Abstract
The study of Epigenetics is intimately linked and inseparable from developmental biology. Many of the genes that imprint epigenetic information on chromatin, function during the specification of cell lineages in the developing embryo. These include the histone methyltransferases and their co-factors of the Polycomb and Trithorax gene families. How histone methylation is established and what regulates the tissue and locus specificity of histone methylation is an emerging area of research. The embryonic kidney is used as a model to understand how DNA binding proteins can specify cell lineages and how such proteins interact directly with the histone methylation machinery to generate a unique epigenome for particular tissues and cell types. In adult tissues, histone methylation marks must be maintained for normal gene expression patterns. In chronic and acute renal disease, epigenetic marks are being characterized and correlated with the establishment of metabolic memory, in part to explain the persistence of pathologies even when optimal treatment modalities are utilized. Thus, the state of the epigenome in adult cells must be considered when attempting to alleviate or alter gene expression patterns in disease.
Introduction
How epigenetic marks are imprinted on the genome of a multi-cellular organism can best be understood within the context of embryonic development where such marks are first established. Indeed, many of the genes and proteins that imprint epigenetic information were first discovered in genetic screens of developing Drosophila embryos, long before the biochemical functions of such proteins were known. The term “epigenetics” is best defined as the study of heritable traits that are not directly encoded within the DNA sequence of the genome. In a conventional sense, heritability refers to the passing of a trait from one organism to its offspring. However, at the cellular level, we often think of specific traits, such as patterns of gene expression or cellular fates, as being passed on from a mother cell to a daughter cell. This heritability or cellular memory is essential to maintaining cell lineages in development and in the stability of the differentiated state. That the differentiated state has innate stability is exemplified by the difficulty and inefficiency of cloning by nuclear transfer [1]. In other words, the genome of a differentiated cell is not easily erased or reprogrammed by the cytoplasmic environment of a stem cell. Thanks to the pioneering work of Yamanaka [2], we now know that the epigenetic imprints of differentiated cells can be erased by a defined set of stem cell factors and selecting for clones whose characteristics resemble embryonic stem cells. Such induced pluripotent stem cells (iPSCs) can be reintroduced into an early embryo and contribute to all cell lineages, in part bypassing the need for creating embryonic stem cells from human embryos, a process fraught with ethical controversy. However, iPS cells are an in vitro phenomenon and do not appear sporadically during development or in normal adult tissues. Rather, the differentiated state is generally stable, even among dividing adult stem cells whose fates are restricted to a particular lineage or among cancer cells that rarely convert to phenotypes outside of their original lineages.
The question then becomes at what point is a cell epigenetically imprinted with a specific fate and what are the molecular mechanisms that remember that fate through hundreds of rounds of cell division? Within the last 15 years, the convergence of two, seemingly disparate, areas of biology have begun to address these questions. The first is the study of chromatin, which is generally defined as the DNA double helix and its associated proteins. The second is the genetic analyses of embryonic development in model organisms as diverse as flies and mice.
The genome within a cell must be tightly packaged to fit within a confined space and yet allow for the myriad of functions, such as transcription, replication, and repair. The DNA double helix is wrapped approximately 1.6 times around a core histone octamer, which consists of 2 molecules of the histones H2A, H2B, H3 and H4, and constitutes the nucleosome. Between each nucleosome is a spacer region, often bound by the histone H1 (Fig. 1). This primary structure is referred to as beads on a string. The genome can be compacted further into higher density nucleosomal arrays to create a fiber. Ultimately, the mitotic chromosome represents the most compacted form of the genome. The realization that histone tails, protruding out of the nucleosome, were subject to post-translational modifications and that distinct modifications correlated with the degree of compaction or higher order chromatin structure was a defining breakthrough in understanding epigenetic imprints [3, 4]. For example, tightly compacted chromatin such as the inactive X-chromosome in females, the micronucleus of Tetrahymena, or yeast telomeres and centromeres all had high levels of histone H3 lysine 9 methylation (H3K9me) [5, 6]. Conversely, chromatin that was accessible, more DNAse sensitive, and often transcriptionally active contained alternate histone marks, including H3K4me and H3K36me. That individual lysine residues could be mono-, di-, or trimethylated further increased the complexity of this potential histone code [7]. Thus, differential histone methylation, particularly for histone H3 and H4, could mark regions of chromatin and compartmentalize the genome into compact or silent domains and accessible or active domains.
Figure 1. The Dynamic Structure of Chromatin.
The basic unit of chromatin is the nucleosome, a histone octamer represented by the tan colored balls on the 11 nm string. The DNA helix is wrapped around the nucleosome and a spacer region, bound with histones H1 or H5, separates adjacent nucleosomes. The histone tails extend out of the nucleosome and can be modified by methylation or acetylation. The beads-on-a-string can condense into a solenoid fiber, a theoretical structure thought to be about 30 nm in diameter. Increased condensation leads to a chromatin fiber, several hundred nm in diameter, and associated with the nuclear scaffold. The mitotic chromosomes are the most compact form of chromatin in the cell.
