Introduction
Allergic rhinitis (AR) affects up to ∼14% of adults1 and ∼22% of children2 in the United States, and its prevalence can be as high as 60-80% among children with asthma3,4. In Europe, a large multi-center study that combined survey and physical examination data reported that AR affects ∼13-23% of the population5; that study reported up to ∼45% under-diagnosis. We recently reported up to ∼75% AR under-diagnosis among Puerto Rican (PR) children6, who bear a disproportionate burden of asthma7.
The presence and severity of AR have been associated with several comorbidities in children, including asthma, otitis media, and adenoid hypertrophy8. It has also been associated with higher risk of chronic sinusitis in adults9. AR may worsen asthma severity and control by enhancing lower airway inflammation10 and may delay recovery of lung function after asthma exacerbations11. Adequate treatment with nasal corticosteroids may improve lung function in children with asthma12, although some studies have shown no improvement on asthma-related airway inflammation13.
Exposure to higher levels of mouse urinary protein (Mus m 1, hereinafter called ‘mouse allergen’) has been previously linked with mouse sensitization and indicators of asthma severity or control in some studies14,15 but not in others16,17. To date, little is known about the association between mouse allergen exposure and allergic rhinitis (AR). We hypothesized that mouse allergen exposure would be associated with decreased prevalence of AR in PR children, and explored potential mechanisms by analyzing possible correlated microbe-associated molecular patterns (MAMPs).
Methods
Study Population
The details of the study recruitment and protocol have been previously described6,18,19. In brief, from March 2009 to June 2010, children in San Juan (Puerto Rico) were chosen from randomly selected households using a multistage probability sample design scheme similar to that of a prior study20. Primary sampling units (PSUs) were randomly selected neighborhood clusters based on the 2000 U.S. census, and secondary sampling units were randomly selected households within each PSU. A household was eligible if one or more residents was a child aged 6 to 14 years. In households with more than one eligible child, one child was randomly selected for screening20. After selection and screening, we attempted to enroll a total of 783 children. Parents of 105 of these 783 eligible households refused to participate or could not be reached; there were no significant differences in age, sex, or area of residence between eligible children who did (n=678, ∼87%) and did not (n=105, ∼13%) agree to participate. For the current study, focused on allergic rhinitis and mouse allergen exposure, only those with non-missing data on allergy skin testing and Mus m 1 levels (n=511) were included; there were no significant differences between those included and those excluded from analysis (n=167) (see eTable 1 in the E-Supplement). Written parental consent was obtained for participating children, from whom written assent was also obtained. The study was approved by the Institutional Review Boards of the University of Puerto Rico (San Jan, PR), Brigham and Women's Hospital (Boston, MA), and the University of Pittsburgh (Pittsburgh, PA).
Study Procedures
Study participants completed a protocol that included questionnaires, allergy skin testing, and collection of dust samples. The child's parents completed two questionnaires used in the Genetics of Asthma in Costa Rica Study21. These questionnaires were used to obtain information about the child's general and respiratory health, family history, socio-demographic characteristics, in utero smoke exposure, family history, and household characteristics.
Skin test reactivity (STR) to aeroallergens, histamine and saline diluent, was assessed using a Multi Test device (Lincoln Diagnostics, Decatur, IL) on the skin of the forearm (in a site free of eczema). Aeroallergens tested included house dust mites (D. pteronyssinus and D. farinae), B. tropicalis, German cockroach (B. germanica), mouse pelt, dog dander, cat dander, mold mix, Alternaria tenuis, mixed tree pollen, mixed grass pollen, mugwort sage, and ragweed (Alk-Abello, Round Rock, TX). A skin test was considered positive if the maximum wheal diameter exceeded the diluent wheal diameter by ≥3 mm.
Dust samples were obtained from three areas in the home: the one in which the child sleeps (usually his/her bedroom), the living room/television room, and the kitchen. The dust was sifted through a 50-mesh metal sieve, and the fine dust was reweighed, extracted, and aliquoted for analysis of allergens from mouse (mouse urinary protein [Mus m 1]), dust mite (Dermatophagoides pteronyssinus [Der p 1]), cockroach (Blatella germanica [Bla g 2]), dog dander (Can f 1), and cat dander (Fel d 1), as well as microbe-associated molecular patterns (MAMP) levels of glucan, peptidoglycan and endotoxin (see E-Supplement for details). Levels of allergens and MAMPs were transformed to a log-10 scale for analysis, and are reported as geometric means for clarity.
