Abstract
Background
This study was designed to test the effects of induced pluripotent stem cell (iPSC) in the treatment of chronic myocardial ischemia.
Methods
The reprograming of passage 3 myocardial fibroblasts was performed by using the lentiviral vector containing 4 human factors: OCT4, SOX2, KLF4, and c-MYC. The iPSC Colonies at P12–17 were allogeneically transplanted into ischemic myocardium of ten swine by direct injection. Cohorts of two animals were sacrificed at 2, 4, 6, 8, and 12 weeks after injection.
Results
No signs of graft versus host disease were evident at any time points. At 2 weeks, clusters of SSEA-4-positive iPSCs were detected in the injected area. At 4 to 8 weeks, these cells started to proliferate into small spheres surrounded by thin capsules. At 12 weeks, the cell clusters still existed, but decreased in size and numbers. The cells inside these masses were homogeneous with no sign of differentiation into any specific lineage. Increased smooth muscle actin or vWF positive cells were found inside and around the iPSC clusters, compared with non-injected areas. By RT-PCR, the levels of VEGF, FGF, and ANRT expression were significantly higher in the iPSC treated myocardium compared to untreated areas. These results suggest that iPSCs contributed to angiogenesis.
Conclusions
Allogeneically transplanted pig iPSCs proliferated despite an ischemic environment in first two months and survived for at least three months in immunocompetent hosts. Transplanted iPSCs were also pro-angiogenic and thus might have beneficial effects on the ischemic heart diseases.
Keywords: Cell Transplantation, Stem cells, Myocardial ischemia, Angiogenesis, Animal model
Introduction
Marrow derived stem or progenitor cells have been used for patients with ischemic myocardial diseases since 2003 (1–8). Functional improvement has been reported by most clinical trials, however, no evidence has shown that marrow derived cells can differentiate into beating myocytes in vitro, even though several studies claimed myocyte-like characteristics or markers after treating these cells with certain chemicals or factors (9–11). Thus, most investigators believe that the beneficial improvement is mainly due to paracrine effects of implanted cells (12–16). Induced pluripotent stem cells (iPSCs) have been recently generated in murine, human, and several other species since 2006 (17–19). These iPSCs have become exciting tools for understanding the mechanisms of diseases and for potentially treating diseases through cell replacement therapy. In order to apply this new technology for future clinical use, it needs to be tested in a large animal model. Recently, pig iPSCs have been derived from fetal fibroblasts by ectopic expression of four human transcriptional factors, OCT4, SOX2, KLF4, and c-MYC using lentiviral vectors (20). These pig iPSCs showed pluripotency via in vitro differentiation assays and teratoma formation assays. However, these cells have failed to differentiate into beating myocytes in vitro. Recent studies have suggested that iPSCs seem to retain epigenetic memory because they have a higher tendency to differentiate into the lineages which they were reprogrammed from (21–23). Therefore, we sought to derive iPSCs from porcine myocardial fibroblasts and hope these iPSCs can facilitate our efforts to generate cardiomyocytes. In this study, we performed allogeneic transplantation of the cardiac fibroblast-derived pig iPSCs into chronic ischemic myocardium, and compared their effects with fetal fibroblast-derived iPSCs (20). The results were compared with those using bone marrow derived mesenchymal stem cell (MSC) previously reported by our laboratory (24,25).
Material and Methods
Pig myocardium-derived fibroblasts preparation and culture
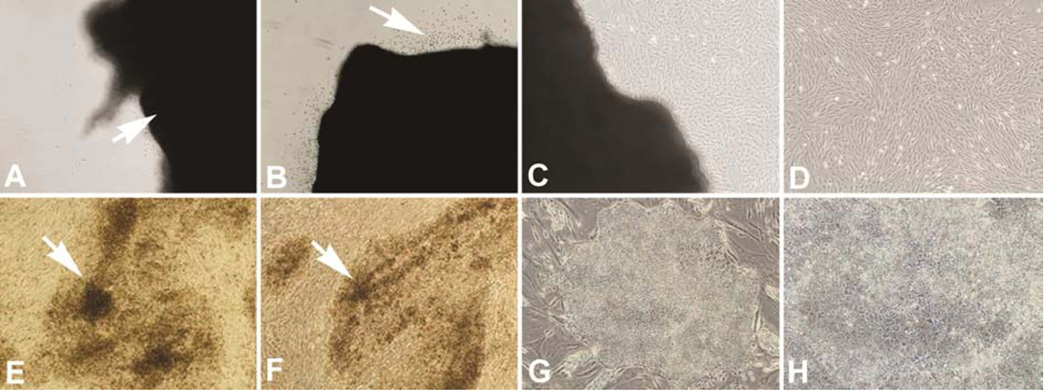
A tissue explant method was used for the myocardium-derived fibroblast culture. Briefly, a full length of left ventricular tissue (1×1 cm) was obtained from a healthy adult donor (Yorkshire, three months old). After washing three times in serum-free 2× penicillin-streptomycin solution containing Dulbecco's Modification of Eagle's Medium (DMEM), the tissue was cut into small pieces (2 mm cube) and then washed twice. The small tissue clumps were transferred into 10 cm-culture dish, and cultured with DMEM supplemented with 10% fetal bovine serum, 1× penicillin-streptomycin solution. Fibroblasts began to migrate out of the tissue fragments and into the surrounding area after 3–7 days in culture. These fibroblasts were harvest by trypsinization and passaged in DMEM with 10% fetal bovine serum (FBS) (Figure 1a–d).
