Abstract
Maintenance of hematopoietic progenitors ensures a continuous supply of blood cells during the lifespan of an organism. Thus, understanding the molecular basis for progenitor maintenance is a continued focus of investigation. A large pool of undifferentiated blood progenitors are maintained in the Drosophila hematopoietic organ, the larval lymph gland, by a complex network of signaling pathways that are mediated by niche-, progenitor-, or differentiated hemocyte-derived signals. In this study we examined the function of the Drosophila fibroblast growth factor receptor (FGFR), Heartless, a critical regulator of early lymph gland progenitor specification in the late embryo, during larval lymph gland hematopoiesis. Activation of Heartless signaling in hemocyte progenitors by its two ligands, Pyramus and Thisbe, is both required and sufficient to induce progenitor differentiation and formation of the plasmatocyte-rich lymph gland cortical zone. We identify two transcriptional regulators that function downstream of Heartless signaling in lymph gland progenitors, the ETS protein, Pointed, and the Friend-of-GATA (FOG) protein, U-shaped, which are required for this Heartless-induced differentiation response. Furthermore, cross-talk of Heartless and target of rapamycin signaling in hemocyte progenitors is required for lamellocyte differentiation downstream of Thisbe-mediated Heartless activation. Finally, we identify the Drosophila heparan sulfate proteoglycan, Trol, as a critical negative regulator of Heartless ligand signaling in the lymph gland, demonstrating that sequestration of differentiation signals by the extracellular matrix is a unique mechanism employed in blood progenitor maintenance that is of potential relevance to many other stem cell niches.
Keywords: Drosophila, Lymph gland, Myeloid, Progenitors, FGFR, Perlecan
Introduction
The maintenance of hematopoietic stem cells is a crucial process for the normal production of blood cells, but in addition, understanding its molecular basis could enhance the therapeutic benefits of this cell population. Studies of hematopoiesis and the regulation of hematopoietic progenitors have identified a variety of molecular mechanisms that regulate progenitor/stem cell maintenance (Arai and Suda, 2007; Seita and Weissman, 2010; Teitell and Mikkola, 2006). As in vertebrate systems, Drosophila hematopoiesis requires a population of multipotent progenitor cells that give rise to all differentiated hemocyte lineages (Mandal et al., 2007). Previous studies of Drosophila larval hematopoiesis have uncovered a complex network of signaling pathways that cooperate to regulate hemocyte progenitor maintenance. These include niche-derived Hedgehog signaling (Krzemien et al., 2007; Mandal et al., 2007), an adenosine deaminase growth factor A-mediated signal emanating from differentiated hemocytes (Mondal et al., 2011), and Wingless signaling, which autonomously regulates progenitor cell maintenance (Sinenko et al., 2009). In contrast, the signal(s) required for differentiation of Drosophila hemocyte progenitors during larval development remain largely unknown.
One wave of hematopoiesis in Drosophila occurs in the larval lymph gland (Lebestky et al., 2000; Tepass et al., 1994). Studies of the lymph gland have allowed genetic dissection of signaling networks that operate in a niche-, progenitor- or differentiated hemocyte-dependent manner to maintain blood homeostasis, owing to the ability to perform cell-type specific genetic manipulation with direct in vivo imaging of its effects on these distinct cell populations. In the lymph gland, multipotent blood progenitors termed prohemocytes express high levels of Drosophila (d)E-cadherin, or Shotgun, and are compactly arranged in a medial region of both primary lobes termed the medullary zone (MZ; Fig. 1A) (Jung et al., 2005). These prohemocytes give rise to all mature, myeloid-like hemocyte lineages (Jung et al., 2005): macrophage-like plasmatocytes, platelet-like crystal cells, and lamellocytes, which are not normally present in a healthy larva but are induced to differentiate upon parasitic wasp infection (Lanot et al., 2001; Sorrentino et al., 2002). Differentiated hemocytes that arise from lymph gland prohemocytes populate the peripheral cortical zone (CZ; Fig. 1A) (Jung et al., 2005) and do not enter circulation until the onset of metamorphosis (Grigorian et al., 2011b). Finally, the posterior signaling center (PSC; Fig. 1A), a small group of cells at the posterior tip of each primary lobe, functions as a hematopoietic niche by supplying pro-maintenance signals, such as Hedgehog, to progenitors (Krzemien et al., 2007; Lebestky et al., 2003; Mandal et al., 2007). The strict dependence of Drosophila progenitor maintenance on the PSC (Krzemien et al., 2007; Mandal et al., 2007) highlights the unique role within the lymph gland of a hematopoietic niche devoted to the maintenance of myeloid lineage-restricted progenitors.
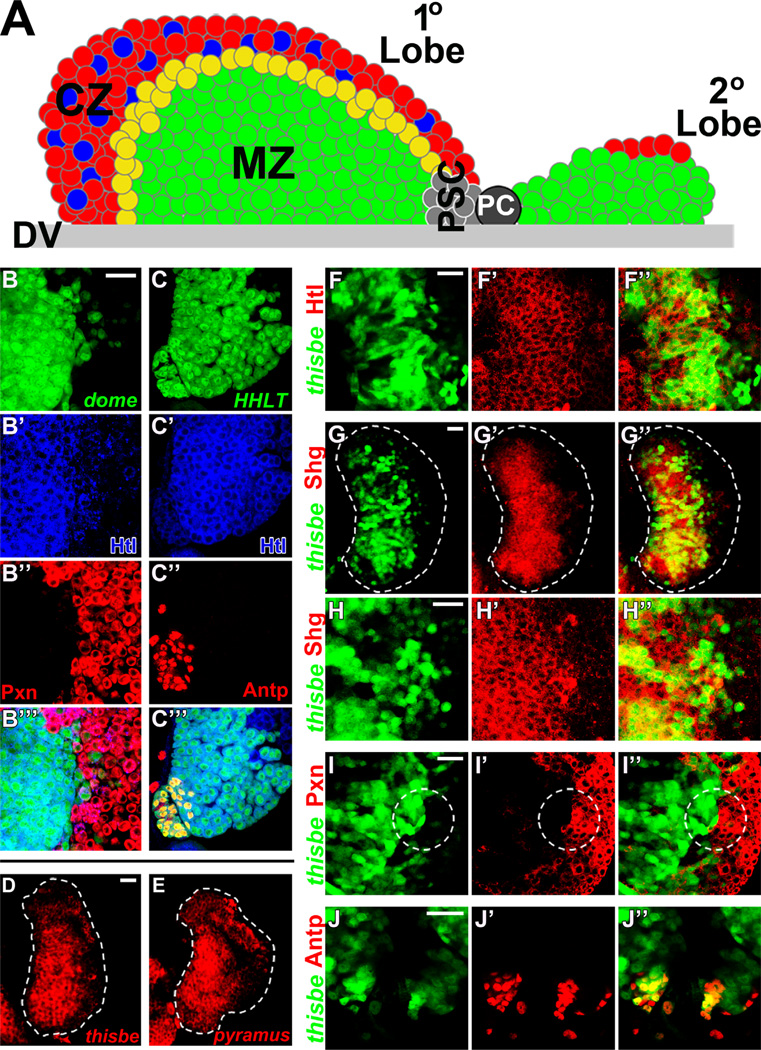
Fig. 1. Expression patterns of Heartless and its ligands, Thisbe and Pyramus, in the lymph gland.
All panels represent wild-type lymph glands. In panels B–B”’ dome-Gal4 is used to drive UAS-2xEGFP (green) expression in MZ prohemocytes. In panels C–C”’ HHLT (hand-gal4, hml-gal4, UAS-2xEGFP, UAS-FLP; A5C–FRT-STOP-FRT-gal4) is used to clonally express UAS-2xEGFP (green) in lymph gland hemocytes. In panels F–J” thisbe-Gal4 drives UAS-2xEGFP (green) expression in thisbe-expressing hemocytes. Peroxidasin (Pxn) expression is shown in red in panels B”-B”’ and I’-I”. Antennapedia (Antp) expression is shown in red in panels C”-C” and J–J”. Heartless (Htl) expression is shown in blue in panels B–C” and in red in panels F–F”. The MZ marker, Shotgun (Shg), is shown in red in panels G–G”. Thisbe transcript expression is shown in red in panel D, and pyramus transcript expression is shown in red in panel E. (A) Schematic diagram of a wandering third instar lymph gland primary and secondary lobe. Differentiated plasmatocytes (red) and crystal cells (blue) localize to the peripheral cortical zone (CZ). Prohemocytes (green) are compactly arranged in the Medullary Zone (MZ). Posterior signaling center (PSC) cells (gray) localize to the posterior tip of the primary lobe. Secondary lobes mostly consist of undifferentiated progenitors (green) with few differentiated hemocytes (red). Pericardial cells (PC) intercalate between the primary and secondary lobes. DV, dorsal vessel. (B–B”’) Heartless (Htl, blue) is strongly expressed in dome+ hemocyte progenitors of the MZ (green), and is largely reduced in Pxn+ (red) differentiated hemocytes of the peripheral CZ. (C–C”) Heartless (blue) is detected in Antp+ cells of the PSC (red) at lower levels than adjacent hemocytes of the lymph gland. (D–E) Fluorescence in situ hybridization against thisbe (D) or pyramus (E) mRNA transcripts demonstrates highest expression in MZ hemocytes. (F–F”) Thisbe (green) expression overlaps (yellow) with Heartless (red) expression in medial lymph gland regions, although not all Heartless+ cells are thisbe+. Thisbe > GFP is strongly expressed in a subset of MZ cells. (G–H”) Thisbe (green) expression overlaps (yellow) with Shotgun (Shg, red) in the MZ, confirming highest thisbe expression in MZ prohemocytes and reduced or negative thisbe expression in Shotgun-negative hemocytes of the peripheral CZ. (I–I”) High thisbe (green) expression rarely overlaps (yellow) with high Pxn expression, although thisbe expression often extends to the most medial Pxn+ hemocytes of the CZ. Co-expression of low levels of thisbe and low levels of Pxn are also observed in some scattered hemocytes. (J–J”) Most, but not all, Antp+ PSC cells express thisbe (green, overlap in yellow). Scale bars=50 µm. Scale bar in A corresponds to A–B”’; scale bar in I corresponds to I–J.
Similar to the vertebrate aorta-gonadal mesonephros (AGM), wherein hematopoietic and vascular lineages are derived from a common progenitor cell called the hemangioblast (Ema et al., 2003; Medvinsky and Dzierzak, 1996), a common origin of Drosophila vascular cells and hematopoietic lymph gland progenitors has been described (Mandal et al., 2004). Specifically, the Drosophila lymph gland, vascular cardioblasts (heart and aorta), and pericardial nephrocytes all arise from the cardiogenic mesoderm late in embryonic development. Clonal analysis demonstrated that a single cardiogenic mesoderm cell can give rise to both lymph gland and vascular cells, providing evidence for a Drosophila hemangioblast, akin to the vertebrate AGM (Mandal et al., 2004). Amongst the signaling pathways required for development of all three lineages derived from the cardiogenic mesoderm is the fibroblast growth factor receptor (FGFR) homolog, Heartless (Htl) (Beiman et al., 1996; Frasch, 1995; Mandal et al., 2004). Loss-of-function mutations in Heartless result in the absence of a heart/cardioblasts (Shishido et al., 1997), and impair lymph gland progenitor development (Mandal et al., 2004). In contrast, constitutive activation of Heartless in the cardiogenic mesoderm increases the number of blood progenitors, nephrocytes, and cardioblasts (Grigorian et al., 2011a).
Heartless is one of only two Drosophila FGFRs and is activated by two FGF-8-like ligands, Pyramus (Pyr) and Thisbe (Ths) (Gryzik and Müller, 2004; Stathopoulos et al., 2004). Heartless signaling has been studied extensively in the context of mesoderm development, migration during gastrulation, and differentiation of mesodermal lineages (Beiman et al., 1996; Gisselbrecht et al., 1996; Klingseisen et al., 2009; McMahon et al., 2010). It has also been shown to play a major role in glial cell migration and differentiation in the eye (Franzdottir et al., 2009). The second Drosophila FGFR, Breathless, is activated by a single FGF ligand, branchless, and is critical for morphogenesis of the trachea during embryonic, larval and pupal developmental stages (reviewed in Cabernard et al., 2004). With only three potential FGF-FGFR combinations, Drosophila FGFR signaling is less complex than in vertebrates, wherein 22 FGF ligands and four FGFRs contribute to over 120 potential FGF-FGFR interactions (Zhang et al., 2006). Despite this apparent simplicity, recent studies have provided evidence for overlapping functions of Pyramus and Thisbe, in which they act redundantly to provide robust signaling, as well as distinct functions due to their differential effects on Heartless activation (Franzdottir et al., 2009; Kadam et al., 2009; Klingseisen et al., 2009).