Outcome Definition
AR was defined by having both current symptoms suggestive of AR and STR to ≥1 allergen; this definition is consistent with Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 guidelines22. A child was considered to have current symptoms suggestive of AR if there were affirmative answers to two questions (taken from the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire23): 1) “Has your child ever had hay fever or a runny or stuffy nose accompanied by sneezing and itching at a time when he/she did not have a cold or flu?”, and 2) “Has your child had these symptoms in the last 12 months?”. Asthma was defined as physician-diagnosed asthma and wheeze in the previous year.
Statistical Analysis
Bivariate analyses were conducted using Fisher's exact tests for categorical variables, and two-sample two-sided t tests for continuous variables. Logistic regression was used for the multivariable analyses. Because of their known relation to AR, all models included age, sex, and type of health insurance (private/employer-based vs. others). Other covariates (see Table 1) were also included in the initial multivariate models if p≤0.20 in bivariate analyses; these additional covariates remained in the final models if they were associated with AR at p<0.05 or if they changed the parameter estimates by ≥10%. The Pearson correlation coefficient was used to assess the relationship between levels of MAMPs and mouse allergen. Parental education was dichotomized into at least one parent completed high school vs. none. Household income was dichotomized into above or below $15,000/year, as that was the approximate median income in Puerto Rico during the study. Eczematous rash referred to ever having a prolonged, itchy, scaly or weepy skin rash. All analyses were done using Stata/SE 12.1 (College Station, TX).
Table 1. Characteristics of Study Participants.
|
No AR (n = 264, 51.7%) |
AR (n = 247, 48.3%) |
|
|---|---|---|
| Baseline characteristics | ||
| Age (years) | 10.2 (9.9, 10.5) | 10.5 (10.1, 10.8) |
| Female sex | 126 (47.7%) | 113 (45.8%) |
| Parental education (>high school) | 205 (77.7%) | 204 (82.6%) |
| Higher household income (>$15K/year) | 80 (31.6%) | 89 (36.8%) |
| Private/employer-based health insurance | 77 (29.2%) | 94 (38.1%)* |
| Asthma | 92 (34.9%) | 176 (71.3%)** |
| Leukotriene inhibitor in prior 6 months | 19 (7.2%) | 49 (19.8%)** |
| Inhaled corticosteroids in prior 6 months | 24 (9.1%) | 64 (25.9%)** |
| Atopic history | ||
| Eczematous rash, ever | 59 (22.4%) | 104 (42.1%)** |
| Family history | ||
| Parental history of allergic rhinitis | 40 (15.2%) | 57 (23.2%)* |
| Parental history of asthma | 111 (42.2%) | 145 (58.7%)** |
| Parental history of eczema | 4 (1.5%) | 8 (3.3%) |
| Home environment | ||
| Number of older siblings | 1.8 (1.6, 2.0) | 1.6 (1.4, 1.8) |
| Shared bedroom | 155 (58.7%) | 121 (49.0%)* |
| History of daycare | 48 (18.3%) | 63 (25.9%)* |
| Signs of mold/mildew | 88 (33.5%) | 119 (48.2%)** |
| Dog at home | 132 (50.4%) | 131 (53.0%) |
| Cat at home | 32 (12.3%) | 21 (8.5%) |
| Seeing rats or mice | 81 (30.7%) | 57 (23.1%) |
| Skin test reactivity | ||
| ≥1 allergen | 143 (54.2%) | 247 (100.0%)** |