While the implications for histone methylation in defining chromatin structure and transcriptional activity were apparent, the connection to development and epigenetic imprinting was not clear until the discovery that epigenetic modifier genes discovered in Drosophila encoded proteins with either histone methyltransferase activity or co-factors required for histone methyltransferase activity. Genetic screens identified families of epigenetic regulators that fell into the Polycomb [8] or Trithorax [9] families, depending on whether they repressed or activated homoetic (HOX) gene expression during development (Table 1). That the Polycomb family had histone methyltransferase activities associated with compact and silent chromatin (H3K9me and H3K27me) correlated well with the known biological functions as repressors. Conversely, the Trithorax proteins encoded histone methylation activities generally associated with active gene expression (H3K4me or H3K36me), again consistent with their biological functions as activators. Thus, the convergence of chromatin biochemistry and developmental genetics, laid the foundation for our understanding of epigenetic events that drive cell lineages, gene expression, and cellular memory [10].
Table 1.
The Proteins of the Polycomb and Trithorax Complexes
| Complex | Drosophila | Human | Function |
|---|---|---|---|
| PRC1 | Pc | CBX2 (4,6,7,8) | chromo-domain, methyl-histone binding |
| Ph | PHC(1-3) | zinc-finger | |
| Psc, Su(z)2 | Bmi1, Mel18 | ring domain | |
| Sce (Ring1) | Ring1a,b (Rnf1, 2) | ring domain, E3 ubiquitin ligase | |
| Scm | Scmh1, Scml2 | zinc-finger | |
| PRC2 | E(z) | Ezh1,2 | H3K27 methylase |
| Esc, Escl | Eed | WD40 domains | |
| Su(z)12 | Suz12 | zinc-finger | |
| Caf1 | Rbbp4, 7 | WD40 domains | |
| Jing | Aebp2 | zinc-finger | |
| Trithorax | Trx | Mll1/2 | H3K4 methylase |
| Ash2 | Ash2L | DNA binding | |
| Rbbp5 | Rbbp5 | Mll binding co-factor | |
| WD5 | WDR5 | WD40 domains, H3K4me binding | |
| Dpy30 | hDpy30 | co-factor for H3K4me3 | |
| Menin | Menin | scaffold, recruitment to DNA | |
| Trithorax- related | Trr | Mll3/4 | H3K4 methylase |
| Ash2 | Ash2L | DNA binding | |
| Rbbp5 | Rbbp5 | Mll binding co-factor | |
| WD5 | WDR5 | WD40 domains, H3K4me binding | |
| Dpy30 | hDp3y30 | co-factor for H3K4me3 | |
| PTIP | PTIP (Paxip1) | scaffold, recruitment to DNA | |
| Utx | Utx | H3K27 demethylase |
In addition to lysine methylation, there are many other types of histone modifications, including acetylation, phosphorylation and ubiquitination. In fact, histone acetylation was a well-studied phenomena with respect to gene expression and nucleosome dynamics long before the significance of histone methylation was fully realized. Acetylation neutralizes the positively charged lysine residues and alters the affinity of histones to DNA [11]. Multiple lysine residues can be acetylated, including H3K4, 9, 14, 18, 23 and 27 and H4K5, 8, 12 and 16. Studies in yeast suggested that the total net charge neutralization was most critical for gene expression and not the specific position of the acetylated lysines [12, 13]. Thus reducing the affinity of DNA to the nucleosome could facilitate the dynamic processes of transcription, DNA replication, and DNA repair that require nucleosome displacement or sliding. While acetylation was clearly necessary for gene expression, it is not clear that histone acetylation is a true epigenetic mark. Histone acetylation and deacetylation occurs rapidly in response to gene activation or repression signals such that the half-life of a single acetylation event is estimated to be on the order of minutes [14]. In fact, both acetylases and deacetylases are associated with active genes, suggesting that acetylation-deacetylation may be critical for temporarily loosening the histone-DNA interactions as RNA polymerase complexes elongate down the transcription unit [15, 16]. Thus, whether histone acetylation represents a true epigenetic mark is debatable. Histone acetylation correlates with and is necessary for transcription, but it does not appear to be instructive or heritable. Rather, acetylation appears to be part of the mechanism for unraveling DNA, remodeling nucleosomes, and allowing access. Furthermore, unbiased genetic screens designed specifically to identify epigenetic regulatory genes in Drosophila failed to find histone acetyltransferases or deacetylases, even though multiple histone methylases and their co-factors were identified.
