Abstract
The aim was to characterize placental transfer of some congeners of polychlorinated biphenyls (PCBs) and to relate human in utero exposure to these pollutants to their physicochemical properties. We included into the study 1134 births during the period 2002–2003 from two highly PCB contaminated districts in eastern Slovakia. Concentrations of 15 PCB congeners (IUPAC No. 28, 52, 101, 123+149, 118, 114, 153, 105, 138+163, 167, 156+171, 157, 180, 170, and 189) in umbilical cord (C) and maternal serum (M) were determined. The C/M ratios were significantly related, either positively or inversely depending on parameter, to the logarithm of partition coefficient octanol-water (KOW), to fusion enthalpy at the melting point, molecular weight, water solubility, total surface area of the molecule, solvent accessible surface area, melting point, molar volume, and molecular electronegativity distance vector. We found an inverse association between log KOW and lipid adjusted log C/M (const= 1.078, b1 = −0.179, p < 0.001, R2 = 0.039). Parameters evaluated were interrelated except fusion enthalpy at the melting point and electron affinity vs. solubility. We discuss the possible role of cholesterol as a transplacental transporter of PCBs.
Keywords: polychlorinated biphenyls, placenta, placental transfer, partition coefficient octanol water, physicochemical parameters
Introduction
The sensitivity of developing organism to the effects of environmental pollutants during the prenatal period has been amply documented (Fox et al., 2012; Winneke, 2011; Parent et al., 2011; Gore, 2010; Dickerson et al., 2011; Wigle et al., 2008; Sly and Flack, 2008). Polychlorinated biphenyls (PCBs) (ATSDR, 2000) are members of the group of persistent organic pollutants (POPs) and are important with respect to bioaccumulation in environmental media, persistence in the environment and toxic properties. They have been detected in fetuses (De Koning and Karmaus, 2000; Berg et al., 2010) where they can exert adverse effects (Ulbrich and Stahlmann, 2004; Boucher et al., 2009). To reach the fetus they must cross the placenta. PCBs, as a group, easily pass the placental barrier (Park et al., 2008; Linderholm et al., 2007; Correia Carreira et al., 2011; Bergonzi et al., 2009) by simple diffusion due to their electronegativity, high lipophilicity and moderate molecular weight. However PCBs in the environment are a mixture of congeners, each of which is characterized by its own physicochemical properties and toxicity. The knowledge of rules of transplacental transfer is important for protection of developing organism. The speed and the extent of compound-transfer from the maternal to fetal side depend on the physicochemical and structural characteristics of the chemical as well as the physical characteristics of the maternal–placental–embryonic-fetal unit (Giaginis et al., 2009; Giaginis et al., 2011; Myren et al., 2007). Pollutant properties such as molecular weight, lipid solubility and protein binding could also determine the transfer of pollutants from mother to fetus to a great extent (Myllynen et al., 2009). Kinetics of placental transfer of several POPs in humans have only recently been described (Needham et al., 2011; Suzuki et al., 2005; Tsukimori et al., 2013; Porpora et al., 2013), however we did not find any data on correlation of placental transfer of POPs to their physicochemical parameters. In a recent study on placental transfer of POPs any correlation between the maternal/cord serum concentration ratios and chemical properties of these pollutants such as molecular weight, molar volume, number of halogen substituents or log octanol water partition coefficient (Kow) were found (Vizcaino et al. 2014). A close relationship between the physicochemical properties encoded in the molecular structure and the ability of PCBs to mimic natural hormones may reflect toxic responses they elicit in biological systems (Puri et al., 2003). It is known that of these factors the lipophilicity, mostly expressed as the KOW, drives the kinetics of environmental pollutants in many biological systems (Hawker and Connell, 1988; Isnard and Lambert, 1988; Paasivirta et al., 1999; van Gestel et al., 1985; Woodburn et al., 1987). The aim of our study was to determine how is related the placental transfer of individual PCB congeners to their physicochemical properties. Besides transfer by simple diffusion, closely related to lipid solubility, transport of PCBs by carrier lipids was considered. In this connection we discussed which lipid components of serum may be involved in PCB transport across the placenta.
Materials and methods
We included into the study 1134 births during the period 2002–2003 from two districts (Michalovce and Svidnik) in eastern Slovakia highly contaminated by PCBs (Hertz-Picciotto et al., 2003). The characteristics of infants and mothers participating in the study have been described elsewhere (Hertz-Picciotto et al., 2003; Sonneborn et al., 2008; Sonneborn et al., 2008; Park et al., 2008). All women provided written informed consent, and the study protocol was approved by institutional review boards at the University of California-Davis and the Slovak Medical University, Bratislava. Concentrations of 15 PCB congeners (IUPAC No. 28, 52, 101, 105, 114, 118, 123+149, 138+163, 153, 156+171, 157, 167, 170, 180, and 189) in the umbilical cord and maternal serum were determined using solid phase extraction and high resolution gas chromatography with micro electron capture detection as already described (Conka et al., 2005; Petrík et al., 2006). For quality control, a solvent blank and recovery sample (fortified porcine serum) were analyzed with each batch of 10 human serum samples. Recovery was checked using PCB 174 as internal standard and PCB 103 served as a syringe standard. Certified reference material, Mackerel oil (CRM no. 350, Community bureau of reference, Brussels, Belgium) was used to verify the analytical procedure every 3 months as described earlier (Conka et al., 2005). LODs for individual PCB congeners and samples were evaluated using the ratio of noise/peak height in GC chromatogram and standardized for unit sample amount. We restricted our analyses to serum samples with PCB concentrations ≥ LOD. A useful marker of placental transfer of chemicals is the ratio between concentration in cord serum (C) to that in maternal serum (M) (Abballe et al., 2008; Covaci et al., 2002; Patayová et al., 2013; Vizcaino et al. 2014). C/M ratios were calculated for PCB congeners for which a sufficient number of pairs (>133) were available from samples with concentrations ≥ LOD. We considered this number satisfactory as concentrations of PCBs are strongly intercorrelated in maternal and cord serum (Ayotte et al., 2003).
We report both lipid adjusted and wet weight PCB concentrations. We estimated total serum lipids using the enzymatic summation method (Akins et al., 1989). Serum total cholesterol (TC), nonesterified cholesterol (FC), triglycerides (TG), and phospholipids (PL) were assayed by automated, enzymatic methods and total lipids (TL) were calculated from the expression TL = 1.677 * (TC − FC) + FC + TG + PL. We gathered data on physicochemical parameters of individual PCB congeners from available literature sources. We used linear regression to examine the association between log C/M ratio for PCB congeners and their physicochemical parameters (SPSS 16, Softonic International S.L.).
