Abstract
Objectives
To determine the relationship between albuminuria and cardiac structure/function in heart failure with preserved ejection fraction (HFpEF).
Background
Albuminuria, a marker of endothelial dysfunction, has been associated with adverse cardiovascular outcomes in HFpEF. However, the relationship between albuminuria and cardiac structure/function in HFpEF has not been well studied.
Methods
We measured urinary albumin-to-creatinine ratio (UACR) and performed comprehensive echocardiography, including tissue Doppler imaging and right ventricular (RV) evaluation, in a prospective study of 144 patients with HFpEF. Multivariable-adjusted linear regression was used to determine the association between UACR and echocardiographic parameters. Cox proportional hazards analyses were used to determine the association between UACR and outcomes.
Results
The mean age was 66±11 years, 62% were female, and 42% were African-American. Higher UACR was associated with greater left ventricular (LV) mass, lower preload-recruitable stroke work, and lower global longitudinal strain. Higher UACR was also significantly associated with RV remodeling (for each doubling of UACR, RV wall thickness was 0.9 mm higher [95% confidence interval (CI) 0.05–0.14 mm; P=0.001, adjusted P=0.01]) and worse RV systolic function (for each doubling of UACR, RV fractional area change was 0.56% lower [95% CI 0.14–0.98%; P=0.01, adjusted P=0.03]. The association between UACR and RV parameters persisted after excluding patients with macroalbuminuria (UACR > 300 mg/g). Increased UACR was also independently associated with worse outcomes.
Conclusions
In HFpEF, increased UACR is a prognostic marker and is associated with increased RV and LV remodeling, and longitudinal systolic dysfunction.
Keywords: diastolic heart failure, albuminuria, endothelial dysfunction, ventricular remodeling, ventricular function
INTRODUCTION
Albuminuria is associated with cardiovascular morbidity and mortality in diabetics, hypertensives, and the general population (1–5). In patients with heart failure (HF), there is increased prevalence of albuminuria, and a higher urine albumin-to-creatinine ratio (UACR) is associated with greater overall cardiovascular mortality and more frequent hospitalization for HF (6–8). However, these studies have largely been focused on HF with reduced ejection fraction (HFrEF). In HF with preserved EF (HFpEF), a positive urine dipstick for albuminuria has been found to be associated with worse outcomes (9), and urinary albumin excretion preferentially predicts progression to HFpEF (compared to HFrEF) (10).
While albuminuria is known to be associated with increased left ventricular (LV) mass (11), the more specific structural or functional changes in the heart that underlie these phenomena remain unclear. Albuminuria is theoretically related to multiple pathophysiological processes including systemic inflammation and endothelial dysfunction (12). Some evidence suggests that systemic inflammation and endothelial dysfunction play a role in HFpEF (13, 14), and that these abnormalities in HFpEF extends beyond the LV to other cardiovascular structures (15, 16).
For these reasons, we sought to better understand the relationship between elevated UACR and abnormal cardiac structure/function in HFpEF. We hypothesized that increased UACR is associated with multiple measures of cardiac remodeling and dysfunction (including abnormal right ventricular [RV] structure/function), and is predictive of adverse outcomes, in HFpEF. Therefore, we prospectively studied the association between UACR, cardiac structure/function, and outcomes in a well-characterized HFpEF cohort.
METHODS
Study population
Between March 2008 and May 2011, consecutive patients were prospectively enrolled from the outpatient clinic of the Northwestern University HFpEF Program as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier #NCT01030991). All patients were enrolled in the study in the outpatient setting following a hospitalization for HF. Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital using the following search criteria: (1) diagnosis of HF or the words “heart failure” in the hospital notes; or (2) B-type natriuretic peptide (BNP) >100 pg/ml; or (3) administration of 2 or more doses of intravenous diuretics.
Patients were offered post-discharge follow-up in a specialized HFpEF outpatient program if they met the following 3 inclusion criteria: age ≥ 21 years, left ventricular (LV) ejection fraction (EF) ≥ 50% and presence of HF as defined by Framingham criteria (17). The HF diagnosis was confirmed in the post-hospitalization, outpatient HFpEF clinic. Consistent with previously published criteria (18, 19), all patients were found to have at least one of the following 3 diagnostic hallmarks of HFpEF: grade 2 or worse LV diastolic dysfunction on echocardiography; elevated pulmonary capillary wedge pressure or LV end-diastolic pressure on invasive hemodynamic testing; or elevated BNP (> 100 pg/ml). Patients were excluded if they had greater than moderate valvular disease, prior cardiac transplantation, prior LVEF < 40%, LV end-diastolic volume (EDV) > 97 ml/m2, or constrictive pericarditis. All study patients were seen in the outpatient HFpEF program within one month of hospital discharge. All study participants gave written, informed consent, and the institutional review board at Northwestern University approved the study.
Clinical characteristics
We collected the following data in all study participants: demographics, race/ethnicity, New York Heart Association (NYHA) functional class, comorbidities, medications, vital signs, body mass index, and laboratory data, including serum sodium, blood urea nitrogen, creatinine, hemoglobin, and BNP. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation. Chronic kidney disease (CKD) was defined as eGFR < 60 ml/min/1.73 m2. Diabetes mellitus (DM) was defined by the presence of physician-documented history of diabetes or the use of oral hypoglycemic agents or insulin for the treatment of hyperglycemia. Coronary artery disease (CAD) was defined as presence of physician-documented history of CAD, known coronary stenosis >50%, history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, or abnormal stress test results consistent with myocardial ischemia. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, physician-documented history of hypertension, or current use of antihypertensive medications. Hyperlipidemia was defined as physician-documented history of hyperlipidemia or current use of lipid-lowering medications. Obesity was defined as body mass index >30 kg/m2. Anemia was defined as hemoglobin < 11.6 g/dl for women or < 13 g/dl for men; hemoglobin cut-off values were chosen based on lower limits of normal as reported by the laboratory at our institution (Northwestern Memorial Hospital).
Urine albumin-to-creatinine ratio
Urinary albumin excretion was measured on spot urine specimens by immunoturbidimetry using goat anti-human albumin antiserum with a coefficient of variation < 10%. Urinary creatinine was measured using a colorimetric dye-binding technique based on its reaction with picric acid with a coefficient of variation < 6%. Both tests were performed on a laboratory analyzer with standard reagents (Beckman Coulter AU680, Beckman Coulter, Inc., Brea, CA). UACR values were calculated as mg albumin/g creatinine.
