcome variable and year of cohort entry (2005–2012) as the explanatory variable, adjusting for socio-demographic variables and assessing physical health score, body mass index (BMI), and CD4 count as potential mediators of a secular trend in depression symptom severity. There was a statistically significant negative association between year of entry and depression symptom severity, suggesting a 3.1% relative decline in the mean depression symptom severity score at ART initiation in each year of study recruitment after the first year. This trend remained statistically significant after inclusion of baseline socio-demographic characteristics to the model and appeared to be driven by improved physical health scores, but not CD4 count or BMI.
Keywords: Depression, trends, physical health, antiretroviral therapy, HIV, Uganda
Introduction
Depression is a highly prevalent and leading cause of disability worldwide, particularly among people living with HIV (PLHIV) in low- and middle-income countries (LMICs)[1–4]. Among PLHIV, depression has been associated with poorer HIV-related outcomes, including increased transmission risk[5,6], greater CD4 count declines[7,8], reduced anti-retroviral therapy (ART) adherence[9], and more rapid progression to AIDS and death[8,10,11]. More importantly, depression is a modifiable risk factor: depression treatment can result in reduced transmission risk[12] and improved ART adherence and virologic outcomes[13], although other studies have suggested that depression treatment should be combined with behavioral interventions in order to effect changes in these HIV-related outcomes[14,15].
Little is known about trends in depression among PLHIV in LMICs during the early years of dramatic expansions in access to ART[16]. The increasing availability of ART has resulted in patients presenting for care with higher CD4 counts, lower burden of AIDS-related illnesses, and higher health-related quality of life[17–19]. It seems plausible that PLHIV presenting for ART with better physical health would also be less likely to be depressed[20–22], given the known association between physical health and mental health[23,24] and the increasingly understood biologic connections between HIV disease and depression[25]. Thus, although it is unclear whether the overall prevalence of depression among PLHIV has changed, greater access to ART and earlier ART initiation would be expected to result in concomitant declines in depression among PLHIV presenting for ART, although the extent to which this has occurred is unknown.
Uganda, a low-income country in sub-Saharan Africa with a generalized HIV epidemic, offers a window into examining trends in depression among PLHIV presenting for initial treatment. Estimates of the prevalence of depressive disorders or clinically significant symptoms of depression among PLHIV in Uganda have ranged from 13–47%, depending on the measure used[4,26,27]. As in other LMICs, access to ART in Uganda has expanded rapidly in recent years, and PLHIV may be initiating treatment earlier in the course of their disease[28,29]. Whether these changing patterns in the timing of ART initiation are driving changes in depression among PLHIV presenting for treatment in Uganda is unknown.
This study has two aims. The first aim is to estimate secular trends in depression among PLHIV presenting for ART initiation in rural Uganda. The second aim is to understand the factors that may explain these changes. The improved physical health of patients presenting for care may be particularly important under the assumption that earlier ART initiation, by preserving physical health, may prevent the development of depression[23]. Evidence of a role for improved physical health in explaining secular trends in depression among PLHIV at ART initiation may therefore provide an important rationale for expanding access to ART in LMICs.
Methods
Study Design
Data for this analysis were obtained from the Uganda AIDS Rural Treatment Outcomes (UARTO) study, an ongoing cohort of treatment-naïve PLHIV begun in 2005. Participants were recruited from the Mbarara Immune Suppression Syndrome (ISS) Clinic, an HIV clinic located in the rural Mbarara district approximately 275 km southwest of the capital city, Kampala. The clinic is prototypical of HIV clinics in the region that receive funding from bilateral and multilateral programs such as the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)[30]. More than 100 patients are initiated on ART each month.
Patients were eligible for participation in the UARTO study if they were newly initiating ART, were at least 18 years of age, and lived within 60 km of the clinic. At the pre-treatment baseline visit, participants underwent blood draws and structured interviews conducted by a research assistant in the local language, Runyankole. Patients whose baseline visits occurred between 2005 and 2012 were included in this analysis. Recruitment strategies have been unchanged since the inception of the study.
Written consent was obtained from all study participants. If there were cultural literacy reasons why a signature would not be appropriate, participants were allowed to sign consent forms with a thumbprint. Ethical approval for all study procedures was obtained from the University of California at San Francisco Committee on Human Research, the Partners Human Research Committee, and the Institutional Ethical Review Committee at Mbarara University of Science and Technology. Consistent with national guidelines, we received clearance for the study from the Uganda National Council for Science and Technology and from the Research Secretariat in the Office of the President.