Results
We present information on the number of analyzed serum samples and number of samples with concentrations ≥ LOD and geometric means of lipid adjusted (ng/g lipid) and wet weight (ng/mL) concentrations of PCB congeners in cord and maternal blood serum in Table 1. It can be seen that at the low end of M values, with PCB congeners 28, 52, 101, and 189, the lipid adjusted C values > M values. On the other hand, at the high end of M values, C values < M values. The difference between the wet weight adjusted concentrations in cord blood and maternal blood serum reflects much lower lipid content in cord serum (median 2.46 g/L) compared to maternal serum (median 10.17 g/L). For PCB congeners 105, 114, 123, and 157 the percentage of samples with concentrations below the detection limit was unacceptably high and therefore these congeners were not considered for statistical treatment. For the remaining PCB congeners the geometric means of lipid and wet weight C and M concentrations, ranked in order of increasing values of M.
Table 1.
Numbers of analyzed serum samples from cord and maternal blood and numbers of serum samples with concentrations of PCB congeners ≥ LOD (limit of detection) and geometric means of concentration of PCB congeners in cord and maternal blood serum.
| Mother | Cord | Mother | Cord | Mother | Cord | Mother | Cord | Mother | Cord | |
|---|---|---|---|---|---|---|---|---|---|---|
| PCB congener |
28
|
52
|
101
|
105
|
114
|
|||||
| Total number | 1053 | 1063 | 1094 | 1065 | 1101 | 1078 | 1101 | 1076 | 1101 | 1079 |
| Number ≥ LOD | 409 | 136 | 159 | 133 | 212 | 212 | 207 | 77 | 146 | 47 |
| Concentration ng/g lipids | 9.69 | 9.86 | 5.23 | 7.31 | 6.05 | 8.71 | 6.23 | 5.31 | 4.36 | 5.8 |
| Concentration ng/mL | 0.1 | 0.02 | 0.06 | 0.02 | 0.06 | 0.02 | 0.06 | 0.01 | 0.05 | 0.01 |
| PCB congener |
118
|
123+149
|
138+163
|
153
|
156+171
|
|||||
| Total number | 1101 | 1080 | 1101 | 1079 | 1101 | 1080 | 1101 | 1081 | 1101 | 1080 |
| Number ≥ LOD | 920 | 750 | 154 | 133 | 1101 | 1074 | 1101 | 1079 | 1051 | 729 |
| Concentration ng/g lipids | 12.95 | 8.65 | 3.35 | 4.98 | 93.84 | 77.14 | 146.35 | 109.95 | 13.69 | 6.36 |
| Concentration ng/mL | 0.13 | 0.02 | 0.03 | 0.01 | 0.93 | 0.19 | 1.46 | 0.27 | 0.14 | 0.02 |
| PCB congener |
157
|
167
|
170
|
180
|
189
|
|||||
| Total number | 1101 | 1080 | 1101 | 1080 | 1101 | 1080 | 1101 | 1080 | 1101 | 1080 |
| Number ≥ LOD | 208 | 76 | 571 | 194 | 1099 | 1066 | 1101 | 1079 | 339 | 133 |
| Concentration ng/g lipids | 3.57 | 5.25 | 9.3 | 6.64 | 57.29 | 35.76 | 134.33 | 90.09 | 4.41 | 4.92 |
| Concentration ng/mL | 0.04 | 0.01 | 0.1 | 0.02 | 0.57 | 0.09 | 1.33 | 0.22 | 0.05 | 0.01 |
When searching for the physicochemical parameters we have found data on KOW (Hawker and Connell, 1988; Woodburn et al., 1987; De Bruijn and Hermens, 1990; Li et al., 1992; Lü et al., 2007; Mackay et al., 1992), water solubility (Abramowitz and Yalkowsky, 1990; Dunnivant and Elzerman, 1988; Huang and Hong, 2002; Makino, 1998; Yalkowsky and Valvani, 1979; Yalkowsky et al., 1983), total surface area of the molecule (Hawker and Connell, 1988; De Bruijn and Hermens, 1990; Yalkowsky and Valvani, 1979), solvent accessible surface area (Makino, 1998), electron affinity (Makino, 1998), melting point (Abramowitz and Yalkowsky, 1990; Yalkowsky and Valvani, 1979), enthalpy of fusion at the melting point (Puri et al., 2003), molar volume (Yalkowsky and Valvani, 1979; Huang et al., 1993; Liu et al., 2008; Shiu et al., 1986) and molecular electronegativity distance vector (Liu et al., 2008; Qin et al., 2008) (Table 2).
Table 2.
The values of physicochemical parameters of 15 PCB congeners.
| PCB congener | Log octanol-water partition (Hawker and Connell, 1988) | Solubility (Makino, 1998) | Molecular weight | Total surface area (Hawker and Connell, 1988) | Solvent accessible surface area (Makino, 1998) | Electron affinity (Makino, 1998) | Melting point (Abramowitz and Yalkowsky, 1990) | Fusion enthalpy at melting point (Puri et al., 2003) | Molar volume (Shiu et al., 1986) | Molecular electronegativity distance vector (Qin et al., 2008) |
|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 5.67 | −6.22 | 257.54 | 230.83 | 121.44 | −0.703 | 347 | 24.3 | 247.3 | 4.16 |
| 52 | 5.84 | −7 | 291.99 | 235.84 | 130.98 | −0.49 | 329 | 15.8 | 268.2 | 4.62 |
| 101 | 6.38 | −7.8 | 326.43 | 251.62 | 139.72 | −0.721 | 340 | 27 | 289.1 | 5.23 |
| 105 | 6.65 | −7.52 | 326.43 | 259.41 | 137.6 | −0.855 | 398 | 20.8 | 5.13 | |
| 114 | 6.65 | −7.5 | 326.43 | 259.41 | 137.87 | −0.942 | 392 | 19.9 | 4.97 | |
| 118 | 6.74 | −7.33 | 326.43 | 262.04 | 139.3 | −0.922 | 392 | 20.4 | 289.1 | 5.37 |
| 123+149 | 6.74 | −7.42 | 326.43 | 262.04 | 137.6 | −0.855 | 398 | 26 | 310 | 5.25 |
| 138+163 | 6.83 | −8.38 | 360.90 | 264.76 | 146.86 | −0.747 | 382 | 21 | 310 | 5.58 |
| 153 | 6.92 | −8.49 | 360.88 | 267.39 | 148.61 | −0.773 | 412 | 19.2 | 310 | 5.84 |
| 156+171 | 7.11 | −8.64 | 395.32 | 273.15 | 153.14 | −0.869 | 425 | 310 | 5.56 | |
| 157 | 7.18 | −8.25 | 360.88 | 275.01 | 146.79 | −0.954 | 414 | 29.1 | 310 | 5.6 |
| 167 | 7.27 | −8.21 | 360.88 | 277.64 | 148.25 | −1.009 | 408 | 22.5 | 5.85 | |
| 170 | 7.27 | −8.9 | 395.32 | 277.74 | 154.41 | −0.826 | 405 | 23.1 | 5.72 | |
| 180 | 7.36 | −9.1 | 395.32 | 280.37 | 155.95 | −0.884 | 372 | 22.1 | 6 | |
| 189 | 7.71 | −8.72 | 395.32 | 290.61 | 156.02 | −1.072 | 431 | 31.3 | 6.03 |
We found by bivariate regression analysis (Table 3) that C/M values are statistically significantly related to the physicochemical parameters found, except electron affinity. We illustrate the relationship between lipid adjusted log C/M and log KOW in Figure 1 as this parameter is playing a central role in the behavior of many xenobiotics in biological systems (Hawker and Connell, 1988). It shows that the placental transfer of PCB congeners is decreasing with their increasing lipophilicity (const = 1.078, b1 = −0.179, p < 0.001, R2 = 0.039).