Echocardiography
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging (TDI) using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500, Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp., Waukesha, WI) at the time of follow-up in the outpatient HFpEF clinic. Blood pressure was recorded at the time of echocardiography using a digital blood pressure monitor (Omron HEM-907XL, Omron Healthcare Inc, Vernon Hills, IL). Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (20–22). All measurements were made by an experienced research sonographer (L.B.) using ProSolv 4.0 echocardiographic analysis software (ProSolv CardioVascular; Indianapolis, IN), and verified by an experienced investigator with expertise in echocardiography (S.J.S.). Echocardiograms were quantitated and interpreted blinded to the laboratory data, clinical outcomes, and results of the study.
Comprehensive diastolic function assessment was performed according to published guidelines (21) and graded according to mitral inflow characteristics, tissue Doppler e′ velocities, and E/e′ ratio (23). All Doppler and tissue Doppler measurements were averaged over 3 beats (5 beats for patients in atrial fibrillation).
We used tissue Doppler s′ velocity as a marker of longitudinal LV systolic function. Preload-recruitable stroke work (PRSW), a marker of global LV contractility, was estimated on echocardiography using a fully non-invasive, single-beat method (24). PRSW describes the myocardial contractile response to increasing preload (i.e. greater end-diastolic volume), and is thus a marker of contractile performance (25, 26).
Echocardiographic markers of RV structure and function, including RV end-diastolic and end-systolic area (indexed to body size) (27), RV basal diameter, RV wall thickness, RV fractional area change (RVFAC), and tricuspid annular plane systolic excursion (TAPSE) were measured using 2-dimensional echocardiography and quantified as recommended by the American Society of Echocardiography (22). RV hypertrophy was defined as RV end-diastolic free wall thickness >5.0 mm (measured in the subcostal view); abnormal TAPSE was defined as <1.6 cm; and abnormal RVFAC was defined as < 35% (22). Pulmonary artery pressure (PASP) and right atrial pressure (RAP) were estimated using echocardiography as previously described (28, 29).
Strain analysis
In a subset of 29 randomly selected HFpEF study participants, we performed further echocardiographic imaging and speckle-tracking analysis to calculate LV strain values to determine the association between UACR and cardiac mechanics. All 29 patients underwent echocardiographic imaging with a GE Vivid 7 system (GE Medical Systems, Milwaukee, WI) with dedicated views of the LV in the parasternal short axis and apical views. The depth and sector width were optimized to ensure a frame rate of 50–70 fps. The images obtained were then analyzed using EchoPAC software (GE Medical Systems, Milwaukee, WI), and global longitudinal, circumferential, and radial strain were quantified.
Outcomes
After enrollment, study participants were evaluated in the Northwestern HFpEF Program at least every 6 months or as clinically indicated. At each visit, inter-current hospitalizations were documented, reviewed, and categorized as due to cardiovascular or non-cardiovascular causes. For cardiovascular hospitalizations, specific causes (e.g., HF, acute coronary syndrome, arrhythmia) were identified. Every 6 months, participants (or their proxy) were contacted to determine vital status with verification of deaths through query of the Social Security Death Index. Outcomes were adjudicated by 2 investigators, blinded to all other collected data and independent of each other. Discrepant adjudication of events was resolved by committee discussion. Enrollment date was defined as the first visit to the outpatient HFpEF clinic. Date of last follow-up was defined as date of death or last HFpEF clinic visit. Follow-up was complete in all patients. Our primary endpoint was a combined outcome of cardiovascular hospitalization and death, which included hospitalization for any cardiovascular cause (including HF) and death from any cause.
Statistical analysis
For descriptive purposes only, subjects were split into UACR quartiles. For all regression models, UACR (predictor variable) was log-transformed to normalize its distribution (raw UACR was right-skewed). We described clinical characteristics, laboratory data, and conventional echocardiographic parameters by UACR quartile. Categorical variables are displayed as count and percentage and continuous data with a normal distribution are displayed as mean±standard deviation. Right-skewed data are presented as median and interquartile range. To test for trends in clinical characteristics, laboratory data, and conventional echocardiographic parameters across quartiles, we examined the association between UACR and each parameter using linear regression, logistic regression, or ordered logistic regression where appropriate. We treated UACR as a continuous (log-transformed) variable in all regression models. To correct for multiple testing among univariate comparisons, false discovery rate (FDR) methods were applied using all calculated univariate p-values (Tables 1 and 2). Q-values were calculated as previously described (30). FDR < 5% corresponded to p-values < 0.011. Only comparisons meeting this criterion were advanced to multivariable comparisons.
Table 1.
