Abstract
We treated mucopolysaccharidosis IVA (MPS IVA) mice to assess the effects of long-term enzyme replacement therapy (ERT) initiated at birth, since adult mice treated by ERT showed little improvement in bone pathology (1).
To conduct ERT in newborn mice, we used recombinant human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) produced in a CHO cell line. First, to observe the tissue distribution pattern, a dose of 250 units/g body weight was administered intravenously in MPS IVA mice at day 2 or 3. The infused enzyme was primarily recovered in liver and spleen, with detectable activity in bone and brain. Second, newborn ERT was conducted after tissue distribution study. The first injection of newborn ERT was performed intravenously, the second to fourth weekly injections were intraperitoneal, and the remaining injections from 5th to 14th week were intravenous into the tail vein.
MPS IVA mice treated with GALNS showed clearance of lysosomal storage in liver, spleen, and sinus lining cells in bone marrow. The column structure of the growth plate was organized better than adult mice treated with ERT; however, hyaline and fibrous cartilage cells in femur, spine, ligaments, discs, synovium, and periosteum still had storage materials to some extent. Heart valves were refractory to the treatment. Levels of serum keratan sulfate were kept normal in newborn ERT mice.
In conclusion, the enzyme, which enters the cartilage before the cartilage cell layer becomes mature, prevents disorganization of column structure. Early treatment from birth leads to partial remission of bone pathology in MPS IVA mouse.
Keywords: enzyme replacement therapy, fibrous cartilage, hyaline cartilage, mucopolysaccharidosis IVA, N-acetylgalactosamine-6-sulfate sulfatase
Introduction
Lysosomal storage disorders (LSDs) result from a deficiency of one or more degradative enzymes necessary for normal cell metabolism. Mucopolysaccharidosis IVA (MPS IVA; Morquio A syndrome) is an inherited autosomal recessive LSD (2). Two MPS IV subtypes result from deficiencies in different lysosomal enzymes that sequentially degrade keratan sulfate (KS): N-acetylgalactosamine-6-sulfate sulfatase (GALNS) for MPS IVA and β-galactosidase for MPS IVB (2). MPS IV occurs in 1 in 200,000 live births in British Columbia (3). MPS IVA occurs in 1 in 76,000 live births in Northern Ireland (5), 1 in 450,000 live births in The Netherlands (6), 1 in 201,000 in Australia (7), 1 in 450,000 live births in Portugal (8), 1 in 263,000 live births in Germany (9), and 1 in 500,000 live births in Japan [Orii et al., personal communication].
MPS IVA is characterized by severe systemic bone disease resulting in progressive bone deformity through excessive storage of KS. After a period of seemingly normal development, patients exhibit a range of initial symptoms including short stature, odontoid dysplasia, protrusion of the chest, genu valgum, kyphoscoliosis, hyperlaxity of joints, and abnormal gait before 3 years of age (2, 10, 11, 12, 13, 14, 15). Gibbus deformity has been noticed in some of patients even at birth, and radiographs show signs of the abnormal lumbar vertebrae (16). Most patients will be diagnosed before 5 years of age. The series of surgeries occur around 10 years of age: neck, hip, knee, and leg regions. These patients will be wheelchair bound as teenagers. Death occurs in severely affected patients in third decade of life, primarily as the result of cervical instability, complications of surgery, and pulmonary and/or cardiac compromise (17, 18).
Mouse models of MPS IVA that lack any detectable GALNS activity have been produced (19, 20, 21). MPS IVA mice exhibit widespread intracellular storage in a variety of cell types. These MPS IVA mouse models share biochemical and histopathologic characteristics with the human counterpart disease, including the storage materials in the liver, spleen, bone marrow, kidneys, heart valves, brain, osteoblasts, chondrocytes, ligaments, periosteum. The storage materials (vacuoles) are observed in chondrocytes of the growth plate region in affected newborns. By 8 weeks of age, MPS IVA mice accumulate the lysosomal storage materials in various tissues. The column structure of the growth plate is disorganized. In spite of clear storage materials in these tissues and cells, MPS IVA mice do not develop synovial hyperplasia, cartilage degeneration, or pannus formation over the articular cartilage. After backcrossing onto the C57/B6 strain, MPS IVA-affected mice had an upward angled calcaneus at age 6–7 months (22). Thus, the clinical phenotypes observed in MPS IVA mice are very mild compared with human patients (14, 15, 21). The primary reason for less clinical features in the current MPS IVA mice is KS synthesis in mice is limited compared with human KS synthesis. Nevertheless, these mouse models with pathological features of MPS IVA make it feasible to assess the therapeutic efficacy.
The concept of treating LSDs by enzyme replacement therapy is widely known; its success relies on the cellular uptake of enzyme by receptor-mediated endocytosis. The first attempt at ERT using macrophage-targeted recombinant β-glucocerebrosidase was successful in treating the nonneuronopathic form of Gaucher disease (23, 24). Successively, ERT mediated by the mannose-6-P receptor was also approved for use in patients with Fabry disease (25, 26), MPS I (27), MPS II (28), and MPS VI (29, 30, 31). ERT for MPS IV A has been also approved by FDA (32), although it is thought that ERT has only a limited effect on bone involvement, especially in avascular cartilage area.
