Abstract
Objectives
Our objective was to test the hypothesis that nulliparous women with a history of miscarriage have an increased risk of depression during late pregnancy, and at 1, 6, and 12 months postpartum compared to women without a history of miscarriage.
Methods
We conducted secondary analysis of a longitudinal cohort study, the First Baby Study, and compared 448 pregnant women with a history of miscarriage to 2343 pregnant women without a history of miscarriage on risk of probable depression (score >12 on the Edinburgh Postnatal Depression Scale). Logistic regression models were used to estimate odds ratios at each time point and generalized estimating equations were used to obtain estimates in longitudinal analysis.
Results
Women with a history of miscarriage were not more likely than woman without a history of miscarriage to score in the probable depression range during the third trimester or at 6 or 12 months postpartum but were more likely at 1 month postpartum, after adjustment for sociodemographic factors (OR 1.66, 95% CI 1.03 – 2.69).
Conclusions
Women with a history of miscarriage may be more vulnerable to depression during the first month postpartum than women without prior miscarriage, but this effect does not appear to persist beyond this time period. We support the promotion of awareness surrounding this issue and recommend that research is planned to identify risk factors that may position a woman with a history of miscarriage to be at higher risk for depression.
Keywords: Perinatal loss, Perinatal depression, Pregnancy, Postpartum depression, Miscarriage
Introduction
Miscarriage, the spontaneous loss of a pregnancy before completion of 20 weeks gestational age (1), may result in intense grief for women who experience it (2). Emotional experiences during subsequent pregnancy may then be impacted by previous miscarriage experiences. These emotional effects of previous miscarriage during and after subsequent pregnancy are often studied in combination with women with a history of other types of perinatal loss including stillbirth, electively induced abortion, and neonatal death. Very few studies of the effects of previous miscarriage alone on subsequent pregnancy and birth are available. Therefore, this introduction to the extant literature includes what is known about the effect of perinatal loss history in general.
In the United States, perinatal loss occurs in 12–20% of confirmed pregnancies (3). The immediate reaction to perinatal loss for women and their partners is commonly one of grief comparable in intensity to the grief following other types of loss via death (2). After experiencing a perinatal loss, over 85% of women will become pregnant again within 18 months (4). Depressive symptoms can continue to manifest for women with a history of perinatal loss even during a subsequent pregnancy (5, 6). For example, several researchers found that women who are pregnant following a perinatal loss have increased levels of depressive symptoms compared to women who have not experienced such loss (5–13). Women with a history of perinatal loss may perceive a subsequent pregnancy as a threat (14), stress is increased (8), and they describe alterations in their concept of self (15). In contrast, others found no difference in the levels of depression for these two groups of women (16–23).
Although the relationship between a history of perinatal loss and depressive symptoms in subsequent pregnancy has some supportive evidence, it is even more unclear whether depressive symptoms may ameliorate after the birth of a healthy infant. In a sample of women with a history of perinatal loss, Armstrong and colleagues (24) found that clinical levels of depressive symptoms were present during the 3rd trimester of pregnancy; however, more normal levels were typical after the birth of a healthy infant (at 3 and 8 months postpartum), and symptoms of depression decreased over time. Hughes and colleagues (12) found that depression rates in women with a history of perinatal loss were comparable to controls at 12 months postpartum. However, Blackmore and colleagues (9) reported that depressive symptoms were significantly related to perinatal loss during pregnancy and up to 33 months postpartum.
Not only is perinatal depression a concern for the health of the woman affected, it can also have a substantial negative impact on the health of her fetus or newborn. For example, depression during pregnancy may put a woman at greater risk for preterm birth and a low birth weight or small for gestational age baby (25, 26). In the postpartum period, mothers with depression report a wide range of negative emotions and may be fearful or overly intrusive in their interactions with their infant (27, 28). These suboptimal interactions may also have long term consequences for the children, including deficits in cognitive-linguistic functioning and an increase in negative affect or behavior (29, 30). Reduction of depression in the perinatal time period is of utmost importance to assuring the health of childbearing women and their children.
Although the available literature points to a decrease in depressive symptoms over time for pregnant and postpartum women with a history of perinatal loss, no conclusion can be made regarding whether these women are at higher risk for depression after the birth of a healthy infant. The objective of our study was to determine the longitudinal relationship between a history of miscarriage and probable depression during pregnancy and throughout the first year postpartum in a sample of women giving birth for the first time. Our hypothesis was that women with a history of miscarriage have an increased risk of depression during late pregnancy, and at 1 month, 6 months, and 12 months postpartum compared to women without a history of miscarriage. An exploratory secondary objective of our study was to examine the potential moderating effects of social support and maternal stress on the relationship between previous miscarriage and probable depression.
Methods
Study Design and Population
This study utilized secondary analysis of a longitudinal cohort study, the First Baby Study (FBS). The FBS enrolled 3006 pregnant women planning to deliver their first live-born baby in the state of Pennsylvania, USA from January 2009–April 2011. Exclusion criteria were not speaking English or Spanish, more than one fetus, a previous stillbirth that occurred at more than 20 weeks gestation, a previous cesarean delivery regardless of length of gestation, a gestational or surrogate carrier, planned to give the baby up for adoption, planned to have a tubal ligation while hospitalized for delivery, did not have a telephone or were not able to commit to participation in the study for a period of 3 years. These exclusion criteria were related to the primary aim of the FBS, to examine mode of first childbirth and relationship to subsequent pregnancy and delivery. The study was approved by the Institutional Review Board at the Penn State Hershey Medical Center and at participating study hospitals and written informed consent was obtained from each participant. A detailed description of the sampling design and recruitment is described elsewhere (31).