Despite the functional requirement of Heartless signaling during development of early lymph gland progenitors in the late embryo, a role for Heartless signaling in the larval lymph gland is currently unknown. Here, we provide evidence that activation of Heartless signaling by both of its ligands, Thisbe and Pyramus, is both required and sufficient for differentiation of hemocyte progenitors in the lymph gland. We demonstrate that these effects are Ras-mediated, and dependent on two downstream transcriptional effectors, Pointed and U-Shaped, as well as on cross-talk with the target of rapamycin (TOR) growth signaling pathway. Finally, we identify the Drosophila heparan sulfate proteoglycan, Trol, as a crucial modulator of Heartless signaling in the lymph gland, demonstrating that sequestration of differentiation signals by the extracellular matrix is a unique mechanism utilized to maintain blood progenitors, in addition to niche-, progenitor-, and differentiated hemocyte-generated maintenance signals.
Materials and methods
Drosophila stocks and crosses
All RNAi lines used in the described experiments were obtained from the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria). The following Drosophila stocks were used: UAS-heartlessDN, UAS-[d4EBP(LL)] and thisbe-Gal4 (Bloomington); UAS-heartlessAct (A. Michelson); UAS-thisbeWT and UAS-pyramusWT (A. Stathopoulos); FRT82B heartlessAB42 and FRT42B pyramusEx36 (unpublished reagents; kindly provided by A. Stathopoulos); UAS-rasDN, UAS-ras85BAct, UAS-rolled (MAPK)Act, UAS-pointedP2Act, and Trol-GFP protein trap line ZCL1973X (V. Hartenstein); UAS-u-shapedWT (N. Fossett); FRT82B ras85BΔC40B and FRT82B pointedΔ88 (J. Bateman); UAS-vein, UAS-secreted-spitz, UAS-pvf1, and UAS-pvf2 (U. Banerjee); UAS-secreted-gurken and UAS-secreted-keren (A. Simcox); UAS-jelly-belly (J. Weiss); UAS-branchless (M. Krasnow); hh-F4f-GFP (R. Schulz).
Hemocyte progenitor-specific gene expression was performed using dome-gal4, UAS-2xEGFP (S. Noselli). Expression of UAS-pyramusWT, UAS-u-shapedWT or UAS-pointedRNAi genetic constructs in hemocyte progenitors was performed using dome-gal4; P{tubP-gal80[ts20]} (U. Banerjee) to permit larval viability; larvae were shifted from the permissive temperature (18 °C) to the restrictive temperature (29 °C) at the onset of the second instar stage.
Clonal analysis
FLP-out clones in the lymph gland were generated using HHLT (hand-gal4, hml-gal4, UAS-2xEGFP, UAS-FLP; A5C-FRT-STOP-FRT-gal4) as described (Evans et al., 2009). Genotypes for MARCM FRT clones (Mosaic Analysis with a Repressible Cell Marker) (Lee and Luo, 1999) are as follows: hs-flp FRT82B heartlessAB42 FRT82B Tub-mCD8-GFP; hs-flp FRT82B pointedΔ88 FRT82B Tub-mCD8-GFP; and hs-flp FRT82B rasΔC40B FRT82B Tub-mCD8-GFP. Genotypes for FLP-FRT clones are as follows: FRT42B pyramusEx36 FRT42B UbiGFP; hs-flp. Clones were induced by a 2-hour heat shock at 37 °C in late embryos or early first instar larvae.
Larval staging
Staging was performed at 25 °C and the following time-points after egg hatching (AEH) were used for larval stages: 54 h AEH (second instar, L2), 72 h AEH (early third instar, eL3), 116 h AEH (wandering third instar, wL3).
Immunohistochemistry
Primary antibodies used: mouse anti-Peroxidasin (Pxn, 1:300; J. Fessler), rabbit anti-prophenoloxidase (PPO, 1:300; M. Kanost), mouse anti-Pi and anti-L1 (1:20; I. Ando), rabbit anti-p4EBP (1:300; Cell Signaling), rat anti-Heartless and anti-PVR (1:400, B. Shilo), rat anti-Shotgun (1:400, V Hartenstein), rat anti-Sprouty (1:400, M. Krasnow), and mouse anti-Antp (1:20, DSHB). Lymph glands were dissected from staged larvae in 1xPBS and then fixed for 20 min in 3.7% formaldehyde. Lymph glands were blocked in 10% NGS with 0.4% TritonX-100 in 1xPBS (PBST) for 1 h at room temperature. Primary antibodies were diluted in PBST and incubated with lymph glands in 96-well MicroWell plates (Thermo Scientific) overnight at 4 °C in a humidified chamber. Lymph glands were then washed 4 × 15 min in PBST with gentle shaking. Secondary antibodies (Jackson Immunoresearch) were prepared in 10% NGS in 0.4%PBST at a concentration of 1:400 and incubated with lymph glands overnight in a dark, humidified chamber at 4 °C. Lymph glands were then washed 4 × 15 min in PBST with gentle shaking and 1 × 15 min in 1 × PBS. Lymph glands were mounted using Vectashield (Vector Laboratories). Samples were imaged using a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope.
In situ hybridization
Tissue dissection, in situ hybridization and signal amplification with fluorescent-labeled antibodies were performed as in Nagaraj et al. (2012). For the anti-pyramus probe, a 561-base-pair DNA fragment from genomic DNA was amplified via PCR with Pyramus forward (5’-CGATGCTGATATGCAACTGG-3’) and Pyramus reverse primers (5’-TAATACGACTCACTATAGGCTGAGGCCACGAAGAAGTTT-3’), which has the RNA polymerase T7 promoter (sequence designed by Young Bae, A. Stathopoulos Lab). For the anti-thisbe probe, a 559-base-pair DNA fragment from genomic DNA was amplified via PCR with Thisbe forward (5’-CTCCCAGCTGGTGATAGAGC-3’) and Thisbe reverse primers (5’-TAATACGACTCACTATAGGATCGATGTCGATATCTCCGC-3’), which has the RNA polymerase T7 promoter (sequence designed by Young Bae, A. Stathopoulos Lab).
Rapamycin treatment
Crosses were set up on 15 µM rapamycin food plates and larvae were grown at 25 °C until wandering third instar stage.
Quantification of Pxn+ hemocyte population distribution (% area) in the lymph gland
Methodology to determine the proportion of Pxn+ hemocytes in the lymph gland was performed as in Dragojlovic-Munther and Martinez-Agosto (2012). Statistical significance of data was assessed with Student’s t-test.
Results
Heartless, Thisbe and Pyramus expression in the lymph gland
Although Heartless signaling is required to generate lymph gland progenitors in the embryo, a role for Heartless signaling in the larval lymph gland remains unknown. We reasoned that a role for Heartless in hematopoiesis would extend to later stages of lymph gland development, given its essential role in the embryo. We therefore first examined Heartless expression in the lymph gland at distinct larval stages. Undifferentiated hemocyte progenitors in the lymph gland are identified by positive expression of the Janus tyrosine kinase/signal transducers and activators of transcription (JAK/STAT) receptor domeless (dome), and negative expression of the early differentiation marker, Peroxidasin (Pxn) (dome+/Pxn−), a component of extracellular matrix (Nelson et al., 1994). As progenitors begin to differentiate, they co-express both dome and Pxn at low levels (dome+/Pxn+) and represent a population of intermediate progenitors (Dragojlovic-Munther and Martinez-Agosto, 2012; Krzemien et al., 2010). During the second larval instar stage (L2) the lymph gland is largely undifferentiated, but a small population of differentiating progenitors (dome+/Pxn+) begin to emerge at the lymph gland periphery (Fig. S1A). Heartless expression is detected throughout the entire second instar lymph gland (Fig. S1A’), and overlaps completely with the progenitor marker, dome (Fig. S1A”). By early third instar stage (eL3), the number of Pxn+ hemocytes expands, forming an early CZ (Fig. S1B). Heartless expression is still detected throughout the lymph gland (Fig. S1B’) but is most strongly expressed in dome+ progenitors (Fig. S1B”). By the wandering third instar stage (wL3), at the end of larval development, a mature CZ is observed at the lymph gland periphery (Fig. S1C). Heartless expression is still detected at high levels throughout the population of dome+ progenitors but is absent in a subset of dome−/Pxn+ hemocytes in the peripheral CZ (white arrows, Fig. S1C-C” and 1B–B”’). In addition to high Heartless expression in dome+ MZ prohemocytes (Fig. 1B–B”’), Heartless expression is also detected in Antennapedia+ (Antp+) cells of the posterior signaling center (PSC) (Fig. 1C–C”’).
Unlike the homogenous expression of Heartless during the second and early third instar stages, expression of its ligand thisbe is more restricted in the lymph gland, and is not found in all Heartless+ hemocytes (Fig. S1D–E’). Interestingly, thisbe-negative hemocytes often correlate with Pxn expression in the lymph gland periphery, suggesting that thisbe expression may turn off upon hemocyte differentiation (Fig. S1G–I’). By wandering third instar stage, high thisbe expression is observed in medial, Heartless+ lymph gland regions (Fig. S1F–F’ and F-F”). We confirmed the expression of thisbe in hemocyte progenitors of the MZ by demonstrating overlap of thisbe expression and the MZ marker, Shotgun (Jung et al., 2005) (Fig. 1G–H”), as well as by the detection of endogenous transcripts in the MZ (Fig. 1D). A similar pattern of expression was observed for pyramus transcripts (Fig. 1E). Also at this late stage, thisbe expression remains largely absent in peripheral Pxn+ differentiated hemocytes of the mature CZ (Fig. S1I–I’ and I-I”). In addition to peripheral thisbe−/Pxn+ hemocytes (white arrows, Fig. S1H–I’), scattered thisbe− cells are also sometimes observed in medial lymph gland regions, which are undifferentiated (yellow arrows, Fig. S1H–I’). Finally, thisbe expression is also detected in a subset of Antp+ PSC cells (Fig. 1J–J”). These patterns of expression for Heartless and its ligands in the lymph gland suggested a potential role for Heartless signaling in regulating lymph gland hemocyte progenitors.
Heartless signaling in lymph gland prohemocytes regulates their differentiation
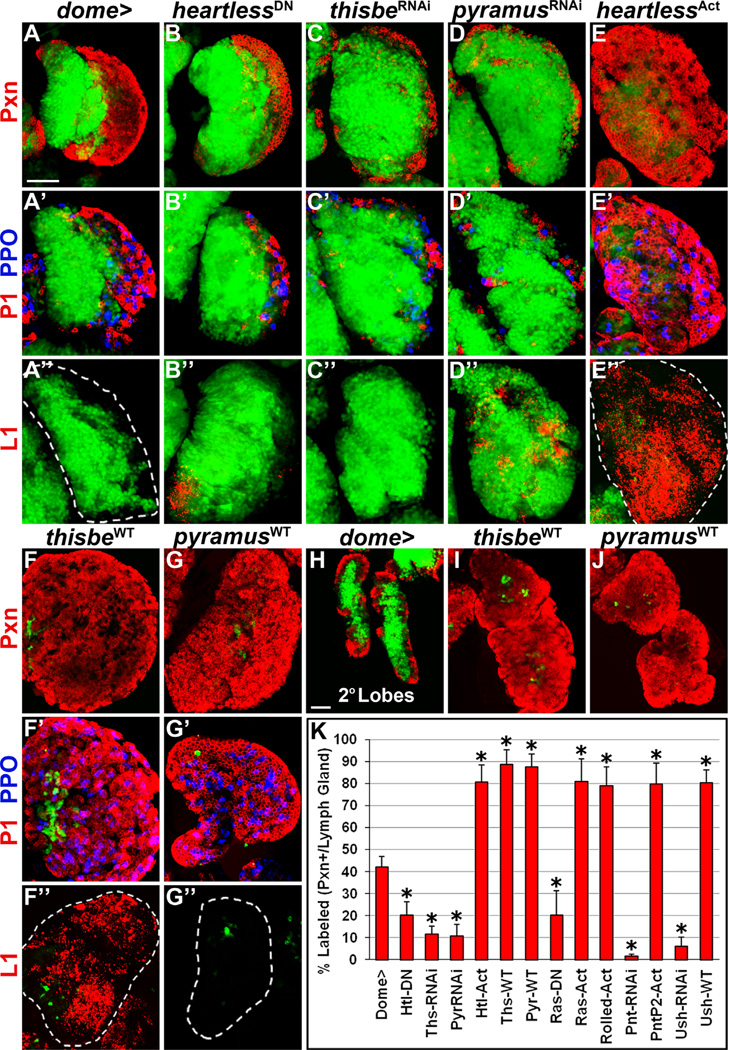
We next examined the functional role of Heartless in prohemocytes by performing progenitor-specific loss-of-function and gain-of-function analyses in the lymph gland. Wild-type (WT) lymph glands at wandering third instar stage are composed of 42 ± 5% Pxn+ hemocytes (Fig. 2A and K). Measuring the proportion of Pxn-labeled hemocytes in the lymph gland is a good indicator of relative changes in CZ size and lymph gland differentiation status (Dragojlovic-Munther and Martinez-Agosto, 2012; Krzemien et al., 2010; Shim et al., 2012). Specifically decreasing Heartless signaling in prohemocytes by overexpression of a dominant-negative (DN) heartless allele significantly decreases the proportion of Pxn+ differentiated hemocytes in the lymph gland to 20 ± 6% (p < 0.0001, Fig. 2B and K). Terminally differentiated plasmatocytes (labeled with P1) and crystal cells (labeled with Prophenoloxidase, PPO) are restricted to a thin peripheral layer compared to wild-type (compare Fig. 2B’ to A’). In addition, a small number of lamellocytes (L1+) are observed (42% of lymph glands, n = 150, Fig. 2B”), unlike in wild-type (Fig. 2A”).