| Total number positive skin tests | 2.8 (2.3-3.3) | 5.6 (5.1-6.0)** |
| Dust mite mix | 47 (17.9%) | 108 (43.9%)** |
| B. tropicalis | 81 (31.5%) | 179 (73.7%)** |
| Cockroach | 47 (18.0%) | 106 (43.6%)** |
| Mouse | 39 (14.8%) | 72 (29.2%)** |
| Dog | 42 (15.9%) | 82 (33.2%)** |
| Cat | 68 (25.8%) | 103 (41.7%)** |
| Mold mix | 25 (9.5%) | 37 (15.0%) |
| Alternaria | 44 (16.7%) | 75 (30.4%)** |
| Mixed trees | 59 (22.4%) | 83 (33.6%)* |
| Mixed grasses | 28 (12.2%) | 49 (21.9%)* |
| Mugwort sage | 22 (9.6%) | 43 (19.8%)** |
| Ragweed | 73 (27.7%) | 131 (53.0%)** |
| Home allergen levels1 | ||
| Log Der p (μg/g) | 4.2 (3.7, 4.9) | 4.8 (4.1, 5.5) |
| Log Bla g 2 (U/g) | 1.9 (1.6, 2.3) | 1.8 (1.5, 2.2) |
| Log Mus m 1 (ng/g) | 12.7 (9.7, 16.6) | 8.5 (6.3, 11.4)* |
| Log Can f 1 (μg/g) | 0.19 (0.15, 0.25) | 0.16 (0.12, 0.21) |
| Log Fel d 1 (μg/g) | 0.04 (0.03, 0.06) | 0.03 (0.02, 0.04) |
| Microbial associated molecular patterns1 | ||
| Log glucan (μg/mg) | 0.14 (0.11, 0.17) | 0.15 (0.12, 0.19) |
| Log peptidoglycan (μg/mg) | 0.014 (0.009, 0.02) | 0.015 (0.01, 0.02) |
| Log endotoxin (EU/mg) | 34.8 (30.6, 39.6) | 36.2 (31.1, 42.2) |
Values are presented as number (%) or mean (95% CI).
Presented as geometric means (95% CI). Difference between subjects with and without AR on univariate analysis at
P<0.05,
P<0.005.
Results
The characteristics of participants (n=511) who did and did not have AR is shown in Table 1. AR was present in 247 (48.3%) of participating children. Variables significantly and positively associated with AR included asthma, an eczematous rash, parental AR, parental asthma, history of daycare, reported signs of mold/mildew in the home, STR ≥1 allergen, total number of positive skin tests, and STR to dust mite, B. tropicalis, cockroach, mouse, dog, cat, Alternaria, mixed trees, mixed grasses, mugwort sage and ragweed. Mus m 1 and shared bedroom were significantly and inversely associated with AR.
Table 2 shows the multivariate analysis of mouse allergen exposure and AR. After adjustment for age and sex (Model 1A in Table 2), private/employer-based health insurance and asthma were significantly associated with increased odds of AR, whereas Mus m 1 level and shared bedroom were significantly associated with decreased odds of AR: each log10-unit increment in Mus m 1 level decreased the odds of AR by 25% (95 confidence interval [CI] = 8% - 38%). Among children sensitized to mouse (Model 1B in Table 2), there was a similar trend for a reduction in the odds of AR with increasing Mus m 1 level, though it did not reach statistical significance (p=0.15); and asthma was still associated with increased odds of AR. Among children not sensitized to mouse (Model 1C in Table 2), Mus m 1 level was significantly associated with decreased odds of AR after adjustment for relevant covariates: each log10-unit increase in Mus m 1 level decreased the odds of AR by ∼21% (95% CI = 1% - 37%). Since asthma was such a strong predictor of AR in our sample, we then explored whether there was an interaction between mouse allergen and asthma on AR, without significant results (data not shown).
Table 2. Multivariate analysis of mouse allergen level and AR by mouse sensitization status.