If true epigenetic marks are heritable, the mechanisms of inheritance from mother to daughter cells are still poorly understood. The methylation of DNA at CpG dinucleotides is a hertibable epigenetic mark whose mode of transmission is well studied [17]. The patterns of DNA methylation are replicated due to the semi-conservative nature of DNA strand replication, whereby one methylated strand serves as a template for the newly synthesized strand. However, for histone methylation, the mechanisms of replication are less clear. The most recent data suggests that the histone methyltransferases themselves, but not the methylated histones, are directly associated with the newly synthesized DNA strands and function to rapidly mark the assembled histone octamers [18, 19]. Binding of the Polycomb protein Eed to trimethylated histone H3K27 is thought to mediate this association, providing a mechanism for replicating the pattern of repressive epigenetic marks [20].
Nowhere are the concepts of epigenetics and cellular memory more apparent than in the development of the embryo. Indeed, we know that intrinsic cellular memory is imprinted very early in stem and progenitor cells, long before the eventual cellular phenotype is fully manifested. Yet these stem and progenitor cells often undergo hundreds of rounds of cell division and must still remember their eventual fates. A landmark study in embryonic stem cells showed that many key regulatory genes have low levels of both activating (H3K4me3) and repressive (H3K27me3) epigenetic marker, the so called bivalent chromatin domains [21]. Upon differentiation of ES cells to specific lineages, these marks resolve into either fully activating or fully repressing, depending on the types of progenitor cells made. Thus, histone methylation can compartmentalize the genome into active or silent domains, unique to individual cell lineages. The key question in development is how specific DNA sequences are recognized by the histone methyltransferase complexes, which generally do not contain specific DNA binding proteins. Such questions have begun to be addressed in the developing kidney. Moreover, we now appreciate that epigenetic modifications are can be altered in both chronic and acute disease, the implications of which will be discussed.
The Epigenetics of Kidney Development
The development of the mammalian kidney is a great model system to understand cell lineage specification, complex patterning, and epigenetic events. Furthermore, many of the pathways critical for development also contribute to regeneration of renal cells or to the initiation or progression of renal disease. The adult kidney is composed of a large number of specialized epithelial, stromal and endothelial cells. Renal epithelial and stromal cells share a common lineage that is specified early in development, shortly after gastrulation in a region of mesoderm, called the intermediate mesoderm that lies between the paraxial mesoderm and the lateral plate mesoderm along the medio-lateral axis (Fig. 2A) [22, 23]. The kidney develops sequentially along the anterior posterior axis. Early anterior kidney structures include the pro- and mesonephros. The adult, or metanephric kidney, forms at the posterior end of this intermediate mesoderm through inductive interactions between the ureteric bud, an outgrowth of the nephric duct, and the adjacent metanephric mesenchyme (Fig. 2B). As the ureteric bud invades the mesenchyme it emits signals that promote aggregation of the mesenchyme around the tips of the ureteric bud. Reciprocally, the ureteric bud is induced to undergo repeated branching, with each branch point accumulating more mesenchyme at the tips. The mesenchymal aggregates at the tips, commonly called cap mesenchyme, are the progenitor cells of the nephron. The tip of the branching ureter and its associated cap mesenchyme constitutes a stem cell niche that generates all of the renal epithelial cell types (Fig. 2C). A portion of cap mesenchyme undergoes a mesenchymal to epithelial conversion to create the renal vesicle, a primitive epithelial sphere abutting the stalk of the growing ureteric branching network. Through a series of well-orchestrated morphogenetic events, the renal vesicle forms a cleft, which is invaded by endothelial precursor cells, to create the glomerular tuft (Fig. 2D). On the opposing side, the epithelia fuses with the ureteric stalk derived collecting duct epithelia to create a continuous tubule from proximal to distal ends. Epithelial cells within this newly forming nephron continue to proliferate and lengthen the tubules as the descending and ascending limbs of Henle's loop grow into the medullary zone.
Figure 2. The Development of the Mammalian Kidney.
A) In amniotes, the kidney arises from the intermediate mesoderm, located between the paraxial mesoderm (PM) and the lateral plate mesoderm, shown in a cross section through a mouse embryo at embryonic day 8.5 (E8.5) at approximately the 6th somite. B) A series of images and schematics show the development of the intermediate mesoderm in a mouse embryo from embryonic day 9.5 to 11.5. Pax2 expression is marked by EGFP on the right panels. A single epithelial tube, the nephric duct (nd) extends caudally from the rudimentary pronephros (p). By E10.0, mesonephric tubules (mt) are formed more rostrally and the Pax2 positive metanephric mesenchyme (m) begins to aggregate adjacent to the caudal nephric duct. By E10.5, the ureteric bud (ub) begins to grow out of the posterior nephric duct and invades the metanephric mesenchyme. By E11.5, the ureteric bud has invaded and begins to branch. The cap mesenchyme (cm) is seen condensing around the tips of the branching ureteric bud. C) A higher magnification of the renal stem cell niche is shown schematically, with an adjacent immunostained whole mount on the right. Pax2 is stained in orange, the epithelial basement membrane protein laminin is stained blue, and the ureteric bud specific cytokeratin is green. Expression of Wnt9b and Fgf2 promote induction and survival of the cap mesenchyme, whereas Six2 expression in the mesenchyme promotes self-renewal. A portion of the cap mesenchyme forms the Wnt4 positive cells of the renal vesicle, which abuts the stalk of the growing ureteric bud and begins the development of a new nephron. Stromal cells provide survival and proliferation signals for the cap mesenchyme. D) The renal vesicle forms a proximal cleft, which is invaded by endothelial precursor cells, and fuses with the ureteric stalk at the distal end to form a continuous epithelial lumen. This s-shaped body is patterned along the proximal-distal axis with glomerular podocytes most proximally and distal tubule precursors at the point of fusion. The mature nephron takes shape as the glomerular tuft develops and the proximal and distal tubules proliferate and extend.