Measures
The primary outcome of interest in this analysis was depression symptom severity, measured as a continuous variable using the 15-item depression sub-scale of the Hopkins Symptom Checklist (HSCL-D)[31]. This sub-scale was modified for the local context with the addition of a 16th item (“feeling like I don’t care about my health”)[32,33]. We removed the four somatic items (“feeling low in energy, slowed down,” “feeling fidgety,” “poor appetite,” and “having difficulty falling or staying asleep”) and averaged across the remaining 12 cognitive-affective items to avoid possible overlap between symptoms of depression and symptoms of HIV infection[34]. For the purposes of characterizing the sample to compare to other studies, participants were classified as having probable depression using the conventional threshold of greater than 1.75[35]. At baseline, the estimated scale reliability coefficient for the modified HSCL-D was 0.86.
Explanatory variables of interest measured at ART initiation included socio-demographic variables as well as three measures of physical health: the Medical Outcomes Study-HIV Health Survey (MOS-HIV) Physical Health Summary (PHS) score[36], CD4+ T-lymphocyte cell count, and body mass index (BMI). BMI was chosen as a surrogate for nutritional status, with lower values suggesting possible wasting or malnutrition. Socio-demographic variables included age in years, gender, educational attainment (higher than primary level vs. primary or none), marital status (legally married/not separated or cohabiting with a primary partner vs. other), household asset wealth, employment status, and heavy alcohol use. Among women, pregnancy status was determined by patient self-report. For the asset wealth measure, we applied principal components analysis to a series of 25 binary variables for household-owned assets and housing characteristics[37]. The first principal component was retained and used to define the wealth index and was entered into the regression models as a continuous variable, with higher values indicating greater asset wealth. History of heavy alcohol use was measured by the three-item consumption subset of the Alcohol Use Disorders Identification Test (AUDIT-C)[38].
Statistical analysis
We used descriptive statistics to characterize the sample. To summarize changes in depression by year of cohort entry, we estimated mean HSCL-D scores among subjects with complete data on the variables of interest. Categorical variables were compared with chi-square tests and continuous variables were compared with t-tests. To estimate changes in depression severity scores over time, a linear regression model was fitted with HSCL-D score as the outcome variable and year of cohort entry as the explanatory variable. A statistically significant and negative regression coefficient was considered evidence that depression symptom severity among PLHIV at ART initiation was declining over time. As an alternative parameterization, we fitted a simple linear regression model specifying time as a series of dummy variables for each 1-year period, using a Wald-type F test to test the joint statistical significance of the time dummy variables. For this and all other analyses, a gender interaction term was included in the models to test for evidence of variation in association by gender and separate models were fitted for men and women. We did this for two reasons. First, previous studies have demonstrated that correlates of depression are gender-specific[32,39]. Second, the majority of participants in HIV treatment studies in sub-Saharan Africa, including UARTO, are women[40]. The regression models for women initially included all women regardless of pregnancy status, but in a subsequent sensitivity analysis we excluded pregnant women from the models.
We then returned to the model with HSCL-D as the outcome variable and year of cohort entry as a continuous explanatory variable. To obtain an adjusted estimate for the regression coefficient for year of entry, we fitted a multivariable linear regression model with the inclusion of socio-demographic characteristics measured at ART initiation including age, gender (for the analysis of the overall cohort), educational attainment, marital status, household asset wealth, employment status, and heavy alcohol use. Using this multivariable model, we then sought to identify mediators of any changes in depression symptom severity at presentation by adding the explanatory variables of interest (BMI, CD4 count, and PHS) to the model and re-assessing the statistical significance of the regression coefficient for year of cohort entry. Reduced statistical significance of the regression coefficient was considered evidence for a variable’s role in explaining the trends in depression symptom severity at ART initiation.
For any variables that were shown to possibly explain changes over time in depression symptom severity, we fitted a new regression model with that variable as the outcome and year of cohort entry as a continuous explanatory variable, adjusting for baseline socio-demographic characteristics. A statistically significant regression coefficient for year of cohort entry was considered evidence for a meaningful trend over time for the variable in question.
All analyses were performed using SAS software (Version 9.3, SAS Institute, Cary, NC). Regression diagnostic procedures yielded no evidence of collinearity or overly-influential outliers in any of the models.
