Abstract
Background
It has been suggested that all patients with Parkinson’s disease (PD) who undergo functional neurosurgery have difficulties in slowing down in high conflict tasks. However, it is unclear whether concomitant dopaminergic medication is responsible for this impairment.
Objective
To assess perceptual decision making in PD patients with bilateral deep brain stimulation.
Methods
We tested 27 PD patients with bilateral deep brain stimulation on a task in which participants had to filter task relevant information from background noise. Thirteen patients were treated with Levodopa monotherapy and 14 patients were treated with Levodopa in combination with a dopamine agonist. Results were compared to healthy matched controls.
Results
We found that all PD patients who were treated with a dopamine agonist made faster decisions than controls and PD patients who were not exposed to a dopamine agonist. Further, all patients made more errors than controls, but there was no difference between the two patient groups.
Conclusions
Our results suggest that dopamine agonist therapy rather than deep brain stimulation is likely responsible for the inability to slow down in high conflict situations in PD. These results further strengthen the need to reduce dopamine agonists in PD patients undergoing functional neurosurgery in order to prevent them making inadvisable decisions.
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is commonly used in patients with advanced Parkinson’s disease (PD) to improve motor handicap [1]. Whether STN-DBS can cause or improve impulsivity in PD is, however, the subject of ongoing debate. Some neurobehavioural tests have shown that PD patients with STN-DBS have difficulty slowing down in high conflict situations [2], whereas other studies have shown that impairments on information sampling tasks are induced by dopamine agonist therapy and not DBS [3]. Similarly, some clinical studies suggest that STN-DBS can either cause [4] or improve [5] addictive behaviours in PD. Variable electrode placement or differential reduction in dopaminergic medication may contribute to differences in outcome [5, 6]. The STN has been suggested to act as a “brake” influencing cortico-striatal pathways to allow more time to elapse before committing to a decision [7]. Dopamine agonists on the other hand have been shown to reduce prefrontal cortical function, and at the same time increase activity of the mesolimbic dopaminergic neurons during reward processing [8].
To clarify the role of STN-DBS and dopamine agonist therapy in high conflict decisions we tested PD patients on a perceptual decision making task. In perceptual decision making tasks participants are required to select relevant information from a noisy background. For example in the “random dot motion task”, participants need to report in which direction the majority of dots are moving. A recent study using this random dot task showed an acute effect of STN-DBS stimulation on task performance. On STN-DBS participants responded faster in high conflict situations compared to off stimulation, demonstrating that the STN plays a key role in decision threshold [9]. However, the effects of STN-DBS stimulation under stable conditions and in combination with dopamine agonist therapy on perceptual decision making tasks are unclear.
Therefore, we recruited two PD groups, both of whom had undergone bilateral STN-DBS. One group was treated with Levodopa with a dopa decarboxylase inhibitor (L-dopa) in combination with a dopamine agonist, whereas the other group was treated with L-dopa monotherapy.
We hypothesized that PD patients with STN-DBS and dopamine agonist therapy would respond quicker than those STN-DBS patients who were just on L-dopa monotherapy. Further, we speculated that those patients who were on L-dopa monotherapy would make fewer errors than those who were treated in addition with a dopamine agonist and that both patient groups would make more errors than healthy control subjects.
Methods
Only participants who scored above 26/30 points on the Mini-Mental state examination were included [10]. All participants provided written informed consent according to the declaration of Helsinki and had full capacity to consent. The study was approved by the UCLH Trust Research Ethics Committee.
All PD patients were recruited from the National Hospital for Neurology and Neurosurgery London, fulfilled the Queen Square Brain Bank criteria for the diagnosis of PD [11] and were treated with L-dopa. We recruited 27 PD patients who had previously undergone bilateral STN-DBS. Fourteen of these PD patients were treated with L-dopa in combination with a dopamine agonist and 13 were treated with L-dopa monotherapy having never been exposed to a dopamine agonist previously. None of the PD patients had a history of impulsive or compulsive behaviours. Results were compared to 17 healthy matched controls.