This study utilized data collected during four telephone interviews with participants during the third trimester of pregnancy (at least 30 weeks gestation- baseline interview), and at 1, 6, and 12 months postpartum. At 12 months postpartum, 6.6% of the participants were lost to follow-up.
Measures
The independent variable, a history of miscarriage, was measured via self-report. Women who were enrolled in the FBS and reported a history of elective abortion were excluded from analysis. Women with one or more missing responses for the variables of interest at 1 month postpartum (n= 63) were also excluded. The analytic sample included 448 women with a history of one or more miscarriages and 2343 women experiencing their first pregnancy.
Sociodemographic variables were obtained during the baseline interview. Poverty was measured using the US Census Bureau classification system to categorize participants based on household income and family composition – poverty, near poverty and not poverty. Those with household incomes ≥ 200% above the threshold are classified as “not poverty”, those with household incomes that are 100% to 200% of the poverty threshold are “near poverty”, and those with household incomes < 100% of the poverty threshold are classified as “poverty”. For 127 women, regression methods were used to impute missing income values and create a poverty status category. Analysis completed with and without the imputed values revealed no difference in the results. Thus, imputed values were retained in the analysis.
During the baseline interview, data were also collected on the following potential confounding variables: Use of fertility advice or treatment, and history of anxiety or depression. Women were said to have used fertility advice or treatment if they had planned the pregnancy and responded affirmatively to the question, “Did you and/or your partner use any type of fertility advice, testing, or treatment before you became pregnant?” Women who reported that they had a doctor or nurse tell them that they had anxiety or depression prior to this pregnancy were considered to have a history of anxiety or depression.
At the 1-month postpartum interview, potential confounding factors of mode of delivery, infant hospitalization after birth, postpartum mental health visits, and birth experience were measured. Birth experience was measured using a 16-item scale with a potential range of scores from 16–80. A higher score indicates a more positive birth experience (32).
Potential confounding factors of maternal stress and social support were measured at each time point and were utilized as time-varying covariates. Maternal stress was measured using the Psychosocial Hassles Scale (33), an 11-item instrument which measures perceived maternal stress due to common stressors, such as “money worries like paying bills”. Several of the items were modified to fit the study population and one item was added"Problems with the baby”, for a total of 12 items. In this study, the Cronbach’s alpha was 0.71 at 1 month postpartum and higher scores indicated higher levels of stress. Social support was measured using 5 items from the MOS Social Support Survey (34) and 4 items were added specifically concerning support for a new mother (i.e. “Someone to help you take care of the baby”). The Cronbach’s alpha was 0.88 at 1 month postpartum and higher scores indicated higher levels of social support.
The outcome variable, probable depression, was measured at each time point using the Edinburgh Postnatal Depression Scale (EPDS) (35). Two of the original items were modified: “Things have been getting on top of me” was changed to “I have had trouble coping” and “The thought of harming myself has occurred to me” was changed to “The thought of harming myself or others has occurred to me”. The Cronbach’s alpha in this study was 0.82 at 1 month postpartum. A dichotomous variable was created with probable depression defined as EPDS >12, as suggested by a recent systematic review (36).
Our relatively large sample size enabled us to detect the difference in proportion of probable depression between the two groups of women as small as 3.6% with at least 80% statistical power. A difference more than 4.3% between groups could be detected with at least 90% statistical power.
Analytic Approach
Data analysis was completed using SPSS 20 and verified independently by the study statistician (JZ) using SAS 9.3. Chi-square and Student’s t-tests were used to compare variables by miscarriage status at baseline. Then, a univariate logistic regression model (model 1) was created for each of the four time points followed by multiple logistic regression models as follows: 2) the addition of factors significantly related to the independent variable: maternal age and use of fertility advice or treatment; 3) in addition to model 2 factors, sociodemographic factors marital status, race and ethnicity, education and poverty status; 4) in addition to model 3 factors, obstetric factors including mode of delivery, infant hospitalization after birth, and birth experience; and 5) in addition to model 4 factors, psychosocial factors including postpartum mental health visits, history of anxiety or depression, maternal stress, and social support. At baseline, model 4 was not completed because these variables were measured at the 1-month postpartum interview. Next, longitudinal analysis was completed using generalized estimating equations (GEE) with probable depression as a repeated outcome measure, and adjusting for maternal age and fertility treatment or advice. Finally, interaction analysis was completed for maternal stress and social support at each time point using a logistic regression model adjusted for maternal age, fertility treatment or advice, maternal stress or social support, and the appropriate interaction term.
Results
Women in our sample were a mean age of 27.3 years old (SD= 4.3) at the baseline interview. The majority of women were married (72.1%), non-Hispanic White (84.8%), not living in poverty (80.8%), and had completed a 4-year degree or greater (57.8%). Characteristics of the study sample by miscarriage history are shown in Table 1. Compared to women without a history of miscarriage, women with a history of miscarriage were older (27.1 vs. 28.2 years) and more likely to have received fertility advice or treatment (9.7% vs. 20.3%). The two groups of women did not differ significantly on any other variables.
Table 1.