A great deal is known regarding the genes and proteins that regulate early kidney development and patterning, much of which is summarized in recent reviews [24, 25] [26]. Indeed, the kidney is among the best characterized developing tissues, with thousands of gene expression patterns catalogued spatially and temporally and searchable on public databases (see: www.gudmap.org). In this article, I will focus only on those genes and pathways that have been linked to epigenetic mechanisms. Induction of the metanephric mesenchyme is mediated by WNT signals emanating from the ureteric bud. However, Saxen [23] noted early on that these inductive signals are permissive, not instructive, meaning that renal epithelial cells are induced regardless of the source of the inductive signal. This implies that the renal progenitors, from the region of intermediate mesoderm, have some instruction already imprinted. If such instructions are epigenetic imprinted, then genes acting very early within the intermediate mesoderm must play a role.
Among the most critical genes that define the intermediate mesoderm and the renal progenitor cells are Osr1, Lhx1, and Pax2/Pax8. Lhx1 null mice lack the nephric duct, although Pax2 expression is observed in cells at the boundary between the paraxial and lateral plate mesoderm shortly after gastrulation [27]. In the intermediate mesoderm, Pax2 expression marks all of the cells fated to become epithelia and is necessary to establish and maintain the epithelial phenotype [28]. Pax2 null mutants do develop a nephric duct [28, 29], but the duct is completely absent in a Pax2/8 double mutant, suggesting that these Pax genes function redundantly in this early IM domain [30]. Pax2/8 double mutants also do not express Lhx1. Mice homozygous for an Osr1 null allele, the expression of which precedes that of Pax2/8, still exhibit nephric duct formation and Pax2 expression in the anterior IM, yet lack more developed mesonephric tubules and the metanephric mesenchyme in the posterior IM [31, 32]. Within the metanephric mesenchyme, Pax2 mutants cannot respond to inductive signals nor does the mutant mesenchyme aggregate into early renal vesicles [29].
The Pax genes encode nuclear, DNA binding proteins that are evolutionary conserved, demarcate boundaries or regions in the embryo, and specify progenitor cells during development. To study early cell lineage specification at the level of chromatin and epigenetic modifications is difficult. The standard tools of genetics are unlikely to yield all the answers because the biological readouts, i.e. the failure to develop, are not very specific. Chromatin structure and gene specific histone modifications cannot be visualized directly in the embryo, as can gene expression patterns for comparison. These problems are not unique to kidney development. To understand the biochemical function of a nuclear protein, its interaction with DNA and with other cellular factors must be defined within the appropriate context. Yet, the context is often inaccessible to standard biochemical purification or manipulation because cells corresponding to that progenitor state, in which these proteins function, are not available as stable lines.
Recent studies indicate that Pax2 may function as part of an epigenetic network that specifies early renal cell lineages. The Pax2 protein contains an amino-terminal DNA binding domain and a carboxy-terminal transactivation domain [33, 34]. This carboxy-terminal domain was found to interact with PTIP (Pax-transactivation domain interacting protein or Paxip1) a BRCT domain protein essential for early embryonic development [35, 36]. The interactions with PTIP, link Pax2 to an MLL3/4 Trithorax-like protein complex that promotes histone H3K4 methylation to mark regions of active chromatin [37]. Thus, Pax2 can provide the DNA binding specificity to recruits the MLL proteins such that positive epigenetic marks are established at genes slated for transcription activation. Similar observations were made with the related protein Pax5, which is essential for specifying B cell fate. In B cells, the Pax/PTIP complex can promote long-distance enhancer and promoter interactions through chromatin looping [38], again suggesting effects on chromatin structure to activate gene expression. However, Pax proteins also interact with other proteins, such as the co-repressor Grg4/Tle4, to recruit Polycomb complexes that establish repressive epigenetic marks on chromatin [39]. Thus, this dual potential for activation and repression may be spatially and temporally regulated by the availability of co-factors. These data suggest that Pax2 may provide some locus and tissue specificity to imprint a kidney specific epigenetic fate by partitioning the genome of the IM into active and inactive domains unique for the renal lineage. Whether H3K4 trimethylation promotes gene expression or merely inhibits Polycomb mediated repression still needs to be clarified, as genetic evidence suggests that repression is the default state in the absence of Trithorax mediated derepression [40]. In any case, more definitive proof of this concept awaits better technology that could characterize chromatin modifications at single genes in a spatial and temporal manner, in small numbers of cells, during development.