Clinical Characteristics and Laboratory Data by Urinary Albumin-to-Creatinine Ratio Quartile
| Characteristic | All patients (N=144) | Q1 (N=35) | Q2 (N=37) | Q3 (N=36) | Q4 (N=36) | P-value* |
|---|---|---|---|---|---|---|
| UACR (mg/g) | <7.0 | 7.0–16.5 | 16.5–94.5 | >94.5 | ||
|
| ||||||
| Age (years) | 66±11 | 67±10 | 65±10 | 69±13 | 64±9 | 0.38 |
| Female | 89 (62) | 27 (77) | 21 (62) | 21 (58) | 19 (53) | 0.11 |
| Race | ||||||
| White | 66 (46) | 16 (46) | 22 (65) | 12 (33) | 16 (44) | 0.41 |
| Black | 60 (42) | 12 (34) | 11 (32) | 19 (53) | 15 (42) | 0.58 |
| Other | 18 (13) | 7 (20) | 1 (3) | 5 (14) | 5 (14) | 0.67 |
| NYHA class | 0.40‡ | |||||
| I | 6 (4) | 2 (6) | 1 (3) | 2 (6) | 1 (3) | |
| II | 55 (38) | 16 (46) | 15 (44) | 10 (28) | 13 (36) | |
| III | 83 (58) | 17 (49) | 18 (53) | 24 (67) | 22 (61) | |
| Comorbidities | ||||||
| Coronary artery disease | 72 (50) | 14 (40) | 15 (44) | 21 (58) | 22 (61) | 0.07 |
| Hypertension | 129 (90) | 29 (83) | 31 (91) | 33 (92) | 33 (92) | 0.46 |
| Hyperlipidemia | 104 (72) | 22 (63) | 27 (79) | 24 (67) | 28 (78) | 0.33 |
| Diabetes mellitus | 65 (45) | 11 (31) | 13 (38) | 17 (47) | 22 (61) | 0.02 |
| Chronic kidney disease | 56 (39) | 11 (31) | 7 (21) | 13 (36) | 24 (67) | <0.001† |
| Smoker | 56 (39) | 17 (49) | 9 (26) | 19 (53) | 10 (28) | 0.18 |
| Atrial fibrillation | 36 (25) | 4 (11) | 8 (24) | 13 (36) | 10 (28) | 0.19 |
| Obesity | 88 (61) | 21 (60) | 23 (68) | 23 (64) | 19 (53) | 0.33 |
| COPD | 66 (46) | 23 (66) | 13 (38) | 14 (39) | 14 (39) | 0.05 |
| Anemia | 69 (48) | 12 (34) | 15 (44) | 16 (44) | 25 (69) | 0.01† |
| Medications | ||||||
| ACE-inhibitor or ARB | 91 (63) | 21 (60) | 19 (56) | 27 (75) | 21 (58) | 0.95 |
| β-blocker | 106 (74) | 25 (71) | 21 (62) | 26 (72) | 32 (89) | 0.02 |
| Calcium channel blocker | 63 (44) | 16 (46) | 13 (38) | 18 (50) | 15 (42) | 0.91 |
| Vasodilators | 23 (16) | 2 (6) | 5 (15) | 4 (11) | 12 (33) | 0.001† |
| Nitrate | 21 (15) | 5 (14) | 2 (6) | 3 (8) | 10 (28) | 0.01† |
| Loop diuretic | 93 (65) | 15 (43) | 19 (56) | 26 (72) | 31 (86) | <0.001† |
| Loop diuretic total daily dose (mg furosemide equivalents)** | 40 (30–80) | 40 (20–50) | 40 (40–60) | 60 (20–80) | 80 (40–80) | <0.001† |
| Thiazide diuretic | 37 (26) | 11 (31) | 8 (24) | 10 (28) | 7 (19) | 0.37 |
| No diuretic | 25 (17) | 11 (31) | 7 (19) | 4 (11) | 3 (8) | 0.01† |
| Statin | 88 (61) | 16 (46) | 21 (62) | 22 (61) | 28 (78) | 0.005† |
| Aspirin | 63 (44) | 11 (31) | 13 (38) | 17 (47) | 22 (61) | 0.01 |
| Heart rate (beats per minute) | 71±13 | 67±11 | 72±10 | 69±13 | 74±16 | 0.02 |
| Systolic BP (mmHg) | 128±19 | 123±16 | 129±17 | 127±19 | 131±23 | 0.06 |
| Diastolic BP (mmHg) | 71±12 | 69±9 | 75±12 | 71±12 | 70±16 | 0.97 |
| Pulse pressure (mmHg) | 56±16 | 54±13 | 54±14 | 57±16 | 60±18 | 0.02 |
| Body mass index (kg/m2) | 35±10 | 33±8 | 35±9 | 35±10 | 34±12 | 0.81 |
| Weight (kg) | 97±31 | 90±26 | 102±32 | 97±26 | 100±36 | 0.61 |
| Serum sodium (mEq/l) | 139±3 | 139±3 | 139±2 | 138±3 | 138±3 | 0.07 |
| Blood urea nitrogen (mg/dl) | 25±16 | 23±15 | 18±7 | 26±17 | 33±17 | <0.001† |
| Serum creatinine (mg/dl) | 1.4±0.8 | 1.1±0.3 | 1.1±0.3 | 1.4±0.5 | 1.9±1.3 | <0.001† |
| Estimated GFR (ml/min/1.73m2) | 58±23 | 61±20 | 70±20 | 55±23 | 45±23 | <0.001† |
| Fasting glucose (mg/dl) | 134±70 | 115±50 | 130±65 | 145±77 | 144±80 | 0.09 |
| Hemoglobin (g/dl) | 12.0±1.9 | 12.5±1.7 | 12.4±1.7 | 11.8±2.1 | 11.2±1.8 | 0.002† |
| B-type natriuretic peptide (pg/ml)** | 171 (72–433) | 92 (20–247) | 115 (65–197) | 290 (92–577) | 410 (145–999) | <0.001† |
P-value by linear trend across UACR quartiles using linear regression for continuous variables and logistic regression for categorical variables
Values expressed as median (25th–75th percentile)
Remains significant after correction using false discovery rate < 5%
P-value by ordered logistic regression against log-transformed UACR
UACR = urinary albumin-to-creatinine ratio; NYHA = New York Heart Association; COPD = chronic obstructive pulmonary disease; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; GFR = glomerular filtration rate
Table 2.