Baseline Demographic and Obstetric Characteristics of Study Participants
| Total N=2791 |
No history of miscarriage N=2343 |
History of miscarriage N=448 |
P-value | |
|---|---|---|---|---|
| Maternal Age | 27.3 ±4.3 | 27.1 ±4.3 | 28.2 ±4.3 | p<0.001*** |
| Marital Status | p=0.063 | |||
| Married | 2011 (72.1) | 1672 (71.4) | 339 (75.7) | |
| Not Married | 780 (27.9) | 671 (28.6) | 109 (24.3) | |
| Fertility Advice or Treatment | p<0.001*** | |||
| No | 2473 (88.6) | 2116 (90.3) | 357 (79.7) | |
| Yes | 318 (11.4) | 227 (9.7) | 91 (20.3) | |
| Race/Ethnicity | p=0.477 | |||
| Non-Hispanic White | 2366 (84.8) | 1982 (84.6) | 384 (85.7) | |
| Non-Hispanic Black | 180 (6.4) | 148 (6.3) | 32 (7.1) | |
| Hispanic | 144 (5.2) | 127 (5.4) | 17 (3.8) | |
| Other | 101 (3.6) | 86 (3.7) | 15 (3.3) | |
| Education | p=0.814 | |||
| High school graduate or GED or less | 452 (16.2) | 376 (16.0) | 76 (17.0) | |
| Some college or vocational programs | 726 (26.0) | 614 (26.2) | 112 (25.0) | |
| Completed 4 year college degree or greater | 1613 (57.8) | 1353 (57.7) | 260 (58.0) | |
| Poverty | p=0.905 | |||
| Poverty | 227 (8.1) | 190 (8.1) | 37 (8.3) | |
| Near Poverty | 308 (11.0) | 256 (10.9) | 52 (11.6) | |
| Non-poverty | 2256 (80.8) | 1897 (81.0) | 359 (80.1) | |
| Mode of delivery† | p=0.130 | |||
| Vaginal delivery | 1977 (70.8) | 1673 (71.4) | 304 (67.9) | |
| Cesarean delivery | 814 (29.2) | 670 (28.6) | 144 (32.1) | |
| Infant Hospitalization after Birth† | p=0.089 | |||
| No | 2723 (97.6) | 2291 (97.8) | 432 (96.4) | |
| Yes | 68 (2.4) | 52 (2.2) | 16 (3.6) | |
| Postpartum Mental Health Visits† | p=0.292 | |||
| No | 2662 (95.4) | 2239 (95.6) | 423 (94.4) | |
| Yes | 129 (4.6) | 104 (4.4) | 25 (5.6) | |
| History of Anxiety or Depression | p=0.161 | |||
| No | 2152 (77.1) | 1818 (77.6) | 334 (74.6) | |
| Yes | 639 (22.9) | 525 (22.4) | 114 (25.4) | |
| Edinburgh Postnatal Depression Scale | p=0.495 | |||
| No probable depression | 2635 (94.4) | 2209 (94.3) | 426 (95.1) | |
| Probable depression | 156 (5.6) | 134 (5.7) | 22 (4.9) | |
| EPDS Continuous Score (Range 0–27) | 5.8 ±3.7 | 5.8 ±3.9 | 5.8 ±3.7 | p=0.987 |
| Birth Experience† | 68.7 ±6.4 | 68.7 ±6.4 | 68.5 ±6.4 | p=0.512 |
| Maternal Stress | 18.6 ±4.4 | 18.6 ±4.4 | 18.5 ±4.4 | p=0.750 |
| Social Support | 22.3 ±2.9 | 22.3 ±2.9 | 22.3 ±2.9 | p=0.920 |
All results reported as n (%) or mean ± SD.
p<0.05,
p<0.01,
p<0.001
Measured at 1 month postpartum
During pregnancy, 5.7% of women without a history of miscarriage and 4.9% of women with a history of miscarriage reported symptoms of probable depression. Mean EPDS scores at baseline are shown in Table 1 and did not differ significantly between groups. Mean EPDS scores at 1 month postpartum were 4.3 (SD 3.9) for women without a history of miscarriage and 4.5 (SD 4.1) for women with a history of miscarriage, and did not differ significantly between groups. Likewise, scores did not differ significantly between groups at 6 months or 12 months postpartum and were similar in value to the 1 month postpartum scores. Analysis of the relationship between a history of miscarriage and probable depression during pregnancy revealed no significant relationship in any of the five models (all p>0.05) (Table 2). Likewise, at 6 months postpartum and 12 months postpartum, a history of miscarriage was not significantly associated with risk of probable depression (all p>0.05).
Table 2.