A second essential gene for early kidney development is the tumor suppressor WT1. The WT1 gene was identified by positional cloning using pedigrees that carried a familial form of the kidney embryonal carcinoma, Wilm's tumor. Loss of heterozygosity among individuals that carry one mutant WT1 allele is associated with Wilm's tumors, a newborn kidney tumor composed of blastemal and differentiated cell types. However, homozygous null mouse embryos fail to develop any kidneys at all. The WT1 gene is expressed in the early metanephric mesenchyme and is essential for its survival and ability to respond to inductive signals. The WT1 protein may control target gene expression, such as the WNT4 locus, by establishing open and accessible chromatin domains, rather than by direct activation of transcription. Thus, WT1 is implicated in setting chromatin boundaries, marked by specific histone modifications.
Epigenetics and Kidney Disease
The study of epigenetic modifications in renal disease is an emerging area of research that merits significant investment for a variety of reasons. Chronic and acute renal diseases are a major burden on public health. More than 20 million Americans are estimated to have a chronic renal disease. Every year, more than 110,000 patients progress to end-stage renal disease, which requires dialysis. In 2009, the cost of treating end stage disease was more than 40 billion dollars [41]. More than 70% of end stage renal disease is linked directly to diabetes or hypertension. In patients with diabetes, even when blood glucose levels are well controlled, diabetic nephropathy can progress, suggesting that the hyperglycemic environment has altered the gene expression patterns that drive pathogenesis. This process, termed metabolic memory, may involve epigenetic modifications of regulatory domains driving the transcription of key inflammatory genes [42].
In several model systems of diabetes, epigenetic changes correlate with the changes in gene expression that accompany metabolic memory. In Zebrafish, hyperglycemia can be induced by streptozocin, which kills insulin producing beta cells. However after withdrawl and recovery, pancreatic beta cells are regenerated and the normal, euglycemic state is reestablished. Fin regeneration and wound healing are impaired in hyperglycemic fish, but this impairment is not alleviated even in the euglycemic state [43-45]. The altered expression of genes that control repair persists even after the initial insult is removed. Furthermore, such altered gene expression patterns correlate with changes in DNA methylation, strongly suggesting that epigenetic mechanisms maintain the transcriptional program established in the hyperglycemic state. Similar effects of hyperglycemia are observed in diabetic nephropathy but the effects on H3K4me were noted. The association of the lysine demethylase LSD1 at the Sod2 gene persisted in diabetic rats even after establishing glycemic control and this correlated with continued low expression of Sod2 [46, 47]. In kidney disease, DNA methylation has been profiled among diabetic patients with or without nephropathy [48, 49]. In kidney fibrosis, TGF-β increases the expression of the histone methyltransferases Set7/9 which leads to increased H3K4me at critical pathogenic genes [50]. The data suggest that patterns of DNA and histone methylation can persist long after the initial insult, the hyperglycemic state, is alleviated. Similarly, treating hypertension in animal models of metabolic syndrome was not sufficient to reverse all of the epigenetic changes associated with vascular complications [51]. These finding may have broad implications for treatment, as new and better pharmaceuticals are developed that target histone modification or DNA methylation pathways specifically. Thus, treating the initial insult may not be enough to halt disease progression, but resetting the epigenetic imprint may also be required.
Whether chronic renal diseases, in the absence of metabolic syndrome, are impacted by epigenetic mechanisms is also under investigation. Renal interstitial fibrosis is a common pathology, often associated with repeated bouts of acute injury and repair or with reduced nephron numbers [52]. DNA methylation increased in models of renal fibrosis that were in part alleviated by a reduction of the DNA methyltransferase gene Dnmt1 in heterozygous mice [53]. Micro RNAs are also implicated in the progression of renal fibrosis, of which mir-192 appears critical for regulating pro-fibrotic gene expression [54, 55]. However, whether regulation of disease progression by mir-192 is truly an epigenetic phenomenon remains to be determined. Podocyte specific mutations in the H3K4 methyltransferase co-factor PTIP, result in glomerular sclerosis and mesangial expansion, but such mutations in epigenetic pathways have not been found in patient populations [56]. The real problem with chronic disease states is determining whether such epigenetic changes are truly instructive, whether they predetermined the course of the disease, or whether they are merely the result of or indicators of disease progression. This will be an extremely difficult question to address in patient samples, since longitudinal biopsies and control tissues are difficult to collect.