An ERT preclinical trial in 3-month-old adult MPS IVA mice resulted in marked reduction of storage material in the visceral organs, but provided a limited effect in hyaline and fibrous cartilage cells in the femur, ligaments, and synovium. The column structure of the growth plate region remained disorganized (1). While it has been well known that adult ERT provides a limited pathological and clinical impact to animal models with other types of MPS, newborn ERTs on animal models with MPS were attempted. ERT from birth in MPS VI cats showed that body weights in treated cats were heavier than those in untreated cats and these cats had greatly reduced or no spinal cord compression. Skeletal pathology was reduced, with more normalized bone dimensions and more uniform bone density and trabecular pattern clearly visible on radiographs by 5 to 6 months of age, although no reduction in lysosomal vacuolation was observed in cartilage cells (33). The growth plate region in 1- or 2-day-old MPS VII mice or even in mice fetus had abundant ballooned and vacuolated chondrocytes in resting and proliferative zones (34, 35). ERT in newborn MPS VII mice showed that vacuolated epiphyseal and articular cartilage cells remained refractory to therapy, although clinical improvement in bone was observed (36, 37, 38). An alternative approach to treatment is gene therapy. Neonatal gene therapy for MPS VII resulted in reduced storage in chondrocytes of both the growth plate and the articular surface (39, 40).
Dierks et al. (2003) (41) and Cosma et al. (2003) (42). identified formylglycine-generating enzyme (FGE) and its gene, named sulfatase-modifying factor (SUMF1). FGE converts cysteine, which is commonly present in the active site of all sulfatases, to formylglycine and is thought to be essential for sulfatase activity, including GALNS. Normally, the necessary amount of FGE required to modify sulfatases is constitutively expressed in the cell. However, when a specific recombinant sulfatase, namely, GALNS in our study, is excessively expressed, the relative amount of FGE is anticipated to decrease, making it necessary to coexpress FGE. Transient co-expression of GALNS with SUMF1 in COS cells has been shown to increase the enzyme activity of GALNS (42, 43).
Stably transfected Chinese hamster ovary (CHO) cells secrete large amounts of recombinant human GALNS (native-GALNS), with a subunit size of 57 kD (44). The mannose-6-phosphate receptor was shown to mediate uptake of CHO native GALNS in skin fibroblasts by inhibition studies with mannose-6-phosphate (44). In the present study, we co-transfected SUMF1 in the overexpressed GALNS cell line, produced, and purified the SUMF1-GALNS enzyme.
Here we show the response to ERT from birth in MPS IVA mice to evaluate implications of ERT at an early stage.
Materials and Methods
Enzyme production and purification
GALNS enzyme was purified from the media of transfected CHO cells by a two-column procedure (44). Specific activity of enzyme was determined before each of the 14 weekly injections. SUMF1-GALNS had an average of specific activity (270,715 ± 25,063 U/mg) toward the artificial fluorogenic substrates. The enzyme used here was the same as that used in the adult mice experiments described previously (1). The results of adult mice treated by ERT were compared to the newborn studies here.
Experimental mice
Homozygous knockout mutant (Galns−/−) mice were obtained from the MPS IVA mouse colony (17). All mice were identified at birth as normal or mutants by obtaining genomic DNA from tissue obtained by a toe clip and amplifying with respective primer. Briefly, the genotyping of the mice was as follows (19). The presence of the C76S point mutation in exon 2 of the Galns gene was detected by PCR of the mouse tail DNA using a forward primer in intron 1(mGMO1F: 5′-CCTGTGTCATTTGCATGTGACTATT-3′) and a reverse primer in intron 2 (mGMO2R: 5′-TTGTCCTGTGACCAGGAAGTGCAG-3′), which amplified a 549-bp fragment of the mouse Galns gene. Digested with Sac I, the 189- and 360-bp restriction fragments present in the mutant allele are distinguished from the uncleaved 549-bp PCR fragment from the normal allele.
Mouse studies were approved by the appropriate institutional ethics committee. All experimental procedures were conducted at Saint Louis University.
GALNS distribution in MPS IVA newborn mice
A previous study characterized the tissue distribution pattern of native GALNS in MPS IVA mice (44). In this study, four MPS IVA newborn mice received an infusion of 250 units/g (1.0 mg/kg) body weight of SUMF1-GALNS into the superficial temporal vein on day 2 or 3 of life. Two mice were euthanized at each time point 2 h and 24 h after injection. Two untreated mice were used as controls. Tissues such as brain, liver, spleen, kidney, lung, heart, and bone (left arm and calvaria) were removed and assayed for GALNS activity. Organs were weighed and homogenized immediately in 1 ml of homogenization buffer consisting of 25 mM Tris-HCl, pH 7.2 and 1 mM phenylmethylsulfonyl fluoride. GALNS assays on dilutions of tissue extracts were incubated for 24 h, and the activity was expressed as units/mg protein. GALNS enzyme activity was assayed by using 4-methylumbelliferyl-β-D-galactopyranoside-6-sulfate as a substrate (Moscerdam Substrate, Rotterdam, The Netherlands). The enzyme assay was performed with two steps as described previously (45).