Relationship between Miscarriage and Probable Depression from logistic regression models at baseline, 6 month postpartum, and 12 months postpartum
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Baseline: | |||||
| No history of miscarriage | Ref | Ref | Ref | - | Ref |
| History of miscarriages | 0.85 (0.54–1.35) | 0.99 (0.62–1.58) | 0.89 (0.55–1.44) | 0.84 (0.50–1.42) | |
| 1 month Postpartum: | |||||
| No history of miscarriage | Ref | Ref | Ref | Ref | Ref |
| History of miscarriages | 1.69 (1.05–2.70) | 1.70 (1.05–2.75) | 1.66 (1.03–2.69) | 1.63 (0.99–2.69) | 1.70 (0.99–2.92) |
| 6 Months Postpartum: | |||||
| No history of miscarriage | Ref | Ref | Ref | Ref | Ref |
| History of miscarriages | 1.02 (0.59–1.77) | 1.18 (0.68–2.05) | 1.08 (0.61–1.88) | 1.06 (0.60–1.88) | 0.90 (0.46–1.78) |
| 12 Months Postpartum: | |||||
| No history of miscarriage | Ref | Ref | Ref | Ref | Ref |
| History of miscarriages | 1.17 (0.69–2.01) | 1.40 (0.81–2.41) | 1.25 (0.72–2.17) | 1.25 (0.71–2.19) | 1.35 (0.72–2.53) |
All results reported as OR (95% CI)
Model 1 is unadjusted
Model 2 adjusted for maternal age and use of fertility advice or treatment
Model 3 adjusted for variables in model 2 plus marital status, race/ethnicity, education, and poverty status
Model 4 adjusted for variables in model 3 plus mode of delivery, infant hospitalization after birth, and birth experience
Model 5 adjusted for variables in model 4 plus postpartum mental health visits, history of anxiety or depression, maternal stress, and social support
We found a significant relationship between a history of miscarriage and probable depression at 1 month postpartum where women with a history of miscarriage were 1.69 times (95% CI 1.05–2.70) more likely to report symptoms of probable depression than the comparison group of women (Table 2). This increased odds remained significant after adjustment for sociodemographic factors (models 2 & 3). After further adjustment for obstetric and psychosocial factors (models 4 & 5), the odds ratio was no longer significant. However, even after full adjustment, the odds ratio remained stable across all models and still indicated a 1.70 times higher odds (95% CI 0.99–2.92) of probable depression for women with a history of miscarriage.
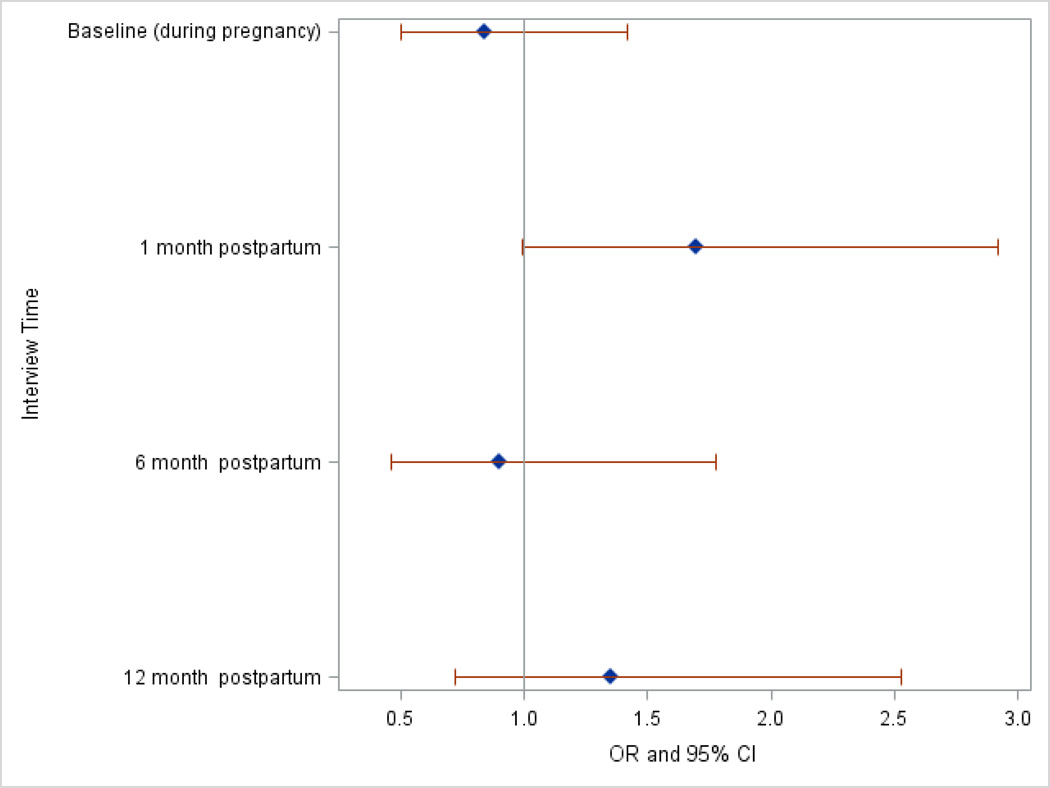
Longitudinal analysis of the relationship between a history of miscarriage and probable depression revealed no significant relationship over time (Table 3). Notably, interview time was a significant predictor of probable depression, as all postpartum time periods were associated with reduced odds of probable depression compared to during pregnancy. Additionally, there was no evidence that the pattern of probable depression risk differed between the two groups of women over the four time periods, as the interaction between miscarriage history and interview time was not significant (data not shown). In women with a history of miscarriage, the highest risk for depression occurred at the 1 month postpartum period (Figure 1).
Table 3.
Longitudinal Relationship between Previous Miscarriage and Probable Depression from Generalized Estimating Equations
| OR (95% CI) | |
|---|---|
| Perinatal Loss | |
| No history of miscarriage | Ref |
| History of miscarriages | 1.27 (0.93–1.75) |
| Time | |
| Pregnancy | Ref |
| 1 month | 0.63 (0.49–0.79) |
| 6 months | 0.64 (0.51–0.80) |
| 12 months | 0.64 (0.50–0.81) |
| Age | 0.90 (0.87–0.93) |
| Fertility Advice or Treatment | |
| No | Ref |
| Yes | 0.90 (0.59–1.36) |
Figure 1.