Figure 1.
Top panel shows tissue explant method to obtain myocardial fibroblasts: A. Arrow indicating a tissue clump attached to the culture plate; B. Arrow indicating cells migrating out from tissue clump after three days in culture; C. Migrated cells starting to proliferate to localized confluence; D. Cells derived from the myocardial tissue clump showing homogeneous fibroblast morphology after splitting and subculturing. Bottom panel-Reprograming of myocardial fibroblasts: E and F. Arrows indicating preliminary colonies five days after transfection; G. a mature iPSC colony at passage 5 at day 44; H. Higher magnification of G. Magnifications: A–G = 100×; H = 200×.
Pig iPSC derivation and culture
Passage 3 myocardium-derived fibroblasts were seeded in six-well culture plates at a density of approximately 1 × 105 per well. In the next day, they were transduced with StemCCA lentiviral vectors (Millipore, Billerica, MA) containing the four human factors: OCT4, SOX2, KLF4, and c-MYC, following the manufacturer provided protocol. After viral transduction, the fibroblasts were cultured in DMEM medium with 10% FBS for five more days, and then trypsinized and plated on 10 cm tissue culture dishes with irradiated mouse embryonic fibroblasts (MEF). The next morning, the culture medium was switched to standard human ES cell medium and changed daily afterwards. The iPSC-like colonies began to appear 5 days after switching to ES medium (Figure 1e and f). These colonies were manually picked about a week later into 24-well plates, and subsequently passed and expanded following standard human ESC/iPSC cultural protocol. Passage 12–17 pig iPSCs were used for the allogeneic transplantation study.
Animals
Yorkshire domestic pigs, initially weighing 15–20 kg were used as allogeneic iPSC recipients for this study. The experimental protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute, and all procedures conformed to the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996, Washington, D.C.).
Ameroid placement and iPSC transplantation
To create a chronic ischemic model, all animals underwent ameroid constrictor placement around the proximal left circumflex coronary artery (26). Four weeks later, a second left thoracotomy was performed on each animal, the circumflex territory (ischemic zone) was exposed and injected with an average of 1.6 × 107 iPSCs (from four 10-cm culture dishes with confluent iPSC colonies, suspended in 2.5 ml of normal saline, with 25 injection sites). Cohorts of two animals were sacrificed at 2, 4, 6, 8 weeks and 3 months after injection and the hearts were harvested and sectioned to study the differentiation of the injected cells.
To test if different stem cell lines behave differently in terms of their differentiation and proliferation capabilities, iPSCs kindly provided by Dr. Robert’s laboratory were used for allogeneic transplantation using the same methods. Before injection, these cells were expanded using the methods suggested by the provider (20).
Histological and immunohistochemistry analysis
Myocardial tissues with the iPSCs transplantation were cut into 5 × 5 mm-thick pieces, either collected in cassettes and fixed with 10% buffered formalin for paraffin embedding or in O.C.T. for frozen sections with no fixation. Lung, liver, kidney and spleen samples were also collected for possible cell tracking. Paraffin embedded sections were stained with hematoxylin and eosin (H&E) for morphological analysis. The immunofluorescent staining was performed using rabbit polyclonal antibody against human OCT4 and SSEA4 to detect pluripotent marker genes. Antibody against vWF was used to detect vascular endothelial cells, and mouse monoclonal antibody against human smooth muscle actin to detect vascular smooth muscle cells. Mouse monoclonal antibody against human desmin was used to detect myocytes. All above mentioned antibodies were purchased from Dako North America (Carpinteria, CA). The incubations of primary antibodies were followed by detections of fluorescein isothiocyanate conjugated anti-mouse IgG or Rhodamine conjugated anti-rabbit IgG and the nuclei were labeled with 4',6-diamidino-2-phenylindole..