One unit is defined as the enzyme catalyzing 1 nmol of 4 methylumbelliferyl-β-D-galactopyranoside-6-sulfate per hour. Total protein was determined by micro-Lowry protein assay (46) with bovine serum albumin used as a standard.
ERT procedure
SUMF1-GALNS treated mice (n = 9) were compared with untreated MPS IVA mice (n = 7). Mice with MPS IVA received weekly injections from birth to 14 weeks of age. Mice were weighed before enzyme injection; dose was calculated according to body weight and the enzyme solution was diluted in PBS (pH 7.2) to give a concentration of 250 units/g body weight of GALNS. The initial injection into the superficial temporal vein of newborn mice was intravenous. The second to fourth injections were intraperitoneal. The remaining injections from fifth to 14th were intravenous into the tail vein.
Pathological response to ERT
For morphological evaluation, the liver, spleen, kidney, brain, heart, femur, spine, and bone marrow from 15-week-old MPS IVA mice treated with 250 units/g of SUMF1-GALNS (n = 9) or PBS buffer (n = 7) were collected at necropsy, immersion-fixed in 4% paraformaldehyde/2% glutaraldehyde in PBS, postfixed in osmium tetroxide, and embedded in Spurr's resin. The autopsy was conducted one week later after the last injection of the enzyme. No enzyme activity was detected in any tissue of autopsied mice. For evaluation of lysosomal storage by light microscopy, toluidine blue-stained 0.5-μm-thick sections were examined. Tissues from the treated and untreated mice were evaluated for reduction in storage without knowledge of treatment. The amount of storage materials in each cell type was scored. “No storage or very slight” was 0 (−), “slight but obvious” was 1 (+), “moderate” was 2 (++), and “marked” was 3 (+++). A minimum of three fields were examined in each cell type. We averaged the score in a group of mice per tissue. Mann–Whitney U test was used to compare the score between untreated and treated mice.
KS assay
Liquid chromatography tandem mass spectrometry (LC-MS/MS) method was used for the analysis of the disaccharides produced from KS digestion (45, 46). API-4,000 mass spectrometer equipped with a turbo ionspray was used (Applied Biosystems, Foster City, CA). The blood was drawn at 5, 7, 8, and 9 weeks of age before the enzyme injection, and at 15 weeks of age during necropsy. KS in mouse serum was digested to the disaccharides by keratanase II (Seikagaku Corp. Tokyo, Japan). Analysis of disaccharides was performed by LC-MS/MS using multiple reactions monitoring in negative ion mode. Separation of LC was performed on a Hypercarb (2.0 mm i.d. ×150 mm, 5 μm) with a gradient elution of acetonitrile-0.01 M ammonium bicarbonate (pH 10). Flow rate of the mobile phase was 0.2 mL/min. This method was applied to the determination of KS in serum of mouse samples.
RESULTS
GALNS distribution in MPS IVA mice
Twenty-four hours after injection into the superficial temporal vein of newborn MPS IVA mice, 5.35, 0.18, 0.70, 0.86, 0.36, 0.77, and 0.68 units/mg of GALNS was detected in the liver, bone (left arm), calvaria, spleen, kidney, heart, and lung, respectively, while 0.1 units/mg of GALNS was detected in the brain (Table 1). The amount of enzyme detected 24 h after injection into the liver, calvaria, brain, and left arm was nearly 68%, 12%, 7%, and 6% of that observed in wild-type mice (Table 1). The amount of enzyme detected 24 h after injection into the liver, left arm, and brain was nearly 40%, 13%, and 15% of that observed at 2 h after injection (data not shown).
Table 1.
Distribution of GALNS in newborn MPS IVA mice
| Enzyme activity (units/mg) | Wild-type mice (n = 3) | MPS IVA treated mice (n = 2) |
|---|---|---|
| Left arm | 3.13 ± 1.11 | 0.18 ± 0.05 |
| Calvaria | 6.08 ± 0.14 | 0.70 ± 0.06 |
| Spleen | 4.98 ± 0.17 | 0.86 ± 0.19 |
| Brain | 1.38 ± 0.10 | 0.10 ± 0.06 |
| Kidney | 21.86 ± 0.90 | 0.36 ± 0.20 |
| Heart | 1.33 ± 0.09 | 0.77 ± 0.26 |
| Lung | 5.48 ± 0.70 | 0.68 ± 0.02 |
| Liver | 7.89 ± 0.56 | 5.35 ± 0.73 |
GALNS: N-acetylgalactosamine-6-sulfate sulfatase; MPS: mucopolysaccharidosis: SE, standard error.
*GALNS (250 units/g SUMF1-GALNS) was injected into the superficial temporal vein of MPS IVA mice on day 1 of life. Detectable levels of GALNS were observed in the brain, kidney, heart, and lung as well as bone at post-24 h injection in newborn mice. ±: SE.
In comparison, 24 h after injection with 250 units/g in 12-week-old adult MPS IVA mice, 0.9 and 0.7 units /mg were detected in the liver and spleen, while almost undetectable amounts were observed in the bone, brain, heart, kidney, and lung (data not shown). GALNS enzyme was more widely delivered to the bone, brain, heart, kidney, and lung in newborn than that in adult MPS IVA mice. Tissue distribution pattern of native enzyme without SUMF1 was previously conducted, showing the similar pattern (44).