Pixel task
In the perceptual inference task [12] participants were shown a circle in which a proportion of the pixels were red and the rest were blue. Participants then had to guess whether there were more blue or more red pixels presented on the screen. Sixty trials were performed in total, 20 of which contained a high conflict 60/40 distribution of red and blue pixels, 20 an easier 70/30 distribution and a further 20 trials starting with a 60/40 condition gradually changing to an 80/20 distribution of coloured pixels after 2.5 seconds.
Participants were told to press the labelled keys whenever they thought they knew the answer.
Feedback (“correct”/ “wrong”) was given instantly. Correct choices were rewarded with 0.25 units, incorrect choices were unrewarded. Participants were told that faster responses did not lead to higher rewards.
The majority of these patients- (7 DBS+DA, 10 DBS-DA) and all controls also performed a baseline reaction time (RT) task, in which they were presented with a solid blue or red circle and had to respond as quickly as they could. At the end of the task participants received a modest amount of money depending on their final score, usually around £10–£15.
Statistics
Data analyses were performed using SPSS 21 using a mixed model Anova. Demographic variables were analysed using ANOVA, or χ2 tests. RTs and baseline RTs were log transformed and residuals were normally distributed. Condition (60/40, 70/30/, morphing to 80/20) and group were modelled as fixed factors. Errors were analyzed using a non-parametric Kruskal Wallis ANOVA.
Results
Demographic characteristics
There were no significant differences on any demographic characteristics between the groups (Table 1).
Table 1.
Demographic characteristics
| Controls | DBS-DA | DBS+DA | t statistic χ2 or F- statistic |
p-value | |
|---|---|---|---|---|---|
| Participants (no.) | 17 | 13 | 14 | ||
| Gender (male) | 14 | 12 | 11 | χ2 =1.0 | 0.6 |
| Age (years) | 59.9±10.4 | 60.0±7.2 | 55.9±10.0 | F=0.8 | 0.4 |
| Age PD of diagnosis | 46.1±7.5 | 40.1±8.1 | t=1.8 | 0.08 | |
| PD Disease duration (years) | 13.3±4.8 | 15.0±4.9 | t=0.9 | 0.37 | |
| DBS (years) | 3.6±2.4 | 3.9±2.3 | t=0.3 | 0.7 | |
|
LEU dose(mg/day) L-dopa (mg/day) |
613.3±379.0 558.6±308.4 |
794.3±292.2 600.0±327.5 |
t=1.8 t=0.3 |
0.085 0.7 |
|
| UPDRS on | 16.5±1.2 | 15.2±1.2 | t=1.1 | 0.2 |
UPDRS = Unified Parkinson’s Disease Rating Scale; LEU = L-dopa equivalent units;
All values are mean ± SD.
Parkinson’s disease patients who underwent deep brain stimulation and were treated with (DBS+DA) and without a dopamine agonist (DBS-DA).
Baseline reaction time
We first analysed baseline RT and found a significant group difference (F2,23=4.1, p=0.027). Post hoc comparison showed that both DBS groups were significantly slower than controls (p<0.001). There was no difference between the two patient groups (p=0.67) (see Figure 3-supplementary material).
Next we analysed errors on the baseline RT task and found a significant group difference (F2,132=5.2, p=0.01). Pairwise comparison showed that controls made significantly less errors than both patient groups (p<0.001). Furthermore, DBS-DA made less errors than DBS+DA (p<0.001) (See Figure 4-supplementary material).