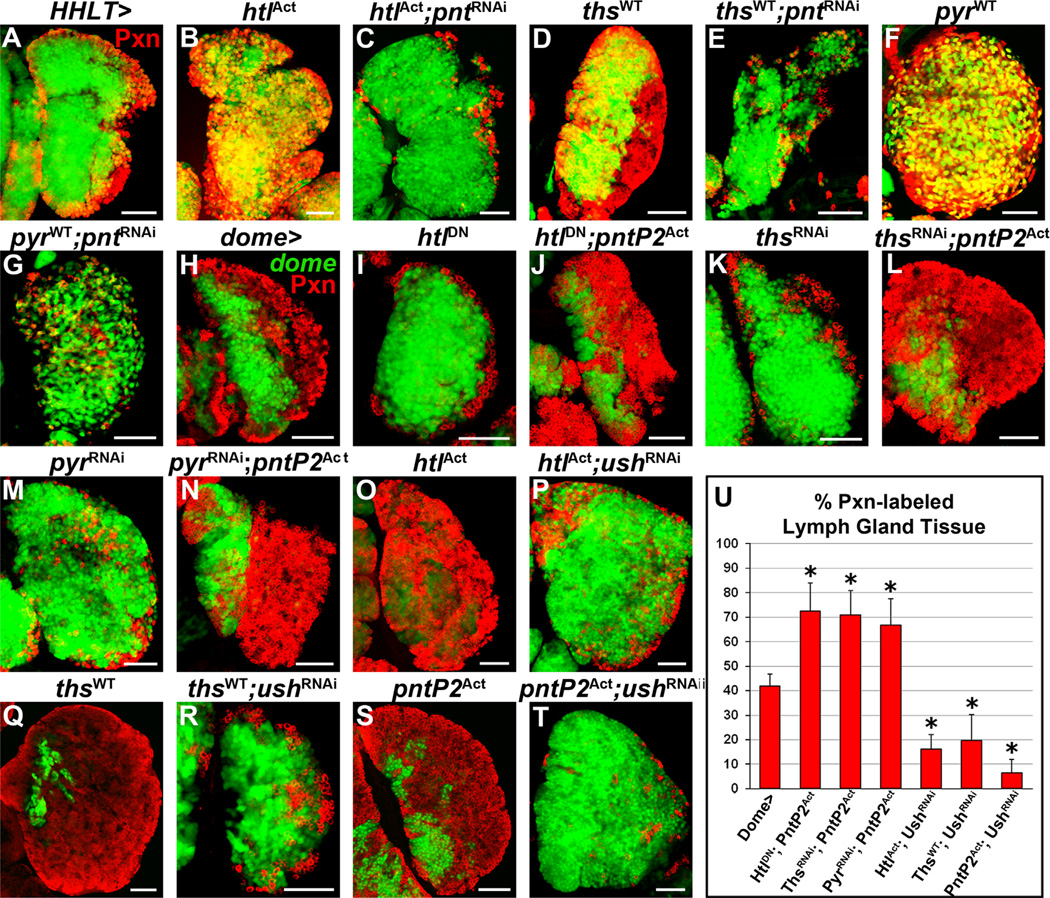
Fig. 2. Heartless and its ligands regulate progenitor differentiation in the lymph gland.
All panels represent wandering third instar. In all panels, dome-Gal4, UAS-2xEGFP (wild-type, A–A”, H) is used to express UAS-heartlessDN (B–B”), UAS-thisbeRNAi (C-C”), UAS-pyramusRNAi (D–D”), UAS-heartlessAct (E–E”), UAS-thisbeWT (F–F”, I), and UAS-pyramusWT(G-G”, J), in prohemocytes (green). Differentiation markers are labeled to the left of corresponding rows. (A–A”) Wild-type lymph gland primary lobe. Peroxidasin (Pxn, red, A) is expressed in differentiated hemocytes (dome) as well as in a small population of dome+/Pxn+ intermediate progenitors at the MZ/CZ boundary. P1 (red, A’) and prophenoloxidase (PPO, blue, A’) label terminally differentiated plasmatocytes and crystal cells, respectively. L1 (red, A”) marks lamellocytes, not present in wild-type. (B–D”) Overexpression of heartlessDN (B) or downregulation of thisbe (C) or pyramus (D) in prohemocytes impairs CZ formation. Plasmatocytes and crystal cells are restricted to a thin peripheral layer compared to wild-type (compare B’, C, and D’ to A’). Reduced Heartless function in prohemocytes induces rare lamellocytes in the lymph gland (42% of lymph glands, n = 150, B”), but thisbe downregulation does not (C”), while pyramus downregulation induces many lamellocytes to differentiate in the lymph gland (78% of lymph glands, n=64, D”). (E–G”) Overexpression of heartlessAct (E), thisbeWT (F), or pyramusWT (G) is sufficient to induce almost the complete loss of dome+/Pxn prohemocytes due to premature differentiation into plasmatocytes and crystal cells (E’, F’, and G’). While robust lamellocyte differentiation occurs upon heartlessAct (82% of lymph glands, n=60, E”) or thisbeWT (88% of lymph glands, n=42, F”) overexpression in prohemocytes, lamellocytes are not observed upon pyramusWT overexpression (G”). (H) Wild-type lymph gland secondary lobes. Lymph gland secondary lobes consist mostly of undifferentiated dome+/Pxn hemocytes, although a few differentiating Pxn+ hemocytes are observed at the periphery. (I–J) Overexpression of thisbeWT (I) or pyramusWT (J) in dome+ hemocytes is sufficient to induce severe hypertrophy and differentiation of lymph gland secondary lobes. (K) Quantification of Pxn+ hemocyte population distribution (% area) in the lymph gland. Data are represented as mean ± s.d., n=10. Asterisks denote statistically significant results compared to wild-type (dome >), measured by Student’s t-test. List of abbreviations: Heartless (Htl), Thisbe (Ths), Pyramus (Pyr), Pointed (Pnt), U-shaped (Ush). Scale bars=50 µm. Scale bar in A corresponds to A–G” except for the following additional magnifications: X0.8 for F and F’ and X0.7 for E” and F”. Scale bar in H corresponds to H–J.
Surprisingly, downregulating the expression of the Heartless ligands, thisbe or pyramus, in lymph gland progenitors had an even stronger effect on hindering progenitor differentiation, such that lymph glands are composed of only 12 ± 4% or 11 ± 5% Pxn+ differentiating hemocytes, respectively (p < 0.0001, Fig. 2C, D, and K). Plasmatocytes and crystal cells are likewise restricted to a very thin peripheral lymph gland layer (Fig. 2C and D’). Lamellocytes are not observed upon thisbe downregulation (Fig. 2C”), but are detected throughout the lymph gland upon pyramus downregulation, intermixed among the expanded progenitor population (Fig. 2D”).
In contrast to the reduced differentiation that occurs upon decreasing Heartless signaling, progenitor-specific overexpression of a constitutively active heartless allele (heartiesAct) expands Pxn expression in the lymph gland (81 ± 8%) compared to wild-type (p < 0.0001, Fig. 2E and K). Terminally differentiated plasmatocytes, crystal cells, and lamellocytes are all observed throughout the lymph gland (Fig. 2E’-E”). Likewise, forcibly overexpressing thisbe or pyramus in prohemocytes is sufficient to induce almost complete differentiation of the lymph gland, such that the proportion of Pxn+ differentiated hemocytes in the lymph gland increases to 89 ± 7% and 88 ± 6%, respectively (p < 0.0001, Fig. 2F, G and K). Terminally differentiated plasmatocytes and crystal cells are also detected throughout the lymph gland (Fig. 2F’ and G’), and large numbers of lamellocytes are induced to differentiate upon thisbe, but not pyramus, overexpression (Fig. 2F” and G”). The increased differentiation that occurs upon thisbe or pyramus overexpression in progenitors is also accompanied by increased size of the lymph gland primary lobes, compared to wild-type (compare Fig. 2F–G to A). Likewise, lymph gland secondary lobes, which consist mostly of undifferentiated dome+ progenitors in wild-type (Fig. 2H), are severely hypertrophied and differentiated upon both thisbe and pyramus overexpression in dome+ progenitors (Fig. 2I–J). Altogether these data demonstrate that Heartless and its ligands, Thisbe and Pyramus, are necessary and sufficient to promote hemocyte progenitor differentiation in the lymph gland, with distinct opposing effects on lamellocyte differentiation.
Ras-MAPK, Pointed and U-shaped regulate lymph gland progenitor differentiation
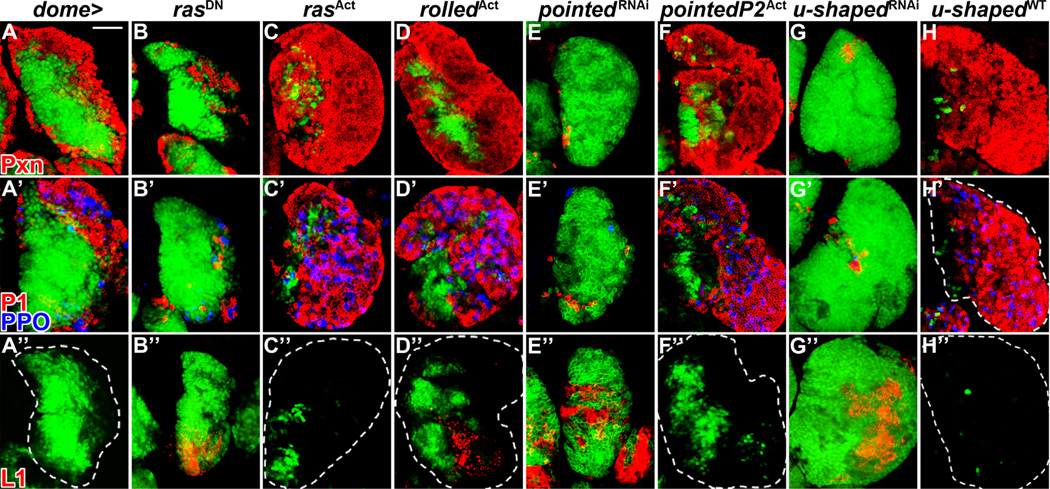
We next assessed potential downstream regulators of Heartless in the lymph gland which might mediate the observed differentiation response of hemocyte progenitors. Similar to other receptor tyrosine kinases, ligand-dependent activation of FGFRs results in the recruitment of adaptor proteins which can activate multiple signal transduction pathways (Eswarakumar et al., 2005; Turner and Grose, 2010). Perhaps the best characterized effector of activated receptor tyrosine kinase signaling is Ras, which activates the mitogen activated protein kinase (MAPK) growth signaling pathway (McKay and Morrison, 2007). We assessed the role of Ras signaling in the lymph gland via progenitor-specific overexpression of a dominant-negative ras allele. Similar to heartless loss-of-function in prohemocytes (Fig. 2B–B”), rasDN overexpression reduces the proportion of Pxn+ differentiated hemocytes in the lymph gland compared to wild-type (20 ± 11%, p < 0.0001, Figs. 2K and 3B). The populations of plasmatocytes and crystal cells are also limited to a thin peripheral layer (Fig. 3B’). In addition, rare lamellocytes are sometimes observed (27% of lymph glands, n = 115, Fig. 3B”), similar to heartless loss-of-function (Fig. 2B”). In contrast, overexpressing activated ras or its downstream effector, rolled, the Drosophila MAPK, in lymph gland prohemocytes expands the populations of Pxn+ hemocytes, terminally differentiated plasmatocytes and crystal cells throughout the lymph gland (Fig. 2K, and Fig. 3C–C and D–D’), at the expense of dome+ progenitors. While lamellocytes are not observed in the lymph gland upon progenitor-specific Ras activation (Fig. 3C”), a small number of lamellocytes differentiate upon Rolled MAPK activation (Fig. 3D”).