Histopathology
Tissues including visceral organs, heart valves, bone (femur and spine), and cerebral cortex sections were assessed from mice in each of the treatment groups at 15 weeks of age.
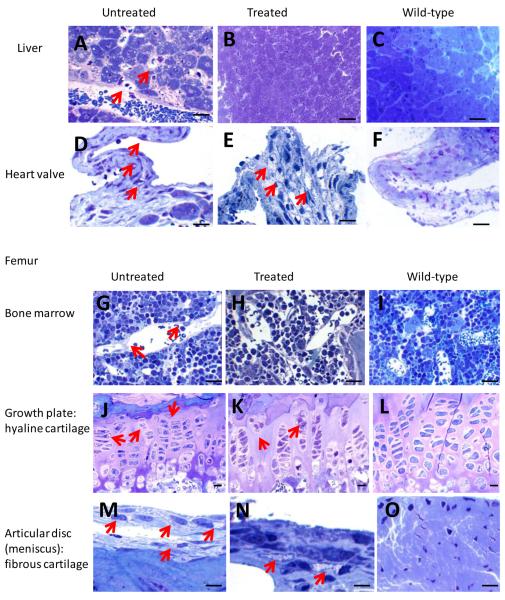
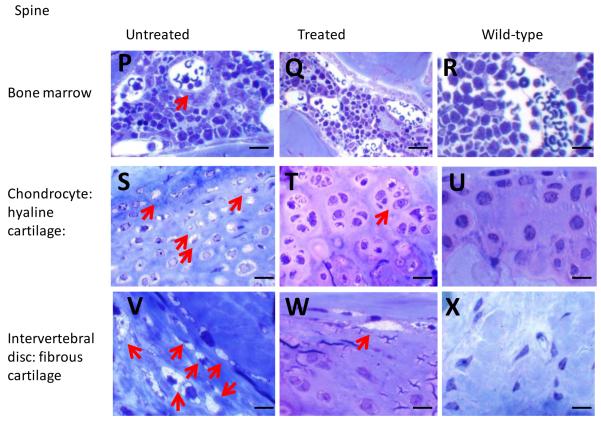
Untreated MPS IVA knockout mice exhibited GAG storage vacuoles in multiple tissues: Kupffer cells and sinus lining cells in the liver (Fig. 1A), mitral heart valve (Fig. 1D), sinus lining cells in bone marrow of the femur (Fig. 1G), growth plate of the femur (hyaline cartilage) (Fig. 1J), articular disc (Fig. 1M), ligament, and periosteum surrounding the femur (fibrous cartilage), sinus lining cells in bone marrow of the spine (Fig. 1P), spine (hyaline cartilage) (Fig. 1S), intervertebral disc (Fig. 1V) and ligament surrounding the spine (fibrous cartilage), neuron cells in the cortex and hippocampus, and meningeal cells (data not shown). Articular disc, intervertebral disc, and ligaments in untreated mice had abundant vacuolated fibrous cartilage cells. The growth plate exhibited an irregular structure with ballooned, vacuolated chondrocytes (Fig. 1G), and chondrocytes in the spine also had many vacuolated cells (Fig. 1K).
Figure 1. Histopathology in ERT-treated mouse.
Left panel shows an untreated MPS IVA mouse, middle panel shows an ERT-treated mouse, and right panel shows a wild-type control mouse.
Shown are liver (A, B, C), heart valve (D, E, F), bone marrow in femur (G, H, I), growth plate in femur (hyaline cartilage) (J, K, L), articular disc (fibrous cartilage) surrounding femur (M, N, O), bone marrow in spine (P, Q, R), growth plate in spine (hyaline cartilage) (S, T, U), and intervertebral disc (fibrous cartilage) surrounding vertebrae (V, W, X). The sections were stained with toluidine blue. The arrows show the vacuolated cells.
A, B, and C: Kupffer cells and sinus lining cells in an untreated mouse contain vacuoles, while no vacuole is seen in a treated or wild-type mouse. Figs. 1D and E show abundant vacuoles at the base in an untreated mouse and at the heart valve in a treated mouse. G, H, and I: Sinus lining cells in bone marrow of femur contain vacuoles in an untreated mouse, while no vacuole is seen in a treated or wild-type mouse. J, K, and L: all chondrocytes at growth plate of femur are vacuolated in an untreated mouse, while less vacuolated in a treated mouse. M, N, and O: Articular disc (meniscus) in femur contains abundant vacuolated cells in both untreated and treated mice. P, Q, and R: Sinus lining cells in bone marrow of spine in an untreated mouse, while no vacuole is seen in a treated or wild-type mouse. S, T, and U: All chondrocytes are vacuolated in an untreated mouse, while less numbers of chondrocytes are vacuolated in a treated mouse. V, W and X: Intervertebral disc in spine contains abundant vacuolated cells in untreated mice and less in treated mice.
Magnifications: x100 except G, H, and I (x40). Bar in the picture indicates 100 μm.
MPS = mucopolysaccharidosis; ERT = enzyme replacement therapy.