Table 3.
Regression analysis between cord/maternal serum PCB concentration ratios (C/M) and physicochemical parameters characteristic for individual PCB congeners. Included were serum samples with concentrations ≥ LOD (limit of detection) of 11 PCB congeners. R2 stands for coefficient of determination and p for statistical significance.
| Physicochemical parameters | C/M | R2 | Constant | Slope | p |
|---|---|---|---|---|---|
| Log octanol-water partition coefficient | Wet weight | 0.032 | 6.455 | −0.126 | <0.001 |
| Lipid adjusted | 0.014 | 6.425 | −0.010 | <0.001 | |
| Electron affinity | Wet weight | 0.026 | −0.739 | 0.030 | <0.001 |
| Lipid adjusted | 0.014 | −0.732 | 0.003 | <0.001 | |
| Fusion enthalpy at the melting point | Wet weight | 0.016 | 21.012 | 0.652 | <0.001 |
| Lipid adjusted | 0.010 | 21.14 | 0.066 | <0.001 | |
| Melting point | Wet weight | 0.062 | 371.635 | −10.985 | <0.001 |
| Lipid adjusted | 0.035 | 369.27 | −1.047 | <0.001 | |
| Total surface area | Wet weight | 0.032 | 253.787 | −3.673 | <0.001 |
| Lipid adjusted | 0.014 | 252.913 | −0.305 | <0.001 | |
| Molecular weight | Wet weight | 0.019 | 324.314 | −7.361 | <0.001 |
| Lipid adjusted | 0.007 | 322.445 | −0.549 | <0.001 | |
| Solubility | Wet weight | 0.011 | −7.599 | 0.121 | <0.001 |
| Lipid adjusted | 0.003 | −7.566 | 0.008 | <0.001 | |
| Molar volume | Wet weight | 0.019 | 287.812 | −4.466 | <0.001 |
| Lipid adjusted | 0.007 | 286.679 | −0.333 | <0.001 | |
| Solvent accessible surface area | Wet weight | 0.017 | 138.702 | −1.773 | <0.001 |
| Lipid adjusted | 0.006 | 138.245 | −0.129 | <0.001 | |
| Molecular electronegativity distance vector | Wet weight | 0.018 | 31.71 | −0.509 | <0.001 |
| Lipid adjusted | 0.007 | 31.594 | −0.041 | <0.001 |
Figure 1.
Relationship between the logarithm of the ratio of cord/maternal PCB congener lipid adjusted serum concentration (C/M) and log octanol-water partition coefficient (KOW). The numbers in the figure denote PCB congener. The relationship is characterized by slope = −0.179, p < 0.001, R2 = 0.039 and. R2 stands for coefficient of determination and p for significance.
Discussion
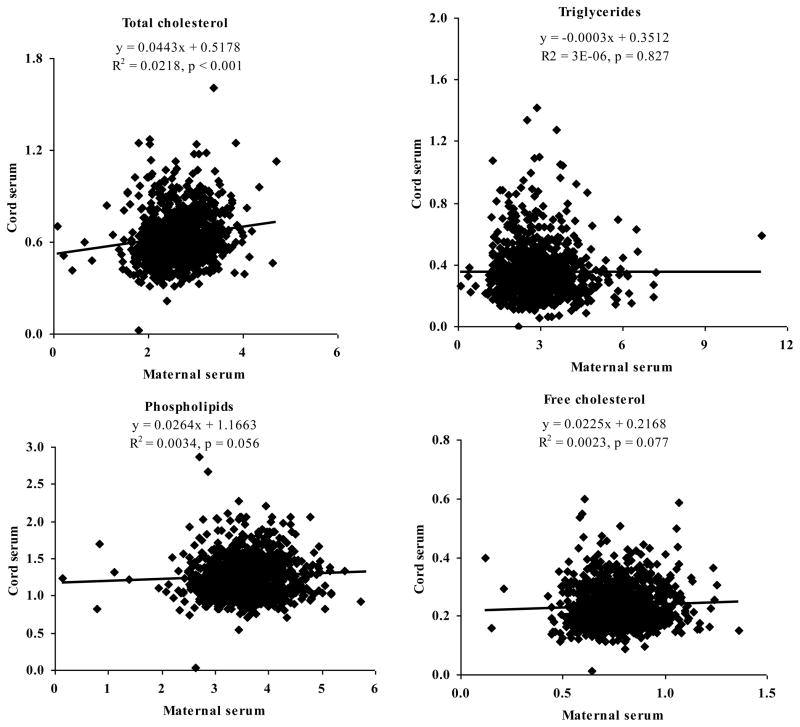
In the current study we have shown that the ratio of cord to maternal serum concentration of PCB congeners is inversely related to their lipophilicity expressed as KOW. We expected a positive relationship between these variables in agreement with rules governing passage of lipid soluble xenobiotics through lipid bilayer membranes (Balaz, 2009) and assumptions on placental transfer of lipophilic substances by diffusion (Suzuki et al., 2005; Tsukimori et al., 2013). We confirmed findings that the overall effect of the physicochemical properties on behavior in biological systems is difficult to predict (Needham et al., 2011) and transfer of PCB congeners from maternal to fetal side is more complex than the simple diffusion of the free fraction governed by parameters as molecular weight, pKa, protein binding and lipid solubility (Pacifici and Nottoli, 1995; Audus, 1999). The predictive potency of lipid solubility has already been questioned in human toxicokinetics (Tonnelier et al., 2012). A clue for explaining this controversy may be at least partly in a variety of transporters expressed in the placenta which can facilitate transfer of xenobiotics (Evseenko et al., 2006). Depending on the localization and function, transporters may either increase or decrease xenobiotic transfer towards fetal circulation (Myllynen and Vähäkangas, 2013; Vähäkangas and Myllynen, 2009). Due to structural resemblance of PCBs to thyroxin the most important candidates are transporters of thyroxin. Thyroid hormone can cross placenta and maternal thyroxin is crucial for normal development of fetal brain and other organs. To cross placenta thyroxin uses various transport mechanisms (Patel et al., 2011; Mortimer et al., 2012; Landers et al., 2013a; 2013b; Landers et al., 2009; Feldt-Rasmussen and Rasmussen, 2007; Richard et al., 2012). With regard to other potential transporters we found data on binding of PCBs to albumin (Han et al., 2013; Becker and Gamble, 1982), serum transport proteins-transthyretin and thyroid-binding globulin (Cheek et al., 1991; Marchesini et al., 2008) and plasma lipoproteins and proteins (Borlakoglu et al., 1990; Becker and Gamble, 1982). Fatty acids have been suggested as a transporter for structurally related dioxins and furans (Koppe et al., 1992). Lipid transport is complex, involves a variety of molecules present on the syncytiotrophoblast surface, and requires their coordinated interaction with other intracellular molecules in different placental cellular compartments. Currently, by far, all of the molecules and processes involved in transplacental lipid transfer have not been identified (Desoye et al., 2011; Larqué et al., 2013; Gil-Sánchez et al., 2012; Herrera et al., 2006). We discuss the hypothesis of transplacental transport by lipids carrying PCB molecules by analyzing behavior of concentration of serum lipid components in maternal and infant’s blood in Figure 2 and Table 4. From the lipid components only total cholesterol in cord serum significantly correlated with that in maternal serum (rs=0.045, p<0.001) and can thus be considered as transporter of PCBs.