| All Subjects | Unadjusted (n = 511) |
Adjusted Model 1A (n = 511) |
Adjusted Model 2A (n = 328) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | P-val | OR (95% CI) | P-val | OR (95% CI) | P-val | |
| Mus m 1 (ng/g)1 | 0.84 (0.70, 1.00) | .049 | 0.75 (0.62, 0.92) | .005 | 0.72 (0.57, 0.92) | .009 |
| Endotoxin1 | 1.11 (0.64, 1.92) | .703 | --- | --- | 1.22 (0.66, 2.24) | .528 |
| Health insurance type | 1.49 (1.03, 2.16) | .034 | 1.66 (1.10, 2.52) | .017 | 1.61 (0.91, 2.83) | .100 |
| Asthma | 4.63 (3.19-6.74) | <.001 | 6.40 (4.20, 9.73) | <.001 | 5.08 (3.01, 8.59) | <.001 |
| Shared Bedroom | 0.68 (0.48, 0.96) | .028 | 0.50 (0.33, 0.76) | .001 | 0.44 (0.26, 0.73) | .002 |
|
| ||||||
| Sensitized to Mouse |
Unadjusted (n = 111) |
Adjusted Model 1B (n = 111) |
Adjusted Model 2B (n = 76) |
|||
|
| ||||||
| Mus m 1 (ng/g)1 | 0.78, 0.52, 1.19) | .256 | 0.71 (0.44, 1.13) | .150 | 0.79 (0.41, 1.51) | .470 |
| Endotoxin1 | 0.83 (0.28, 2.52) | .748 | --- | --- | 0.89 (0.26, 3.05) | .858 |
| Health insurance type | 0.94 (0.41, 2.15) | .881 | 0.99 (0.39, 2.50) | .976 | 0.86 (0.24, 3.12) | .823 |
| Asthma | 4.79 (2.07, 11.12) | <.001 | 6.56 (2.48, 17.37) | <.001 | 4.62 (1.48, 14.47) | .009 |
| Shared Bedroom | 0.78 (0.35, 1.71) | .531 | 0.61 (0.24, 1.52) | .286 | 0.63 (0.21, 1.96) | .428 |
|
| ||||||
| Not Sensitized to Mouse |
Unadjusted (n = 400) |
Adjusted Model 1C (n = 400) |
Adjusted Model 2C (n = 252) |
|||
|
| ||||||
| Mus m 1 (ng/g) 1 | 0.88 (0.72, 1.07) | .204 | 0.79 (0.63, 0.99) | .040 | 0.74 (0.56, 0.98) | .033 |
| Endotoxin1 | 1.27 (0.66, 2.44) | .475 | --- | --- | 1.42 (0.68, 2.95) | .344 |
| Health insurance type | 1.72 (1.13, 2.61) | .011 | 1.97 (1.22, 3.17) | .005 | 2.10 (1.10, 4.02) | .025 |
| Asthma | 4.80 (3.12, 7.37) | <.001 | 6.84 (4.21, 11.14) | <.001 | 5.54 (2.96, 10.38) | <.001 |
| Shared Bedroom | 0.64 (0.43, 0.95) | .026 | 0.44 (0.27, 0.72) | .001 | 0.35 (0.19, 0.65) | .001 |
All models adjusted for age, sex, and variables listed; model 2 additionally adjusted for endotoxin level (EU/mg).
Per each log10 unit increment.
Given that prior studies have shown an inverse association among exposure to microbial components and asthma or allergic sensitization24, we then investigated whether findings for mouse allergen and AR were due to correlated microbial components detected in house dust (endotoxin, peptidoglycan and glucan). Figure 1 shows scatterplots for glucan, peptidoglycan, and endotoxin vs Mus m 1. Of the three, only endotoxin was significantly correlated with Mus m 1 (r=0.184, p<0.001).
Figure 1. Scatterplots of Microbe-Associated Molecular Patterns (MAMPs) vs. Mus m 1.
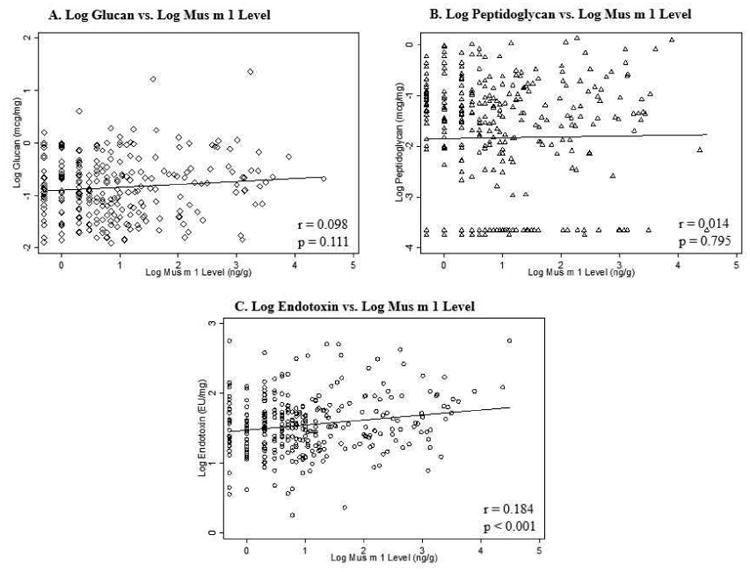
Log10 microbe-associated molecular patterns and log10 Mus m 1 levels are plotted with regression lines. Pearson correlation coefficient (r) and associated p-values are shown for each plot.