Epigenetics and the Control of Phenotypic Stability in Adult Tissues
Given that histone methylation patterns are imprinted in development, one might ask what roles do such epigenetic pathways play in terminally differentiated, non-dividing cell types? With the exception of adult stem cells, heritability is not an issue in most differentiated adult cell types. If stable epigenetic marks define a genome, one might assume that once the genome is imprinted those enzymes and mechanisms are no longer required for maintaining cellular phenotypes. However, both gain and loss of epigenetic modifiers are associated with a variety of disease states, including cancer. Studies in mice have shown that reducing histone H3K4 methyltransferase activity can alter cellular phenotypes. In the kidney for example, deleting the MLL co-factor PTIP in podocytes leads to changes in gene expression patterns and ultimately a glomerular sclerosis phenotype [56]. Similarly, deletion of PTIP, in cardiac myocytes alters H3K4me3 levels, gene expression patterns, and the electrophysiology, ultimately sensitizing cells to arhythmia [57]. These data suggest that there is a maintenance function to H3K4 methylation that stabilizes the gene expression patterns in terminally differentiated cells. Disruption of such epigenetic pathways could lead to slowly progressing, chronic diseases, as gene expression patterns are destabilized.
Epigenetic regulation may also be important for regeneration of renal epithelial cells after injury. In humans acute renal failure is a common result of nephrotoxicity or ischemia, however the injured kidneys will recover and repopulate the damaged tubules if the degree of injury is limited. The origin of these regenerating proximal tubule cells has been studied in some detail. Recent cell lineage tracing methods demonstrate that adult regenerating proximal tubule cells are derived primarily from preexisting, surviving proximal tubular epithelia and not from a preexisting population of renal stem cells [58, 59]. What promotes these surviving epithelial cells to enter the mitotic cycle and repopulate the damaged tubules? Reactivation of developmental genes such as Pax2 has been described [58, 60], suggesting that an embryonic program may drive regeneration.
The genetics of Polycystic Kidney Disease are particularly illuminating with regard to epigenetic control and regeneration. In mouse models, loss of PKD1 function in dividing cells leads to rapid and early cystogenesis in embryos or post-natal animals. However, if PKD1 is deleted in non-dividing cells of adults, cyst formation is rare and focal [61], suggesting a developmental window in which PKD1 function is essential. Yet subsequent renal injury can promote cyst formation in adults that have deleted PKD1 in adults, after this critical window has passed [62], again suggesting that cell division is necessary for the cystic phenotype. The point is further illustrated in mutations of HNF1β, a critical developmental regulator that activates, among others, the PKD2 gene. If HNF1β is lost in development, PKD2 is not activated and renal cysts form early. However, if HNF1b is lost in adult epithelial cells, PKD2 is unaffected and cyst formation is not observed, suggesting that HNF1β is not necessary to maintain PKD2 expression. Then, if the adult HNF1β mutants are injured, PKD2 is lost in the newly regenerating epithelial cells [63], indicating that HNF1β is needed to reset the epigenetic imprint after mitosis. These elegant experiments point to an epigenetic memory that maintains PKD2 expression in the absence of the activator HNF1β. Re-entry into the cell cycle must reset the epigenetic switch to re-establish the requirement for HNF1β in PKD2 activation. Whether DNA replication and cell division somehow dilute out the epigenetic marks that maintain PKD2 expression remains to be seen. If histone methylation marks are replicated, as DNA and associated methylation at CpG islands, then there would be no need for the activators that reset the marks. However, recent data in Drosophila suggests that it is the proteins and enzymes that set the marks that are bound to chromatin during replication and may be involved in passing the marks onto daughter cells [19].
Conclusions
Epigenetics imprints are established during embryonic development and must be maintained in adult cells for appropriate gene transcription and phenotypic stability. DNA binding proteins that control embryonic patterning and cell lineage specification may be responsible for providing locus and tissue specificity for histone and DNA methylation. Such cell lineage specific epigenomes can explain much about how various cell types respond to similar signals with different outputs. For example, a common nuclear hormone receptor binds almost exclusively to sites on accessible chromatin, but those sites are different in unrelated cell types [64]. Thus, the epigenome encodes a secondary level of information, layered upon the DNA sequence, to compartmentalize the genome into potentially active and silent domains. Common nuclear effectors of signaling pathways, acting upon different epigenomes in different cell types, thus can up or down regulate genes to fine tune expression patterns or respond to external stimuli. Investigations into the mechanisms of transcriptional control have focused primarily upon this final level of up or down regulation. Whether we are talking about normal or pathogenic states, it is becoming increasingly clear that we must consider the epigenome upon which this level of regulation acts.