Fig. 3. Ras, Pointed and U-shaped regulate lymph gland progenitor differentiation.
All panels represent wandering third instar. In all panels, dome-Gal4, UAS-2xEGFP (wild-type, A–A”) is used to express UAS-rasDN (B–B”), UAS-rasAct (C–C”), UAS-rolledAct (D–D”), UAS-pointedRNAi (E–E”), UAS-pointedP2Act (F–F”), UAS-u-shapedRNAi (G–G”) and UAS-u-shapedWT(H-H”) in prohemocytes (green). Differentiation markers are indicated in A–A” for corresponding rows. (A–A”) Wild-type lymph gland. The populations of peroxidasin (Pxn+) hemocytes (red, A), plasmatocytes (P1, red) and crystal cells (prophenoloxidase, PPO, blue) (A’) and lamellocytes (L1, red, A”) are labeled. (B–B”) Overexpression of rasDN in prohemocytes reduces CZ size (B), limiting terminal plasmatocyte and crystal cell differentiation (B’) while allowing rare lamellocytes to differentiate (27% of lymph glands, n =115, B”). (C-D”) Overexpression of rasAct (C–C”) or rolledAct (D–D”) in prohemocytes increases CZ size at the expense of the MZ (C and D) and causes plasmatocyte and crystal cell differentiation in medial lymph gland regions (C and D’). Lamellocytes are not observed upon Ras activation (C”), but a small number of lamellocytes differentiate upon Rolled activation (37% of lymph glands, n=52, D”). (E–H”) Downregulation of pointed (E) or u-shaped (G) impairs CZ formation and dramatically reduces plasmatocyte and crystal differentiation (E’ and G’), while inducing significant lamellocyte differentiation throughout the lymph gland for both pointedRNAi (86% of lymph glands, n=66, E”) and u-shapedRNAi (83% of lymph glands, n=47, G”). Overexpression of pointedP2Act (F) or u-shapedWT (H) in prohemocytes increases CZ size at the expense of the MZ. While plasmatocytes and crystal cells are observed throughout the lymph gland (F’ and H’), lamellocytes are not observed (F” and H”). Scale bar=50 µm and corresponds to all panels except for the following additional magnifications: X0.9 for F, X0.8 for D and H’, and X0.7 for F”.
A general inhibitor of receptor tyrosine kinase signaling is Sprouty, which can modulate the pathway at multiple steps (Mason et al., 2006), and more specifically has also been shown to negatively modulate Heartless signaling in Drosophila (Franzdottir et al., 2009). Sprouty expression is detected throughout most of the lymph gland at late third instar stages, where it is expressed in the dome+ MZ progenitor population and most of the Pxn+ CZ population (Fig. S2A–A”’). Interestingly, downregulation of sprouty in dome+ prohemocytes increases differentiation in the lymph gland (Fig. S2C) and also causes a robust increase in lamellocytes (Fig. S2E, compare to D), similar to progenitor-specific Heartless activation (Fig. 2E”). These data are consistent with a potential role for Sprouty as a negative modulator of Heartless signaling in lymph gland progenitors.
Transcriptional effects of Heartless and Breathless signaling in Drosophila can be mediated by the ETS-domain protein, Pointed (Pnt) (Cabernard and Affolter, 2005; Franzdottir et al., 2009). We thus hypothesized that Pointed could mediate the strong differentiation phenotypes that occur upon manipulation of Heartless signaling in prohemocytes downstream of Ras signaling. The Drosophila pointed gene encodes two protein isoforms, PointedP1 and PointedP2, arising from the use of two alternative promoters that are separated by 50 kb of genomic sequence (Klambt, 1993; Scholz et al., 1993). Whereas PointedP1 is a constitutive transcriptional activator, phosphorylation of PointedP2 at Thr151 by MAPK potently activates PointedP2 transcriptional activity (Brunner et al., 1994; O’Neill et al., 1994). Consistent with a role for Pointed downstream of Heartless signaling, downregulation of pointed expression in dome+ prohemocytes completely blocks CZ formation, such that the proportion of Pxn+ hemocytes in the lymph gland is only 2 ± 1% (Figs. 2K and 3E). While few scattered plasmatocytes and crystal cells are observed by wandering third instar stage (Fig. 3E’), a significant induction of lamellocytes occurs upon pointed downregulation (Fig. 3E”), similar to pyramus downregulation (Fig. 2D”). Conversely, overexpression of activated PointedP2 in prohemocytes increases the proportion of Pxn+ lymph gland hemocytes (80 ± 10%, Figs. 2K and 3F) and increases plasmatocytes and crystal cells (Fig. 3F’) while not affecting lamellocyte numbers (Fig. 3F”).
We next examined the function of the transcriptional regulator U-shaped (Ush), the single Drosophila Friend-of-GATA (FOG) zinc finger protein, in lymph gland prohemocytes. Similar to Heartless, U-shaped function is required for mesoderm migration early in embryonic development (Fossett et al., 2000). U-shaped has previously been shown to be expressed within lymph gland prohemocytes of the MZ as well as within a subset of differentiated plasmatocytes and crystal cells (Gao et al., 2009), yet prohemocyte-specific manipulation of u-shaped expression has not been examined. Remarkably, progenitor-specific downregulation of u-shaped in lymph gland prohemocytes potently inhibits lymph gland CZ formation, as the proportion of Pxn+ hemocytes in the lymph gland is only 6 ± 4% (Figs. 2K and 3G). Few plasmatocytes and rare crystal cells are observed (Fig. 3G’), whereas induction of lamellocytes occurs throughout the lymph gland (Fig. 3G”), phenocopying pyramus and pointed downregulation (Figs. 2D–D” and 3E–E”). Importantly, these data also phenocopy u-shaped trans heterozygous mutant lymph glands, which have previously been shown to have decreased plasmatocyte and crystal cell numbers but increased lamellocytes (Gao et al., 2009). Interestingly, u-shaped trans heterozygous mutant lymph glands demonstrate ectopic expression of hedgehog (hh), a critical PSC-derived maintenance signal, in non-PSC cells of the lymph gland, demonstrating a role for U-shaped in negatively regulating hedgehog expression in lymph gland hemocytes (Tokusumi et al., 2010). Consistent with these previous findings, we also observed a robust increase in hedgehog expression in lymph gland hemocytes upon progenitor-specific downregulation of u-shaped (Fig. S3A–D”). While in wild-type lymph glands, hedgehog expression is observed only in Antp+ cells of the PSC (Fig. S3A–B”), ectopic expression is observed upon prohemocyte-specific u-shaped downregulation in Antp-negative (Fig. S3C–C”) and Pxn-negative hemocytes (Fig. S3D–D”), which represents the population of dome+ hemocyte progenitors (Fig. 3G–G”). While we cannot rule out that this increased expression of hedgehog throughout the lymph gland may contribute to the increased progenitor maintenance observed upon u-shaped downregulation (Fig. 3G–G”), previous studies have already demonstrated that increasing hedgehog expression in the MZ, outside of the context of the PSC, is not sufficient to expand the progenitor population (Tokusumi et al., 2010). Converse to u-shaped downregulation, overexpression of wild-type u-shaped in dome+ progenitors increases the proportion of Pxn+ differentiated hemocytes in the lymph gland to 80 ± 6% (Figs. 2K and 3H). The populations of plasmatocytes and crystal cells expand to more medial lymph gland regions (Fig. 3H–H), whereas lamellocytes are not observed (Fig. 3H”). Collectively, these findings demonstrate a role for Ras signaling and Pointed/ETS and U-shaped/FOG transcription factor function in blood cell differentiation.
Clonal disruption of Heartless-Ras-Pointed signaling affects lymph gland hemocyte differentiation
Our results demonstrate robust effects on lymph gland differentiation and CZ formation upon manipulation of Heartless, its ligands (Fig. 2), and its potential downstream intracellular pathway components (Fig. 3) within the total population of prohemocytes. We next confirmed our findings by manipulating Heartless signaling in lymph gland-specific clonal populations of hemocytes. HHLT(hand-hml lineage-traced Gal4) (Evans et al., 2009) generates wild-type clones that are often very large, and span most of the Pxn− MZ (Fig. S4A–A”) as well as a proportion of Pxn+ CZ cells (yellow cells, Fig. S4A”). Clonally overexpressing heartlessDN in lymph gland hemocytes impairs differentiation, such that clonal cells minimally overlap with the early differentiation marker, Pxn (Fig. S4B–B”). Likewise, lymph gland-specific clonal downregulation of thisbe or pyramus strongly impairs differentiation, limiting it to surrounding wild-type cells at the lymph gland periphery (red, Pxn+) and demonstrating absence of overlap of Pxn with mutant cells (Fig. S4C–D”). In addition to generating large clones that often span the entire MZ (Fig. S4C–D”), we also generated small FLP-FRT mutant clones for pyramus, in order to assess whether activation of Heartless signaling by its ligands could be cell-autonomous. Pyramus mutant clones in peripheral lymph gland regions did not block differentiation (Fig. S5B–B”’), whereas more medial pyramus mutant clones remain undifferentiated (Fig. S5C–C”). These data are consistent with the diffusible nature of FGF growth factors, and suggests that adjacent wild-type cells that express the ligand are sufficient to rescue the mutant clone cells. In contrast, downregulation of the ligands throughout the entire progenitor population (dome-gal4, Fig. 2C–D”) or in large lymph gland-specific clones (HHLT, Fig. S4C–D”) is sufficient to hinder differentiation. Conversely, activation of Heartless signaling in lymph gland hemocytes via clonal overexpression of heartlessAct, thisbeWT, or pyramusWT strongly induces differentiation, such that Pxn expression is robustly upregulated within mutant cells (yellow, Fig. S4E–G”), confirming their role in promoting hemocyte differentiation.
Similar to reduced Heartless signaling (Fig. S4B–D”), clonally impairing Ras activity in the lymph gland blocks differentiation of mutant cells, while surrounding wild-type cells differentiate (Fig. S4H–H”). Conversely, clonal activation of Ras or Rolled MAPK in the lymph gland strongly induces differentiation within clonal cells (yellow cells, Fig. S4I–J”). Similarly, the requirement for Pointed activity in regulating differentiation of lymph gland progenitors (Fig. 3E–E”) was further demonstrated by the strong block in differentiation of clonal cells expressing pointedRNAi, which populate almost the entire lymph gland (Fig. S4K–K”). Furthermore, lymph gland-specific clonal downregulation of u-shaped blocks differentiation in clonal cells, whereas adjacent wild-type cells differentiate (Fig. S4M–M”). Finally, clonal activation of PointedP2 (Fig. S4L–L”) or overexpression of wild-type u-shaped (Fig. S4N–N”) robustly induces differentiation in clonal cells.