Sinus lining cells in the liver had complete reversal of lysosomal storage in newborn ERT mice. Figures 1A and B show light microscopy of the liver from untreated MPS IVA mice and SUMF1-GALNS treated mice. Kupffer cells in the liver also had complete clearance of storage in all treated mice (Table 2). There was no storage in the spleen in all treated mice (data not shown).
Table 2.
Pathological score in treated and untreated mice
| Mouse | Liver | Heart | Femur | Spine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLC | Kupffer cells | Valve | SLC in bone marrow | Growth plate: hyaline cartilage | Articular disc: fibrous cartilage | SLC in bone marrow | Growth plate: hyaline cartilage | Intervertebral disc: fibrous cartilage | ||
| Treated | Ave | 0.44 | 0.44 | 2 | 0 | 1.5 | 2 | 0 | 1 | 1 |
| group | SD | 0.882 | 0.882 | 0.837 | 0.000 | 0.378 | 0.707 | 0.000 | 0.651 | 0.795 |
| Untreated | Ave | 2.36 | 2.36 | 2.25 | 1.42 | 2.14 | 2.60 | 0.80 | 2.00 | 1.93 |
| group | SD | 0.556 | 0.556 | 0.274 | 0.585 | 0.378 | 0.548 | 0.671 | 0.548 | 0.345 |
| Mann-Whitney | ||||||||||
| U | P-value | < 0.005 | < 0.005 | 0.45830 | < 0.005 | < 0.005 | 0.12450 | < 0.005 | < 0.01 | < 0.03 |
SLC: sinus lining cells; SD = standard deviation
Treated: mitral heart valve, femur (hyaline cartilage), and spine (fibrous cartilage) (n = 8); other tissues (n = 9).
Untreated: mitral heart valve, femur (SLC), spine (fibrous cartilage) (n = 6); femur (fibrous cartilage), spine (SLC) (n = 5); other tissues (n = 7).
Score: “No storage or very slight” was 0 (−), “slight but obvious” was 1 (+), “moderate” was 2 (++), and “marked” was 3 (+++). Score was averaged with the number of mice investigated.
In heart valves, most treated mice showed slight reduction in storage, and storage persisted at the base of the valve itself, suggesting that ERT was less effective in heart valves compared with other tissues (Fig. 1D, Table 2).
In two mice treated with SUMF1-GALNS, the growth plate, ligaments, articular cartilage, and articular disc in the femur had a substantially reduced amount of storage with organized column structure, although vacuolated chondrocytes were observed focally in hyaline and fibrous cartilage cells. Three additional mice had a moderate reduction of storage materials in growth plate region. Seven other mice still had substantial storage materials in fibrous cartilage of ligaments, articular disc, and periosteum in the femur (Fig. 1B, E, H, K, N), and vacuolated chondrocytes remained widely observed. Thus, fibrous cartilage did not show a significant change compared with untreated mice, while the growth plate showed a significant difference. In the spine, all treated mice except for one had a moderate reduction of storage materials in hyaline cartilage cells (Fig. 1Q). All mice had a clearance of storage materials in SLC of bone marrow (Fig. 1T). Two mice still had moderate storage materials in fibrous cartilage cells of intervertebral disc and ligament, while other mice had a reduced amount of storage materials (Fig. 1W).
In MPS IVA mice treated from birth by ERT with SUMF1-GALNS, reduced storage was observed in neurons in the hippocampus (data not shown), compared with untreated MPS IVA mice. Meningeal cells of untreated MPS IVA mice contained numerous vacuoles, which were absent in newborn ERT mice (data not shown).
Overall, newborn ERT on MPS IVA mice provided nearly complete clearance in the liver, spleen, and bone marrow, partial response in hyaline cartilage cells of the femur and spine, and refractory response in fibrous cartilage cells surrounding the spine and heart valves (Table 2).
Concentration of Serum KS in the MPS IVA Mouse
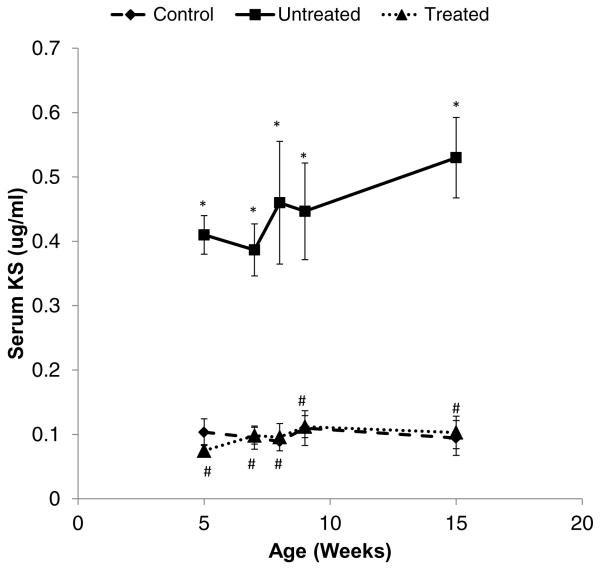
Serum samples from untreated, treated, or wild-type control mice were analyzed for KS (Fig. 2). The baseline serum KS in wild-type controls was relatively constant, showing an average of 0.098 ± 0.019 μg/mL (n = 3). The serum KS in untreated MPS IVA before treatment was elevated at an average level of 0.41 ± 0.088 μg/mL (p < 0.0001). Serum KS concentrations in untreated mice did not change significantly with age and remained elevated.