RNA preparation and real-time reverse-transcription polymerase chain reaction
To test the different gene expression profiles of the tissues with or without iPSC injection, total RNAs from these samples were isolated using RNeasy Kit (Qiagen, Valencia, CA) for real-time reverse-transcription polymerase chain reaction (qRT-PCR) analysis. The first-strand cDNAs were synthesized from 500 ng of total RNA using SuperScript™ III kit (Invitrogen Life Technologies, Carlsbad, CA) and random hexamers. The cDNA templates were then mixed with PCR reaction solution containing 100 nM of both forward and reverse primers, Universal PCR master mix with SYBR Green 1 (QuantiTect™ SYBR Green RT-PCR Kit, QIAGEN Inc, Valencia, CA). The PCR amplification was performed on a multicolor qRT-PCR detection system (IQ™5, Bio-Rad Lab. Inc. Hercules, CA) as follows: 10 min of initial denaturation at 95°Cfollowed by 45 cycles of 15 sec. of denaturation at 95°C and 1 min of annealing/extension at 60°C. Each sample was analyzed in duplicates. Three qRT-PCR reactions were performed for the examination of each signal gene. For normalization, the average of the expression level of two housekeeping genes, swine GAPDH and β2-microglobulin were used. Analysis of relative quantification was performed as previously described (27). The sequences of PCR primers are listed as following:
| Genes | Primer sequences |
| FGF F | GGA GAA GAG CGA CCC TCA CA |
| FGF R | CGG TTT GCA CAC ACT CCT TTG |
| VEGF F | CGC CAT GCA GAT TAT GCG GAT C |
| VEGF R | ACT CAA GCT GCC TCG CCT TGC A |
| ARNT (HIF1B) F | AGC CAT TGT TCA GAG GGC TA |
| ARNT (HIF1B) R | CCG CCG TTC AAT TTC ACT AT |
| OCT 3/4 F | AGT GAG AGG CAA CCT GGA GA |
| OCT 3/4 R | TCG TTG CGA ATA GTC ACT GC |
Statistical analysis
Data is presented as means ± standard deviation (SD). Standard student T-test was used for gene expression comparison and a p value of less than 0.05 was considered significant.
Results
Pluripotency tests of cardiac fibroblast-derived iPSCs
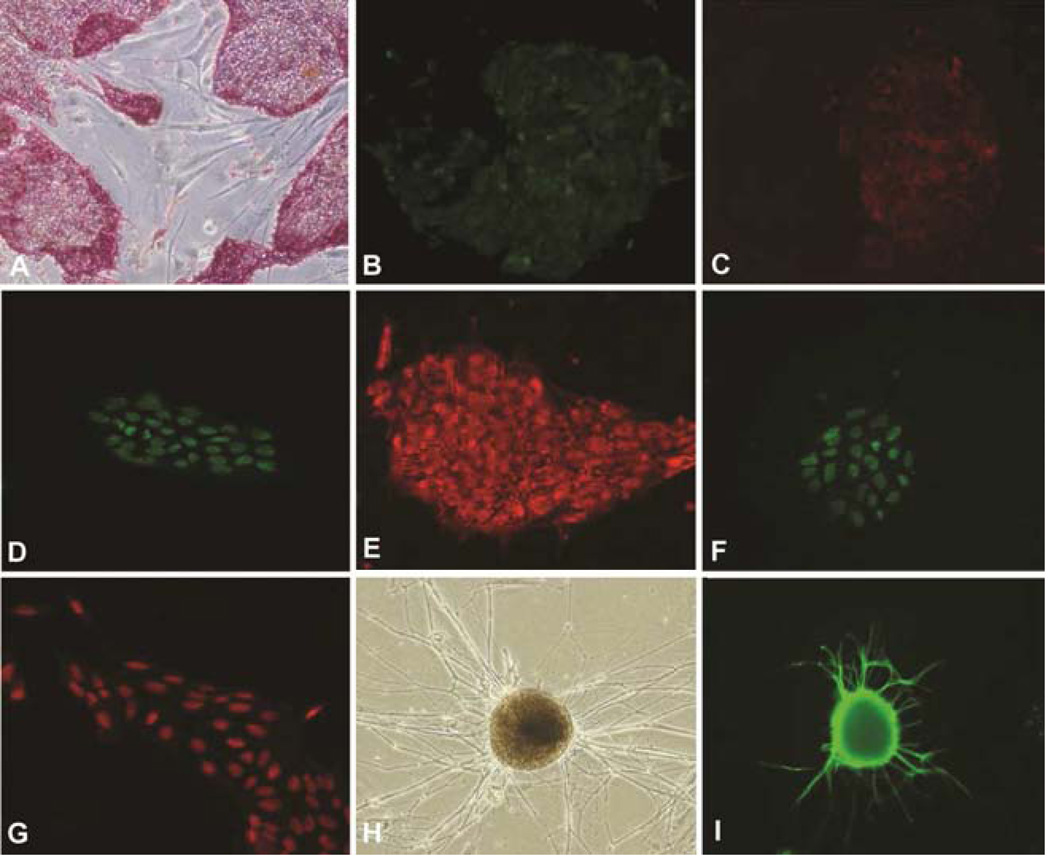
After 5 passages in cultures on MEF feeder layers, the colonies became uniform in appearance with morphology resembling mature human iPSC colonies (Figure 1g and h). They were positive for live staining with Tra-1–60 and Tra-1–81 antibodies. They were also positive for alkaline phosphatase staining, as well as SSEA-4, OCT4, Nanog, and Sox2 antibody staining (Figure 2a–g). After culturing selected large colonies for 4 weeks in ultra-low attachment plates, embryoid bodies, containing the typical three differentiated germ layers, were found. After injecting into immunocompromised mice, these cells formed teratomas. These cells can also differentiate into neuronal lineages as evidenced by strong staining of Tuj-1 (Figure 2h and i). After culturing selected large colonies for 7 days in ultra-low attachment plates and reseeding on MEF feeder layers, positive GATA-4 staining were found in some of the reseeded cells.