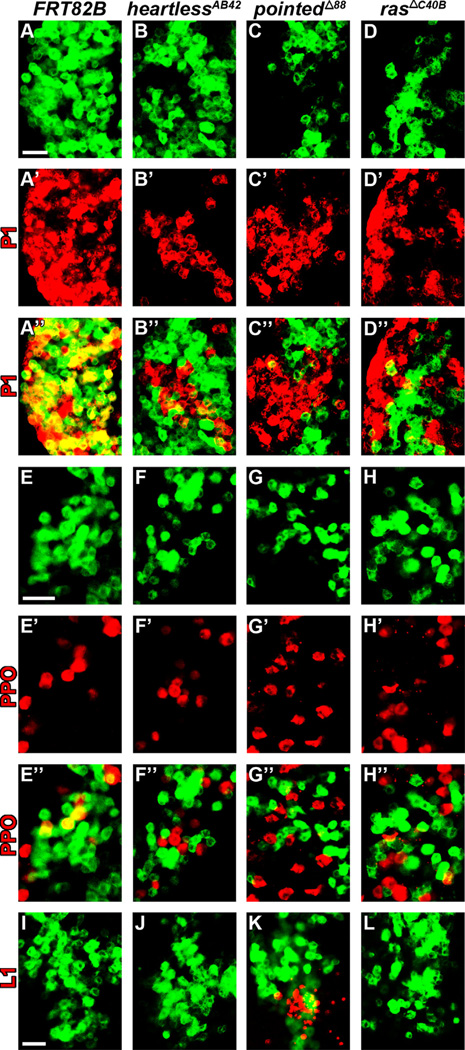
We next explored the cell-autonomous requirement of Heartless and its downstream components in regulating differentiation by generating homozygous loss-of-function MARCM mutant clones for heartless, ras, or pointed. In all three cases mutant clones demonstrated an absence of terminal differentiation markers for plasmatocytes (P1) and crystal cells (PPO) in peripheral lymph gland regions, where adjacent wild-type cells differentiate (Fig. 4B–D” and F-H”). In contrast, wild-type clonal cells demonstrate overlap of both P1 (Fig. 4A–A”) and PPO (Fig. 4E–E”) in peripheral lymph gland regions. Only pointed mutant clones demonstrated lamellocyte differentiation (Fig. 4K), consistent with the effects on lamellocyte differentiation induced by pointed downregulation in progenitors (Fig. 3E”). While disruption of heartless or ras function in clones (Fig. 4J and L) did not induce lamellocyte formation, this is likely due to the smaller number of hemocytes genetically disrupted, compared to dome-gal4-mediated disruption of the entire progenitor population (Figs. 2B” and 3B”). Taken together, our HHLT and MARCM clonal analyses demonstrate the cell-autonomous requirement of Heartless and its potential downstream effectors Ras, Pointed and U-shaped in regulating lymph gland hemocyte differentiation.
Fig. 4. Heartless-Ras-Pointed mutant clonal analysis demonstrates impaired plasmatocyte and crystal cell differentiation.
All panels represent wandering third instar. The plasmatocyte marker, P1, is labeled in red in panels A–D”, whereas the crystal cell marker prophenoloxidase (PPO) is labeled in red in panels E–H”, and the lamellocyte maker L1 is labeled in red in panels I–L Wild-type (A–A”, E–E” and I) and mutant (B–D”, F–H” and J–L) MARCM clones are shown in green. (A–A”, E–E” and I) Wild-type MARCM clones (hs-flp FRT82B Tub-mCD8-GFP) in peripheral lymph gland regions demonstrate overlap (yellow) with terminally differentiated plasmatocyte (A–A”) and crystal cell (E–E”) lineage markers, but are negative for lamellocytes (I). (B–D”, F–H” and J–L) MARCM clones for heartlessAB42 [hs-flp FRT82B heartlessAB42 FRT82B Tub-mCD8-GFP (B–B”, F–F” and J)], pointedΔ88 [hs-flp FRT82B pointedΔ88 FRT82B Tub-mCD8-GFP (C–C”, G–G” and K)] or rasΔC40B [hs-flp FRT82B rasΔc40B FRT82B Tub-mCD8-GFP (D–D”, H-H” and L)] in peripheral lymph gland regions demonstrate minimal overlap with terminally differentiated plasmatocyte (B-D”) and crystal cell (F–H”) lineage markers. Whereas lamellocytes are observed in pointedΔ88 clones (K), no lamellocytes are detected in heartlessAB42 (J) or rasΔC40B (L) clones. Scale bars=50µm. Scale bar in A corresponds to panels A–D”; scale bar in E corresponds to panels E–H”; scale bar in I corresponds to I–L.
Receptor tyrosine kinase signaling effects on differentiation are specific to Pyramus and Thisbe
Our findings suggest that canonical Ras-MAPK signaling through Pointed may mediate the effects of activated Heartless signaling in the lymph gland. Previous studies have demonstrated functional roles of other receptor tyrosine kinases in circulating hemocytes (Munier et al., 2002; Zettervall et al., 2004) as well as in the lymph gland, including epidermal growth factor receptor (EGFR) (Sinenko et al., 2012) and the Drosophila platelet derived growth factor/vascular endothelial growth factor (PDGF/VEGF) receptor homolog, PVR (Mondal et al., 2011). In order to assess the specificity of Thisbe and Pyramus in inducing progenitor differentiation, we monitored the effects on cell populations upon forcibly overexpressing other receptor tyrosine kinase ligands in lymph gland progenitors.
Progenitor-specific overexpression of branchless, the third Drosophila FGF ligand that specifically activates the Breathless receptor, does not alter lymph gland differentiation (Fig. S6B and K), demonstrating specificity for Heartless and its ligands in mediating lymph gland progenitor differentiation. While the receptor tyrosine kinase EGFR is expressed in the lymph gland (JA Martinez-Agosto, unpublished results, and Sinenko et al., 2012), forcibly overexpressing the EGFR ligands, vein, spitz, gurken, or keren in dome+ prohemocytes is not sufficient to affect lymph gland differentiation (Fig. S6C–F and K), and neither is overexpression of the anaplastic lymphoma kinase (ALK) ligand, jelly-belly (Fig. S6G and K). Finally, recent studies have demonstrated PVR expression in MZ prohemocytes (Mondal et al., 2011). The PVR ligand, Pvf1, is expressed in PSC cells, whereas Pvf2 is expressed in PSC and the most medial MZ cells (Mondal et al., 2011). While overexpression of pvf1 in lymph gland progenitors does not induce a differentiation response (Fig. S6H and K), pvf2 overexpression is sufficient to induce almost complete differentiation of the lymph gland (Fig. S6I and K). In contrast, downregulation of pvf2 in the MZ has no effect on progenitor differentiation (Mondal et al., 2011), suggesting that Pvf2 is not required to induce differentiation during normal lymph gland development, but is sufficient to induce a differentiation response upon increased ligand expression. Pvf2 signals independently from Heartless in blood progenitors, as reducing Heartless function in prohemocytes upon pvf2 overexpression does not impair the differentiation response observed upon pvf2 overexpression alone (Fig. S6J, compare to I). Our data thus identify two independent positive effectors of differentiation in lymph gland prohemocytes, one that is necessary and sufficient for normal hemocyte differentiation and the other able to promote differentiation exclusively upon overexpression. From these experiments, we conclude that the requirement of Thisbe and Pyramus in lymph gland progenitors for their differentiation is not a general response of activating receptor tyrosine kinase signaling, but is specific to Heartless signaling.
Pointed and U-shaped function downstream of Heartless in lymph gland progenitors
We next determined the function of Pointed downstream of Heartless signaling in mediating lymph gland hemocyte differentiation by performing epistasis analysis. Lymph gland-specific downregulation of pointed upon overexpression of heartlessAct is sufficient to block the differentiation response of Heartless activation alone (compare Fig. 5B–C) and hinder hemocyte differentiation, similar to pointed downregulation alone (Fig. S4K”). Likewise, downregulation of pointed upon thisbe or pyramus overexpression also effectively hinders hemocyte differentiation (Fig. 5D–G), suggesting that the differentiation response downstream of activated Heartless signaling in the lymph gland is dependent on Pointed activity.
Fig. 5. Pointed and U-shaped function downstream of Heartless in lymph gland progenitors.
All panels represent wandering third instar. In panels A–G, HHLT (hand-gal4, hml-gal4, UAS-2xEGFP, UAS-FLP; A5C–FRT-STOP-FRT-gal4) is used to clonally express the specified genetic constructs in lymph gland hemocytes. In panels H–T, dome-Gal4, UAS-2xEGFP is used to express the specified genetic constructs in dome+ prohemocytes. GFP (green) labels clonal cells in A–G and prohemocytes in H–T. Peroxidasin (Pxn) expression is shown in red. (A) Wild-type lymph gland. Wild-type clonal cells (green) express Pxn (red) only in peripheral CZ regions (overlap in yellow). (B–G) Overexpression of heartlessAct (htlAct, B), thisbeWT (thsWT, D), or pyramusWT (pyrWT, F) in lymph gland hemocytes robustly increases differentiation in the lymph gland. Downregulation of pointed (pnt) upon overexpression of heartlessAct (htlAct; pntRNAi ,C), thisbeWT (thsWT; pntRNAi, E), or pyramusWT (pyrWT; pntRNAi, G) is sufficient to dramatically block the differentiation response of hemocytes normally observed upon overexpression of heartlessAct (B), thisbeWT (D), or pyramusWT (F) alone. (H) Wild-type lymph gland. Wild-type expression of dome (green) in the MZ and Pxn (red) in the CZ. (I–N) Overexpression of heartlessDN (htlDN, I) or downregulation of thisbe (thsRNAi, K) or pyramus (pyrRNAi; M) in prohemocytes impairs prohemocyte differentiation and CZ formation. Overexpression of pointedPAct (pntP2Act) upon heartlessDN (htlDN; pntP2Act, J), thisbeRNAi (thsRNAi; pntp2Act, L) or pyramusRNAi (pyrRNAt; pntP2Act, N) expression in prohemocytes is sufficient to induce their precocious differentiation. (O–T) Progenitor-specific overexpression of heartlessAct (htlAct, O), thisbeWT (thsWT, Q), or pointedP2Act (pntP2Act, S) increases differentiation in the lymph gland at the expense of prohemocytes. Downregulation of u-shaped (ush) upon overexpression of heartlessAct (htlAct; ushRNAi, P), thisbeWT (thsWT; ushRNAi, R), or pointedP2Act (pntP2Act; ushRNAi, T) in prohemocytes impairs their differentiation, hindering CZ formation. (U) Quantification of Pxn+ hemocyte population distribution (% area) in the lymph gland. Data are represented as mean ± s.d., n=10. Asterisks denote statistical significance compared to wild-type (dome >), measured by Student’s t-test. Scale bars=50 µm.
We next assessed the importance of Pointed function downstream of Heartless signaling by examining the sufficiency of PointedP2 activation to induce differentiation in conditions of reduced Heartless signaling. Co-expression of pointedP2Act with heartlessDN, thisbeRNAi, or pyramusRNAi in dome+ prohemocytes is sufficient to significantly increase lymph gland differentiation in these backgrounds (Fig. 5I–N). Importantly, the proportion of Pxn+ hemocytes in these backgrounds (72 ± 11%, 71 ± 10%, or 67 ± 11%, respectively; Fig. 5U) is similar to progenitor-specific pointedP2Act overexpression alone (80 ± 10%; Fig. 2K).
Given the lack of differentiation observed upon u-shaped downregulation (Figs. 3G–G” and S4M–M”) and reduction in Heartless signaling (Figs. 2B–D” and S4B–D”), we examined whether U-shaped also functions downstream of Heartless signaling in the lymph gland. Downregulating u-shaped upon overexpression of heartlessAct or thisbeWT in dome+ hemocyte progenitors significantly reduces progenitor differentiation in the lymph gland (Fig. 5P and R) compared to overexpression of heartlessAct or thisbeWT alone (Fig. 5O and Q). The proportion of Pxn+ hemocytes in these backgrounds is dramatically decreased (16 ± 6% and 20 ± 10%, respectively; Fig. 5U), compared to heartlessAct or thisbeWT over-expression alone (81 ± 8% and 89 ± 7%, respectively; Fig. 2K). Having demonstrated that reducing either pointed or u-shaped expression in the lymph gland is sufficient to rescue differentiation phenotypes induced by activated Heartless signaling, we next examined the epistatic relationship of U-shaped and Pointed in MZ prohemocytes. Interestingly, downregulation of u-shaped upon pointedP2Act overexpression in dome+ progenitors dramatically impairs progenitor differentiation in the lymph gland (Fig. 5S–T), phenocopying u-shapedRNAi alone (Fig. 3G), and dramatically reduces the proportion of Pxn+ hemocytes in the lymph gland to only 7 ± 5% (Fig. 5U).
Altogether, our epistasis analyses demonstrate that Pointed/ETS activation downstream of Heartless signaling mediates the effects of Thisbe/Pyramus-Heartless signaling on hemocyte progenitor differentiation. Our data also describes U-shaped/FOG as a potent effector of differentiation, which either functions downstream of Pointed or is required for Pointed activity in mediating the Heartless-induced differentiation response of hemocyte progenitors.