Figure 2. Level of serum KS in the MPS IVA mouse.
The serum was collected at 5, 7, 8, and 9 weeks old before the enzyme injection, and at 15 weeks old at necropsy. ◆ with a dash line: Wild-type control group, ■ with a solid line; Untreated MPS IVA group; ▲ with a dot line; Treated MPS IVA group. Each group comprises three mice and shows the average value with SD. Statistical analyses by Student's t-test show as follows. *: control vs. untreated p < 0.01, #: untreated vs. treated p < 0.01.
MPS = mucopolysaccharidosis; SD = standard deviation
Serum KS levels in treated mice were the same as those in control wild-type mice during the 14 weeks of treatment. The average level of serum KS concentration in SUMF1-GALNS treated mice was 0.1 ± 0.018 μg/mL (n = 3). Thus, the final serum KS in treated mice was similar to that in normal control mice.
DISCUSSION
This study has allowed us to evaluate and compare the efficacy of newborn ERT in MPS IVA mice, compared with adult ERT. The results showed that 1) the storage materials in hyaline and fibrous cartilage cells in treated mice were reduced to some extent, while heart valves were more refractory to therapy, and that 2) the normal level in serum KS was observed up to 15 weeks in MPS IVA mice treated with ERT. These findings suggest that newborn ERT works more effectively, compared with adult ERT, in MPS IVA mice (1).
Severe systemic bone deformities like protrusion of the chest, kyphoscoliosis, and knock-knee are noticed in children with MPS IVA at the ages of 1–3 years because of progressive accumulation of KS. Patients have marked short stature, hypermobile joints, and weak muscular strength, leading to frequent falling (2, 10, 11, 12, 14, 15, 16). The data of adult ERT in MPS IVA mice showed that the response to pathological improvement in bone lesions is refractory and that the column structure with vacuolated chondrocytes remains disorganized (1). The finding of this previous preclinical trial demonstrates that there is little possibility to reverse bone pathology once a certain period has passed after birth. This is mainly because of avascular cartilage tissue, where the drug cannot penetrate readily.
The enzyme administered intravenously to newborn mice could access the cartilage cell layer more efficiently because of a more vascular-rich status, as well as being more efficient in the brain because of a permeable blood-brain barrier in the first 2 weeks of life. Weekly injections with SUMF1-GALNS from birth led to better organization of column structure of the growth plate and reduced storage material to some extent in bone at 15 weeks of age. There was less clearance of storage material in fibrous cartilage cells of the articular disc, ligaments, periosteum, and synovium surrounding the femur of MPS IVA mice. There was also a limited response in heart valves, since heart valves are avascular as well. Although further detailed studies are required, reduced accumulation of storage material and better organized column structure in the growth plate indicate that more benefits are provided for the MPS IVA patients clinically as well, if treatment starts at birth.
We used serum KS level as a biomarker. At 5 week of age, newborn ERT mice showed a significantly lower serum KS level than untreated mice. The level of serum KS was kept normal up to 15 weeks of age. The detection of a small amount of KS at an early stage is quite useful for monitoring the clinical course and response to treatment in individual MPS IVA mice and patients as well as for newborn screening. Thus, the present LC-MS/MS method to assay KS is a powerful tool for monitoring and screening MPS IVA (13).
Enzyme distribution studies in MPS IVA mice demonstrated the presence of GALNS in the brain of newborn mice, but not in the brain of 12-week-old adult mice with 250 units/g body weight dose (1, 44). Although MPS IVA patients and mice have no clinical signs and symptoms involving the central nervous system (CNS), MPS IVA mice have obvious vacuoles in meningeal cells and neurons in the cortex, specifically the hippocampus. A correction of pathology in the brain of MPS VII mice was required to treat affected mice at the newborn stage (44, 45, 46), or to use a high dose of enzyme if adult mice (49). This similar finding was seen with newborn ERT MPS IIIA mice (50). Two hours after intravenous injection of GALNS in newborn MPS IVA mice, the enzyme was detected in the brain. In contrast, no enzyme was detected in the adult brain at any time point including 2 h after infusion (44). It is likely that only the first two to three enzyme injections reached the CNS of mice that were treated from birth, resulting in almost no lysosomal storage in brain.
ERT with SUMF1-GALNS on newborn MPS IVA mice was fully effective in preserving the column structure, while clearing (or preventing) storage material in chondrocytes was partially effective. These findings are comparable with the results of other newborn ERTs on MPS animal models (33, 34, 36, 37, 51), suggesting the clinical improvement in bone lesions and column structure of the growth plate. The limited number of injections of enzyme in MPS IVA mice penetrates the bone (especially, cartilage cell layer) at this early stage of life, leading to better remission of bone lesions in MPS IVA mice.
Limitations of the current results are: 1) the current mouse model does not have severe clinical features in bone, and therefore, we cannot assume how much the bone lesions can be improved clinically in human patients, even if pathological improvement is achieved, and 2) pathology in hyaline and fibrous cartilage remains unsolved in spite of newborn ERT, suggesting that an additional unique strategy to provide more impact to the bone lesions, such as high dose administration (49), bone targeting (52), or long-circulating enzyme (34) are required. Since the bone lesion is already seen clinically in a newborn MPS IVA patient (16) and storage materials are observed in chondrocytes of an affected fetus (53) and newborn MPS IVA mice (17), achieving reversal of pathology and clinical improvement in bone lesions should be addressed carefully in future experiments and clinical trials.