Odds ratio and 95% confidence interval of risk of probable depression at each time point for women with a history of miscarriage obtained using logistic regression model 5.
Analysis of interaction between a history of miscarriage and either maternal stress or perceived social support at each time period did not reveal significant interaction (data not shown). This indicates that the relationship between miscarriage and probable depression does not differ significantly based on a woman’s level of social support or stress.
Discussion
We did not observe any difference in odds of probable depression between the women with a history of miscarriage when compared to women without a history of miscarriage over time, however, women with a history of miscarriage were 1.66 times more likely to have probable depression at 1 month postpartum compared to women with no history of miscarriage.
For both groups of women, depressive symptoms followed the same pattern over time and were highest during pregnancy and remained consistently low after the birth of the infant. Our results concur with those of Blackmore and colleagues, although these authors examined perinatal loss including both miscarriage and stillbirth (9). The idea that depressive symptoms are highest during pregnancy and lower in the postpartum period was also reported in studies by Armstrong and colleagues (24) and Hughes and colleagues (12).
We found that at 1month postpartum, women with a history of miscarriage were 1.66 times more likely to report probable depression compared to women without a history of perinatal loss. Although this relationship did not maintain significance after adjustment for all potential confounders, we believe the result deserves comment. Results of similar studies during the early postpartum period were mixed. Blackmore and colleagues (9) found higher rates of depression in women with a history of perinatal loss compared to controls at 2 months postpartum, while Hughes and colleagues (12) found no difference between these two groups of women at 6 weeks postpartum. It is possible that the increased risk of depression we found at 1 month postpartum may not be of strong clinical significance, as the mean EPDS scores differed only by 0.2 points between groups.
In our study, we found no evidence that women with a history of miscarriage had increased risk of depression at 6 months or 12 months postpartum compared to women without a history of miscarriage. This is consistent with much of the published research in women with a history of perinatal loss during the second half of the postpartum year. Although Carerra and colleagues (37) found a positive association between a history of stillbirth and increased depressive symptoms at 6 and 12 months postpartum, several other researchers found no difference during this same time period (12, 16, 38).
We found that women with a history of miscarriage did not differ from women without a history of miscarriage with respect to probable depression during pregnancy. There is much reported in the literature on depression in pregnancy subsequent to perinatal loss, however, there is no clear consensus. Nearly half of the studies that we reviewed reported that women with a history of perinatal loss have increased levels of depressive symptoms in a subsequent pregnancy compared to pregnant women without a history of perinatal loss (5, 6, 8–13) while the other half report no difference in depressive symptoms (17–23). The heterogeneity of these studies is great, as they differ on the definition of perinatal loss, the timing of measurement of depressive symptoms, the type of instrument used to measure depression, and sample parity. However, one factor that we believe may specifically impact the validity of studies of perinatal loss is the potential problem of sample selection bias.
Armstrong and colleagues, whose publications provide much of the support for a relationship between perinatal loss and depression in subsequent pregnancy, consistently used recruitment methods that included advertisement of the study in perinatal loss support groups (online or local) (5, 8, 16). This method may introduce bias because women who seek support after a perinatal loss may be different from those women who do not. For example, it is plausible that using recruitment methods such as advertisements and flyers that state the study purpose may deter participants who are not emotionally distressed by the loss and do not wish to further explore the issue. Conversely, women who experience severe emotional distress may not be willing to participate in a research study. However, Armstrong (2004) reported that parents who attended a support group after a perinatal loss had higher depression scores than those who did not, suggesting that the former explanation may be more likely. Therefore, it is possible that studies using this potentially biased sampling method may overestimate the impact of previous perinatal loss. One method of decreasing this selection bias is to study these women through samples that are obtained without the aim of selecting women with a history of perinatal loss.
Two recent population-based studies examined depression in women with a history of perinatal loss. Blackmore and colleagues analyzed the Avon Longitudinal Study of Parents and Children, based in England, to determine whether depression was related to a history of miscarriage or stillbirth (9). These authors found that the number of perinatal losses significantly predicted depressive symptoms at both prenatal and postpartum time points. In contrast, a study using the Early Childhood Longitudinal Study, Birth Cohort based in the United States found no association between a history of one perinatal loss and depression at 9 months postpartum (38). These authors found that having two or more perinatal losses was associated with higher depression when compared to women with no history of loss; however, the difference amounted to 1 point on the depression scale (range 12–48), which may not be clinically significant. The results of our study, along with the results of these population-based studies, suggest that a history of perinatal loss may not be directly related to negative emotional outcomes for the majority of women. Price (2008) suggests that the normative response to perinatal loss may not be an increased risk for emotional impairment; rather, other social and emotional contextual features may contribute significantly in those women who develop depression or other emotional difficulties after perinatal loss.
We examined two of these potential contextual factors by investigating the effect of the interaction between miscarriage history and a woman’s level of stress and her perceived available social support. Neither maternal stress nor perceived social support affected the relationship between a history of miscarriage and depressive symptoms. Although to our knowledge general stress and social support have not been examined in this context previously, Armstrong and colleagues (16) found that the more stress women associated with their previous perinatal loss, the higher their depressive symptoms in a subsequent pregnancy. This suggests that perinatal loss-specific stress, rather than general stress as measured in our study, may contribute to a woman’s increased risk of depression in subsequent pregnancy after perinatal loss.