Echocardiographic Characteristics by UACR Quartile
| Echocardiographic parameter | All patients (N=144) | Q1 (N=35) | Q2 (N=37) | Q3 (N=36) | Q4 (N=36) | P-value* |
|---|---|---|---|---|---|---|
| Left heart parameters | ||||||
| LV end-systolic volume index (ml/m2) | 15.0±5.3 | 14.9±3.7 | 13.9±5.3 | 15.6±5.5 | 15.1±5.2 | 0.58 |
| LV end-diastolic volume index (ml/m2) | 38.6±9.2 | 37.9±6.7 | 37.3±9.3 | 39.9±8.3 | 38.3±10.9 | 0.61 |
| LV mass index (g/m2.7) | 52.3±15.6 | 47.3±9.3 | 50.1±14.7 | 51.9±16.9 | 59.7±18.1 | 0.001† |
| Relative wall thickness | 0.52±0.14 | 0.49±0.09 | 0.50±0.13 | 0.50±0.12 | 0.58±0.20 | 0.003† |
| LV mass/volume ratio (g/ml) | 2.7±1.1 | 2.5±0.7 | 2.7±0.9 | 2.5±0.8 | 3.3±1.5 | 0.001† |
| LV ejection fraction (%) | 62±7 | 61±5 | 63±8 | 62±7 | 61±6 | 0.47 |
| Left atrial volume index (ml/m2) | 31.9±11.3 | 30.7±11.2 | 31.2±11.3 | 33.7±11.8 | 31.7±11.2 | 0.78 |
| Stroke volume (ml) | 83.9±25.3 | 46.5±11.7 | 49.3±14.3 | 51±10.6 | 49.7±17 | 0.48 |
| Cardiac index (L/min/m2) | 2.8±0.8 | 1.5±0.4 | 1.7±0.4 | 1.7±0.4 | 1.7±0.5 | 0.16 |
| Mitral regurgitation | 0.06‡ | |||||
| None | 88 (61) | 26 (74) | 22 (65) | 20 (56) | 18 (50) | |
| Mild | 36 (25) | 6 (17) | 7 (21) | 10 (28) | 13 (36) | |
| Moderate | 18 (13) | 3 (9) | 5 (15) | 6 (17) | 4 (11) | |
| Preload recruitable stroke work (mmHg) | 72±24 | 79±22 | 76±23 | 71±24 | 63±26 | 0.003† |
| Tissue Doppler s′ velocity (cm/s) | 8.2±2.5 | 8.8±2.2 | 8.7±2.9 | 7.7±1.4 | 7.6±3.0 | 0.01† |
| E/A ratio | 1.4±0.7 | 1.2±0.6 | 1.5±0.9 | 1.5±0.7 | 1.4±0.7 | 0.43 |
| E deceleration time (ms) | 219±55 | 237±55 | 216±39 | 211±66 | 214±52 | 0.18 |
| Tissue Doppler e′ velocity (cm/s) | 8.6±3.0 | 8.7±2.4 | 8.1±2.3 | 8.8±2.7 | 8.7±4.2 | 0.79 |
| E/e′ ratio | 15.2±7.0 | 12.8±6.8 | 16.3±7.1 | 14.7±5.5 | 17.4±8.1 | 0.01 |
| Diastolic function grade | 0.03‡ | |||||
| Normal diastolic function | 11 (8) | 9 (26) | 1 (3) | 0 (0) | 1 (3) | |
| Grade I (mild) diastolic dysfunction | 12 (8) | 4 (11) | 4 (11) | 0 (0) | 4 (11) | |
| Grade II (moderate) diastolic dysfunction | 61 (42) | 15 (43) | 14 (38) | 16 (44) | 16 (44) | |
| Grade III (severe) diastolic dysfunction | 49 (34) | 6 (17) | 13 (35) | 16 (44) | 14 (39) | |
| Indeterminate diastolic function | 11 (8) | 1 (3) | 5 (14) | 4 (11) | 1 (3) | |
|
| ||||||
| Right heart parameters | ||||||
|
| ||||||
| RV end-diastolic area index (cm2/m2) | 13.5±3.7 | 12.8±2.2 | 12.9±3.7 | 13.6±3.5 | 14.5±4.6 | 0.10 |
| RV end-systolic area index (cm2/m2) | 7.9±2.8 | 7.1±1.7 | 7.5±2.4 | 8.1±2.6 | 8.9±3.6 | 0.02 |
| RV basal diameter (cm) | 3.9±0.6 | 3.7±0.5 | 3.8±0.6 | 4.0±0.6 | 4.1±0.7 | 0.05 |
| RV maximal diameter (cm) | 4.5±0.8 | 4.4±0.7 | 4.4±0.8 | 4.6±0.9 | 4.7±1.0 | 0.69 |
| RV wall thickness (mm) | 5.0±0.8 | 4.7±0.5 | 4.8±0.8 | 5.0±0.6 | 5.5±1.1 | 0.001† |
| RV fractional area change (%) | 42±7 | 45±6 | 42±7 | 41±7 | 40±8 | 0.01† |
| TAPSE (cm) | 2.0±0.6 | 2.1±0.6 | 2.1±0.8 | 1.9±0.5 | 2.0±0.6 | 0.59 |
| Pulmonary artery systolic pressure (mmHg) | 43.4±15.1 | 38.6±14.1 | 46.8±18.3 | 41±11.8 | 46.9±14.1 | 0.04 |
| Right atrial pressure (mmHg) | 7.3±3.9 | 5.8±2.3 | 7.9±4.1 | 7.2±4.1 | 8.3±4.4 | 0.20 |
P-value by linear trend across log-transformed UACR
Remains significant after correction using false discovery rate < 5%
P-value by ordered logistic regression against log-transformed UACR
LV = left ventricular; E = early mitral inflow; A = late (atrial) mitral inflow; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion
For the multivariable analyses, covariates were chosen based on a combination of clinical relevance and association with both the predictor (UACR) and outcome (echocardiographic parameters). The associations between UACR and cardiac parameters of interest were adjusted for: (1) Model 1: age, sex, and African-American race; (2) Model 2: age, sex, African-American race, pulse pressure, DM, eGFR, CAD and LV mass; and (3) Model 2 in the subset of subjects without macroalbuminuria to determine whether associations were being driven by those with the highest levels of UACR. Linear models with and without log UACR were compared and differences in regression R2 were reported.
For survival analyses, we used Cox proportional-hazards regression to evaluate the unadjusted relationship between UACR (log-transformed) and outcomes. Models were then adjusted for covariates chosen based on a combination of clinical relevance and association with adverse outcomes in HFpEF. These covariates were age, sex, African-American race, DM, CKD, CAD, anemia, and various markers of cardiac disease severity including, BNP, LV mass index, E/e′ ratio, and NYHA class. Area under the curve (AUC) and integrated discrimination improvement (IDI) analyses were used to determine the incremental risk prediction provided by UACR. All analyses were performed using Stata 12 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics
Clinical characteristics for the entire cohort (N=144), both in aggregate and stratified by UACR quartile, are shown in Table 1. The average age was 66 years, 62% were female, and just over half were non-white. Microalbuminuria (UACR = 30–300 mg/g) was present in 36/144 (25%), while macroalbuminuria (UACR > 300 mg/g) was present in 20/144 (14%). Comorbidities, including CAD, hypertension, hyperlipidemia, DM, CKD, obesity, and smoking were common. Subjects with higher UACR levels were more likely to have anemia and CKD, which was reflected by higher creatinine and blood urea nitrogen in the higher UACR quartiles. Chronic obstructive pulmonary disease was non-significantly less common in subjects with higher UACR values. There was a significant linear correlation between higher log UACR and higher log BNP (r=0.43; P<0.0001). The relationship between UACR and BNP persisted after adjustment for comorbidities and when restricted to those without macroalbuminuria (Table 3).