Pixel task
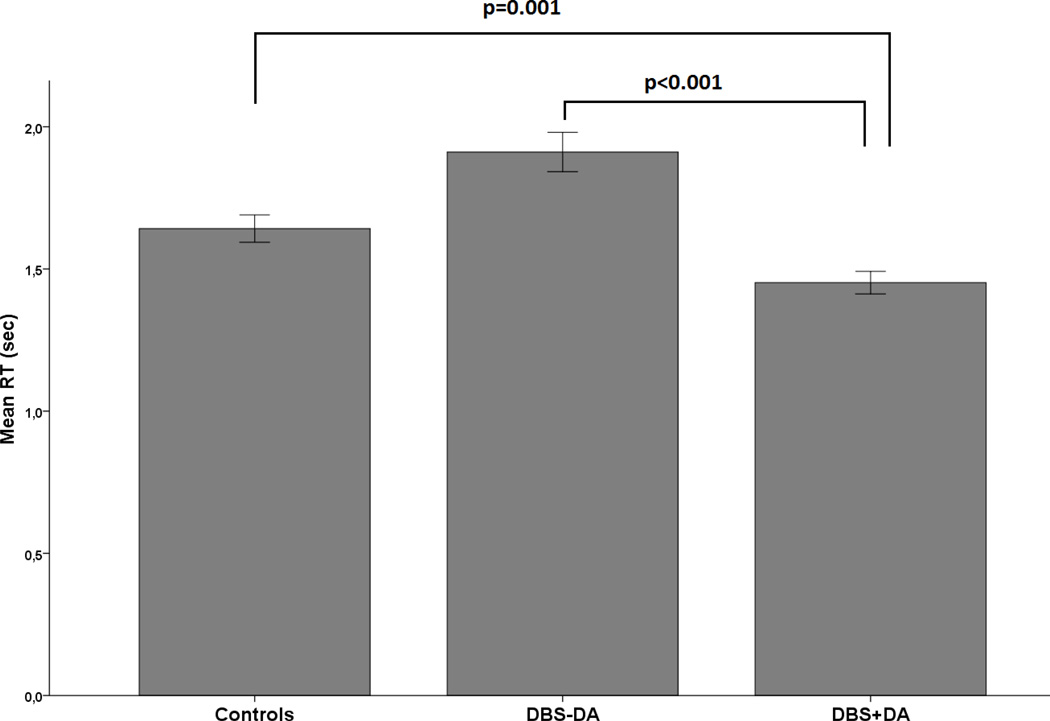
A three (control, DBS-DA, DBS+DA) by three (condition 1(60/40), condition 2(70/30), condition 3(80/20) mixed ANOVA (Greenhouse-Geisser corrected) revealed a significant group difference in RT (F2,153=15.0, p<0.001). Post hoc comparison showed that DBS+DA were significantly faster than controls (p=0.001) and DBS-DA (p<0.001). There was, however, no difference between controls and DBS-DA (p=0.14) (Figure 1). There was also a significant effect of condition (p<0.0001) but no interaction of group and condition (p>0.7).
Figure 1.
Mean reaction time across all trials. Controls, Parkinson’s disease patients, who underwent deep brain stimulation without (DBS-DA) and with dopamine agonist therapy (DBS+DA). All error bars are +/− 1 standard error.
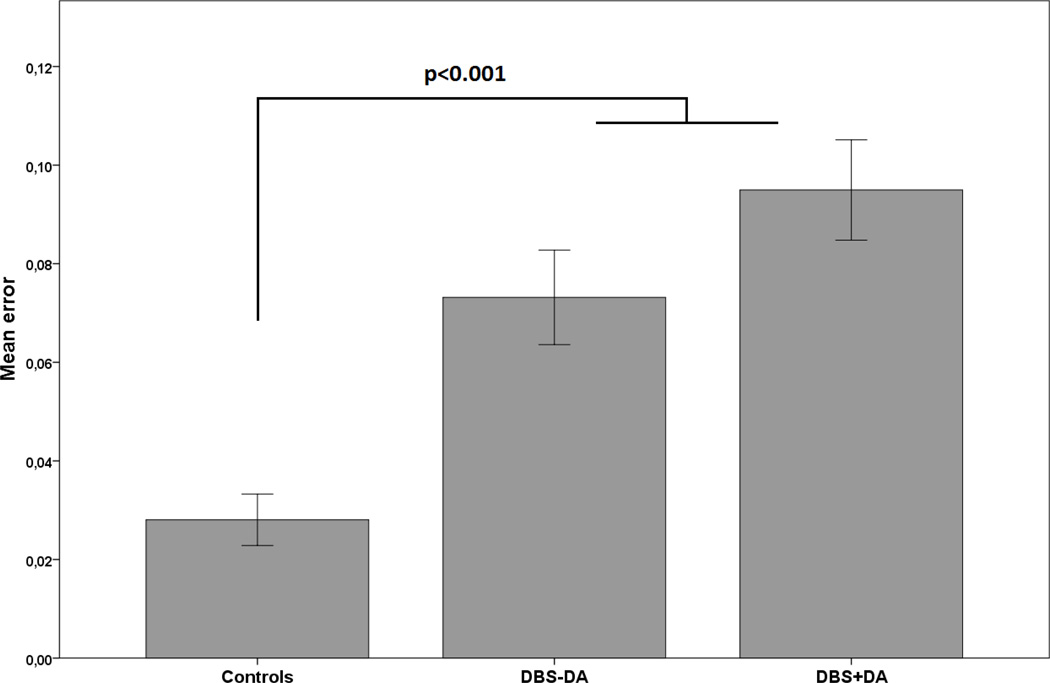
We then examined the total amount of errors and found a significant group effect (F2,60=15.4, p<0.001). Pairwise comparison showed that controls made less errors than DBS-DA (p=0.002) and DBS+DA (p<0.001). There was no difference between the two PD groups (p=0.2) (Figure 2). There was also a significant effect of condition (F2,87=10.9, p<0.001) but no group by condition interaction.
Figure 2.
Mean errors across all trials. All error bars are +/− 1 standard error.
Discussion
We found that all participants were faster and made fewer errors in the easier 70/30 condition. Further, PD patients who underwent bilateral STN-DBS and who were treated with L-dopa in combination with dopamine agonists made significantly faster decisions than STN-DBS patients who were on L-dopa monotherapy and matched volunteers.
Previous reports in PD patients treated with bilateral DBS suggested that STN-DBS in general impairs the ability to slow down during high conflict tasks [2, 13] leading to impulsive choice [7].However, in these studies it is not clear whether PD patients were treated with dopamine agonists. Our findings expand these results as they suggest that dopamine agonist therapy and not STN-DBS is likely responsible for the inability to slow down in high conflict situations.