Figure 2.
Relationship between concentration (g/L) of main lipid components in cord blood serum and in maternal blood serum.
Table 4.
Descriptive statistics on concentration of main lipid components (g/L) in cord and mother serum.
| Total cholesterol g/L | Phospholipids g/L | Free cholesterol g/L | Triglycerides g/L | Total lipids g/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cord | Mother | Cord | Mother | Cord | Mother | Cord | Mother | Cord | Mother | |
| Number | 1075 | 1066 | 1075 | 1067 | 1075 | 1067 | 1075 | 1067 | 1065 | 1065 |
| Mean | 0.63 | 2.61 | 1.26 | 3.55 | 0.23 | 0.77 | 0.35 | 2.83 | 2.52 | 10.23 |
| Standard Deviation | 0.16 | 0.53 | 0.26 | 0.58 | 0.07 | 0.15 | 0.17 | 1.01 | 0.52 | 1.98 |
| Median | 0.62 | 2.57 | 1.23 | 3.54 | 0.22 | 0.76 | 0.31 | 2.70 | 2.46 | 10.17 |
| Minimum | 0.02 | 2.70 | 0.03 | 0.15 | 0.01 | 0.12 | 0.05 | 0.10 | 0.13 | 1.75 |
| Maximum | 1.61 | 4.71 | 2.87 | 5.72 | 0.60 | 1.36 | 2.02 | 11.05 | 5.34 | 20.17 |
| Geometric Mean | 0.62 | 2.54 | 1.23 | 3.49 | 0.23 | 0.76 | 0.32 | 2.65 | 2.47 | 10.03 |
The C/M ratio, a marker of placental transfer of PCBs used in this study, suffers from potential disadvantage as inter-individual variations due to endogenous and/or exogenous factors. Indeed in our previous study (Patayová et al., 2013) we have shown that the anthropometric, socioeconomic, and maternal health factors are associated with functioning of the important part of the body system, the placenta. The strong interrelation between the different physicochemical parameters makes difficult the identification of a “primer” association between one of them and the measured outcomes (materno-fetal transfer rate). With this objective we analyzed our data with multiple linear regression. However interrelation between variables precluded to obtain valid results. Extremely high values of the Variance Inflation Factor (how2stats, 2011) confirmed multicollinearity. Lipophilicity determines the behavior of many chemicals in biological systems (Balaz, 2009) and in agreement bioconcentration of PCBs in environmental media is dependent on KOW values (Eisler and Belisle, 1996; Paasivirta et al., 1999; Hawker and Connell, 1985a; 1985b; Noegrohati and Hammers, 2008; Padmanabhan et al., 2006; Hope et al., 1998). The role of KOW in functioning of the blood-brain barrier, one of the most important and sophisticated biological systems, was confirmed in a recently published model relating transfer parameters across it to the physical chemical properties of 70 structurally diverse compounds (Zhang et al., 2010). For placenta a similar general model has not yet been published. Moreover, most modeling efforts have focused on placental drug transfer (Hutson, 2011; Hutson et al., 2011) with less attention to environmental toxins (Needham et al., 2011).
Our main finding that the transfer of PCB congeners across human placenta decreases with increasing KOW contrasts to enhanced passage of substances across the blood-brain barrier (Hou and Xu, 2003; Levin, 1980) with increasing KOW. It has to be noted however, that KOW of substances studied in blood brain barrier transfer were several orders of magnitude smaller than KOWs of PCB congeners evaluated currently.
There is an indication from our data that PCB congeners occurring in serum at low concentrations in a typically environmental PCB congener mixture have a C/M ratio >1 compared to more abundant congeners with a C/M ratio <1. However, the significance of this observation may be questioned due to detection limits in the current study. A similar greater exposure to offspring from lower than from higher doses has been described (Chen et al., 2001) for placental transfer of non-ortho substituted PCB congeners.
Our observation on inverse relationship between transfer of PCB congeners through the human placental barrier and lipophilicity, is in agreement with PCB mother fetus transfer data in marine mammals. In grey seal (Halichoerus grypus) the transfer from inner blubber to maternal serum was selective and strongly depended on the log Kow value of the compounds, with less lipophilic compounds being more efficiently released. These results indicate that compounds with a high log Kow and thus with a high lipophilicity are less easily transferred into the bloodstream (van den Berghe et al., 2012). In southern elephant seals (Mirounga leonina) lactational transfer rates were dependent on the log Kow values of the analytes measured, less lipophilic compounds being more readily transferred to the pups by the lactational route (Miranda Filho et al., 2009). In sea lions the fetus blubber to mother partition ratio of PCBs decreased with increasing KOW (Greig et al., 2007). In common dolphin the percentage of transfer declines inversely with the number of chlorines paralleled by increase of lipophilicity (Borrell and Aguilar, 2005). In a study on arctic beluga whales (Delphinapterus leucas) a single physicochemical parameter, log KOW, largely explained the transplacental transfer for PCBs with congeners having a log KOW <6.5 preferentially transferred to the fetus (Desforges et al., 2012). This parameter has also been examined in Zebrafish (Brachydanio rerio), in which a curvilinear relationship was observed between bioconcentration of PCBs and log KOW based upon data covering the log KOW range 5.06–8.18. Highest bioconcentration resulted at about a log KOW of 7.38, above which the degree of bioconcentration decreased (Fox et al., 1994). The physicochemical background to these events can be found in the diffusion limitation of the exchange between adipose and blood, which steeply decreased as a function of the KOW for the 13 PCBs studied (Levitt, 2010). In agreement with our findings was found a decrease of partition ratio between lipid-based concentrations of PCB congeners in milk and maternal serum in regard to the number of chlorine substitutions of each congener measured (Needham et al., 2011). A similar trend was observed with cord/maternal ratios (Vizcaino et al., 2014).