Activation of target of rapamycin (TOR) signaling upon Heartless activation
Target of rapamycin (TOR) complex 1 (TORC1) signaling in Drosophila lymph gland progenitors regulates their early proliferation during second and early third instar stages and later differentiation (Dragojlovic-Munther and Martinez-Agosto, 2012). Interestingly, activation of TORC1 through disruption of the TORC1 pathway inhibitor, tuberous sclerosis complex 1/2 (tsc1/2), in lymph gland progenitors induces significant lamellocyte differentiation (Dragojlovic-Munther and Martinez-Agosto, 2012), similar to the activation of Heartless or overexpression of thisbe in progenitors (Fig. 2E” and F”). Given that lamellocytes are not observed in the lymph gland upon progenitor-specific Ras or Pointed activation (Fig. 3C” and F”), we reasoned that a distinct intracellular signaling pathway might mediate the lamellocyte differentiation induced by Thisbe-Heartless signaling and examined the possibility that it could be TORC1-mediated. In wild-type lymph glands, phosphorylated translation initiation factor 4E–binding protein (p4EBP), a marker of active TORC1 signaling, is expressed at low levels in the lymph gland (Fig. 6A–A”, Dragojlovic-Munther and Martinez-Agosto, 2012). Overexpression of heartlessAct or thisbeWT in hemocyte progenitors robustly increases p4EBP expression throughout the lymph gland, demonstrating activation of mTORC1 signaling upon Thisbe-mediated Heartless signaling (Fig. 6B–C”). In contrast, overexpression of pyramusWT in hemocyte progenitors does not significantly change p4EBP levels in the lymph gland compared to wild-type (compare Fig. 6D–D” to A–A”), demonstrating a lack of TORC1 activation downstream of pyramus overexpression.
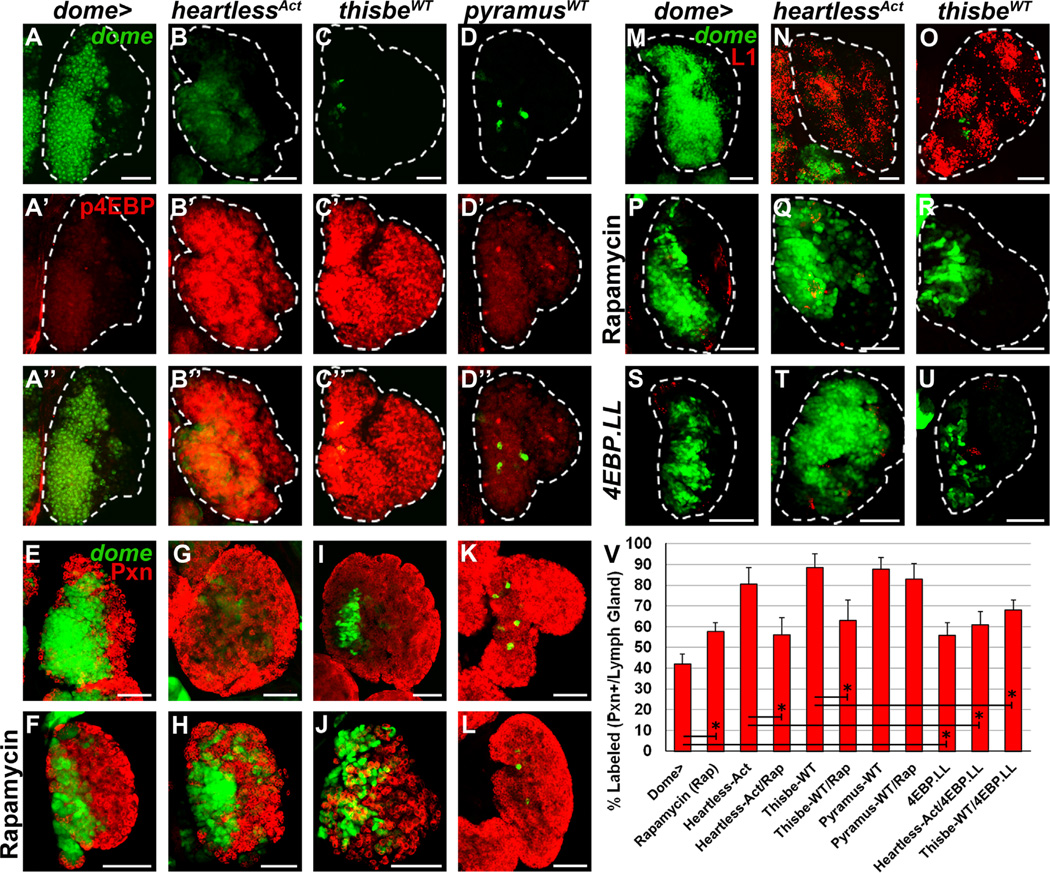
Fig. 6. Activation of target of rapamycin (TOR) signaling by Thisbe and Heartless.
All panels represent wandering third instar. In all panels, dome-Gal4, UAS-2xEGFP (wild-type, A–A”, E and M) is used to express the specified genetic constructs in prohemocytes (green). Phoshphorylated 4EBP (p4EBP) is labeled in red in panels A’-D”, peroxidasin (Pxn) is labeled in red in panels E–L, and the lamellocyte marker L1 is labeled in red in panels M–U. (A–A”) Wild-type lymph gland. P4EBP (red) is expressed at low levels in medial regions of the lymph gland. (B–D”) Overexpression of heartlessAct (B–B”) or thisbeWT (C–C”) in prohemocytes robustly increases p4EBP levels (red) throughout the lymph gland, whereas pyramusWT overexpression (D–D”) does not. (E) Wild-type lymph gland. (F) Reducing target of rapamycin complex 1 (TORC1) signaling via systemic rapamycin treatment increases CZ (red) size at the expense of the MZ. (G–J) Overexpression of heartlessAct (G) or thisbeWT (I) in prohemocytes robustly increases differentiation in the lymph gland, which is partially reduced following rapamycin treatment (H and J). (K–L) Overexpression of pyramusWT in hemocyte progenitors robustly increases differentiation throughout the lymph gland (K), which is not affected by systemic rapamycin treatment (L). (M–O) Whereas lamellocytes are not observed in wild-type lymph glands (M), overexpression of heartlessAct (N) or thisbeWT (O) in prohemocytes robustly induces lamellocyte differentiation throughout the lymph gland. (P–U) A small number of lamellocytes are sometimes observed upon systemic rapamycin treatment (48% of lymph glands, n=50, P) or progenitor-specific overexpression of d4EBP(LL) (36% of lymph glands, n=44, S), yet both conditions are sufficient to strongly impair the robust increase in lamellocyte differentiation (seen in N and O) in both heartlessAct (Q and T) and thisbeWT (R and U) overexpressing lymph glands. (V) Quantification of Pxn+ hemocyte population distribution (% area) in the lymph gland. Data are represented as mean ± s.d., n=10. Asterisks denote statistical significance between indicated genotypes/treatments, measured by Student’s t-test. Scale bars=50 µm.
We next examined the TORC1-dependence of Heartless- and Thisbe-induced phenotypes by treating larvae with the TORC1 inhibitor, rapamycin. Systemic rapamycin treatment of wild-type larvae increases differentiation in the lymph gland (compare Fig. 6F to E) (Dragojlovic-Munther and Martinez-Agosto, 2012; Shim et al., 2012), significantly increasing the proportion of Pxn+ hemocytes in the lymph gland to 58 ± 4% (p < 0.0001 compared to wild-type, Fig. 6V). Interestingly, rapamycin treatment partially restores lymph gland zonation upon overexpression of heartlessAct in hemocyte progenitors (compare Fig. 6H to G), significantly reducing the proportion of Pxn+ hemocytes in the lymph gland (56 ± 8%) compared to heartlessAct overexpression alone (p < 0.0001, Fig. 6V). While rapamycin treatment also reduces the proportion of Pxn+ hemocytes in the lymph gland upon thisbe overexpression (63 ± 10%), compared to thisbe overexpression alone (p < 0.0001, Fig. 6I–J and V), rapamycin does not affect differentiation under conditions of pyramus overexpression (p > 0.05, Fig. 6K–L and V). Importantly, rapamycin treatment is sufficient to largely block the lamellocyte differentiation normally observed upon progenitor-specific heartlessAct or thisbeWT overexpression (compare Fig. 6Q–R to N–O), despite the small lamellocyte differentiation that occurs in the lymph gland upon rapamycin treatment of wild-type larvae (Fig. 6P). Similar to pyramus overexpression alone (Fig. 2G”), lamellocytes are never observed in the lymph gland following rapamycin treatment (data not shown).
Our previous studies identified 4EBP as a potent effector of TORC1 -dependent phenotypes in Drosophila hemocyte progenitors (Dragojlovic-Munther and Martinez-Agosto, 2012). Phosphorylation of 4EBP by TORC1 promotes growth by relieving translation initiation factor 4E (IF-4E) from inhibitory 4EBP binding (Gingras et al., 2001). Similar to systemic rapamycin treatment, progenitor-specific overexpression of a mutant form of Drosophila 4EBP [d4EBP(LL)] that binds more tightly to IF-4E and thus inhibits TOR-dependent effects increases the proportion of Pxn+ hemocytes in the lymph gland to 55 ± 6% (p < 0.0001 compared to wild-type, Figs. 6V and S7B). Overexpression of d4EBP(LL) upon heart-lessAct or thisbeWT overexpression in progenitors is also sufficient to impair lamellocyte differentiation (Fig. 6T–U) and affects the proportion of Pxn+ hemocytes (Figs. 6V and S7C–D), similar to rapamycin treatment. Together, these results suggest that the dramatic expansion of lamellocytes observed upon progenitor-specific Heartless activation or thisbe overexpression is mediated by active TORC1 signaling through 4EBP, rather than Ras-Pointed signaling, which is required for plasmatocyte differentiation. The failure of pyramus overexpression to induce a similar TORC1-dependent response highlights the context-specific signaling of Heartless ligands in Drosophila.
Regulation of Heartless signaling in the lymph gland by heparan sulfate proteoglycans
Intriguingly, thisbe and pyramus expression in the lymph gland is highest in the MZ (Fig. 1), and overexpressing thisbe or pyramus ligands in the progenitor population is sufficient to induce almost complete differentiation of the lymph gland (Fig. 2F–G and K). We reasoned that Heartless ligand signaling in lymph gland progenitors must be tightly regulated, both to allow the progression of differentiation of peripheral progenitors that go on to populate the maturing CZ during development, and also to prevent the premature differentiation of medial prohemocytes before the onset of metamorphosis, when MZ progenitors differentiate and enter circulation (Grigorian et al., 2011b). One candidate modulator of Heartless signaling in the lymph gland is the Drosophila Perlecan homolog, Terribly Reduced Optic Lobes (Trol), a member of the heparan sulfate proteoglycan family (Park et al., 2003). Perlecans are mainly distributed in the extracellular matrix and exclusively have heparan sulfate chains (Perrimon and Bernfield, 2000). Fibroblast growth factor signaling is among the most well characterized pathways regulated by heparan sulfate proteoglycans, which can serve at least two context-dependent functions in regulating signaling through FGF receptors. First, heparan sulfate proteoglycans can serve as a co-factor that stabilizes the FGF/FGFR interaction. Secondly, binding of FGF ligands to highly sulfated heparan sulfate sequences can sequester FGFs, regulate their movement and diffusibility in the extracellular matrix, and also protect them from proteolytic degradation (Dowd et al., 1999; Saksela et al., 1988).