Further, our results are in line with previous studies showing that PD patients who are treated with dopamine agonists make faster decisions [14] and sample less information, regardless of whether they were treated with bilateral STN-DBS or not [3]. It is possible that dopamine agonists in combination with STN-DBS sensitize brain areas that are involved in reward processing [15] such as the ventral striatum. These higher mesolimbic dopamine levels then cause incentive salience, where previously neutral stimuli trigger motivational value and can lead to faster responses [16, 17].
An alternative explanation is that here we tested PD patients under stable conditions, to more closely resemble their real-life clinical situations, whereas previous tests were done in acute “on/off” changes. Acute changes of STN-DBS increase impulsivity and reduce the ability of slowing down in high conflict situations whereas under stable conditions STN-DBS reduces impulsive action [18].
Further, we found that both PD groups made more errors than controls, but there was no group difference between patients. Preliminary results in a small cohort of DBS patients on the baseline RT task showed that those treated with dopamine agonists made significantly more errors than those not using dopamine agonists, which is generally consistent with our previous study demonstrating poorer task performance in STN-DBS patients on dopamine agonist therapy [3]. It is, however, important to acknowledge that the sample size for the baseline reaction time was too small to draw any definite conclusions.
Whether dopamine agonists reduce accuracy in STN-DBS patients in a simple reaction time tasks needs to be explored in a larger cohort of patients. Accuracy is reduced in PD patients “on” STN-DBS compared to “off” STN-DBS [9] and thus, “off” stimulation testing may have reduced error rates. However, “off medication” testing can cause dysphoria and anxiety [19] which can interfere with task performance.