That PCB congener specific C/M values are related to several physicochemical parameters was expected as many physicochemical parameters of PCB congeners are interrelated (Hawker and Connel, 1988; De Bruijn and Hermens, 1990; Shiu and Mackay, 1986; Patil, 1991; Mackay et al., 1980; Inoue et al., 2006; Miller et al., 1985; Banerjee et al., 1980; Chiou et al., 2005; Opperhuizen et al., 1988; Silla et al., 1992). We confirmed this by pairwise Spearman rank correlation (Table 5) except electron affinity and fusion enthalpy at the melting point which were partly or completely unrelated to other parameters.
Table 5.
Pairwise Spearman rank correlation between physicochemical parameters characterizing 15 PCB congeners. N stands for number, rs for correlation coefficient and p for statistical significance.
| Physico-chemical parameters | Molecular weight | Log octanol- water partition coefficient | Solubility | Total surface area | Solvent accessible surface area | Electron affinity | Melting point | Fusion enthalpy at the melting point | Molar volume | Molecular electronegativity distance vector | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | N | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 14 | 9 | 15 |
| rs | 0.908 | −0.962 | 0.916 | 0.955 | −0.446 | 0.607 | 0.284 | 0.862 | 0.855 | ||
| p | . | <0.001 | <0.001 | <0.001 | <0.001 | 0.095 | 0.016 | 0.326 | 0.003 | <0.001 | |
| Log octanol-water partition coefficient | N | 15 | 15 | 15 | 15 | 15 | 15 | 14 | 9 | 15 | |
| rs | −0.856 | 0.999 | 0.909 | −0.667 | 0.683 | 0.320 | 0.880 | 0.956 | |||
| p | . | <0.001 | <0.001 | <0.001 | 0.007 | 0.005 | 0.265 | 0.002 | <0.001 | ||
| Solubility | N | 15 | 15 | 15 | 15 | 15 | 14 | 9 | 15 | ||
| rs | −0.866 | −0.951 | 0.336 | −0.522 | −0.244 | −0.844 | −0.839 | ||||
| p | . | <0.001 | <0.001 | 0.221 | 0.046 | 0.401 | 0.004 | <0.001 | |||
| Total surface area | N | 15 | 15 | 15 | 15 | 14 | 9 | 15 | |||
| rs | 0.913 | −0.652 | 0.681 | 0.322 | 0.880 | 0.952 | |||||
| p | . | <0.001 | 0.008 | 0.005 | 0.262 | 0.002 | <0.001 | ||||
| Solvent accessible surface area | N | 15 | 15 | 15 | 14 | 9 | 15 | ||||
| rs | −0.463 | 0.573 | 0.257 | 0.780 | 0.912 | ||||||
| p | . | 0.082 | 0.026 | 0.374 | 0.013 | <0.001 | |||||
| Electron affinity | N | 15 | 15 | 14 | 9 | 15 | |||||
| rs | −0.659 | −0.306 | −0.633 | −0.565 | |||||||
| p | . | 0.008 | 0.288 | 0.067 | 0.028 | ||||||
| Melting point | N | 15 | 14 | 9 | 15 | ||||||
| rs | 0.326 | 0.780 | 0.608 | ||||||||
| p | . | 0.255 | 0.013 | 0.016 | |||||||
| Fusion enthalpy at the melting point | N | 14 | 8 | 14 | |||||||
| rs | 0.255 | 0.297 | |||||||||
| p | . | 0.542 | 0.303 | ||||||||
| Molar volume | N | 9 | 9 | ||||||||
| rs | 0.844 | ||||||||||
| p | . | 0.004 | |||||||||
| Molecular electronegativity distance vector | N | 15 | |||||||||
| rs | |||||||||||
| p | . |
Acknowledgments
This project has been funded by the U.S. National Institutes of Health grants R01-CA96525 and K12-ES019852, the European Commission through the 7FP project OBELIX (No. 227391) and the project “Center of Excellence of Environmental Health”, ITMS No. 26240120033, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund.
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abballe A, Guarino M, Taggi F, Traina ME, Urbani E, Valentini S, De Felip E. Maternal blood levels of persistent organic pollutants can be used to estimate in utero exposure. Ann Ist Super Sanita. 2008;44:281–291. [PubMed] [Google Scholar]
- Abramowitz R, Yalkowsky SH. Estimation of aqueous solubility and melting point of PCB congeners. Chemosphere. 1990;21:1221–1229. [Google Scholar]
- Agency for Toxic Substances and Disease Registry U.S. Department of Health and Human Services Public Health Service. Toxicological profile for polychlorinated biphenyls (PCBs) 2000 Nov; Available on: http://www.atsdr.cdc.gov/toxprofiles/tp17.pdf. [PubMed]
- Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Audus KL. Controlling drug delivery across the placenta. Eur J Pharm Sci. 1999;8:161–165. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Inuit Cohort Study. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit Cohort Study. Environ Health Perspect. 2003;111:1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaz S. Modeling kinetics of subcellular disposition of chemicals. Chem Rev. 2009;109:1793–1899. doi: 10.1021/cr030440j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Yalkowsky SH, Valvani SC. Water solubility and octanol-water partition coefficient of organics: limitations of the solubility-partition coefficient correlation. Environ Sci Technol. 1980;14:1227–1229. [Google Scholar]
- Becker MM, Gamble W. Determination of the binding of 2,4,5,2′,4′,5′-hexachlorobiphenyl by low density lipoprotein and bovine serum albumin. J Toxicol Environ Health. 1982;9:225–234. doi: 10.1080/15287398209530157. [DOI] [PubMed] [Google Scholar]
- Berg V, Lyche JL, Gutleb AC, Lie E, Skaare JU, Aleksandersen M, Ropstad E. Distribution of PCB 118 and PCB 153 and hydroxylated PCB metabolites (OH-CBs) in maternal, fetal and lamb tissues of sheep exposed during gestation and lactation. Chemosphere. 2010;80:1144–1150. doi: 10.1016/j.chemosphere.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Bergonzi R, Specchia C, Dinolfo M, Tomasi C, De Palma G, Frusca T, Apostoli P. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: data from an Italian polluted urban area. Chemosphere. 2009;76:747–754. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Borlakoglu JT, Welch VA, Wilkins JP, Dils RR. Transport and cellular uptake of polychlorinated biphenyls (PCBs)--I. Association of individual PCB isomers and congeners with plasma lipoproteins and proteins in the pigeon. Biochem Pharmacol. 1990;15:265–272. doi: 10.1016/0006-2952(90)90687-g. [DOI] [PubMed] [Google Scholar]
- Borrell A, Aguilar A. Mother-Calf Transfer of Organochlorine Compounds in the Common Dolphin (Delphinus delphis) Bull Environ Contam Toxicol. 2005;75:149–156. doi: 10.1007/s00128-005-0731-y. [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek AO, Kow K, Chen J, McLachlan JA. Potential mechanisms of thyroid disruption in humans: interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ Health Perspect. 1999;107:273–278. doi: 10.1289/ehp.99107273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hamm JT, Hass JR, Birnbaum LS. Disposition of polychlorinated dibenzo-p-dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in pregnant Long Evans rats and the transfer to offspring. Toxicol Appl Pharmacol. 173:65–88. doi: 10.1006/taap.2001.9143. 200F1. [DOI] [PubMed] [Google Scholar]