How much dose of the enzyme is required for newborn ERT; how possible it is to achieve complete remission of storage materials in epiphyseal growth plate, articular cartilage, and fibrous cartilage; how often and how long is the administration of ERT required; and how are clinical improvements correlated with pathological improvements and reduction of KS are questions that remain unanswered.
In conclusion, the current and previous preclinical ERT studies on newborn and adult MPS IVA mice demonstrate that newborn ERT improves bone pathology partially, while adult ERT provides little impact (1). This conclusion is supported by ERT studies on other types of MPS animal models (33, 34, 36, 37, 51). Early diagnosis of newborns and early treatment should be recommended to ameliorate or remit devastating skeletal dysplasia (13, 14, 15).
Highlight.
This is the first newborn ERT on the mouse model with MPS IVA.
Newborn ERT provides a better therapeutic efficacy.
Bone pathology is not resolved completely.
Heart valve is refractory to ERT.
ERT improves the pathology but does not cure the disease.
Acknowledgements
This work was supported by grants from the Austrian MPS Society, National MPS Society, Jacob Randoll Foundation, Bennett Foundation, and International Morquio Organization (Carol Ann Foundation). S.T. is supported by National Institutes of Health grant 8P20 GM103464-08. The content of the article has not been influenced by the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest with any co-author from the industry. Any industry has neither a patent nor a licensing agreement for the LC-MS/MS method on mucopolysaccharidosis IVA described here. The corresponding author will provide the enzyme for KS assay upon request. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Compliance with Ethics
Conflict of Interest: All the authors contributed to this “Original Article” and have no conflict of interest with any other party.
Shunji Tomatsu, Adriana M. Montaño, Hirotaka Oikawa, Vu Chi Dung, Amiko Hashimoto, Toshihiro Oguma, Tatsuo Takahashi, Tsutomu Shimada, Tadao Orii, and William S. Sly declare that they have no conflict of interests.
Informed Consent: Not applicable for this manuscript.
This article does not contain any studies with human subjects performed by the any of the authors.
Animal Rights: All institutional and national guidelines for the care and use of laboratory animals were followed.
Contributions to the Project: Shunji Tomatsu: He is a Principal Investigator responsible for this project. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Adriana M. Montaño: She has contributed to the planning, performance of mouse experiments (purification of enzyme and injections), data analysis, and reporting of the work described in the article.
Hirotaka Oikawa: He has contributed to the planning, performance of mouse experiments (mouse care, breeding, and injections), and reporting of the work described in the article.
Vu Chi Dung: He has contributed to the planning, performance of pathological studies of mice, and reporting of the work described in the article.
Amiko Hashimoto: She has contributed to the planning, performance of pathological studies of mice, and reporting of the work described in the article.
Toshihiro Oguma: He has contributed to the planning, performance of keratan sulfate assay of mice, and reporting of the work described in the article.
Monica L. Gutiérrez: She has contributed to the planning, performance of mouse experiments (purification of enzyme), and reporting of the work described in the article.
Tatsuo Takahashi: He has contributed to the planning, performance of mouse experiments (mouse care, breeding), and reporting of the work described in the article.
Tsutomu Shimada: He has contributed to conduct data and statistical analyses and reporting of the work described in the article.
Tadao Orii: He has contributed to the planning and reporting of the work described in the article.
William S. Sly: He has contributed to the planning and reporting of the work described in the article.