In summary we have shown distinct differences in perceptual decision making in PD patients treated with STN-DBS depending on whether they were exposed to dopamine agonist therapy or not. It is possible that dopamine agonists in combination with STN-DBS cause sensitization of the mesolimbic dopamine levels resulting in reduced decision threshold in perceptual decision making tasks. As was the case in our cohort, a significant proportion of people with PD undergoing DBS have a younger age of PD onset, which in itself is a risk factor for the development of impulsive-compulsive behaviours (ICBs)[20]. Additionally, younger PD patients are often prescribed dopamine agonists more frequently than L-dopa, and dopamine agonists are generally accepted to be more strongly associated than levodopa with the development of most ICBs. Whether STN-DBS itself is a risk factor or protective factor in the development of ICBs is still unclear. It is, however, important for treating clinicians to be aware of the effects of dopamine agonists on decision-making in PD patients with DBS.
Supplementary Material
Footnotes
The authors report no conflict of interest.
References
- 1.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 2.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 3.Djamshidian A, O'Sullivan S, Foltynie T, Aviles-Olmos I, Limousin P, Noyce A, Zrinzo L, Lees A, Averbeck BB. Dopamine agonists rather than deep brain stimulation cause reflection impulsivity in Parkinson’s disease. Journal of Parkinson's disease. 2013;3:139–144. doi: 10.3233/JPD-130178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moum SJ, Price CC, Limotai N, Oyama G, Ward H, Jacobson C, Foote KD, Okun MS. Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PLoS One. 2012;7:e29768. doi: 10.1371/journal.pone.0029768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lhommee E, Klinger H, Thobois S, Schmitt E, Ardouin C, Bichon A, Kistner A, Fraix V, Xie J, Aya Kombo M, Chabardes S, Seigneuret E, Benabid AL, Mertens P, Polo G, Carnicella S, Quesada JL, Bosson JL, Broussolle E, Pollak P, Krack P. Subthalamic stimulation in Parkinson's disease: restoring the balance of motivated behaviours. Brain. 2012;135:1463–1477. doi: 10.1093/brain/aws078. [DOI] [PubMed] [Google Scholar]
- 6.Lim SY, O'Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O'Sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson's disease. J Clin Neurosci. 2009;16:1148–1152. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Z, Hammer A, Camara E, Munte TF. Pramipexole modulates the neural network of reward anticipation. Hum Brain Mapp. 2011;32:800–811. doi: 10.1002/hbm.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green N, Bogacz R, Huebl J, Beyer AK, Kuhn AA, Heekeren HR. Reduction of influence of task difficulty on perceptual decision making by STN deep brain stimulation. Curr Biol. 2013;23:1681–1684. doi: 10.1016/j.cub.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo M, Lee E, Averbeck BB. Action selection and action value in frontal-striatal circuits. Neuron. 2012;74:947–960. doi: 10.1016/j.neuron.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulthard EJ, Bogacz R, Javed S, Mooney LK, Murphy G, Keeley S, Whone AL. Distinct roles of dopamine and subthalamic nucleus in learning and probabilistic decision making. Brain. 2012;135:3721–3734. doi: 10.1093/brain/aws273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, Fernandez H, Potenza MN, Dolan RJ, Hallett M. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demetriades P, Rickards H, Cavanna AE. Impulse control disorders following deep brain stimulation of the subthalamic nucleus in Parkinson's disease: clinical aspects. Parkinsons Dis. 2011;20:658415. doi: 10.4061/2011/658415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy H, Levy-Gigi E, Somlai Z, Takats A, Bereczki D, Keri S. The effect of dopamine agonists on adaptive and aberrant salience in Parkinson's disease. Neuropsychopharmacology. 2012;37:950–958. doi: 10.1038/npp.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WP. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain. 2010;133:3611–3624. doi: 10.1093/brain/awq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SY, Evans AH, Miyasaki JM. Impulse control and related disorders in Parkinson's disease: review. Ann N Y Acad Sci. 2008;1142:85–107. doi: 10.1196/annals.1444.006. [DOI] [PubMed] [Google Scholar]
- 20.Averbeck BB, O'Sullivan SS, Djamshidian A. Impulsive and compulsive behaviors in Parkinson's disease. Annu Rev Clin Psychol. 2014;10:553–580. doi: 10.1146/annurev-clinpsy-032813-153705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.