Results
Missing data for any given variable ranged from <1% to 16%. Due to lack of overlap in missing values, of the 690 persons in the complete UARTO cohort, 534 persons (77.4%) were included in the regression models as complete cases. There were no significant differences between complete cases and persons with missing data for any of the variables of interest, including mean CD4 count (187 vs. 196; t=0.69, p=0.49) and mean HSCL-D score (1.64 vs. 1.58, t=1.11, p=0.27).
There were 379 women (71.0%) and 155 men (29.0%) in the sample. The number of women and men entering the cohort per year are included in Figures 1 and 2. Baseline characteristics are stratified by gender in Table I. Average age was 35 years old. Women were younger than men (33 vs. 38 years, p<0.001), and were less likely to be married (40% vs. 72%, p<0.001) or employed (68% vs. 90%, p<0.001). Women had a higher BMI (22.4 vs. 19.9, p<0.001) and were less likely to screen positive for heavy alcohol use (19% vs. 39%, p<0.001). 33 (8.7%) of the women were pregnant at the time of entry into the cohort.
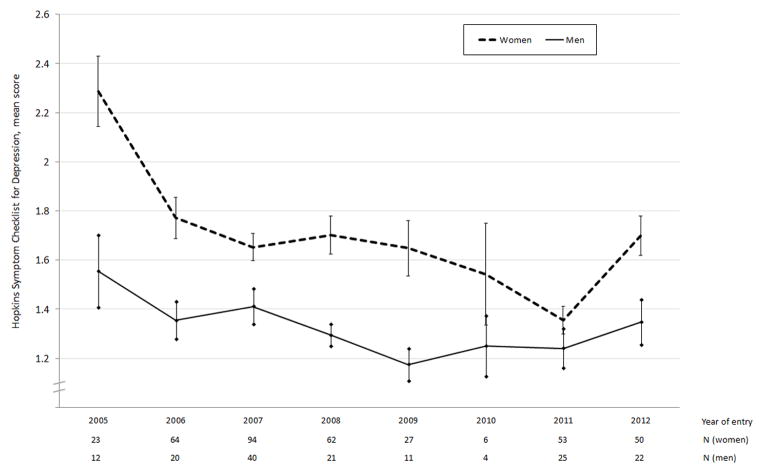
Figure 1. Mean HSCL-D scores by year of entry, stratified by gender.
Bars represent standard errors
Figure 2. Mean PHS scores by year of entry, stratified by gender.
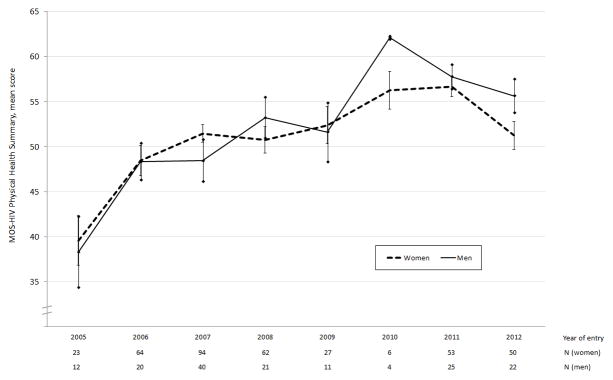
Bars represent standard errors
Table I.
Characteristics of patients by gender
| Characteristic | Overall (n=534) | Women (n=379) | Men (n=155) | T-test / χ2 test statistic | P-value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 34.5 (8.9) | 33.1 (8.8) | 38.0 (8.2) | 5.91 | <0.001 |
| Achieved more than primary education, n (%) | 141 (26.4) | 97 (25.6) | 44 (28.4) | 0.44 | 0.51 |
| Married, % | 263 (49.3) | 152 (40.1) | 111 (71.6) | 43.69 | <0.001 |
| Pregnant, % | 33 (8.7) | ||||
| Household asset index, mean (SD) | 0.03 (2.1) | 0.004 (2.1) | 0.11 (2.1) | 0.53 | 0.60 |
| Employed, % | 396 (74.2) | 256 (67.6) | 140 (90.3) | 29.78 | <0.001 |
| AUDIT-C positive screen for heavy alcohol use, n (%) | 134 (25.1) | 73 (19.3) | 61 (39.3) | 23.63 | <0.001 |
| Body mass index, mean (SD) | 21.6 (3.7) | 22.4 (3.9) | 19.9 (2.3) | 7.42 | <0.001 |
| CD4 cell count, mean (SD) | 186.8 (140.3) | 191.6 (140.3) | 175.0 (140.2) | 1.25 | 0.21 |
| MOS-HIV physical health summary score, mean (SD) | 51.1 (11.6) | 50.9 (11.4) | 51.4 (12.0) | 0.41 | 0.68 |
| HSCL-D score, mean (SD) | 1.58 (0.58) | 1.68 (0.60) | 1.34 (0.39) | 6.49 | <0.001 |
| Probable depression (HSCL-D>1.75), n (%) | 154 (28.8) | 134 (35.4) | 20 (12.9) | 27.0 | <0.001 |
154 (28.8%) respondents, including significantly more women than men (35.4% vs. 12.9%, p<0.001), had HSCL-D scores consistent with probable depression. The mean HSCL-D score was higher for women compared to men (1.68 vs. 1.34, p<0.001). Mean depression scores by year of cohort entry are shown in Figure 1; there appeared to be a downward trend among women, but not among men.