REFERENCES
- 1.Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 2.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A Syndrome: A review. Research and Reports in Endocrine Disorders. 2012a;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowry RB, Applegarth DA, Toone JR, MacDonald E, Thunem NY. An update on the frequency of mucopolysaccharide syndromes in British Columbia. Hum. Genet. 1990;85:389–390. doi: 10.1007/BF00206770. [DOI] [PubMed] [Google Scholar]
- 4.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia. 1969–1996. Pediatrics. 2000;105:e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- 5.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 6.Poorthuis BJ, Wevers RA, Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 7.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 8.Pinto R, Caseiro C, Lemos M, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 9.Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 10.Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013 doi: 10.1016/j.ymgme.2013.04.002. pii: S1096-7192(13)00111-X. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 12.Tomatsu S, Montaño AM, Oikawa H, et al. Handbook of growth monitoring and health and disease. Springer Publications; London: 2012b. Chapter 126: Impairment of Body Growth in Mucopolysaccharidoses. In: Preedy VR, editor. [Google Scholar]
- 13.Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab. 2013a;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomatsu S, Alméciga-Díaz CJ, Barbosa H, et al. Therapies of Mucopolysaccharidosis IVA (Morquio A Syndrome) Expert Opinion on Orphan Drugs. 2013b;10:805–818. doi: 10.1517/21678707.2013.846853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomatsu S, Yasuda E, Patel P, et al. Morquio A Syndrome: Diagnosis and Current and Future Therapies. Pediatr Endocrinol Rev. 2013c in press. [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–769. 910–912. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 17.Lavery C, Hendriksz C. Mortality in Patients with Morquio Syndrome A. JIMD Rep. 2014 Apr 10; doi: 10.1007/8904_2014_298. 2014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol Genet Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Tomatsu S, Orii KO, Vogler C, et al. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns−/−) produced by targeted disruption of the gene defective in Morquio A disease. Hum Mol Genet. 2003;12:3349–3358. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 20.Tomatsu S, Gutierrez M, Nishioka T, et al. Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum Mol Genet. 2005;14:3321–3335. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- 21.Tomatsu S, Vogler C, Montaño AM, et al. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol Genet Metab. 2007a;91:251–258. doi: 10.1016/j.ymgme.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Rowan DJ, Tomatsu S, Grubb JH, Montaño AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J Inherit Metab Dis. 2013;36:235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton NW, Brady RO, Dambrosia JM, et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 24.Barranger JA, O'Rourke E. Lessons learned from the development of enzyme therapy for Gaucher disease. J Inherit Metab Dis. 2001;24(Suppl 2):89–96. doi: 10.1023/a:1012440428282. [DOI] [PubMed] [Google Scholar]
- 25.Schiffmann R, Kopp JB, Austin HA, III, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 26.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A--replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 27.Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 28.Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 29.Harmatz P, Whitley CB, Waber L, et al. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J Pediatr. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Harmatz P, Ketteridge D, Giugliani R, et al. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–e689. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- 31.Harmatz P, Giugliani R, Schwartz I, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin SP, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, STRIVE Investigators. Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2014 May 9; doi: 10.1007/s10545-014-9715-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol Genet Metab. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 34.Rowan DJ, Tomatsu S, Grubb JH, et al. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol Genet Metab. 2012;107:161–72. doi: 10.1016/j.ymgme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogler C, Levy B, Galvin N, Lessard M, Soper B, Barker J. Early onset of lysosomal storage disease in a murine model of mucopolysaccharidosis type VII: undegraded substrate accumulates in many tissues in the fetus and very young MPS VII mouse. Pediatr Dev Pathol. 2005a;8:453–462. doi: 10.1007/s10024-005-0025-8. [DOI] [PubMed] [Google Scholar]
- 36.Sands MS, Vogler C, Kyle JW, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS. Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type VII: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival. Pediatr Res. 1996;39:1050–1054. doi: 10.1203/00006450-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Vogler C, Sands MS, Galvin N, et al. Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis. 1998;21:575–586. doi: 10.1023/a:1005423222927. [DOI] [PubMed] [Google Scholar]
- 39.Macsai CE, Derrick-Roberts AL, Ding X, Zarrinkalam KH, McIntyre C, Anderson PH, Anson DS, Byers S. Skeletal response to lentiviral mediated gene therapy in a mouse model of MPS VII. Mol Genet Metab. 2012;106(2):202–13. doi: 10.1016/j.ymgme.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Lingfei Xu, Robert L, Mango, Sands Mark S., Haskins Mark E., Ellinwood N. Matthew, Katherine Parker Ponder Evaluation of pathological manifestations of disease in mucopolysaccharidosis VII mice after neonatal hepatic gene therapy. Mol Ther. 2002;6:745–758. doi: 10.1006/mthe.2002.0809. [DOI] [PubMed] [Google Scholar]
- 41.Dierks T, Schmidt B, Borissenko LV, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell. 2003;113:435–444. doi: 10.1016/s0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- 42.Cosma MP, Pepe S, Annunziata I, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 43.Landgrebe J, Dierks T, Schmidt B, von Figura K. The human SUMF1 gene, required for posttranslational sulfatase modification, defines a new gene family which is conserved from pro- to eukaryotes. Gene. 2003;316:47–56. doi: 10.1016/s0378-1119(03)00746-7. [DOI] [PubMed] [Google Scholar]
- 44.Tomatsu S, Montaño AM, Gutierrez M, et al. Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol Genet Metab. 2007b;91:69–78. doi: 10.1016/j.ymgme.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 45.van Diggelen OP, Zhao H, Kleijer WJ, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 46.Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- 47.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 48.Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010a Jan 27; doi: 10.1007/s10545-009-9013-x. 2010. doi 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 49.Vogler C, Levy B, Grubb JH, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005b;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gliddon BL, Hopwood JJ. Enzyme-replacement therapy from birth delays the development of behavior and learning problems in mucopolysaccharidosis type IIIA mice. Pediatr Res. 2004;56:65–72. doi: 10.1203/01.PDR.0000129661.40499.12. [DOI] [PubMed] [Google Scholar]
- 51.Vogler C, Sands M, Higgins A, et al. Enzyme replacement with recombinant beta-glucuronidase in the newborn mucopolysaccharidosis type VII mouse. Pediatr Res. 1993;34:837–840. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Tomatsu S, Montaño AM, DunG VC, et al. Enhancement of drug delivery: enzyme replacement therapy for murine Morquio A syndrome. Mol Ther. 2010b;18:1094–10102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck M, Braun S, Coerdt W, Merz E, Young E, Sewell AC. Fetal presentation of Morquio disease type A. Prenat Diagn. 1992;12:1019–1029. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]