Table 3.
Association of Urinary Albumin-to-Creatinine Ratio with Cardiac Parameters: Unadjusted and Multivariable-Adjusted Linear Regression Analyses
| Cardiac parameter | Unadjusted | Model 1 | Model 2 | Model 2 (UACR < 300 mg/g)* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β-coefficient** (95% CI) | P-value | β-coefficient (95% CI) | P-value | β-coefficient (95% CI) | P-value | β-coefficient (95% CI) | P-value | |
| RV wall thickness | 0.09 [0.04, 0.14] | 0.001 | 0.09 [0.04, 0.14] | 0.001 | 0.08 [0.02, 0.13] | 0.01 | 0.14 [0.06, 0.22] | 0.001 |
| RV fractional area change | −0.56 [−0.98,−0.14] | 0.01 | −0.55 [−0.97,−0.13] | 0.01 | −0.52 [−1.01,−0.03] | 0.04 | −0.97 [−1.72,−0.23] | 0.01 |
| PRSW | −2.16 [−3.57,−0.74] | 0.003 | −2.05 [−3.48,−0.62] | 0.01 | −1.62 [−3.1,−0.15] | 0.03 | −2.24 [−4.50, 0.02] | 0.05 |
| Tissue Doppler s′ velocity | −0.19 [−0.34,−0.05] | 0.01 | −0.20 [−0.35,−0.06] | 0.01 | −0.12 [−0.29, 0.04] | 0.14 | −0.07 [−0.33, 0.20] | 0.62 |
| Log BNP | 0.21 [0.14, 0.29] | <0.001 | 0.23 [0.16, 0.30] | <0.001 | 0.17 [0.10, 0.25] | <0.001 | 0.19 [0.07, 0.31] | 0.003 |
Subgroup analysis of study participants without macroalbuminuria (N=124)
All β-coefficients shown represent change in cardiac parameter per doubling of UACR
Model 1: Adjusted for age, sex, and African-American race
Model 2: Adjusted for age, sex, African-American race, pulse pressure, diabetes mellitus, coronary artery disease, estimated glomerular filtration rate, and left ventricular mass
UACR = urinary albumin-to-creatinine ratio; RV=right ventricular, PRSW = preload-recruitable stroke work
Hypertension prevalence was not more prevalent in higher UACR quartiles, and while systolic blood pressure was higher in study participants with higher UACR, the association did not reach statistical significance. Pulse pressure did increase with increasing UACR reflecting increased arterial stiffness in study participants with higher UACR values. However this comparison was not significant after adjustment for multiple comparisons. Participants with higher UACR levels were more likely to be taking vasodilators, nitrates, loop diuretics, and statins (Table 1).
Association of UACR with left heart parameters
Table 2 summarizes the echocardiographic characteristics of the study cohort, in aggregate and stratified by UACR quartile. Higher UACR was associated with indicators of LV hypertrophy such as higher LV mass, increased relative wall thickness, and higher LV mass-to-volume ratio. However, UACR was not associated with LV chamber size. Despite the presence of a preserved EF in all study participants, LV systolic function was lower in the subjects with higher UACR levels: both PRSW (reflective of the ability of the LV to increase stroke work with increasing preload, a load-independent marker of contractility), and longitudinal LV systolic function (i.e., tissue Doppler s′ velocity) were inversely correlated with UACR. In multivariable-adjusted analyses, higher UACR remained associated with lower PRSW, even when subjects with macroalbuminuria were excluded (Table 3). Higher UACR was associated with reduced tissue Doppler s′ velocity after adjustment for age and sex, but not after adjustment for comorbidities.
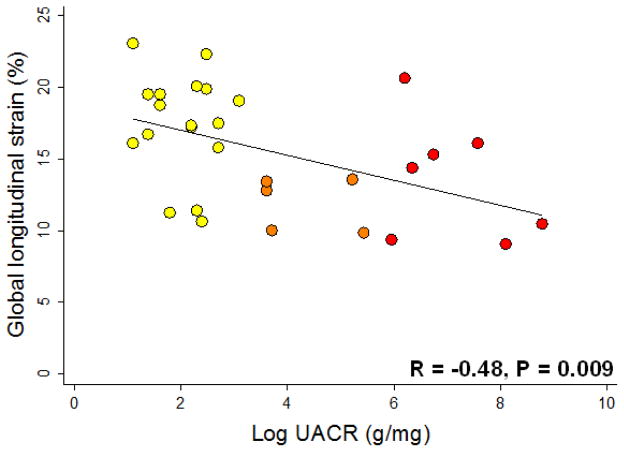
In a subset of the HFpEF study participants on whom we performed speckle-tracking analysis (N=29), log-transformed UACR was significantly correlated with global longitudinal strain (Figure 1; r=−0.48, P=0.009). The inverse association between UACR and absolute values of global longitudinal strain persisted after adjustment for DM, eGFR, and CAD (P=0.004). UACR was not associated with global circumferential strain (r=0.01; P=0.94) or global radial strain (r=−0.04; P=0.84).
Figure 1. Scatterplot of Urinary Albumin-to-Creatinine Ratio vs. Global Longitudinal Strain.
UACR = urinary albumin-to-creatinine ratio
In terms of LV diastolic function, higher UACR was associated with worse diastolic dysfunction grade and elevated E/e′ ratio, but these associations were no longer present after adjusting for multiple comparisons (Table 2). UACR was not significantly associated with e′ tissue velocity, a marker of LV diastolic relaxation.
Association of UACR with right heart parameters
UACR was associated with several right heart parameters, including RV wall thickness, RV end-systolic area index, RV basal diameter, and RVFAC (Table 2). However, only RV wall thickness and RVFAC were significant after adjustment for multiple comparisons. UACR was significantly elevated in those with RV hypertrophy: median UACR 41.5 mg/g (interquartile range [IQR] 12–229 mg/g) vs. UACR 12 mg/g (IQR 6–46 mg/g) for those without RV hypertrophy; P=0.002. Those with abnormal RV systolic function, defined as RVFAC < 35%, had higher median UACR values as well: 67 mg/g (IQR 16–213 mg/g) vs. 13 mg/g (IQR 6–48mg/g); P=0.02. Those with and without abnormal TAPSE did not have significantly different levels of UACR (P=0.70).