To formally test for changes in depression symptom severity at ART initiation, a linear regression model with HSCL-D score as the outcome variable and year of cohort entry as the explanatory variable revealed a statistically significant negative association between year of entry and HSCL-D score (b=−0.05; 95% CI, −0.07 to −0.03). This suggests a 3.1% (5/158) relative decline in the mean depression symptom severity score in each year of study recruitment after the first year. When disaggregated by gender, we found a declining trend among the women (b=−0.05; 95% CI, −0.08 to −0.03) but not the men (b=−0.03; 95% CI, −0.05 to 0.001), although a formal test of interaction by gender did not yield a statistically significant result (t=1.28, p=0.20). The alternative parameterization of a linear regression model specifying time as a series of dummy variables for each 1-year period was consistent with this finding, rejecting the null hypothesis that mean depression symptom severity at ART initiation was constant across time among women (F=6.41, p<0.001) but not among men (F=1.35, p=0.23).
After inclusion of baseline socio-demographic characteristics including age, gender, educational attainment, marital status, household asset wealth, employment status, and heavy alcohol use in a multivariable linear regression model, the adjusted regression coefficient for year of cohort entry remained statistically significant (b=−0.04; 95% CI, −0.06 to −0.02). As in the unadjusted model, the inclusion of a gender interaction term in the model did not yield a statistically significant coefficient for the interaction term (t=1.38, p=0.17). However, when disaggregated by gender, the adjusted regression coefficient for year of cohort entry was statistically significant among the women (b=−0.05; 95% CI, −0.08 to −0.02), but not among the men (b=−0.02; 95% CI, −0.05 to 0.01). A sensitivity analysis excluding pregnant women from the women-only model did not produce a substantively different result for the adjusted coefficient for year of cohort entry (b=−0.05, 95% CI, −0.08 to −0.02). Results of the multivariable linear regression model, stratified by gender, are included in Table II.
Table II.
Linear regression estimates for the association between year of cohort entry and depression symptom severity, with covariates
| Variable | Men | Women | ||
|---|---|---|---|---|
| Adjusted coefficient | 95% CI | Adjusted coefficient | 95% CI | |
| Year of cohort entry | −0.019 | (−0.047, 0.009) | −0.052 | (−0.080, −0.025) |
| Age, per 10 years | 0.005 | (−0.081, 0.071) | −0.036 | (−0.106, 0.034) |
| Achieved secondary education | 0.074 | (−−0.073, 0.222) | 0.078 | (−0.066, 0.222) |
| Unmarried | 0.083 | (−0.058, 0.224) | 0.057 | (−0.072, 0.186) |
| Asset index | 0.0004 | (−0.033, 0.032) | −0.018 | (−0.048, 0.013) |
| Unemployed | 0.187 | (−0.021, 0.394) | 0.095 | (−0.034, 0.225) |
| Heavy alcohol use | 0.065 | (−0.063, 0.193) | 0.051 | (−0.102, 0.205) |
Turning next to the explanatory variables of interest, the adjusted regression coefficient for year of cohort entry remained statistically significant (among the overall cohort and among women) with the addition of BMI and CD4 count. Among women, trends in pregnancy status at ART initiation did not explain the trends in depression symptom severity. However, when PHS was added to the model, it had a statistically significant association with depression symptom severity in the overall cohort (b=−0.02; 95% CI, −0.022 to −0.015), among women (b=−0.023; 95% CI, −0.028 to −0.018), and among men (b=−0.010; 95% CI, −0.015 to −0.004). The regression coefficient for year of cohort entry was reduced in magnitude in both the overall cohort (b=−0.018; 95% CI, −0.038 to 0.002) and among women (b=−0.029; 95% CI, −0.054 to −0.003).