Figure 2.
Pluripotency tests for myocardial fibroblast-derived iPSCs. A. Positive AP staining; B. Tra-1–60; C. Tra-1–80; D. Nanog; E. SSEA4; F. Sox2; G. Oct4; H. Phase contrast image shows neural differentiation; I. Positive Tuj-1 staining. Magnifications: A–C = 100×; D–G = 200×; H and I = 100×.
Animal outcome
After transplantation, there were no signs of adverse side effects or graft versus host disease. Three unexpected animal deaths were caused by severe surgical wound infection or pulmonary edema following ameroid placement determined by autopsy examination and not as a result of cell injections.
Histology analysis
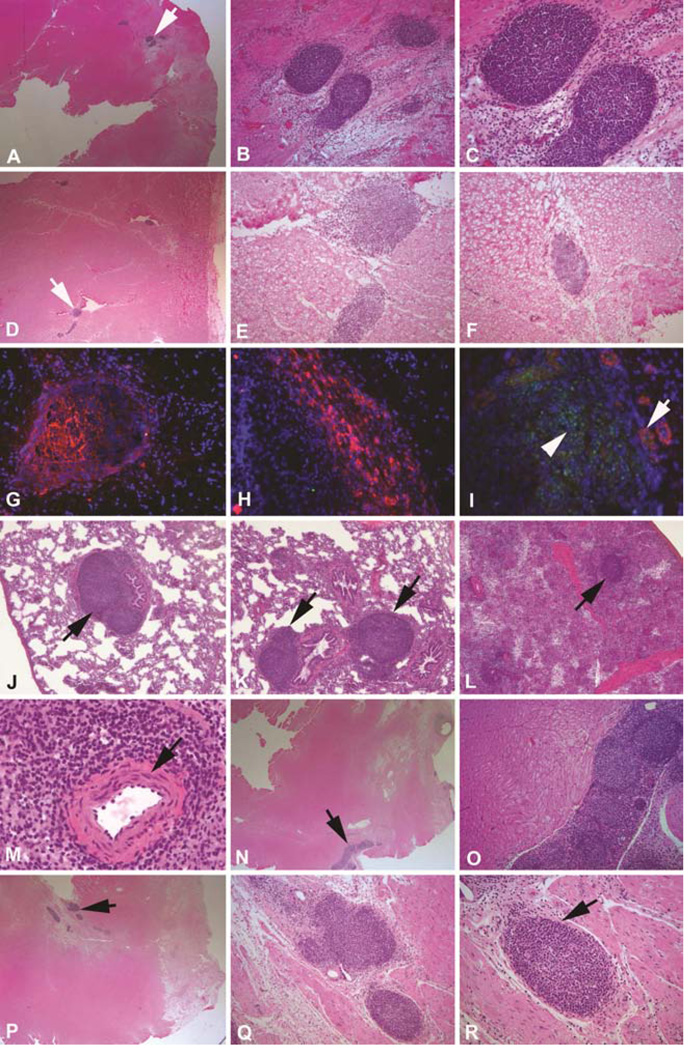
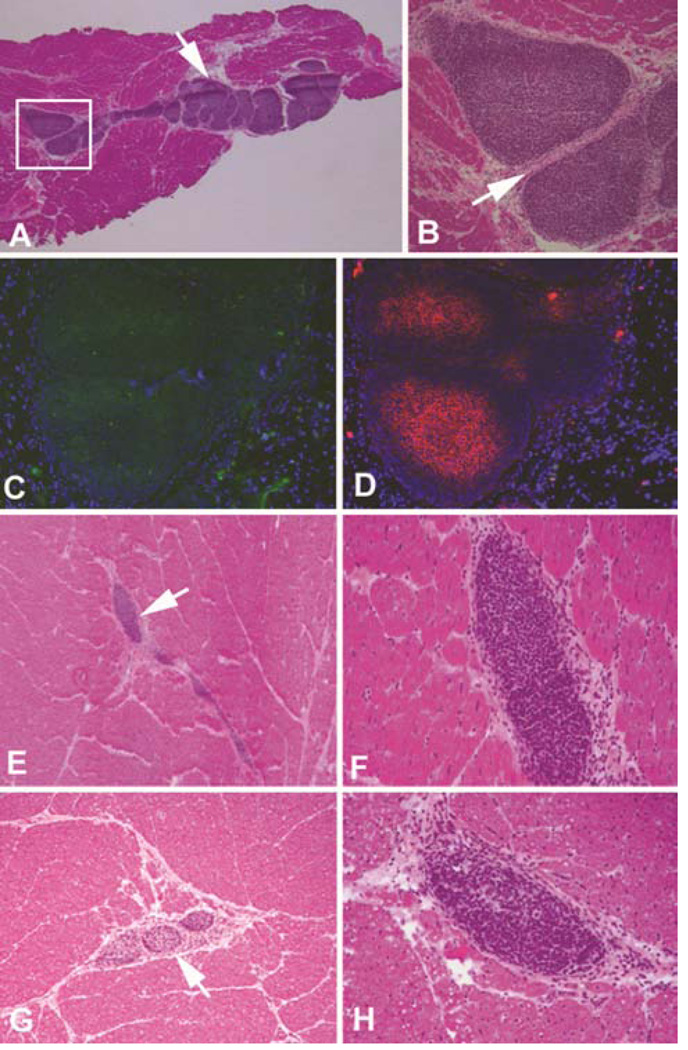
Two weeks after cell injection, clusters of SSEA-4 positive cells were detectable in the frozen sections of the injected area using immunofluorescent staining. However, these cells could hardly be seen in H&E stained paraffin sections. This could be resulted from the small number (one tenth) of injected iPSCs as compared to previous MSC studies. Quite to our surprise, at 4 to 8 weeks post transplantation, these clusters of cells started to proliferate into spherical shaped small tumors surrounded by thin layered capsules within the injected area (Figure 3). The cells inside these tumors showed a homogeneous phenotype with no signs of differentiation into any lineages. Most of these tiny tumors were spherical shaped (figure 3b, c and r) and some of them were irregular or elongated (Figure 3e, n and o), but all had thin layers of capsules. There were multiple spherical shaped tumors within an elongated cell cluster (Figure 3o) suggesting variable growth speed within a single cell cluster. All these small tumors stained positive for Oct4 and SSEA4 suggesting these cells were injected iPSCs (Figure 3g–i). Interestingly, three months after injection, the small