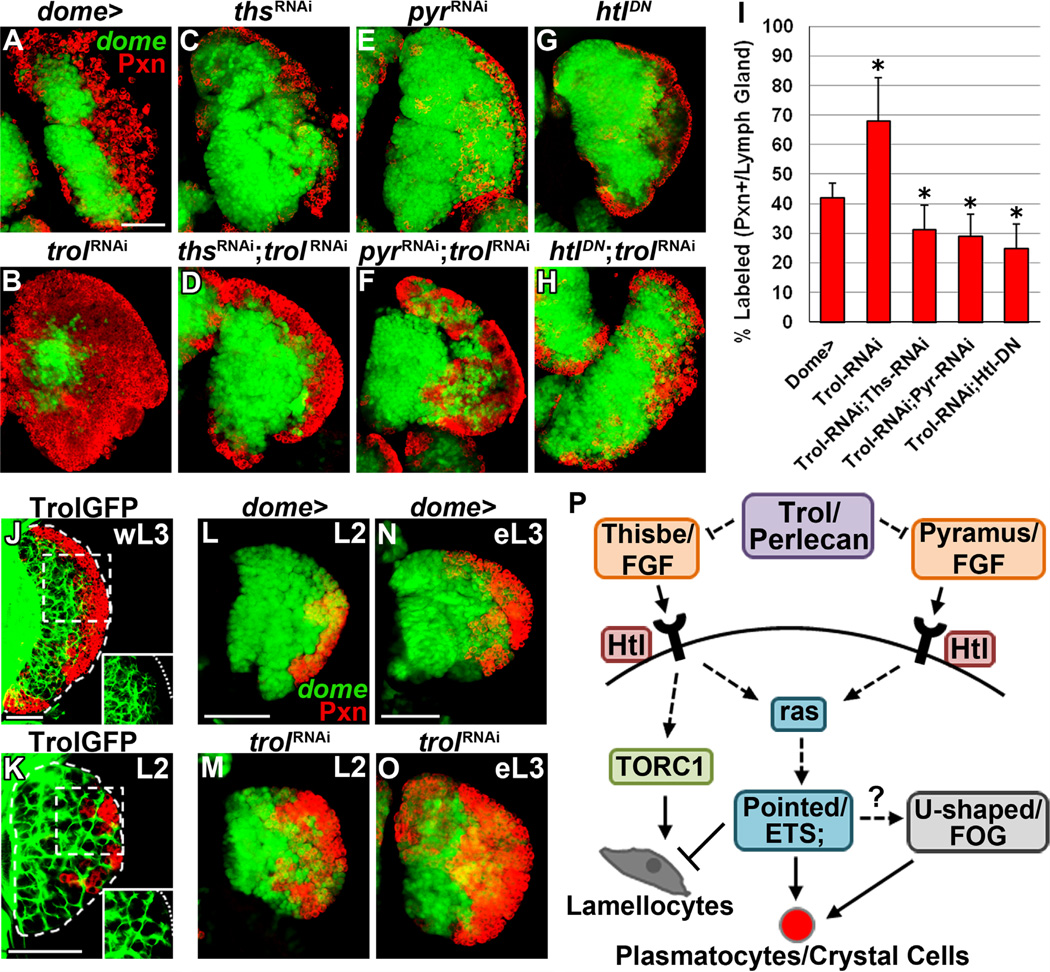
Previous studies have demonstrated that a dense Trol-positive extracellular matrix reticulum surrounds progenitors in the MZ at later third instar stages (Grigorian et al., 2011b). We hypothesized that signaling of FGF ligands in the lymph gland might be modulated spatially and/or temporally through Trol. Surprisingly, trol downregulation in prohemocytes increases differentiation in the lymph gland (68 ± 14% Pxn+ hemocytes, Fig. 7B and I, compared to wild-type, Fig. 7A). Importantly, trol mutant lymph glands also present with robust differentiation and reduction of the undifferentiated progenitor population (Grigorian et al., this issue), supporting our findings that reduced trol expression induces precocious lymph gland differentiation. We next examined whether this increased differentiation can be attributed to excessive Heartless signaling in the lymph gland. Reducing expression of thisbe or pyramus upon downregulation of trol in progenitors is sufficient to significantly reduce the proportion of Pxn+ cells in the lymph gland (31 ± 8% and 29 ± 8%, respectively, Fig. 7D, F and I) compared to wild-type (p < 0.05) or downregulation of trol alone (p < 0.0001). However, the proportion of Pxn+ hemocytes in these backgrounds is also significantly higher than thisbe or pyramus downregulation alone (p < 0.0001, Figs. 2K and 7C and E). These results suggest that the increased availability of one ligand partially compensates for the downregulation of the other in conditions of reduced trol expression, and that Trol sequesters both ligands in the extracellular matrix. Furthermore, overexpression of heartlessDN upon progenitor-specific trol downregulation is sufficient to significantly decrease the proportion of Pxn+ hemocytes (25 ± 8%, Fig. 7H–I) compared to wild-type or trol downregulation alone (p < 0.0001), and is not significantly different than overexpression of heartlessDN alone (20 ± 6%, p > 0.05, Figs. 2K and 7G). This finding further suggests that the differentiation observed upon trol downregulation is mediated by Heartless activation via both its ligands, Thisbe and Pyramus.
Fig. 7. Regulation of FGF signaling in the lymph gland by Trol.
Panels A–H represent wandering third instar. In panels A–H and L–O, dome-Gal4, UAS-2xEGFP (wild-type, A, L and N) is used to express the specified genetic constructs in prohemocytes (green). In panels J–K, the protein trap line ZCL1973X labels endogenous Trol-GFP protein fusion expression. In all panels peroxidasin (Pxn) expression is shown in red. (A) Wild-type lymph gland. (B) Downregulating trol expression in prohemocytes induces their differentiation into Pxn+ hemocytes, increasing CZ size at the expense of the MZ. (C–H) Progenitor-specific downregulation of thisbe (thsRNAi, C) or pyramus (pyrRNAi ,E) or overexpression of heartlessDN (htlDN, G) reduces differentiation (red), impairing CZ formation. Downregulation of thisbe (thsRNAi; trolRNAi, D) or pyramus (pyrRNAi; trolRNAi ,F) or overexpression of heartlessDN (htlDN; trolRNAi, H) upon downregulation of trol is sufficient to block the increased differentiation induced by trol downregulation alone (B). (I) Quantification of Pxn+ hemocyte population distribution (% area) in the lymph gland. Data are represented as mean ± s.d., n=10. Asterisks denote statistical significance compared to wild-type (dome >), measured by Student’s t-test. (J) At wandering third instar stage (wL3), high levels of Trol are detected in the extracellular matrix amidst the population of Pxn hemocytes of the MZ, but Trol expression is not observed among Pxn+ hemocytes (red) of the CZ (inset). (K) During the second larval instar stage (L2), high levels of Trol are observed in the extracellular matrix throughout the lymph gland. Large pockets of Trol-negative tissue outline groups of early differentiating (Pxn+) hemocytes. (L–O) Wild-type lymph glands at second instar (L2, L) contain a small population of early differentiating hemocytes, which increases by early third instar stage (eL3, N) to begin forming the early CZ. Downregulation of trol in prohemocytes expands the population of differentiating hemocytes during second and early third instar stages (M and O, respectively) compared to wild-type. (P) Thisbe/Pyramus-Heartless signaling in Drosophila lymph gland hemocyte progenitors. Thisbe- and Pyramus-induced Heartless (Ht1) activation in lymph gland progenitors is modulated by Tro1/Perlecan heparan sulfate proteoglycan. Heartless activation by its ligands induces plasmatocyte differentiation in a Pointed/ETS-dependent manner through Ras activation, affecting the ratio of MZ progenitors and CZ differentiated hemocytes in the lymph gland. U-shaped/FOG is also required for Heartless- and Pointed-mediated effects on differentiation and functions either downstream of or in parallel to Heartless signaling. Thisbe-specific Heartless activation induces lamellocyte differentiation in a TORC1-dependent manner that is independent of Pointed or U-shaped gain-of-function and is also not observed upon pyramus overexpression. Scale bars=50 µm. Scale bar in A corresponds to panels A–H, except for the following additional magnifications: X0.75 for E, X0.8 for B and H, and X1.2 for D. Scale bar in L corresponds to L–M. Scale bar in N corresponds to N-O.
We also examined Trol expression at earlier stages, when differentiation is just initiating in the developing lymph gland. In contrast to wandering third instar lymph glands, wherein high Trol expression is detected in the extracellular matrix surrounding MZ progenitors (Pxn−) but is absent in the peripheral CZ (Pxn+, inset, Fig. 7J), high Trol levels extend to the lymph gland periphery during the second larval instar stage (Fig. 7K). We also observed large pockets of Trol-negative tissue in the lymph gland periphery which contained groups of early differentiating hemocytes during this stage (inset, Fig. 7K). Interestingly, increased differentiation in the lymph gland associated with progenitor-specific trol down-regulation is already observed by the second and early third instar stages (Fig. 7M and O), compared to wild-type (Fig. 7L and N), culminating in the observed phenotype at wandering third instar (Fig. 7B). Altogether, our data suggest that Trol modulates Heartless signaling in the lymph gland to prevent precocious prohemocyte differentiation. This likely occurs via sequestration of Heartless ligands, Thisbe and Pyramus, in the extracellular matrix until they are required to induce hemocyte differentiation during lymph gland development.
Discussion
In this study we highlight a crucial role for the Drosophila fibroblast growth factor receptor (FGFR), Heartless, in maintaining the homeostatic balance of MZ prohemocytes and differentiated hemocytes of the CZ. Whereas activation of Heartless signaling in blood progenitors dramatically increases differentiation at the expense of the MZ, reducing Heartless signaling in progenitors impairs CZ formation. To date, the signals that induce progenitor differentiation and CZ formation during larval lymph gland development remain largely unknown. Hyperactivation of the target of rapamycin complex 1 (TORC1) signaling pathway by progenitor-specific downregulation of the TORC1 pathway inhibitor tuberous sclerosis complex (tsc) autonomously increases lymph gland differentiation at late larval stages (Dragojlovic-Munther and Martinez-Agosto, 2012). Surprisingly, however, inhibition of TORC1 signaling also increases differentiation in the lymph gland at the expense of prohemocytes, demonstrating that both loss-of-function and gain-of-function of TORC1 signaling promotes progenitor differentiation (Benmimoun et al., 2012; Dragojlovic-Munther and Martinez-Agosto, 2012). Furthermore, while overexpression of the PVR ligand, pvf2, autonomously increases progenitor differentiation (this study), downregulation of pvf2 in progenitors does not affect their differentiation (Mondal et al., 2011). These findings demonstrate that neither Pvf2 nor TORC1 signaling are actually required for lymph gland progenitor differentiation during development. In contrast, our analysis of Pyramus/Thisbe-Heartless signaling through Pointed uncovers a signaling network that is not only sufficient, but is also required, for progenitor differentiation into both plasmatocytes and crystal cells, as well as proper CZ formation in the lymph gland.
Distinct roles of Ras signaling in lymph gland hemocyte populations
Previous studies in Drosophila hematopoiesis have identified Ras as a potent effector of differentiated hemocyte proliferation, demonstrating dramatic increases in circulating hemocyte population numbers upon Ras activation (Asha et al., 2003; Evans et al., 2007; Zettervall et al., 2004). In this study we examined prohemocyte-specific Ras activation in the lymph gland, allowing dissection of a distinct role for Ras activity in Drosophila larval hematopoiesis. Prohemocyte-specific Ras activation in the lymph gland induces their precocious differentiation, expanding CZ size at the expense of the MZ (Fig. 3). In contrast to its proliferative effects on embryo-derived circulating hemocytes, Ras activation in lymph gland prohemocytes does not affect lymph gland size (Fig. 3), as would be expected of a signal that drastically increases cell proliferation in mature hemocytes (Asha et al., 2003; Evans et al., 2007; Zettervall et al., 2004). Unlike prohemocyte-specific Ras activation, clonal Ras activation in the lymph gland autonomously induces hemocyte differentiation and also robustly increases lymph gland tissue size (Fig. S4I–I”). This increase in tissue size is likely due to the sustained expression of the activated ras allele in differentiated clonal hemocytes. Our data thus describe a transition in Ras function in the lymph gland, wherein Heartless-induced Ras activation in hemocyte progenitors initially regulates their differentiation, after which, activation of Ras promotes proliferation of differentiated hemocytes, which may contribute to increasing CZ hemocyte numbers at late larval stages (Jung et al., 2005). Potential candidates for Ras activation in differentiated cortical hemocytes are epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and/or PVR, which all induce differentiated hemocyte proliferation (Zettervall et al., 2004), but do not autonomously regulate progenitor differentiation in the MZ (Fig. S6). Interestingly, however, reducing Ras or Pointed activity in hemocyte progenitors moderately reduces total lymph gland size compared to wild-type, although the sizes of the MZ prohemocyte populations are similar to wild-type (Fig. 3). It is thus likely that the reduced tissue size is not the result of reduced progenitor proliferation, but rather due to the absence of CZ formation, as proliferation of differentiated cortical hemocytes contributes to expansion of the CZ during third instar stages and in turn affects total lymph gland size (Jung et al., 2005).