- Chiou CT, Schmedding DW, Manes M. Improved prediction of octanol-water partition coefficients from liquid-solute water solubilities and molar volumes. Environ Sci Technol. 2005;39:8840–8846. doi: 10.1021/es050729d. [DOI] [PubMed] [Google Scholar]
- Conka K, Drobna B, Kocan A, Petrık J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J Chromatogr A. 2005;1084:33–38. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Correia Carreira S, Cartwright L, Mathiesen L, Knudsen LE, Saunders M. Studying placental transfer of highly purified non-dioxin-like PCBs in two models of the placental barrier. Placenta. 2011;32:283–291. doi: 10.1016/j.placenta.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distributionof PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298:45–53. doi: 10.1016/s0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- De Bruijn J, Hermens J. Relationships between octanol/water partition coefficients and total molecular surface area and total molecular volume of hydrophobic organic chemicals. Quant Struct-Act Relat. 1990;9:11–21. [Google Scholar]
- DeKoning EP, Karmaus W. PCB exposure in utero and via breast milk. A review. J Expo Anal Environ Epidemiol. 2000;10:285–293. doi: 10.1038/sj.jea.7500090. [DOI] [PubMed] [Google Scholar]
- Desforges JP, Ross PS, Loseto LL. Transplacental transfer of polychlorinated biphenyls and polybrominated diphenyl ethers in arctic beluga whales (Delphinapterus leucas) Environ Toxicol Chem. 2012;31:296–300. doi: 10.1002/etc.750. [DOI] [PubMed] [Google Scholar]
- Desoye G, Gauster M, Wadsack C. Placental transport in pregnancy pathologies. Am J Clin Nutr. 2011;94:1896S–1902S. doi: 10.3945/ajcn.110.000851. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnivant FM, Elzerman AW. Aqueous solubility and Henry’s law constant data for PCB congeners for evaluation of quantitative structure-property relationships (QSPRs) Chemosphere. 1988;17:525–541. [Google Scholar]
- Eisler R, Belisle AA. US National Biological Service Biological Report. Vol. 31. U.S. Department of the Interior, National Biological Service; Washington, D.C. 20240: 1996. Planar PCB hazards to fish, wildlife, and invertebrates: a synoptic review. Available on: http://www.pwrc.usgs.gov/infobase/eisler/chr_31_planar_pcbs.pdf. [Google Scholar]
- Evseenko D, Paxton JW, Keelan JA. Active transport across the human placenta: impact on drug efficacy and toxicity. Expert Opin Drug Metab Toxicol. 2006;2:51–69. doi: 10.1517/17425255.2.1.51. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen U, Rasmussen ÅK. Thyroid hormone transport and actions. Pediatric and Adolescent Medicine. 2007;11:80–103. [Google Scholar]
- Fox DA, Grandjean P, de Groot D, Paule MG. Developmental origins of adultdiseases and neurotoxicity: epidemiological and experimental studies. Neurotoxicology. 2012;33:810–816. doi: 10.1016/j.neuro.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Zauke GP, Butte W. Kinetics of bioconcentration and clearance of 28 polychlorinated biphenyl congeners in zebrafish (Brachydanio rerio) Ecotoxicol Environ Saf. 1994;28:99–109. doi: 10.1006/eesa.1994.1038. [DOI] [PubMed] [Google Scholar]
- Giaginis C, Zira A, Theocharis S, Tsantili-Kakoulidou A. Application of quantitative structure-activity relationships for modeling drug and chemical transport across the human placenta barrier: A multivariate data analysis approach. J Appl Toxicol. 2009;29:724–733. doi: 10.1002/jat.1466. [DOI] [PubMed] [Google Scholar]
- Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Assessing drug transport across the human placental barrier: from in vivo and in vitro measurements to the ex vivo perfusion method and in silico techniques. Curr Pharm Biotechnol. 2011;12:804–813. doi: 10.2174/138920111795470930. [DOI] [PubMed] [Google Scholar]
- Gil-Sánchez A, Koletzko B, Larqué E. Current understanding of placental fatty acid transport. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15:265–272. doi: 10.1097/MCO.0b013e3283523b6e. [DOI] [PubMed] [Google Scholar]
- Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones (Athens) 2010;9:16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig DJ, Ylitalo GM, Hall AJ, Fauquier DA, Gulland F. Transplacental transfer of organochlorines in California sea lions (Zalophus californianus) Environ Toxicol Chem. 2007;26:37–44. doi: 10.1897/05-609r.1. [DOI] [PubMed] [Google Scholar]
- Han C, Fang S, Cao H, Lu Y, Ma Y, Wei D, Xie X, Liu X, Li X, Fei D, Zhao C. Molecular interaction of PCB153 to human serum albumin: Insights from spectroscopic and molecular modeling studies. Journal of Hazardous Materials. 2013;248–249:313–321. doi: 10.1016/j.jhazmat.2012.12.056. [DOI] [PubMed] [Google Scholar]
- Hawker DW, Connell DW. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environ Sci Technol. 1988;22:382–387. doi: 10.1021/es00121a006. [DOI] [PubMed] [Google Scholar]
- Hawker DW, Connell DW. Prediction of bioconcentration factor under non-equilibrium conditions. Chemosphere. 1985a;14:1835–1843. [Google Scholar]
- Hawker DW, Connell DW. Relationships between partition coefficient, uptake rate constant, clearance rate constant and time to equilibrium for bioaccumulation. Chemosphere. 1985b;14:1205–1219. [Google Scholar]
- Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res. 2006;65:59–64. doi: 10.1159/000091507. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kocan A, Charles MJ, Ciznar P, Langer P, Sovcikova E, James R. PCBs and early childhood development in Slovakia: Study design and background. Fresen Environ Bull. 2003;12:208–214. [Google Scholar]
- Hope B, Scatolini S, Titus E. Bioconcentration of chlorinated biphenyls in biota from the North Pacific Ocean. Chemosphere. 1998;36:1247–1261. doi: 10.1016/s0045-6535(97)10045-5. [DOI] [PubMed] [Google Scholar]
- Hou TJ, Xu XJ. ADME Evaluation in Drug Discovery. 3 Modeling Blood-Brain Barrier Partitioning Using Simple Molecular Descriptors. J Chem Inf Comput Sci. 2003;43:2137–2152. doi: 10.1021/ci034134i. [DOI] [PubMed] [Google Scholar]
- how2stats. Statisticians do it better p < .05. SPSS, Variance Inflation Factor (VIF) http://www.how2stats.net/2011/09/variance-inflation-factor-vif.html.