To our knowledge, our study is only the second study to examine the pattern of depression over time in women with a history of perinatal loss during subsequent pregnancy and postpartum. Our results confirm that women with a history of miscarriage experience depressive symptomatology during and after a subsequent pregnancy to a similar degree as women without a history of miscarriage. Our study is also one of only a few studies of depression in this population utilizing a large sample size and adjusting for many potential confounders of depression.
Despite the strengths of our study design, there are also some limitations that deserve comment. First, although studies of perinatal loss vary greatly in their definition of loss, our study did not include women with other types of perinatal loss besides miscarriage including stillbirth, neonatal death, or medically-indicated abortion. It is unclear whether the type of perinatal loss has a significant impact on a woman’s emotional health during a subsequent pregnancy, but Armstrong and colleagues (2002) found that the gestational age of a previous perinatal loss was not associated with depressive symptoms during subsequent pregnancy. Also, our version of the EPDS has been slightly modified from the original, and although modifications were minor, validity testing has not be completed on this new version.
Given that women excluded from our sample due to missing data were younger, less likely to be married, non-Hispanic White, educated, or to have a history of miscarriage, and more likely to be living in poverty or depressed, our results may have some selection bias. Additionally, there was selection bias due to differential loss to follow up such that mothers included at 12 months postpartum were older, more likely to be educated, married, non-Hispanic White, and less likely to live in poverty. However, those who were lost to follow-up did not differ from those included on miscarriage history or probable depression and comprised a very small portion of our sample. Also, our study participants were older, more likely to be non-Hispanic White, had higher levels of education and higher household income than the overall population of Pennsylvania (31). Our study population also had a relatively low rate of postpartum depression (6%) compared to other studies (39–41). These differences are indicative of a selection bias that is common in longitudinal research studies where participation is voluntary. As such, although our sample size was large and relatively diverse, the results of the study may not be generalizable to the entire population.
We conclude that although a history of miscarriage may have implications for mental health in subsequent pregnancy and postpartum, it is unlikely that these effects are universal. Each woman who experiences a miscarriage may react to a subsequent pregnancy differently. As depression in the perinatal period may be associated with adverse pregnancy outcomes (25, 26, 42), impairments in maternal-infant bonding (42–47), and developmental impairments in the child (48–54), it is important for researchers to further examine the relationship between a history of miscarriage and depression. Future research should aim to identify risk factors that may position a woman with a history of miscarriage to be at higher risk for depression during a subsequent perinatal period. This should include separate examination of different types of perinatal loss, with the aim of determining if the gestation of a perinatal loss is a significant contributor to health impact. It will also be important for researchers to be mindful of recruitment methods to reduce selection bias. Population-based samples may be ideal.
Based on what is now known about the effect of a history of perinatal loss on depression in a subsequent pregnancy, we recommend that providers for pregnant women remain aware of a woman’s history of previous losses at any gestation. Albeit limited, research has shown that the value a woman places on a pregnancy that is lost, more so than the gestation of the pregnancy, is the most important indicator of her emotional response (5, 15, 23). It is important for providers to openly discuss previous perinatal losses with women who are pregnant, so that they can receive appropriate intervention if they appear to struggle with the experience of subsequent pregnancy. Interventions may include mental health consultation for depressive symptoms and/or bereavement support through local or online support groups. Intervention may be especially valuable in the early postpartum period. Finally, although the research on the effect of a history of perinatal loss on depression in subsequent pregnancy is not definitive, it is clearly evident that many women struggle with the experience, and increasing awareness in the public health and medical communities is an essential primary step in the improvement of health for affected women.
Acknowledgements
This study was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD052990). KHK is the principal investigator for this award. CBK was also supported by a fellowship from the National Institute for Nursing Research (F31 NR013303) and by the Eastern Nursing Research Society and the Council for the Advancement of Nursing Science.