Fitting a model with PHS as the outcome variable and year of cohort entry as a continuous explanatory variable while adjusting for baseline socio-demographic characteristics, we found a statistically significant positive association between year of entry and PHS (b=1.33; 95% CI, 0.90–1.77). This association was observed among both men (b=2.04; 95% CI, 1.23–2.85) and women (b=1.05; 95% CI, 0.53–1.56). The inclusion of a gender interaction term in the model did not yield a statistically significant coefficient for the interaction term (t=1.91, p=0.06). Mean PHS scores by year of cohort entry are shown in Figure 2.
Discussion
In this analysis of data on PLHIV initiating ART in rural Uganda over a seven year period, we observed a decline over time in mean depression severity scores among women (but not men) at ART initiation. The observed decline was not explained by secular changes in the socio-demographic characteristics of persons presenting for ART initiation. Rather, this decline was explained by secular changes in physical health: that is, the mean level of depression symptom severity appeared to be declining among women at ART initiation because they were initiating treatment at a higher level of physical health status. This finding should not be interpreted to suggest that the overall prevalence of depression among PLHIV is declining; rather, it appears that healthier PLHIV who are presenting to care in the era of ART expansion have, on average, lower levels of depression symptom severity. Our findings have important implications for policymakers as they suggest another possible benefit of expansion of ART to healthier populations in LMICs may be the diminution of depression-related sequelae among PLHIV.
To our knowledge, this study is the first to have examined secular trends in depression at ART initiation among PLHIV presenting for ART in LMICs. The association between improved physical health and lower depression severity is conceptually consistent with earlier studies demonstrating an association between HIV-related physical symptoms and psychological distress or depressive symptoms[20–22], which may be mediated in part by biologic pathways such as increased induction of indoleamine 2,3-dioxygenase-1 among patients with advanced disease[25]. The improvement in physical health status among PLHIV in our cohort likely relates to the trend in LMICs of earlier ART initiation, before patients begin experiencing the physical health status declines caused by HIV-related opportunistic infections and wasting[41].
The results of this study demonstrate that patients’ physical health at the time of ART initiation is an important factor explaining secular declines in depression symptom severity among PLHIV presenting for ART initiation. Although further study is needed, the implication is that if PLHIV present to care before they feel sick, they may be less likely to suffer the known negative outcomes associated with co-morbid depression, including risky transmission behaviors[5,6], reduced adherence to therapy[9], and increased rates of morbidity and mortality[7,8,10,11]. Therefore, the findings of this study suggest another important justification for earlier ART initiation for PLHIV in LMICs.
One important caveat is our finding that changes in CD4 count did not explain the observed decline over time in mean depression severity scores. The reason for this is unclear, but may have to do with the large standard deviations for CD4 count in our cohort, which may have reduced the predictive power of this variable compared to PHS. Previous studies on the association between CD4 count and depression or mental health quality of life scores in LMICs have yielded inconsistent findings[4,5,42,43].
There are several limitations to our study. First, although we observed a decline over time in mean depression severity scores among women but not men presenting for ART, the gender interaction terms in our models were not statistically significant, possibly due to the much smaller sample size of men in the UARTO cohort. Thus, our ability to make definitive gender comparisons is limited. Based on the magnitudes of the coefficients for year of cohort entry, it appears that trends in depression at ART initiation are different for men compared to women, but an alternative hypothesis could be that we simply lacked adequate statistical power to estimate gender-specific models. Second, although the Mbarara ISS Clinic is prototypical of ART scale-up clinics in other LMICs, particularly in sub-Saharan Africa, our findings may not generalize to other LMICs. Third, we did not have access to data on DSM-consistent diagnoses of depressive disorders. The HSCL-D measures depression symptom severity and the conventional cutoff of >1.75 only identifies persons with symptoms indicative of probable depression. However, this limitation should not affect our estimates about trends in depression symptom severity, as we have no reason to expect systematic scoring biases in the HSCL-D over time. Furthermore, random measurement error in the dependent variable would tend to bias our results towards the null, thereby strengthening our confidence in the statistically significant findings that were observed.