tumors showed no sign of further growth. Instead, these small tumors became smaller and irregular compared with those seen in the 4–8 week time points. Similar strong SSEA4 positive cell clusters were found in the injected area but in smaller numbers. These results suggested that myocardium-derived iPSCs survived inside the chronic ischemic environment after allogeneic transplantation and started to proliferate after about two weeks. Growth was limited in the immunocompetent host, evidenced by the fact that the small tumors found in the 4–8 week time points were no longer becoming larger at the 12 week time point. In MSC injected animals, cell clusters were also clearly found in the injected area. However, there were more cells in the early time points and the number of MSC decreased gradually over the 3 month period post-injection. Additionally, there was no sign of cell proliferation or evidence of capsules around the MSC clusters.
Figure 3.
Allogeneic transplantation of myocardial fibroblast-derived iPSCs. A. Arrow indicating an area with three spherical shaped tumors at 4 weeks after injection (12.5×); B and C. Higher magnification of A (100× and 200×); D. Arrow indicating an area with three irregular shaped tumors at 6 weeks after injection (12.5×); E&F. Higher magnification of D (100×); G&H. Positive SSEA4 staining (red) of the tumor-like cell clumps at 6 weeks (400×); I. Demonstrates positive Oct 4 (green) and smooth muscle actin staining (red) (400×); J–L. Arrows indicating cell clumps were also found in lung (J&K) and spleen (L) at 6 weeks similar to those found in ischemic heart area (100×); M. A normal splenic nodule with a central artery in the center which was not found in iPSCs formed tumors (400×); N–R. Two sets of photos showing similar spherical shaped small tumors from two different animals at 8 weeks (n and p = 12.5×; o, q and r = 100×).