References
- 1.Zegers-Hochschild F, Adamson G, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility and Sterility. 2009;92(5):1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Brier N. Grief following miscarriage: A comprehensive review of the literature. Journal of Women's Health. 2008;17(3):451–464. doi: 10.1089/jwh.2007.0505. [DOI] [PubMed] [Google Scholar]
- 3.Scotchie J, Fritz M. Early pregnancy loss. Postgraduate Obstetrics and Gynecology. 2006;26(9):1–7. [Google Scholar]
- 4.Cuisinier M, Janssen H, de Graauw C, Bakker S, Hoogduin C. Pregnancy following miscarriage: Course of grief and some determining factors. Journal of Psychosomatic Obstetrics and Gynecology. 1996;17:168–174. doi: 10.3109/01674829609025678. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D. Emotional distress and prenatal attachment in pregnancy after perinatal loss. J Nurs Scholarsh. 2002;34(4):339–345. doi: 10.1111/j.1547-5069.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Franche R, Mikail S. The impact of perinatal loss on adjustment to subsequent pregnancy. Social Science Medicine. 1999;48(11):1613–1623. doi: 10.1016/s0277-9536(98)00438-9. [DOI] [PubMed] [Google Scholar]
- 7.Lamb EH. The impact of previous perinatal loss on subsequent pregnancy and parenting. J Perinat Educ. 2002 Spring;11(2):33–40. doi: 10.1624/105812402X88696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong D. Impact of prior perinatal loss on subsequent pregnancies. J Obstet Gynecol Neonatal Nurs. 2004 Nov-Dec;33(6):765–773. doi: 10.1177/0884217504270714. [DOI] [PubMed] [Google Scholar]
- 9.Blackmore ER, Cote-Arsenault D, Tang W, Glover V, Evans J, Golding J, et al. Previous prenatal loss as a predictor of perinatal depression and anxiety. Br J Psychiatry. 2011 May;198(5):373–378. doi: 10.1192/bjp.bp.110.083105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yilmaz SD, Beji NK. Effects of perinatal loss on current pregnancy in Turkey. Midwifery. 2013;29(11):1272–1277. doi: 10.1016/j.midw.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Gong X, Hao J, Tao F, Zhang J, Wang H, Xu R. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013 Jan;166(1):30–36. doi: 10.1016/j.ejogrb.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Hughes P, Turton P, Evans CD. Stillbirth as risk factor for depression and anxiety in the subsequent pregnancy: cohort study. BMJ. 1999 Jun 26;318(7200):1721–1724. doi: 10.1136/bmj.318.7200.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couto ER, Couto E, Vian B, Gregorio Z, Nomura ML, Zaccaria R, et al. Quality of life, depression and anxiety among pregnant women with previous adverse pregnancy outcomes. Sao Paulo Med J. 2009 Jul;127(4):185–189. doi: 10.1590/S1516-31802009000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cote-Arsenault D. Threat appraisal, coping, and emotions across pregnancy subsequent to perinatal loss. Nurs Res. 2007 Mar-Apr;56(2):108–116. doi: 10.1097/01.NNR.0000263970.08878.87. [DOI] [PubMed] [Google Scholar]
- 15.Cote-Arsenault D, Bidlack D, Humm A. Women's emotions and concerns during pregnancy following perinatal loss. MCN Am J Matern Child Nurs. 2001 May-Jun;26(3):128–134. doi: 10.1097/00005721-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong D. Perinatal loss and parental distress after the birth of a healthy infant. Adv Neonatal Care. 2007 Aug;7(4):200–206. doi: 10.1097/01.ANC.0000286337.90799.7d. [DOI] [PubMed] [Google Scholar]
- 17.Gaudet C, Sejourne N, Camborieux L, Rogers R, Chabrol H. Pregnancy after perinatal loss: Association of grief, anxiety, and attachment. Journal of Reproductive and Infant Psychology. 2010;28(3):240–251. [Google Scholar]
- 18.Theut SK, Pedersen FA, Zaslow MJ, Rabinovich BA. Pregnancy subsequent to perinatal loss: Parental anxiety and depression. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(3):289–292. doi: 10.1097/00004583-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Hutti MH, Armstrong DS, Myers J. Healthcare utilization in the pregnancy following a perinatal loss. MCN Am J Matern Child Nurs. 2011 Mar-Apr;36(2):104–111. doi: 10.1097/NMC.0b013e3182057335. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler SR, Austin J. The loss response list: a tool for measuring adolescent grief responses. Death Stud. 2000 Jan-Feb;24(1):21–34. doi: 10.1080/074811800200676. [DOI] [PubMed] [Google Scholar]
- 21.Bergner A, Beyer R, Klapp BF, Rauchfuss M. Pregnancy after early pregnancy loss: a prospective study of anxiety, depressive symptomatology and coping. J Psychosom Obstet Gynaecol. 2008 Jun;29(2):105–113. doi: 10.1080/01674820701687521. [DOI] [PubMed] [Google Scholar]
- 22.Marcinko VM, Marcinko D, Dordevic V, Oreskovic S. Anxiety and depression in pregnant women with previous history of spontaneous abortion. Coll Antropol. 2011 Jan;35(Suppl 1):225–228. [PubMed] [Google Scholar]
- 23.Hamama L, Rauch SA, Sperlich M, Defever E, Seng JS. Previous experience of spontaneous or elective abortion and risk for posttraumatic stress and depression during subsequent pregnancy. Depress Anxiety. 2010 Aug;27(8):699–707. doi: 10.1002/da.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong D, Hutti M, Myers J. The influence of prior perinatal loss on parents' psychological distress after the birth of a subsequent healthy infant. Journal of Obstetric Gynecologic and Neonatal Nursing. 2009;38:654–666. doi: 10.1111/j.1552-6909.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 25.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosom Med. 2006 Sep-Oct;68(5):747–753. doi: 10.1097/01.psy.0000238212.21598.7b. [DOI] [PubMed] [Google Scholar]
- 27.APA. American Psychiatric Association DSM-V Development. 2010 Available from: http://www.dsm5.org/Pages/Default.aspx.