In conclusion, in this study of PLHIV in rural Uganda, we found evidence for a decline over time in mean depression severity score among women (but not men) presenting for initiation of ART. The observed decline was not explained by secular changes in socio-demographic characteristics, but rather by secular changes in physical health likely related to initiation of ART at earlier stages of disease. These findings suggest an important further rationale for earlier ART initiation in LMICs, as patients may be less likely to suffer the negative outcomes associated with co-morbid depression such as risky transmission behaviors, reduced adherence to therapy, and increased rates of morbidity and mortality. Further study is needed to confirm our findings in other LMICs and the association between earlier ART initiation and diminution of depression-related sequelae.
Acknowledgments
We thank the participants of the Uganda AIDS Rural Treatment Outcomes Study who contributed valuable time and information. The UARTO Study was funded by U.S. National Institutes of Health (NIH) R01MH054907 and P30AI27763. The authors also acknowledge the following additional sources of support: NIH T32AI007433 (Chan), K23MH079713 (Weiser), K23MH087228 (Haberer), U01CA066529 (Martin), K24MH087227 (Bangsberg), K23MH096620 (Tsai), David Brudnoy Scholar Award (Chan), MGH Center for Global Health Travel Award (Chan), Partners Global Health Center of Expertise Travel Grant (Chan), and the Burke Family Foundation (Weiser).
Footnotes
Presented at the 21st Conference on Retroviruses and Opportunistic Infections, March 5, 2014, Boston, MA.
References
- 1.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Nebhinani N, Mattoo SK, Wanchu A. Psychiatric morbidity in HIV-positive subjects: a study from India. J Psychosom Res. 2011;70:449–54. doi: 10.1016/j.jpsychores.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Gaynes BN, Pence BW, Atashili J, O’Donnell J, Kats D, Ndumbe PM. Prevalence and predictors of major depression in HIV-infected patients on antiretroviral therapy in Bamenda, a semi-urban center in Cameroon. PLoS ONE. 2012;7:e41699. doi: 10.1371/journal.pone.0041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaharuza FM, Bunnell R, Moss S, et al. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav. 2006;10:S105–11. doi: 10.1007/s10461-006-9142-2. [DOI] [PubMed] [Google Scholar]
- 5.Olley BO, Seedat S, Stein DJ. Persistence of psychiatric disorders in a cohort of HIV/AIDS patients in South Africa: a 6-month follow-up study. J Psychosom Res. 2006;61:479–84. doi: 10.1016/j.jpsychores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25:433–57. doi: 10.1016/j.cpr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270:2568–73. [PubMed] [Google Scholar]
- 8.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 9.Holzemer WL, Corless IB, Nokes KM, et al. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care STDS. 1999;13:185–97. doi: 10.1089/apc.1999.13.185. [DOI] [PubMed] [Google Scholar]
- 10.Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–40. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44:470–7. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS Behav. 2010;14:252–62. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67:1282–90. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai AC, Karasic DH, Hammer GP, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103:308–15. doi: 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai AC, Mimiaga MJ, Dilley JW, et al. Does effective depression treatment alone reduce secondary HIV transmission risk? Equivocal findings from a randomized controlled trial. AIDS Behav. 2013;17:2765–72. doi: 10.1007/s10461-013-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS. 2012;26 (Suppl 2):S117–35. doi: 10.1097/QAD.0b013e32835bde0f. [DOI] [PubMed] [Google Scholar]
- 17.Wouters E, Van Loon F, Van Rensburg D, Meulemans H. State of the ART: clinical efficacy and improved quality of life in the public antiretroviral therapy program, Free State province, South Africa. AIDS Care. 2009;21:1401–11. doi: 10.1080/09540120902884034. [DOI] [PubMed] [Google Scholar]
- 18.Lessells RJ, Mutevedzi PC, Iwuji CC, Newell M-L. Reduction in early mortality on antiretroviral therapy for adults in rural South Africa since change in CD4+ cell count eligibility criteria. J Acquir Immune Defic Syndr. 2014;65:e17–24. doi: 10.1097/QAI.0b013e31829ceb14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gargano JW, Laserson K, Muttai H, Odhiambo F, Orimba V, Adamu-Zeh M, et al. The adult population impact of HIV care and antiretroviral therapy in a resource poor setting, 2003–2008. AIDS. 2012;26:1545–54. doi: 10.1097/QAD.0b013e328353b7b9. [DOI] [PubMed] [Google Scholar]