- Huang Q, Hong CS. Aqueous solubilities of non-ortho and mono-ortho PCBs at four temperatures. Water Research. 2002;36:3543–3552. doi: 10.1016/s0043-1354(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Huang G, Rengao S, Shugui D. Prediction of aqueous solubility of PCBs based on molecular structure. J Env Sci. 1993;5:30–36. [Google Scholar]
- Hutson JR. Prediction of placental drug transfer using the human placental perfusion model. J Popul Ther Clin Pharmacol. 2011a;18:e533–e543. [PubMed] [Google Scholar]
- Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011b;90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, Takasuga T, Senthilkumar K, Yamashita F, Koizumi A. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ Health Perspect. 2006;114:1179–1185. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard P, Lambert S. Estimating bioconcentration factors from octanol-water partition coefficient and aqueous solubility. Chemosphere. 1998;17:21–34. [Google Scholar]
- Koppe JG, Olie K, van Wijnen J. Placental transport of dioxins from mother to fetus. II PCBs, dioxins and furans and vitamin K metabolism. Dev Pharmacol Ther. 1992;18:9–13. [PubMed] [Google Scholar]
- Landers KA, McKinnon BD, Li H, Subramaniam VN, Mortimer RH, Richard K. Carrier-mediated thyroid hormone transport into placenta by placental transthyretin. Journal of Clinical Endocrinology and Metabolism. 2009;94:2610–2616. doi: 10.1210/jc.2009-0048. [DOI] [PubMed] [Google Scholar]
- Landers KA, Mortimer RH, Richard K. Transthyretin and the human placenta. Placenta. 2013a;34:513–517. doi: 10.1016/j.placenta.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Landers KA, Li H, Subramaniam VN, Mortimer RH, Richard K. Transthyretin-thyroid hormone internalization by trophoblasts. Placenta. 2013b;34:716–718. doi: 10.1016/j.placenta.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Larqué E, Ruiz-Palacios M, Koletzko B. Placental regulation of fetal nutrient supply (2013) Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16:292–297. doi: 10.1097/MCO.0b013e32835e3674. [DOI] [PubMed] [Google Scholar]
- Levin VA. Relationship of Octanol/Water Partition Coefficient and Molecular Weight to Rat Brain Capillary Permeability. J Med Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- Levitt DG. Quantitative relationship between the octanol/water partition coefficient and the diffusion limitation of the exchange between adipose and blood. BMC Clin Pharmacol. 2010;10:1. doi: 10.1186/1472-6904-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Doucette WJ, Andren AW. Solubility of polychlorinated biphenyls in binary water/organic solvent systems. Chemosphere. 1992;24:1347. [Google Scholar]
- Linderholm L, Park JS, Kocan A, Trnovec T, Athanasiadou M, Bergman K, Hertz-Picciotto I. Maternal and cord serum exposure to PCB and DDE methyl sulfone metabolites in eastern Slovakia. Chemosphere. 2007;69:403–410. doi: 10.1016/j.chemosphere.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Qin LT, Liu HL, Yin DQ. Molecular electronegativity distance vector model for the prediction of bioconcentration factors in fish. J Mol Model. 2008;14:83–92. doi: 10.1007/s00894-007-0255-y. [DOI] [PubMed] [Google Scholar]
- Lü W, Chen Y, Liu M, Chen X, Hu Z. QSPR prediction of n-octanol/water partition coefficient for polychlorinated biphenyls. Chemosphere. 2007;69:469–478. doi: 10.1016/j.chemosphere.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Mackay D, Shin WY, Ma KC. Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals. I. Chelsea, Michigan: Lewis Publishers, Inc; 1992. [Google Scholar]
- Mackay D, Mascarenhas R, Shiu WY. Aqueous solubility of polychlorinated biphenyls. Chemosphere. 1980;9:257–264. [Google Scholar]
- Makino M. Prediction of aqueous solubility coefficients of polychlorinated biphenyls by use of computer-calculated molecular properties. Environ Int. 1998;24:653–663. [Google Scholar]
- Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;232:150–160. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Miller MM, Wasik SP, Huang GL, Shiu WY, Mackay D. Relationships between octanol-water partition coefficient and aqueous solubility. Environ Sci Technol. 1985;19:522–529. doi: 10.1021/es00136a007. [DOI] [PubMed] [Google Scholar]
- Miranda Filho KC, Metcalfe CD, Metcalfe TL, Muelbert MM, Robaldo RB, Martinez PE, Colares EP, Bianchini A. Lactational transfer of PCBs and chlorinated pesticides in pups of southern elephant seals (Mirounga leonina) from Antarctica. Chemosphere. 2009;75:610–616. doi: 10.1016/j.chemosphere.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Mortimer RH, Landers KA, Balakrishnan B, Li H, Mitchell MD, Patel J, Richard K. Secretion and transfer of the thyroid hormone binding protein transthyretin by human placenta. Placenta. 2012;33:252–256. doi: 10.1016/j.placenta.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Myllynen P, Vähäkangas K. Placental transfer and metabolism: an overview of the experimental models utilizing human placental tissue. Toxicol In Vitro. 2013;27:507–512. doi: 10.1016/j.tiv.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Myren M, Mose T, Mathiensen L, Knudsen LE. The human placenta– an alternative for studying foetal exposure. Toxicol in Vitro. 2007;21:1332–1340. doi: 10.1016/j.tiv.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, Jr, Sjödin A, Turner WE, Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegrohati S, Hammers WE. Regression models for octanol-water partition coefficients, and for bioconcentration in fish. Toxicol Environ Chem. 2008;34:155–173. [Google Scholar]
- Opperhuizen A, Gobas FAPC, van der Steen JMD, Hutzinger O. Aqueous solubility of polychlorinated biphenyls related to molecular structure. Environ Sci Technol. 1988;22:638–646. [Google Scholar]
- Paasivirta J, Sinkkonen S, Mikkelson P, Rantio T, Wania F. Estimation of vapor pressures, solubilities and Henry’s law constants of selected persistent organic pollutants as functions of temperature. Chemosphere. 1999;39:811–832. [Google Scholar]
- Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- Padmanabhan J, Parthasarathi R, Subramanian V, Chattaraj PK. QSPR models for polychlorinated biphenyls: n-Octanol/water partition coefficient. Bioorg Med Chem Lett. 2006;14:1021–1028. doi: 10.1016/j.bmc.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Parent AS, Naveau E, Gerard A, Bourguignon JP, Westbrook GL. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J Toxicol Environ Health B Crit Rev. 2011;14:328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Bergman A, Linderholm L, Athanasiadou M, Kocan A, Petrik J, Drobna B, Trnovec T, Charles MJ, Hertz-Picciotto I. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia. Chemosphere. 2008;70:1676–1684. doi: 10.1016/j.chemosphere.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patayová H, Wimmerová S, Lancz K, Palkovičová á, Drobná B, Fabišiková A, Kovaáč J, Hertz-Picciotto I, Jusko TA, Trnovec T. Anthropometric, socioeconomic, and maternal health determinants of placental transfer of organochlorine compounds. Environ Sci Poll Res Int. 2013;20:8557–8566. doi: 10.1007/s11356-013-1786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164–170. doi: 10.1016/j.tem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Patil GS. Correlation of aqueous solubility and octanol–water partition coefficient based on molecular structure. Chemosphere. 1991;22:723–738. [Google Scholar]
- Petrık J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J. Serum PCBs and Organochlorine Pesticides in Slovakia: Age, gender, and residence as determinants of organochlorine concentrations. Chemosphere. 2006;65:410–418. doi: 10.1016/j.chemosphere.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Porpora MG, Lucchini R, Abballe A, Ingelido AM, Valentini S, Fuggetta E, Cardi V, Ticino A, Marra V, Fulgenzi AR, Felip ED. Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs. Int J Environ Res Public Health. 2013;10:699–711. doi: 10.3390/ijerph10020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Chickos JS, Welsh WJ. Three-Dimensional Quantitative Structure-Property Relationship (3D-QSPR) Models for Prediction of Thermodynamic Properties of Polychlorinated Biphenyls (PCBs): Enthalpies of Fusion and Their Application to Estimates of Enthalpies of Sublimation and Aqueous Solubilities. J Chem Inf Comput Sci. 43:55–62. doi: 10.1021/ci0200164. [DOI] [PubMed] [Google Scholar]
- Qin LT, Liu SS, Liu HL, Ge HL. A new predictive model for the bioconcentration factors of polychlorinated biphenyls (PCBs) based on the molecular electronegativity distance vector (MEDV) Chemosphere. 2008;70:1577–1587. doi: 10.1016/j.chemosphere.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Richard K, Li H, Landers KA, Patel J, Mortimer RH. Placental Transport of Thyroid Hormone and Iodide. In: Zheng Jing., editor. Recent Advances in Research on the Human Placenta. InTech; 2012. Available from: http://www.intechopen.com/books/recent-advances-inresearch-on-the-human-placenta/placental-transport-of-thyroid-hormone-and-iodide. [Google Scholar]
- Shiu WY, Mackay D. A critical review of aqueous solubilities, vapor pressure, Henry’s law constants, and octanol-water partition coefficients of the polychlorinated biphenyls. J Phys Chem Ref Data. 1986;15:911–929. [Google Scholar]
- Silla E, Tufion I, Villar F, Pascual-Ahuir JL. Molecular surface calculations on organic compounds. Molecular area-aqueous solubility relationships. J Mol Struc-THEOCHEM. 1992;254:1369–377. [Google Scholar]
- Sly PD, Flack F. Susceptibility of children to environmental pollutants. Ann N Y Acad Sci. 2008;1140:163–83. doi: 10.1196/annals.1454.017. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, Nguyen D, Hertz-Picciotto I. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birthweight. Paediatr Perinat Epidemiol. 2008;22:202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, Nguyen DV, Hertz-Picciotto I. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Environ Epidemiol. 2008;18:581–587. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Nakano M, Nakano S. Distribution of PCDDs/PCDFs and Co-PCBs in human maternal blood, cord blood, placenta, milk, and adipose tissue:dioxins showing high toxic equivalency factor accumulate in the placenta. Biosci Biotechnol Biochem. 2005;69:1836–1847. doi: 10.1271/bbb.69.1836. [DOI] [PubMed] [Google Scholar]
- Tonnelier A, Coecke S, Zaldívar JM. Screening of chemicals for human bioaccumulative potential with a physiologically based toxicokinetic model. Arch Toxicol. 2012;86:393–403. doi: 10.1007/s00204-011-0768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimori K, Morokuma S, Hori T, Takahashi K, Hirata T, Otera Y, Fukushima K, Kawamoto T, Wake N. Characterization of placental transfer of polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in normal pregnancy. J Obstet Gynaecol Res. 2013;39:83–90. doi: 10.1111/j.1447-0756.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78:252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Vähäkangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158:665–678. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe M, Weijs L, Habran S, Das K, Bugli C, Rees JF, Pomeroy P, Covaci A, Debier C. Selective transfer of persistent organic pollutants and their metabolites in grey seals during lactation. Environ Int. 2012;46:6–15. doi: 10.1016/j.envint.2012.04.011. [DOI] [PubMed] [Google Scholar]
- van Gestel CAM, Otermann K, Canton JH. Relation between water solubility, octanol/water partition coefficients, and bioconcentration of organic chemicals in fish: A review Review Article. Regulatory Toxicology and Pharmacology. 1985;4:422–431. doi: 10.1016/0273-2300(85)90007-8. [DOI] [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Fernández-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 65:107–115. doi: 10.1016/j.envint.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Bérubé A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Winneke G. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci. 2011;308:9–15. doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Woodburn KB, Doucette WJ, Andren AW. Generator column determination of octanol/water partition coefficients for selected polychlorinated biphenyl congeners. Environ Sci Technol. 1987;18:457–459. doi: 10.1021/es00124a012. [DOI] [PubMed] [Google Scholar]
- Yalkowsky SH, Valvani SC. Solubilities and partitioning 2. Relationships between aqueous solubilities, partition coefficients, and molecular surface areas of rigid aromatic hydrocarbons. J Chem Eng Data. 1979;24:127–129. [Google Scholar]
- Yalkowsky SH, Valvani SC, Mackay D. Estimation of the aqueous solubility of some aromatic compounds. Residue Rev. 1983;85:43–55. [Google Scholar]
- Zhang YH, Xia ZN, Qin LT, Liu SS. Prediction of blood-brain partitioning: a model based on molecular electronegativity distance vector descriptors. J Mol Graph Model. 2010;29:214–220. doi: 10.1016/j.jmgm.2010.06.006. [DOI] [PubMed] [Google Scholar]