The association between RV size, hypertrophy, and systolic function (RVFAC) persisted after adjustment for multiple possible confounders (Table 3). To evaluate the effect of right-sided pressures on the association between UACR and these RV parameters, we ran additional models that adjusted for estimated PASP and estimated RAP. Higher UACR was still associated with increased RV wall thickness after adjustment for PASP and RAP (0.12 mm [95% CI 0.05–0.20 mm] increase in RV wall thickness per doubling of UACR, P=0.002). The association of increased UACR with decreased RVFAC was of borderline statistical significance after multivariable adjustment (−0.78% [95% CI −1.12% to 0.28%] change in RVFAC per doubling of UACR, P=0.050). The increase in regression R2 for each model after adding log UACR is displayed in Supplementary Table S1. As shown in Table 3, the associations between UACR and RV parameters also persisted after excluding study participants with macroalbuminuria (UACR > 300 mg/g). Finally, of the 144 patients included in our study, 97 (67%) underwent right heart catheterization. In these 97 patients, the mean pulmonary capillary wedge pressure was 23.1±9.6 mmHg. Adjustment for LV diastolic pressure (i.e., pulmonary capillary wedge pressure) did not eliminate the association between UACR and RV wall thickness (0.15 mm increase per doubling of UACR, 95% CI 0.06–0.24 mm; P=0.001).
Association of UACR with adverse outcomes
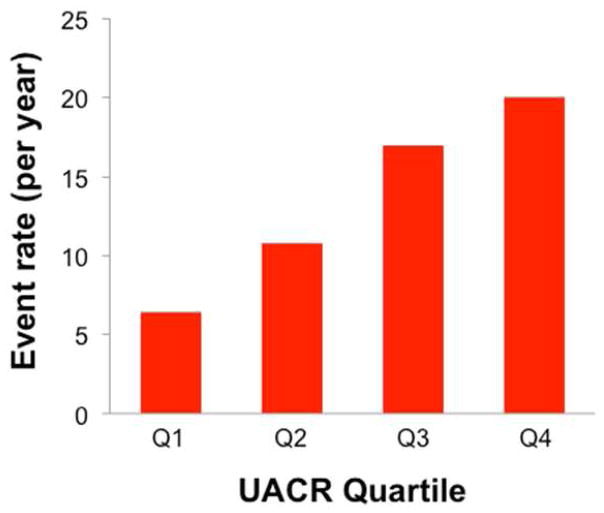
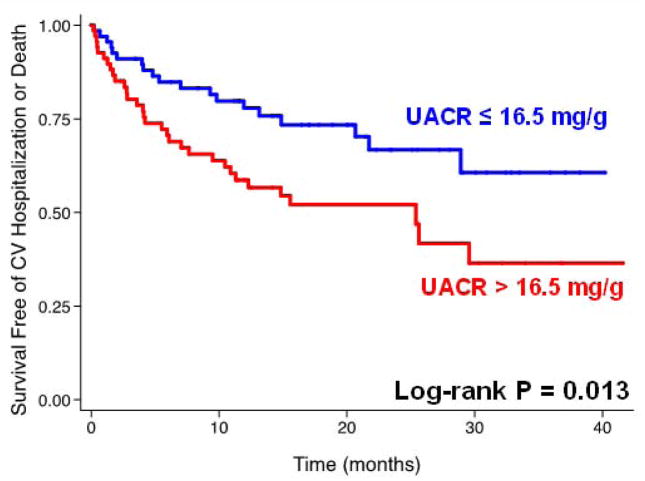
The median follow-up time was 12.1 months (25th–75th percentile 4.1–21.6 months), during which time 58 (40%) of the study participants experienced the composite outcome of cardiovascular hospitalization (including HF hospitalization) or death. Adverse outcomes were increased in a stepwise fashion with each higher UACR quartile, as shown in Figure 2. Figure 3 shows the Kaplan-Meier curves for the combined endpoint of cardiovascular hospitalization or death, stratified by median UACR, demonstrating that higher UACR levels predicted worse outcomes. Table 4 displays Cox-proportional hazard ratios per doubling of UACR for the combined outcome of cardiovascular hospitalization or death. Higher UACR was significantly associated with worse outcomes after adjustment for age, sex, African-American race, DM, CKD, CAD, anemia and several markers of HF severity including E/e′ ratio, LV mass index, and NYHA class. However, adjustment for BNP attenuated the relationship between UACR and outcomes. Supplementary Table S2 displays the AUC before and after inclusion of log UACR, IDI, and relative IDI for each outcomes model.
Figure 2. Event rate for the Combined Outcome of Cardiovascular Hospitalization or Death by Quartile of Urinary Albumin-to-Creatinine Ratio.
UACR = urinary albumin-to-creatinine ratio
Figure 3. Kaplan-Meier Survival Curve for the Composite Outcome of Cardiovascular Hospitalization or Death, Stratified by Median Urinary Albumin-to-Creatinine Ratio.
UACR = urinary albumin-to-creatinine ratio; CV = cardiovascular
Table 4.
Association of Urinary Albumin-to-Creatinine Ratio with the Composite Outcome of Cardiovascular Hospitalization or Death: Unadjusted and Multivariable-Adjusted Cox Proportional Hazards Analyses
| Model | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Unadjusted | 1.13 [1.03, 1.23] | 0.01 |
| Adjusted model | 1.15 [1.04, 1.28] | 0.01 |
| Adjusted model + NYHA class | 1.16 [1.05, 1.30] | 0.01 |
| Adjusted model + E/e′ ratio | 1.17 [1.05, 1.31] | 0.01 |
| Adjusted model + LV mass index | 1.14 [1.02, 1.27] | 0.02 |
| Adjusted model + log BNP | 1.07 [0.95, 1.21] | 0.26 |
Additional covariates in adjusted model: age, sex, African-American race, diabetes mellitus, chronic kidney disease, coronary artery disease, anemia
Hazard ratio shown per doubling of UACR; UACR = urinary albumin-to-creatinine ratio; CI = confidence interval; LV = left ventricular; BNP = B-type natriuretic peptide; NYHA = New York Heart Association
DISCUSSION
In a well-characterized HFpEF cohort, we evaluated the relationship between albuminuria, as measured by UACR, and clinical characteristics, echocardiographic parameters, and outcomes. Higher UACR was associated with higher rates of DM and CKD, and UACR was also associated with higher creatinine, higher blood urea nitrogen, and lower hemoglobin. We also found that higher levels of UACR were associated with markers of biventricular dysfunction and remodeling, even after adjustment for potential confounders including DM and CKD. These cardiac markers included PRSW, log BNP, RV wall thickness, and RVFAC. Furthermore, UACR was associated with the composite outcome (cardiovascular hospitalization, HF hospitalization, or death) even after adjustment for age, sex, DM, CKD, CAD and multiple markers of HF severity (except for BNP). Our study increases understanding of the echocardiographic correlates of albuminuria in HFpEF, particularly parameters of RV remodeling and dysfunction.