In light of this correlation, we added (log10-transformed) endotoxin levels to the multivariable models of mouse allergen and AR (see Models 2A-2C in Table 2). In these models, endotoxin was not significantly associated with AR among all subjects (Model 2A) nor in the subgroups of children sensitized (Model 2B) or not sensitized (Model 2C) to mouse. Adding endotoxin to the models also did not affect the inverse association between Mus m 1 level and AR, although interestingly it did decrease the parameter estimates for asthma by >10%.
Discussion
To the best of our knowledge, this is the first study looking at the association between mouse allergen level and allergic rhinitis in children. We report that exposure to higher levels of mouse allergen are associated with decreased odds of AR, and that these results are driven by children who are not sensitized to mouse.
Exposure to mouse allergen has been linked to increased odds of atopic sensitization, predominantly in inner-city or urban environments16,25. In a cohort of children with parental history of allergy or asthma, Phipatanakul et al. reported that exposure to detectable levels of Mus m 1 in home dust at 2-3 months of age was associated with a twofold increase in the odds of allergic sensitization by age 7 years, compared to those with non-detectable levels25. Recently, the presence of anti-mouse IgE at 3 years of age was found to be associated with increased risk of wheeze, rhinitis, and atopic dermatitis. Thus, studies to date have reported relationships between allergen level and sensitization, and between sensitization and AR or asthma26. Our results suggest that exposure to mouse allergen levels in children who do not develop mouse sensitization may actually have a protective effect against the development of AR, with a ∼21-25% reduction in the odds of AR per each log10-unit increase in house dust Mus m 1 level. This is consistent with our previous report that higher levels of mouse allergen are associated with better lung function among asthmatic children who are not sensitized to mouse17. We speculate that exposure to mouse allergen –or an associated factor– may exert protective, immune-modulatory effects among children who are not allergic to it; whereas this protective effect is lost among children who become allergic to mouse. However, given the smaller number of sensitized vs. non-sensitized children, we cannot fully rule out lack of statistical power to detect an association in the sensitized group.
Based on all of this, we initially hypothesized that mouse allergen level might be a proxy for exposure to other microbial exposures in these children. However, when evaluating the correlation between Mus m 1 and several MAMPs, only endotoxin was significantly (albeit weakly) correlated, and its inclusion in our models did not affect the significance or magnitude of the association between Mus m 1 and AR. Future studies will be needed to elucidate mechanisms by which exposure to mouse allergen may be indeed protective for AR or asthma, or if perhaps certain characteristics of these children make them less likely to develop mouse sensitization and also less prone to developing AR.
Levels of mouse allergen in our study had a mean of 290ng/g (0.29μg/g) and ranged from undetectable (n=58) to 31,227ng/g (or ∼4.5 log10). While these levels are lower than those reported in similar studies performed in inner cities in the U.S. –particularly those from the Northeast– prior studies have found similar variability: one study in Boston reported up to 68% of households had Mus m levels <0.25μg/g25, while other inner-city studies have reported medians of ∼0.3-0.4μg/g27,28. It will be important to determine whether differences in the level of exposure may modify the effect reported. We hypothesize that in environments with relatively low ranges of Mus m 1 levels, the ‘beneficial’ effect among non-sensitized children may predominate (with no effect seen among sensitized children)17, whereas in environments with higher levels of exposure, the ‘deleterious’ effect among sensitized children prevails (with ‘no effect’ detected among non-sensitized children)29,30.