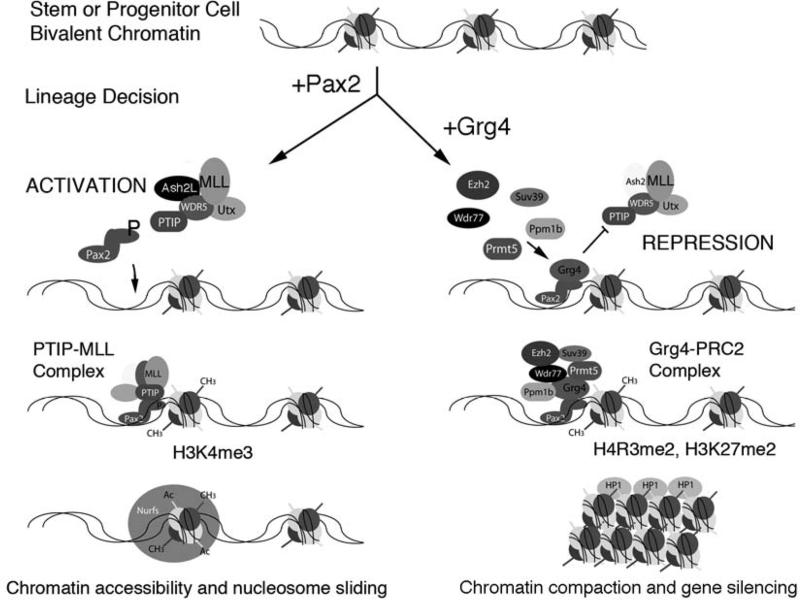
Figure 3. A Chromatin Model for Epigenetic Specification of Cell Lineages.
As cells make lineage decisions, alterations in chromatin structure compartmentalizes the genome into active and inactive domains. In pluripotent embryonic stem (ES) cells, tissue-specific genes and developmental regulators are marked with a bivalent histone code that encompasses low levels of both positive and negative histone methylation marks. As cells become specified and their fates are restricted, cell-type specific DNA binding proteins such as Pax2 could provide locus specificity for the modification of chromatin into active or repressed domains. During intermediate mesoderm specification, the Pax2 protein can interact with a histone H3K4 methyltransferase (PTIP/Mll) complex to prevent repression of kidney specific genes by the Polycomb group (PcG) complexes. High levels of H3K4 trimethylation could then recruit nucleosome remodeling factors (Nurfs) that maintain accessibility of genes and facilitate transcription. In more differentiated cells, increased expression of the repressor Grg4 displaces PTIP and Mll from the Pax2 binding site. Grg4 recruits a histone arginine methyltransferase PRMT and the Polycomb repressor 2 complex (PRC2). PRC2 mediated methylation of histone H3K27 could recruit heterochromatin binding proteins that compact DNA into tightly packaged, silent chromatin. Thus, a single DNA binding protein could recruit either positive or negative epigenetic marks to regions of chromatin. Ash2l, absent small or homeotic like 2; Cbx5, chromobox homolog 5; Kdm6a, 4 lysine (K)-specific demethylase 6A; Mll, mixed-lineage leukemia ; Ptip, Pax trans-activation domain interacting protein; Wdr5, WD repeat domain 5.
Acknowledgments
This work was supported by National Institutes of Health grant DK073722 (G.R.D.) and DK082409 (S.R.P.). We thank the members of our labs for discussion and insights.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest and have read and agree to the journal's authorship agreement.
References
- 1.Narbonne P, Miyamoto K, Gurdon JB. Reprogramming and development in nuclear transfer embryos and in interspecific systems. Curr Opin Genet Dev. 2012;22:450–8. doi: 10.1016/j.gde.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–94. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–3. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 6.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–9. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 7.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–12. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 8.Lanzuolo C, Orlando V. Memories from the polycomb group proteins. Annu Rev Genet. 2012;46:561–89. doi: 10.1146/annurev-genet-110711-155603. [DOI] [PubMed] [Google Scholar]
- 9.Schuettengruber B, Martinez AM, Iovino N, Cavalli G. Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol. 2011;12:799–814. doi: 10.1038/nrm3230. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CL, Fisher AG. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–6. doi: 10.1016/j.gde.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Hong L, Schroth GP, Matthews HR, Yau P, Bradbury EM. Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 “tail” to DNA. The Journal of biological chemistry. 1993;268:305–14. [PubMed] [Google Scholar]
- 12.Dion MF, Altschuler SJ, Wu LF, Rando OJ. Genomic characterization reveals a simple histone H4 acetylation code. Proc Natl Acad Sci U S A. 2005;102:5501–6. doi: 10.1073/pnas.0500136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megee PC, Morgan BA, Smith MM. Histone H4 and the maintenance of genome integrity. Genes Dev. 1995;9:1716–27. doi: 10.1101/gad.9.14.1716. [DOI] [PubMed] [Google Scholar]
- 14.Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci. 2010;35:618–26. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterborg JH. Dynamics of histone acetylation in vivo. A function for acetylation turnover? Biochemistry and cell biology = Biochimie et biologie cellulaire. 2002;80:363–78. doi: 10.1139/o02-080. [DOI] [PubMed] [Google Scholar]
- 17.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Petruk S, Black KL, Kovermann SK, Brock HW, Mazo A. Stepwise histone modifications are mediated by multiple enzymes that rapidly associate with nascent DNA during replication. Nat Commun. 2013;4:2841. doi: 10.1038/ncomms3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petruk S, Sedkov Y, Johnston DM, Hodgson JW, Black KL, Kovermann SK, et al. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell. 2012;150:922–33. doi: 10.1016/j.cell.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, 3rd, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–7. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–29. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 23.Saxen L. Organogenesis of the Kidney. In: Barlow PW, Green PB, White CC, editors. Developmental and Cell Biology Series 19. 19 ed. Cambridge University Press; Cambridge, UK: 1987. [Google Scholar]
- 24.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SR, Dressler GR. The genetics and epigenetics of kidney development. Semin Nephrol. 2013;33:314–26. doi: 10.1016/j.semnephrol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–74. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, et al. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol. 2000;223:77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- 28.Soofi A, Levitan I, Dressler GR. Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys. Dev Biol. 2012;365:241–50. doi: 10.1016/j.ydbio.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–56. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 30.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–70. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–94. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci U S A. 1992;89:1179–83. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechner MS, Dressler GR. Mapping of Pax-2 transcription activation domains. J Biol Chem. 1996;271:21088–93. doi: 10.1074/jbc.271.35.21088. [DOI] [PubMed] [Google Scholar]