Prior study of albuminuria in HFpEF has been limited. Forty-two percent (n=967) of the subjects from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Programme had LVEF >40% (6). The CHARM investigators described a prevalence of 30% and 11% for micro- and macroalbuminuria, respectively, in the combined HFpEF and HFrEF cohort, and showed that continuous UACR was similarly predictive of outcomes in HFpEF and HFrEF. Work from Miura et al. demonstrated the prognostic importance of positive urine dipstick for urine albumin (a dichotomous indicator of albuminuria) in HFpEF, but did not evaluate albuminuria as a continuous marker (9). Recently, albuminuria was reported to predict incident HFpEF rather than HFrEF (10). Like these previous studies, our work confirms the prognostic importance of UACR in HFpEF. However, as shown by our AUC and IDI analyses, the incremental benefit for UACR beyond traditional risk factors is limited. Aside from our results on UACR as a prognostic variable, we also found that the prevalence of micro- and macroalbuminuria in our cohort is similar to those recorded in CHARM HFpEF patients, and associations of greater albuminuria with DM and CKD were similarly present in our study. In the Strong Heart Study, UACR was linked to systolic and diastolic dysfunction in type 2 diabetes (31). However, this study did not utilize tissue Doppler imaging and rather relied on E/A ratio and mitral deceleration time to measure diastolic function.
Our multivariable-adjusted analyses revealed that UACR was independently associated with several parameters indicative of RV remodeling and dysfunction, a novel finding. This association may be due to LV diastolic dysfunction causing pulmonary venous hypertension, in turn leading to RV remodeling, dysfunction, and, ultimately, elevated central venous pressure. The increased renal venous congestion could then lead to increased albuminuria, a phenomenon observed in dogs (32).
However, two items suggest the relationship may be more complex than this. First, the association in the current study between UACR and RV size and function parameters persisted after adjustment for echocardiographic estimates of PASP and RAP, suggesting a pathophysiologic link between causes of increased UACR (such as endothelial dysfunction) and RV dysfunction and remodeling beyond RV preload or after load. In addition, in the subset of patients in our study who underwent invasive hemodynamic testing, adjustment for pulmonary capillary wedge pressure did not attenuate the association between UACR and RV remodeling. Second, albuminuria is linked to cardiac remodeling and dysfunction prior to the development of HF (33, 34). The source of this phenomenon is thus unclear. Albuminuria is theoretically related to multiple pathophysiological processes including comorbidities, systemic inflammation, and endothelial dysfunction (12). Some evidence suggests that systemic inflammation and endothelial dysfunction also play a role in HFpEF (13, 14).
Indeed, Paulus et al. recently posited a paradigm for HFpEF based on these pathophysiological processes (35). The authors suggest underlying HFpEF is a systemic proinflammatory state that leads to endothelial dysfunction, particularly in the coronary arteries, which in turn interrupts cardiomyocyte signaling and, ultimately, function. A corollary of this inflammation-endothelial dysfunction paradigm is myocardial dysfunction beyond the LV, as coronary inflammation and endothelial dysfunction sensitizes other chambers (such as the RV) to stress. Indeed, previous work by our group has shown that right atrial pressures in HFpEF with pulmonary hypertension (WHO Group II pulmonary hypertension) are higher than in those with WHO Group I pulmonary arterial hypertension despite similar pulmonary artery pressures in the two syndromes (36), suggesting greater intrinsic RV dysfunction in HFpEF. Thus, it is possible that the observed associations between higher UACR and greater RV remodeling/dysfunction, independent of comorbidities, RAP, and PASP, are related to inflammation and endothelial dysfunction that may underlie both albuminuria and RV dysfunction in HFpEF.
We also found that higher UACR was independently associated with lower PRSW and global longitudinal strain, markers of LV systolic dysfunction. Higher UACR also correlated with higher E/e′ ratio and worse diastolic function grade, but these associations failed to reach statistical significance. However, the strong association between UACR and BNP in the presence of normal LV volumes and preserved EF suggests that higher UACR is likely associated with increased diastolic wall stress. No other study has demonstrated the relationship between UACR and echocardiographic evidence of LV systolic and diastolic dysfunction in HFpEF.
While diastolic dysfunction is well documented in HFpEF, longitudinal LV systolic dysfunction is being increasingly recognized in HFpEF, though its impact on outcomes is unclear (37). In a community-based cohort of HFpEF patients, stress corrected-midwall fractional shortening was predictive of adverse events (38). Once again, the relationship between higher UACR and worse LV function may be explained by inflammation and endothelial dysfunction. These mechanisms could lead to dysfunction at the level of the cardiomyocyte and simultaneously drive systolic and diastolic dysfunction.
Strengths and limitations
The strengths of our study include the prospective and standardized recruitment of HFpEF patients, and the detailed, quantitative echocardiographic phenotyping used to evaluate cardiac structure and function. For these reasons, we were able to determine the associations between albuminuria and novel markers of LV systolic/diastolic function and RV structure/function. Our study also included detailed adjudication of adverse events, and follow-up was complete on all patients, so we could demonstrate independent associations between UACR and outcomes in HFpEF.
Our study has some potential limitations. First, this study had 144 patients, and would benefit from replication in a larger sample. Additionally, Using a single spot urine test to measure UACR is a limitation, though single-void UACR is known to correlate highly with 24-hour samples (39). Another potential limitation is the recruitment of all patients from a single academic medical center. However, Northwestern Memorial Hospital serves a large, diverse urban environment. While our cohort was younger than those described in epidemiologic and registry HFpEF studies, rates of comorbidities were similar and our cohort was more racially diverse. African-Americans had higher BMI and were younger than whites in our study, which may account for the observed deviations from prior epidemiologic studies. Thus, our data should most likely be generalizable to the broader population of HFpEF patients.