We must acknowledge several limitations. Firstly, the cross-sectional design of the study precludes assessment of temporal relationships between mouse allergen levels and the outcomes assessed. However, positive determinants of mouse allergen load, including urban neighborhoods, lower income homes, older homes, household characteristics and practices31, may all be relatively stable from early-life throughout childhood. This is in contrast to pet ownership, which may be more susceptible to disease-associated modification of exposure. Secondly, we focused on PR children due to their disproportionate burden of asthma and atopy7 and the range of mouse allergen level was lower than those reported elsewhere, as previously discussed. However, mouse allergen is ubiquitous in inner-city homes in the United States16,32, and thus our findings are likely applicable to those environments and its under-served populations. Thirdly, while we adjusted for multiple potential confounders, there may be unmeasured factors at least partly responsible for these results. Of note, additionally adjusting for dust mite (D.pteronyssinus) allergen level and family history of AR did not change our results (data not shown).
In summary, mouse allergen level was inversely associated with AR in PR children, independently of levels of endotoxin in house dust, and the results were driven mainly by children not sensitized to mouse. This finding could be explained by early-life induction of tolerance by mouse allergen or by microbes that are present in mouse feces but were not measured in this study.
Supplementary Material
Acknowledgments
We thank the participating children and their families for their valuable contribution to this study.
Funding: This work was supported by grants HL079966 and HL117191 from the US National Institutes of Health (NIH), and by an endowment from the Heinz Foundation. Dr. Forno's contribution was supported by grant HD052892 from the NIH.
Footnotes
Author Contributions: TSJ, EF, JMB, YH, and JB made substantial contributions to conception, design, analysis and interpretation of data; drafting the article, or revising it critically for important intellectual content. EA, AC, MA, PT, NM and GC made substantial contributions to conception and acquisition of data, and critically reviewed the manuscript drafts. JCC supervised the entire study and made substantial contributions to all of the above. All authors collectively wrote the entirety of the manuscript, and read and approved its final version for submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012 Sep-Oct;33(Suppl 1):S113–41. doi: 10.2500/aap.2012.33.3603. [DOI] [PubMed] [Google Scholar]
- 2.Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008 Nov-Dec;29(6):600–8. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 3.Masuda S, Fujisawa T, Katsumata H, Atsuta J, Iguchi K. High prevalence and young onset of allergic rhinitis in children with bronchial asthma. Pediatr Allergy Immunol. 2008 Sep;19(6):517–22. doi: 10.1111/j.1399-3038.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 4.Everhart RS, Kopel SJ, Esteban CA, et al. Allergic rhinitis quality of life in urban children with asthma. Ann Allergy Asthma Immunol. 2014 Apr;112(4):365–70. e1. doi: 10.1016/j.anai.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004 Nov;24(5):758–64. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs TS, Forno E, Brehm JM, et al. Underdiagnosis of allergic rhinitis in underserved children. J Allergy Clin Immunol. 2014 May 2; doi: 10.1016/j.jaci.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009 Apr;9(2):154–60. doi: 10.1097/aci.0b013e3283292207. Epub 2009/03/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibanez MD, Valero AL, Montoro J, et al. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr Allergy Immunol. 2013 Nov;24(7):678–84. doi: 10.1111/pai.12126. [DOI] [PubMed] [Google Scholar]
- 9.Berrettini S, Carabelli A, Sellari-Franceschini S, et al. Perennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factors. Allergy. 1999 Mar;54(3):242–8. doi: 10.1034/j.1398-9995.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 10.Oka A, Matsunaga K, Kamei T, et al. Ongoing allergic rhinitis impairs asthma control by enhancing the lower airway inflammation. The journal of allergy and clinical immunology In practice. 2014 Mar-Apr;2(2):172–8. doi: 10.1016/j.jaip.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz O, Bakirtas A, Ertoy Karagol HI, Topal E, Demirsoy MS. Allergic rhinitis may impact the recovery of pulmonary function tests after moderate/severe asthma exacerbation in children. Allergy. 2014 May;69(5):652–7. doi: 10.1111/all.12391. [DOI] [PubMed] [Google Scholar]