Angiogenesis related tests
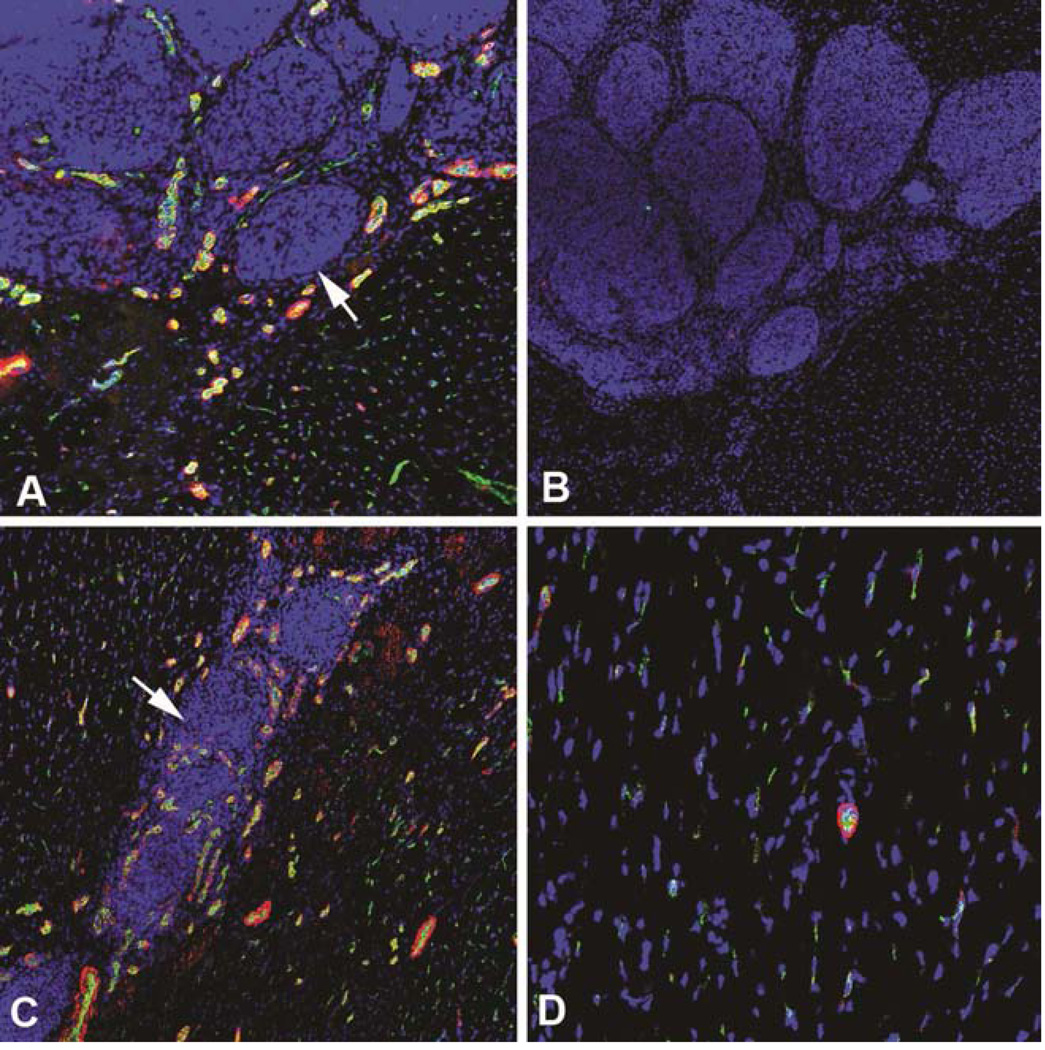
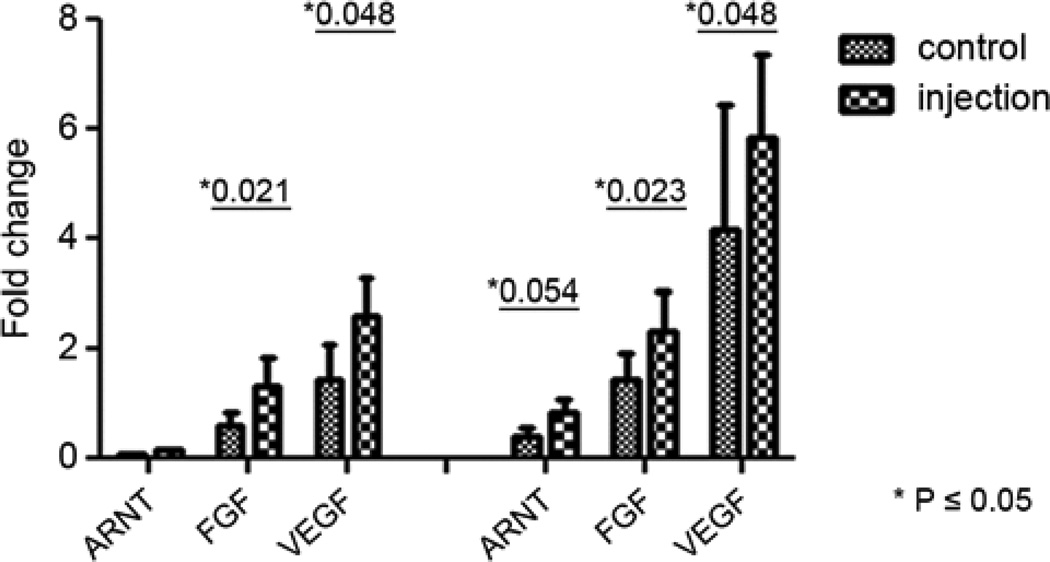
At the 4 and 8 week time points, immunofluorescent staining showed increased smooth muscle actin and vWF positive cells inside and around the iPSC clusters (Figure 4 a, c) compared with those in non-injected areas (Figure 4 d). This finding may suggest that injected iPSCs are associated with angiogenesis. No desmin positive cells were found within or around these cell clusters. The expression of VEGF, FGF, and ANRT, as assessed by qRT-PCR, were significantly higher in the injected myocardium compared with non-injected myocardium both in the 4 week and 3 month time points (Figure 5). These results suggested that injected iPSCs might have contributed to the formation of new blood vessels to a comparable level as those injected with MSCs. This angiogenic effect may have occurred as a result of paracrine like secretion of pro-angiogenesis factors. This finding suggests a beneficial effect of the iPSC transplantation even though injected iPSCs were not sufficient enough to regenerate the damaged heart.