- 20.Maj M, Janssen R, Starace F, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase I. Study design and psychiatric findings. Arch Gen Psychiatry. 1994;51:39–49. doi: 10.1001/archpsyc.1994.03950010039006. [DOI] [PubMed] [Google Scholar]
- 21.Mast TC, Kigozi G, Wabwire-Mangen F, et al. Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS Care. 2004;16:81–94. doi: 10.1080/09540120310001633994. [DOI] [PubMed] [Google Scholar]
- 22.Mello VA, Segurado AA, Malbergier A. Depression in women living with HIV: clinical and psychosocial correlates. Arch Womens Ment Health. 2010;13:193–9. doi: 10.1007/s00737-009-0094-1. [DOI] [PubMed] [Google Scholar]
- 23.Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. 2005;165:1260–6. doi: 10.1001/archinte.165.11.1260. [DOI] [PubMed] [Google Scholar]
- 24.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–9. [PubMed] [Google Scholar]
- 25.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65:456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner GJ, Ghosh-Dastidar B, Garnett J, Kityo C, Mugyenyi P. Impact of HIV antiretroviral therapy on depression and mental health among clients with HIV in Uganda. Psychosom Med. 2012;74:883–90. doi: 10.1097/PSY.0b013e31826629db. [DOI] [PubMed] [Google Scholar]
- 27.Akena D, Joska J, Obuku EA, Stein DJ. Sensitivity and specificity of clinician administered screening instruments in detecting depression among HIV-positive individuals in Uganda. AIDS Care. 2013;25:1245–52. doi: 10.1080/09540121.2013.764385. [DOI] [PubMed] [Google Scholar]
- 28.Kazooba P, Kasamba I, Baisley K, Mayanja BN, Maher D. Access to, and uptake of, antiretroviral therapy in a developing country with high HIV prevalence: a population-based cohort study in rural Uganda, 2004–2008. Trop Med Int Health. 2012;17:e49–57. doi: 10.1111/j.1365-3156.2012.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO, UNAIDS, UNICEF, editor. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. [Internet] World Health Organization; 2010. [cited 2013 Sep 2]. Available from: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. [Google Scholar]
- 30.Geng EH, Bwana MB, Kabakyenga J, et al. Diminishing availability of publicly funded slots for antiretroviral initiation among HIV-infected ART-eligible patients in Uganda. PLoS ONE. 2010;5:e14098. doi: 10.1371/journal.pone.0014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7:79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 32.Tsai AC, Bangsberg DR, Frongillo EA, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74:2012–9. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton P, Ndogoni L. Cross-cultural assessment of trauma-related mental illness (Phase II). [Internet] Baltimore: Johns Hopkins University; 2001. [cited 2013 Nov 15]. Available from: http://www.certi.org/publications/policy/ugandafinahreport.htm. [Google Scholar]
- 34.Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis. 2000;188:662–70. doi: 10.1097/00005053-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Winokur A, Winokur DF, Rickels K, Cox DS. Symptoms of emotional distress in a family planning service: stability over a four-week period. Br J Psychiatry. 1984;144:395–9. doi: 10.1192/bjp.144.4.395. [DOI] [PubMed] [Google Scholar]
- 36.Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29:786–98. doi: 10.1097/00005650-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 38.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 39.Aljassem K, Raboud JM, Benoit A, et al. Differences in severity and correlates of depression between men and women living with HIV in Ontario, Canada. 3rd International Workshop on HIV and Women; Toronto, Canada. 2013. [Google Scholar]
- 40.Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lahuerta M, Wu Y, Hoffman S, et al. Advanced HIV Disease at Entry into HIV Care and Initiation of Antiretroviral Therapy During 2006–2011: Findings From Four Sub-Saharan African Countries. Clin Infect Dis. 2014;58:432–41. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel R, Kassaye S, Gore-Felton C, et al. Quality of life, psychosocial health, and antiretroviral therapy among HIV-positive women in Zimbabwe. AIDS Care. 2009;21:1517–27. doi: 10.1080/09540120902923055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stangl AL, Wamai N, Mermin J, Awor AC, Bunnell RE. Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care. 2007;19:626–36. doi: 10.1080/09540120701203915. [DOI] [PubMed] [Google Scholar]