- 12.Kessel A. The impact of intranasal corticosteroids on lung function in children with allergic rhinitis. Pediatr Pulmonol. 2013 Oct 24; doi: 10.1002/ppul.22912. [DOI] [PubMed] [Google Scholar]
- 13.Pedroletti C, Lundahl J, Alving K, Hedlin G. Effect of nasal steroid treatment on airway inflammation determined by exhaled nitric oxide in allergic schoolchildren with perennial rhinitis and asthma. Pediatr Allergy Immunol. 2008 May;19(3):219–26. doi: 10.1111/j.1399-3038.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsui EC, Eggleston PA, Breysse PN, Rand CS, Diette GB. Mouse allergen-specific antibody responses in inner-city children with asthma. The Journal of allergy and clinical immunology. 2007 Apr;119(4):910–5. doi: 10.1016/j.jaci.2006.12.663. Epub 2007/03/06. eng. [DOI] [PubMed] [Google Scholar]
- 15.Salo PM, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to mouse allergen in U.S. homes associated with asthma symptoms. Environmental health perspectives. 2009 Mar;117(3):387–91. doi: 10.1289/ehp.11847. Epub 2009/04/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106(6):1075–80. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 17.Forno E, Cloutier MM, Datta S, et al. Mouse allergen, lung function, and atopy in puerto rican children. PLoS ONE. 2012;7(7):e40383. doi: 10.1371/journal.pone.0040383. Epub 2012/07/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno E, Acosta-Perez E, Brehm J, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J All Clin Immun. 2013 doi: 10.1016/j.jaci.2013.09.041. Epub Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas-Salazar C, Ramratnam SK, Brehm JM, et al. Prematurity, atopy, and childhood asthma in Puerto Ricans. J Allergy Clin Immunol. 2013 Oct 17; doi: 10.1016/j.jaci.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird HR, Canino GJ, Davies M, et al. A study of disruptive behavior disorders in Puerto Rican youth: I. Background, design, and survey methods. Journal of the American Academy of Child and Adolescent Psychiatry. 2006 Sep;45(9):1032–41. doi: 10.1097/01.chi.0000227878.58027.3d. Epub 2006/08/24. eng. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C. Collaborative studies on the genetics of asthma--National Heart, Lung and Blood Institute. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1995 Nov;25(Suppl 2):29–32. doi: 10.1111/j.1365-2222.1995.tb00416.x. Epub 1995/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Pawankar R, Bunnag C, Khaltaev N, Bousquet J. Allergic Rhinitis and Its Impact on Asthma in Asia Pacific and the ARIA Update 2008. The World Allergy Organization journal. 2012 Apr;5(Suppl 3):S212–7. doi: 10.1097/WOX.0b013e318201d831. Epub 2012/12/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISAAC SC. Phase II modules of the International Study of Asthma and Allergies in Childhood. ISAAC; Munster: 1998. [Google Scholar]
- 24.Sordillo JE, Hoffman EB, Celedon JC, Litonjua AA, Milton DK, Gold DR. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010 Jun;40(6):902–10. doi: 10.1111/j.1365-2222.2010.03509.x. Epub 2010/04/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipatanakul W, Celedon JC, Hoffman EB, Abdulkerim H, Ryan LM, Gold DR. Mouse allergen exposure, wheeze and atopy in the first seven years of life. Allergy. 2008 Nov;63(11):1512–8. doi: 10.1111/j.1398-9995.2008.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donohue KM, Al-alem U, Perzanowski MS, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008 Nov;122(5):914–20. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood RA, Bloomberg GR, Kattan M, et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) The Journal of allergy and clinical immunology. 2011;127(4):913–9. e1–6. doi: 10.1016/j.jaci.2010.12.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106(6):1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia SK, Peng RD, Breysse PN, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013 Oct;132(4):830–5. e1–2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torjusen EN, Diette GB, Breysse PN, Curtin-Brosnan J, Aloe C, Matsui EC. Dose-response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor air. 2013 Aug;23(4):268–74. doi: 10.1111/ina.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. The Journal of allergy and clinical immunology. 2004 Jun;113(6):1167–71. doi: 10.1016/j.jaci.2003.12.592. Epub 2004/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 32.Almqvist C, Garden F, Kemp AS, et al. Effects of early cat or dog ownership on sensitisation and asthma in a high-risk cohort without disease-related modification of exposure. Paediatric and perinatal epidemiology. 2010 Mar;24(2):171–8. doi: 10.1111/j.1365-3016.2010.01095.x. Epub 2010/04/27. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