Figure 4.
A. Smooth muscle actin (red) and vWF (green) staining at 8 weeks. Arrow indicating a cell clump. B. Negative control of A. C. Staining of A at 4 weeks; D. Staining of A at non-injected area at 4 weeks after injection. Magnifications a–d = 100×.
Figure 5.
Expression of VEGF, FGF, ANRT by qRT-PCR were significantly higher in the injected myocardium compared with none injected both in 4 week samples (left panel) and 3 month samples after myocardial-derived iPSC transplantation.
Allogeneic transplantation of another iPSC cell line
Similar results were found from the animals that received another strain of transplanted iPSCs. Spherical and irregular shaped small tumors were found in 4 to 8 week time points and the sizes decreased over 3 months. The small tumors stained positive for Oct4 and SSEA4 suggesting that injected iPSCs may keep their pluripotency markers, but not differentiate in other cell linages. Figure 6 shows the histology images with a larger amount of small tumors in the 8 week time point, which dramatically decrease in the 3 month time point.
Figure 6.
Histologic analysis of the animals that received cell transplantation with the iPSCs strain provided by Michael Robert’s lab. A. Arrow indicating a group of irregular small tumors at 8 weeks (12.5×); B. Higher magnification of the boxed area in A (100×); C. Oct4 staining (400×); D. SSEA4 staining (400×); E. Arrow indicating cell clumps at three months following injection (50×); F. Higher magnification of E (200×); G and H showing images from two separate animals at three months (50× and 200×).
Comment
We report the first in vivo iPSC allogeneic transplantation into the ischemic myocardial environment of a large animal model that focuses on the fate and differentiation abilities of the transplanted cells. We expected myocardium-derived iPSCs developed in our laboratory would preferentially differentiate into the myocyte lineage compared to those derived from skin, blood or other non-muscle lineage cells. The myocardium-derived iPSCs carry myocardial epigenetic memory, which most investigators believe will influence their differentiation capabilities (21–23). Unfortunately, our data did not support this expectation. Rather, the iPSCs derived from pig myocardium only showed limited myocyte differentiation as we only found a few GATA4-positive cells in dissociated embryoid bodies.. No beating myocytes were found when various cultural protocols were performed. The iPSC line provided by Dr. Roberts’ group, which was derived from porcine fetal fibroblasts, also lacked the ability to differentiate into beating myocytes in our hands. The underlining reason for this phenomenon is unclear, but one possibility is that the lentiviral vectors with the four reprogramming genes, which are integrated into the genome, are not completely silenced. The residual levels of these transcription factors may hinder the efficient differentiation into cardiac lineages of the injected iPSCs.
Even though both lines of iPSCs failed to show any signs of differentiation when transplanted into ischemic myocardium by direct injection, this study did provide us with several important findings: 1) when allogeneically transplanted, the iPSCs proliferated in the early stage, reached a peak level at 8 weeks post transplantation, then gradually declined in cell number; 2) once transplanted, iPSCs showed pro-angiogenic effects potentially as a result of paracrine-like secretion of factors which enhanced the pro-angiogenic signal pathways of VEGF, FGF and ANRT; 3) compared with our previous study on MSCs transplantation in the same porcine model, they both showed beneficial effects on angiogenesis, but iPSCs seemed to possess a greater aptitude towards survival and proliferation; 4) The recipient pigs were generally healthy and there were no signs of deleterious tumor formation. However, we did find tumor-like cell clumps in the lung and spleen of one animal at the 6 week time point, suggesting that the injected iPSCs can survive and proliferate in organs other than the heart. This is most likely caused by accidental cell leaking into the blood stream at the time of injection, rather than metastasis. Therefore, extra caution must be exercised to avoid leakage when performing direct injection in future clinical cell-based therapy. The consequences for the leaked iPSCs are not yet clear. More detailed long term studies are needed to investigate whether they will interfere with the function of other organs and whether they will form teratomas, although most of these leaked cells appeared to be short lived. The current study terminates at three months post transplantation. It is very useful for quickly assessing the survival and differentiation potential of iPSCs in vivo, but long term observation and analysis are needed for unequivocally demonstrate whether this method is safe enough for clinical uses.