While our study showed an association between UACR and BNP, there was no such association with other measures of diastolic function including left atrial volume and e′ tissue velocity. Markers such as diastolic function grade and E/e′ ratio failed to meet significance after adjustment for multiple comparisons. These findings may be due to the fact that these markers were abnormal in the majority of our study population, which may have limited our power to detect associations with these parameters. It is additionally important to note that measures of systolic function including PRSW and s′ tissue velocity are not direct, invasive measures of contractility and are instead estimated on echocardiography. However in a study of 45 patients, PRSW by the method used in our study correlated well with invasive measurements of PRSW (r=0.93) (24).
Finally, our study lacks detailed records on how many screened patients failed to meet Framingham criteria for HF on an initial inquiry of hospital records. Furthermore, our study lacks a control group of age-matched healthy volunteers or comorbidity-matched patients without HFpEF; thus it is not possible to determine whether the prognostic utility of UACR is driven by accumulation of comorbidities.
Conclusions
In HFpEF, albuminuria is independently associated with RV remodeling and dysfunction, worse LV systolic and diastolic function, and worse outcomes. UACR may prove to be useful for risk stratification in HFpEF, and the aforementioned associations may allow a deeper understanding of HFpEF pathophysiology.
Supplementary Material
Supplementary Table S1. Change in R2 for adjusted models when log-transformed UACR is added as a covariate
Supplementary Table S2. Comparison of risk prediction models with and without log-transformed UACR
Acknowledgments
Funding Sources: AHA Scientist Development Grant (#0835488N) and National Institutes of Health (R01 HL107557), both to S.J.S.
ABBREVIATIONS
- BNP
B-type natriuretic peptide
- CHARM
Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity Programme
- CKD
chronic kidney disease
- DM
diabetes mellitus
- EDV
end-diastolic volume
- EF
ejection fraction
- eGFR
estimated glomerular filtration rate
- FDR
false discovery rate
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- IDI
integrated discrimination improvement
- LV
left ventricular
- NYHA
New York Hear Association
- PASP
pulmonary artery systolic pressure
- PRSW
preload-recruitable stroke work
- RAP
right atrial pressure
- RV
right ventricular
- RVFAC
RV fractional area change
- TAPSE
tricuspid annular plane systolic excursion
- TDI
tissue Doppler imaging
- UACR
urine albumin-to-creatinine ratio
Footnotes
Disclosures: No relationships with industry related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 2.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–75. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 3.Deckert T, Yokoyama H, Mathiesen E, et al. Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. BMJ. 1996;312:871–4. doi: 10.1136/bmj.312.7035.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miettinen H, Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke. 1996;27:2033–9. doi: 10.1161/01.str.27.11.2033. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Lin J, Solomon CG, et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116:2687–93. doi: 10.1161/CIRCULATIONAHA.107.723270. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–50. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 7.Jackson CE, MacDonald MR, Petrie MC, et al. Associations of albuminuria in patients with chronic heart failure: findings in the ALiskiren Observation of heart Failure Treatment study. Eur J Heart Fail. 2011;13:746–54. doi: 10.1093/eurjhf/hfr031. [DOI] [PubMed] [Google Scholar]
- 8.Masson S, Latini R, Milani V, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805. [DOI] [PubMed] [Google Scholar]
- 9.Miura M, Shiba N, Nochioka K, et al. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail. 2012;14:367–76. doi: 10.1093/eurjhf/hfs001. [DOI] [PubMed] [Google Scholar]
- 10.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 11.Djousse L, Kochar J, Hunt SC, et al. Relation of albuminuria to left ventricular mass (from the HyperGEN Study) Am J Cardiol. 2008;101:212–6. doi: 10.1016/j.amjcard.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 12.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581–90. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama E, Sugiyama S, Matsuzawa Y, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–86. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara J, Sugiyama S, Nozaki T, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861–9. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. 2009;10:733–7. doi: 10.1093/ejechocard/jep052. [DOI] [PubMed] [Google Scholar]
- 16.Morris DA, Gailani M, Vaz Perez A, et al. Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:886–97. doi: 10.1016/j.echo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 19.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:H744–50. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
- 25.Feneley MP, Skelton TN, Kisslo KB, Davis JW, Bashore TM, Rankin JS. Comparison of Preload Recruitable Stroke Work, End-Systolic Pressure-Volume and Dp/Dtmax-End-Diastolic Volume Relations as Indexes of Left-Ventricular Contractile Performance in Patients Undergoing Routine Cardiac-Catheterization. J Am Coll Cardiol. 1992;19:1522–1530. doi: 10.1016/0735-1097(92)90613-r. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi M, Odake M, Takaoka H, Hayashi Y, Yokoyama M. Comparison between preload recruitable stroke work and the end-systolic pressure-volume relationship in man. European Heart Journal. 1992;13:80–84. doi: 10.1093/eurheartj/13.suppl_e.80. [DOI] [PubMed] [Google Scholar]
- 27.Willis J, Augustine D, Shah R, Stevens C, Easaw J. Right ventricular normal measurements: time to index? J Am Soc Echocardiogr. 2012;25:1259–67. doi: 10.1016/j.echo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–9. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- 30.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:479–498. [Google Scholar]
- 31.Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;41:2022–8. doi: 10.1016/s0735-1097(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 32.Wegria R, Capeci NE, Blumenthal MR, et al. The pathogenesis of proteinuria in the acutely congested kidney. J Clin Invest. 1955;34:737–43. doi: 10.1172/JCI103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah AM, Lam CS, Cheng S, et al. The relationship between renal impairment and left ventricular structure, function, and ventricular-arterial interaction in hypertension. J Hypertens. 2011;29:1829–36. doi: 10.1097/HJH.0b013e32834a4d38. [DOI] [PubMed] [Google Scholar]
- 34.Katz DH, Selvaraj S, Aguilar FG, et al. Association of Low-Grade Albuminuria with Adverse Cardiac Mechanics: Findings from the HyperGEN Study. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 36.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 37.Liu YW, Tsai WC, Su CT, Lin CC, Chen JH. Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2009;15:782–9. doi: 10.1016/j.cardfail.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10:414–418. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Change in R2 for adjusted models when log-transformed UACR is added as a covariate
Supplementary Table S2. Comparison of risk prediction models with and without log-transformed UACR