- 28.Beck CT. Postpartum depression: A metasynthesis. Qualitative Health Research. 2002;12(4):453–472. doi: 10.1177/104973202129120016. [DOI] [PubMed] [Google Scholar]
- 29.Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011 Mar;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- 30.NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Dev Psychol. 1999 Sep;35(5):1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- 31.Kjerulff KH, Velott DL, Zhu J, Chuang CH, Hillemeier MM, Paul IM, et al. Mode of First Delivery and Women's Intentions for Subsequent Childbearing: Findings from the First Baby Study. Paediatr Perinat Epidemiol. 2013 Jan;27(1):62–71. doi: 10.1111/ppe.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bicking Kinsey C, Baptiste-Roberts K, Zhu J, Kjerulff K. Effect of previous miscarriage on the maternal birth experience in the First Baby Study. Journal of Obstetric Gynecologic and Neonatal Nursing. 2013;42(4):442–450. doi: 10.1111/1552-6909.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra DP, O'Campo P, Strobino D. Testing a sociomedical model for preterm delivery. Paediatric and Perinatal Epidemiology. 2001;15(2):110–122. doi: 10.1046/j.1365-3016.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 34.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 35.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987 Jun 1;150(6):782–786. doi: 10.1192/bjp.150.6.782. 1987. [DOI] [PubMed] [Google Scholar]
- 36.Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009 May;119(5):350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 37.Carrera L, Diez-Domingo J, Montanana V, Monleon Sancho J, Minguez J, Monleon J. Depression in women suffering perinatal loss. Int J Gynaecol Obstet. 1998 Aug;62(2):149–153. doi: 10.1016/s0020-7292(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 38.Price S. Stepping Back to Gain Perspective: Pregnancy Loss History, Depression, and Parenting Capacity in the Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) Death Studies. 2008;32(2):97–122. doi: 10.1080/07481180701801170. [DOI] [PubMed] [Google Scholar]
- 39.Le Strat Y, Dubertret C, Le Foll B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J Affect Disord. 2011 Dec;135(1–3):128–138. doi: 10.1016/j.jad.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Witt WP, DeLeire T, Hagen EW, Wichmann MA, Wisk LE, Spear HA, et al. The prevalence and determinants of antepartum mental health problems among women in the USA: a nationally representative population-based study. Arch Womens Ment Health. 2010 Oct;13(5):425–437. doi: 10.1007/s00737-010-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witt WP, Wisk LE, Cheng ER, Hampton JM, Creswell PD, Hagen EW, et al. Poor prepregnancy and antepartum mental health predicts postpartum mental health problems among US women: a nationally representative population-based study. Womens Health Issues. 2011 Jul-Aug;21(4):304–313. doi: 10.1016/j.whi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edhborg M, Nasreen HE, Kabir ZN. Impact of postpartum depressive and anxiety symptoms on mothers' emotional tie to their infants 2–3 months postpartum: a population-based study from rural Bangladesh. Arch Womens Ment Health. 2011 Aug;14(4):307–316. doi: 10.1007/s00737-011-0221-7. [DOI] [PubMed] [Google Scholar]
- 43.Taylor A, Atkins R, Kumar R, Adams D, Glover V. A new Mother-to-Infant Bonding Scale: links with early maternal mood. Arch Womens Ment Health. 2005 May;8(1):45–51. doi: 10.1007/s00737-005-0074-z. [DOI] [PubMed] [Google Scholar]
- 44.Reck C, Klier C, Pabst K, Stehle E, Steffenelli U, Struben K, et al. The German version of the Postpartum Bonding Instrument: Psychometric properties and association with postpartum depression. Arch Womens Ment Health. 2006;9:265–271. doi: 10.1007/s00737-006-0144-x. [DOI] [PubMed] [Google Scholar]
- 45.Moehler E, Brunner R, Wiebel A, Reck C, Resch F. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Archives of Women's Mental Health. 2006;9(5):273–278. doi: 10.1007/s00737-006-0149-5. [DOI] [PubMed] [Google Scholar]
- 46.Siu BW, Ip P, Chow HM, Kwok SS, Li OL, Koo ML, et al. Impairment of mother-infant relationship: validation of the Chinese version of Postpartum Bonding Questionnaire. J Nerv Ment Dis. 2010 Mar;198(3):174–179. doi: 10.1097/NMD.0b013e3181d14154. [DOI] [PubMed] [Google Scholar]
- 47.Edhborg M, Lundh W. Some early indicators for depressive symptoms and bonding 2 months postpartum - a study of new mothers and fathers. Archives of Women's Mental Health. 2005;8(4):221–231. doi: 10.1007/s00737-005-0097-5. [DOI] [PubMed] [Google Scholar]
- 48.Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008 Jul;115(8):1043–1051. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 49.Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004 Spring;67(1):63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- 50.Field T, Diego M, Hernandez-Reif M. Depressed mothers' infants are less responsive to faces and voices. Infant Behav Dev. 2009 Jun;32(3):239–244. doi: 10.1016/j.infbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luoma I, Kaukonen P, Mantymaa M, Puura K, Tamminen T, Salmelin R. A longitudinal study of maternal depressive symptoms, negative expectations and perceptions of child problems. Child Psychiatry Hum Dev. 2004 Fall;35(1):37–53. doi: 10.1023/b:chud.0000039319.96151.63. [DOI] [PubMed] [Google Scholar]
- 52.Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, et al. Longitudinal study of maternal depressive symptoms and child well-being. J Am Acad Child Adolesc Psychiatry. 2001 Dec;40(12):1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Milgrom J, Westley DT, McCloud PI. Do infants of depressed mothers cry more than other infants? J Paediatr Child Health. 1995 Jun;31(3):218–221. doi: 10.1111/j.1440-1754.1995.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor TG, Caprariello P, Blackmore ER, Gregory AM, Glover V, Fleming P. Prenatal mood disturbance predicts sleep problems in infancy and toddlerhood. Early Hum Dev. 2007 Jul;83(7):451–458. doi: 10.1016/j.earlhumdev.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]