In summary, in an ischemic environment, pig iPSCs can continue to proliferate in vivo for two months after injection. However, the proliferation ability of the iPSCs was limited within the immunoconpetent hosts. The iPSCs we developed from pig myocardial fibroblasts showed limited ability to differentiate into beating myocytes. For future cell-based therapy, iPSCs with a higher propensity to differentiate into caydiomycytes will be ideal for assessing their potential for treating myocardial infarction. We are planning to use more efficient but non-integrative vectors for reprogramming cardiac fibroblasts into iPSCs.
Acknowledgments
The authors are particularly grateful to Dr. R. Michael Roberts and his colleagues for providing us with the porcine fetal fibroblast-derived iPSCs.
Abbreviations and Acronyms
- ANRT
aryl hydrocarbon receptor nuclear translocator
- bFGF
basic fibroblast growth factor
- DMEM
Dulbecco's Modified Eagle Medium
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- iPSC
induced pluripotent stem cell
- MEF
mouse embryonic fibroblasts
- MSC
mesenchymal stromal cell
- NHLBI
National Heart, Lung, and Blood Institute
- RT-PCR
real-time polymerase chain reaction
- VGEF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study had been presented in the STS 50th Annual Meeting 2014 in Orlando, Florida
References
- 1.Britten MB, Abolmaali ND, Assmus B, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI)-machanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 2.Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction-final one-year results of the TOPCARE-AMI trial. J Am Coll Cardial. 2004;44:1690. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Strauer BE, Brehm M, Zeus T, et al. Repair of infracted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease the IACT study. J Am Coll Cardial. 2005;46:1651. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 5.Perin EC, Dohmann HFR, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs S, Baffour R, Zhou YF, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs S, Satler LF, Kornowski R, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease. J Am Coll Cardial. 2003;41:1721. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardial. 2004;94:92. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho PH, Daibert AP, Monteiro BS, Okano BS, Carvalho JL, Cunha DN, Favarato LS, Pereira VG, Augusto LE, Del Carlo RJ. Differentiation of adipose tissue-derived mesenchymal stem cells into cardiomyocytes. Arq Bras Cardiol. 2013;100(1):82–89. doi: 10.1590/s0066-782x2012005000114. [DOI] [PubMed] [Google Scholar]
- 10.Kang PL, Chen CH, Chen SY, Wu YJ, Lin CY, Lin FH, Kuo SM. Nano-sized collagen I molecules enhanced the differentiation of rat mesenchymal stem cells into cardiomyocytes. J Biomed Mater Res A. 2013;101(10):2808–2816. doi: 10.1002/jbm.a.34589. [DOI] [PubMed] [Google Scholar]
- 11.Mohanty S, Bose S, Jain KG, Bhargava B, Airan B. TGFβ1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2013;163(1):93–99. doi: 10.1016/j.ijcard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento DS, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, Araújo AF, Valente M, Almeida J, Martins JP, Santos JM, Bárcia RN, Cruz P, Cruz H, Pinto-do-Ó P. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling following myocardial infarction by pro-angiogenic, anti-apoptotic and endogenous cell activation mechanisms. Stem Cell Res Ther. 2014;5(1):5. doi: 10.1186/scrt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mureli S, Gans CP, Bare DJ, Geenen DL, Kumar NM, Banach K. Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. Am J Physiol Heart Circ Physiol. 2013;304(4):H600–H609. doi: 10.1152/ajpheart.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29(2):251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 15.Tőgel F, Weiss k, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 16.Rabb H. Paracrine and differentiation mechanisms underlying stem cell therapy for the damaged kidney. Am J Physiol Renal Physiol. 2005;289:F29–F30. doi: 10.1152/ajprenal.00102.2005. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. PNAS. 2009;106(27):10993–10998. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9(1):17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Shtrichman R, Germanguz I, Itskovitz-Eldor J. Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine. Curr Mol Med. 2013;13(5):792–805. doi: 10.2174/1566524011313050010. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Wang S, Yu Z, Hoyt RF, Jr, et al. Direct Injection of Autologous Mesenchymal Stem Cells Improves Myocardial Function. BBRC. 2009;390:902–907. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Wang S, Yu Z, Hoyt RF, Jr, Qu X, Horvath KA. Marrow stromal cells differentiate into vasculature after allogeneic transplantation into ischemic myocardium. Ann Thorac Surg. 2011;91(4):1206–1212. doi: 10.1016/j.athoracsur.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger EF, Banai S, Shou M, et al. A model to assess interventions to improve collateral blood flow: continuous administration of agents into the left coronary artery in dogs, Cardiovasc. Res. 27. 1993:785–791. doi: 10.1093/cvr/27.5.785. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Zhou Y, Seavey CN, Singh AK, Xu X, Hunt T, Hoyt RF, Jr, Horvath KA. Rapid and dynamic alterations of gene expression profiles of adult porcine bone marrow derived stem cell in response to hypoxia. Stem Cell Res. 2010;4(2):117–128. doi: 10.1016/j.scr.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]