- 35.Cho EA, Prindle MJ, Dressler GR. BRCT domain-containing protein PTIP is essential for progression through mitosis. Mol Cell Biol. 2003;23:1666–73. doi: 10.1128/MCB.23.5.1666-1673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechner MS, Levitan I, Dressler GR. PTIP, a novel BRCT domain-containing protein interacts with Pax2 and is associated with active chromatin. Nucleic Acids Res. 2000;28:2741–51. doi: 10.1093/nar/28.14.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SR, Kim D, Levitan I, Dressler GR. The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell. 2007;13:580–92. doi: 10.1016/j.devcel.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab KR, Patel SR, Dressler GR. Role of PTIP in class switch recombination and long-range chromatin interactions at the immunoglobulin heavy chain locus. Mol Cell Biol. 2011;31:1503–11. doi: 10.1128/MCB.00990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SR, Bhumbra SS, Paknikar RS, Dressler GR. Epigenetic mechanisms of Groucho/Grg/TLE mediated transcriptional repression. Mol Cell. 2012;45:185–95. doi: 10.1016/j.molcel.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klymenko T, Muller J. The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins. EMBO Rep. 2004;5:373–7. doi: 10.1038/sj.embor.7400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Systems USRD . USRDS 2009 Annual Reprot: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2009. [Google Scholar]
- 42.Reddy MA, Tak Park J, Natarajan R. Epigenetic modifications in the pathogenesis of diabetic nephropathy. Semin Nephrol. 2013;33:341–53. doi: 10.1016/j.semnephrol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intine RV, Sarras MP., Jr. Metabolic memory and chronic diabetes complications: potential role for epigenetic mechanisms. Curr Diab Rep. 2012;12:551–9. doi: 10.1007/s11892-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen AS, Sarras MP, Jr., Leontovich A, Intine RV. Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression. Diabetes. 2012;61:485–91. doi: 10.2337/db11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarras MP, Jr., Leontovich AA, Olsen AS, Intine RV. Impaired tissue regeneration corresponds with altered expression of developmental genes that persists in the metabolic memory state of diabetic zebrafish. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:320–8. doi: 10.1111/wrr.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowluru RA, Santos JM, Mishra M. Epigenetic modifications and diabetic retinopathy. Biomed Res Int. 2013;2013:635284. doi: 10.1155/2013/635284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong Q, Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol Vis Sci. 2013;54:244–50. doi: 10.1167/iovs.12-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell CG, Teschendorff AE, Rakyan VK, Maxwell AP, Beck S, Savage DA. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genomics. 2010;3:33. doi: 10.1186/1755-8794-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapienza C, Lee J, Powell J, Erinle O, Yafai F, Reichert J, et al. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6:20–8. doi: 10.4161/epi.6.1.13362. [DOI] [PubMed] [Google Scholar]
- 50.Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. Journal of the American Society of Nephrology : JASN. 2010;21:2069–80. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy MA, Sumanth P, Lanting L, Yuan H, Wang M, Mar D, et al. Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int. 2013 doi: 10.1038/ki.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–55. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- 53.Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–50. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–7. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2012;23:458–69. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefevre GM, Patel SR, Kim D, Tessarollo L, Dressler GR. Altering a histone H3K4 methylation pathway in glomerular podocytes promotes a chronic disease phenotype. PLoS Genet. 2010;6:e1001142. doi: 10.1371/journal.pgen.1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein AB, Jones TA, Herron TJ, Patel SR, Day SM, Noujaim SF, et al. Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. The Journal of clinical investigation. 2011;121:2641–50. doi: 10.1172/JCI44641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108:9226–31. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–91. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Imgrund M, Grone E, Grone HJ, Kretzler M, Holzman L, Schlondorff D, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 1999;56:1423–31. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 61.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–5. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet. 2009;18:2523–31. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verdeguer F, Le Corre S, Fischer E, Callens C, Garbay S, Doyen A, et al. A mitotic transcriptional switch in polycystic kidney disease. Nat Med. 2009 doi: 10.1038/nm.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43:264–8. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]