The mechanisms that operate to regulate the population of undifferentiated dome+ hemocyte progenitors in the lymph gland secondary lobes have not been characterized, but maintenance of this hemocyte population provides a reservoir of progenitors during larval development. Our functional analyses of Pyramus and Thisbe in dome+ progenitors demonstrated the sufficiency of these ligands to autonomously induce robust differentiation of the lymph gland secondary lobes, similar to their phenotypic effects in the dome+ progenitor population of the lymph gland primary lobes (Fig. 2). Further, pyramus and thisbe overexpression in dome+ progenitors is sufficient to induce severe hypertrophy of the lymph gland secondary lobes and also causes increased primary lobe size. Since activation of Ras in dome+ hemocyte progenitors does not affect lymph gland size (Fig. 3) but has robust affects on differentiated hemocyte proliferation, we hypothesize that forcibly overexpressing pyramus and thisbe in lymph gland hemocyte progenitors could promote proliferation and expansion of the population of differentiated hemocytes which in turn could contribute to lymph gland tissue overgrowth in the primary and secondary lobes.
Heartless signaling in the lymph gland is mediated by Pointed and U-shaped
Distinct roles in specific hemocyte populations have been identified for the single Drosophila Friend-of-GATA (FOG) transcriptional regulator, U-shaped, during hematopoiesis. These include roles as regulator of crystal cells (Fossett et al., 2001), hemocyte number (Sorrentino et al., 2007), lamellocyte transformation (Avet-Rochex et al., 2010), and a negative regulator of hedgehog expression in lymph gland hemocytes (Tokusumi et al., 2010). U-shaped is strongly expressed in the lymph gland MZ, and it has previously been proposed to regulate prohemocyte maintenance (Gao et al., 2009). In this study we performed prohemocyte-specific downregulation of u-shaped in the lymph gland, which dramatically increases the size of the dome+ MZ prohemocyte population at the nearly complete expense of cortical plasmatocytes and crystal cells, yet also induces lamellocyte differentiation (Fig. 3). These data phenocopy previous loss-of-function studies that have demonstrated a reduction of plasmatocytes and crystal cells but significant induction of lamellocytes in lymph glands from u-shaped trans heterozygous mutant larvae (Gao et al., 2009) but did not confirm their loss to differentiation, unlike previously suggested for u-shaped loss-of-function mutants (Gao et al., 2009). However, the possibility remains that U-shaped could regulate differentiation of a subset of CZ progenitors, as u-shaped overexpression using a CZ driver impairs plasmatocyte formation (Gao et al., 2009).
The similarity of phenotypes induced by u-shaped genetic manipulation in blood progenitors compared to that of heartless, pyramus, and thisbe suggested a potential interaction of U-shaped with Heartless signaling in blood homeostasis. Previous studies have demonstrated a genetic interaction between u-shaped and heartless in the context of early mesodermal cell migration, suggesting that these two genes function in a common genetic pathway (Fossett et al., 2000), although the mechanism remained unclear. Our data demonstrate that U-shaped is required either downstream of or parallel to Heartless signaling to induce differentiation of lymph gland progenitors (Fig. 7P). A link between receptor tyrosine kinase-ras activity and GATA transcriptional networks has also been demonstrated in vertebrates. Nearly half of non-small cell lung cancer (NSCLC) patients contain mutations in the receptor tyrosine kinase/Ras family, and recent studies demonstrated that GATA2 transcriptional activity is requisite for Ras-driven NSCLC cells (Kumar et al., 2012).
We also highlight similar effects of the Drosophila ETS protein Pointed and the FOG homolog U-shaped as transcriptional effectors regulating blood progenitor differentiation, and demonstrate that the differentiation of progenitors induced by activated Pointed requires U-shaped. Intriguingly, differentiation of the vertebrate myeloid megakaryocyte lineage requires cooperativity of ETS and FOG/GATA proteins for transcription of the megakaryocyte-restricted αIIb gene (Wang et al., 2002). Our study suggests that cooperativity between the Drosophila FOG and ETS proteins, U-shaped and Pointed, may also regulate the myeloid-restricted hemocyte differentiation that occurs in the lymph gland (Fig. 7P), similar to vertebrates. U-shaped has been shown to bind to at least two Drosophila GATA factors, Serpent and Pannier (Haenlin et al., 1997; Waltzer et al., 2002), both of which have roles in blood development (Evans et al., 2003; Minakhina et al., 2011), yet it remains unclear how U-shaped and these GATA factors function together in hematopoiesis. Further studies are required to elucidate U-shaped/GATA transcriptional activity, its targets during Drosophila hematopoiesis, and the roles of Pointed in this process.
Roles of TORC1 and other signaling pathways in Thisbe/Pyramus-Heartless signaling
Previous studies in Drosophila hematopoiesis have identified a number of signals that contribute to lamellocyte differentiation, including PVR, Toll, ALK and EGFR (Avet-Rochex et al., 2010; Sinenko et al., 2012; Zettervall et al., 2004), although these studies have been limited to manipulating gene expression in differentiated hemocytes (both circulating and in the lymph gland) or within cells of the lymph gland niche (PSC). Genetic alterations that specifically induce lamellocyte differentiation in the lymph gland prohemocyte population remain poorly characterized. We have recently demonstrated that progenitor-specific TORC1 activation through tsc downregulation is sufficient to induce significant lamellocyte differentiation throughout the lymph gland (Dragojlovic-Munther and Martinez-Agosto, 2012). Here we identify that progenitor-specific overexpression of thisbeWT and heartlessAct, but not pyramusWT, in the lymph gland also promotes significant lamellocyte differentiation (Fig. 2). However, despite the shared requirement for Thisbe and Pyramus on Ras signaling through Pointed in promoting progenitor differentiation and proper CZ formation (Fig. 5), activation of Ras signaling or PointedP2 gain-of-function does not induce lamellocyte differentiation (Fig. 3). Given that neither Ras nor PointedP2 activation in progenitors induces lamellocyte differentiation, we reasoned that Heartless activation via Thisbe might branch from the Ras-Pointed signaling cascade and activate TORC1 signaling. Although FGFR signaling (i.e., either through Heartless or Breathless) has never been shown to signal through TORC1 in Drosophila, recent studies have suggested synergy between Heartless and TORC1 signaling during Drosophila post-embryonic gliogenesis (Avet-Rochex et al., 2012). Our study demonstrates that lamellocyte differentiation induced by Heartless activation and thisbe overexpression in blood progenitors is dependent on TORC1 and 4EBP (Figs. 6 and 7P), suggesting that the TORC1 axis may be particularly important in regulating progenitor-specific lamellocyte differentiation in the lymph gland and confirming the ability of FGF signaling to activate TORC1. In contrast, pyramus overexpression in prohemocytes does not activate TORC1 signaling, and reducing TORC1 signaling upon pyramus overexpression does not affect progenitor differentiation (Fig. 6). Similar ligand-specific downstream effects have been suggested previously for Heartless signaling (Franzdottir et al., 2009), demonstrating the context specificity of its intracellular pathway components.
Our study has also identified downregulation of pyramus, pointed and u-shaped as inducers of lamellocyte formation, while inhibiting plasmatocyte differentiation, suggesting their role as negative regulators of lamellocyte differentiation in lymph gland blood progenitors. This may be a useful mechanism for lineage restriction under circumstances in which progenitors primarily differentiate into lamellocytes at the expense of other lineages, such as upon wasp infection (Krzemien et al., 2010; Rizki and Rizki, 1984). Further studies are required to elucidate the mechanism by which these pathway components impair lamellocyte differentiation, which likely involves repression of a signal that actively promotes lamellocyte differentiation.
Previous studies have identified roles for the Janus tyrosine kinase/signal transducers and activators of transcription (JAK/ STAT) and Wingless signaling pathways in the lymph gland MZ progenitor population (Makki et al., 2010; Sinenko et al., 2009). Specifically, the JAK/STAT ligand, Unpaired 3, has been demonstrated to function in the MZ as part of the immune response, such that wasp infestation induces reduced levels of unpaired 3 transcripts in the lymph gland, which in turn decreases JAK/STAT signaling levels and thus transcription of the target gene, dome (Makki et al., 2010). Latran, a short, non-signaling, cytokine dome-cognate receptor, is expressed in the MZ, and further contributes to the decreased JAK/STAT signaling which follows wasp infestation and is required for the massive lamellocyte differentiation that occurs in response to parasitization (Makki et al., 2010). Akin to what is seen with Latran, we hypothesize that Thisbe-mediated Heartless signaling may cooperate with STAT under immune challenge, particularly in lamellocyte formation. As we discuss below, Thisbe could be a potential mediator of the lamellocyte response upon wasp parasitization. Although regulated JAK/STAT signaling in the MZ is required as part of the lymph gland immune response (Makki et al., 2010), recent studies have suggested that the role for STAT in lymph gland prohemocyte maintenance is actually mediated via non-autonomous signaling in the adjacent CZ (Mondal et al., 2011). Therefore, the robust effects on progenitor differentiation or maintenance that we observe upon prohemocyte-specific gain- or loss-of-function of Heartless signaling, respectively, likely occurs independent of JAK/STAT signaling. However, we cannot rule out that, as previously shown (Gao et al., 2009), JAK/ STAT signaling could regulate u-shaped expression in the lymph gland and in turn contribute to Heartless-induced progenitor differentiation. We do, however, speculate that Wingless signaling, a prohemocyte maintenance signal autonomously required in the MZ (Sinenko et al., 2009), may suppress the pro-differentiation effects mediated by Thisbe and Pyramus, given the similarities between Wingless gain-of-function and reduced Heartless-Ras-Pointed signaling in the progenitor population (Figs. 2 and 3 and Sinenko et al., 2009).
Thisbe/Pyramus-Heartless signaling is modulated by Trol in the extracellular matrix
Given the robust effects on lymph gland differentiation that occur upon forcibly overexpressing the Heartless ligands, thisbe and pyramus, in dome+ hemocyte progenitors, it was surprising that thisbe and pyramus are both highly expressed in the undifferentiated cells of the MZ (Fig. 1 and S1). Furthermore, the impaired CZ formation upon progenitor-specific thisbe or pyramus down-regulation suggested to us that the FGF pathway is required in dome+ cells to restrict the progenitor state. Such highly regulated Heartless signaling would thus require a mechanism by which to regulate Heartless signaling in progenitors, in order to prevent precocious differentiation. One candidate modulator of Heartless signaling that we hypothesized could be functioning in the lymph gland is the Drosophila Perlecan homolog, Trol, as heparan sulfate proteoglycans (HSPGs) are known to modulate the signaling of a number of secreted ligands. Amongst these, HSPGs have the ability to sequester FGF ligands, regulating their movement and diffusibility through the extracellular matrix (Turner and Grose, 2010). In the lymph gland, a dense trol-positive extracellular matrix reticulum surrounds MZ progenitors, whereas in the CZ much of this extracellular matrix has disappeared (Fig. 7J). Reduced expression of trol in the MZ induces precocious prohemocyte differentiation at early and late lymph gland stages, and this effect is rescued by blocking Heartless signaling. Likewise, increased differentiation is observed in trol mutant lymph glands (Grigorian et al., in press). Our findings are consistent with previous studies that have shown gain-of-function phenotypes in fgf mutants with reduced binding affinity to heparan sulfate (Harada et al., 2009; Makarenkova et al., 2009), and suggest that Trol contributes to maintain a reservoir of sequestered Heartless ligands in the lymph gland (Fig. 7P), which become available upon disruption of trol expression.
The role of sequestration of FGF ligands in hematopoiesis is likely multi-faceted. First, the highly regulated release of sequestered Thisbe and Pyramus ligands, perhaps via expression of heparanases, may be responsible for regulating progenitor differentiation and for patterning CZ formation during development. Further studies are required to investigate which signals are responsible for mediating such a stereotypical release of Heartless ligands during lymph gland development. Additionally, the continued presence of thisbe and pyramus in medial lymph gland regions by wandering third instar stages also suggests that Heartless ligands may become available following the onset of metamorphosis, contributing to the rapid differentiation of lymph gland progenitors that occurs before their release into circulation upon pupation (Grigorian et al., 2011b). Consistent with this finding, the strong trol expression observed in the MZ late in larval development is reduced at the onset of pupation, when metamorphosis begins (Grigorian et al., 2011b). Finally, a reservoir of immobilized Heartless ligands, present in lymph gland progenitors throughout development, may also be utilized to induce the rapid differentiation of hemocytes observed in response to larval infection or stress conditions (Krzemien et al., 2010; Lemaitre and Hoffmann, 2007; Shim et al., 2012). Intriguingly, parasitoid infection of Drosophila larvae induces massive lamellocyte differentiation in the lymph gland (Lanot et al., 2001; Sorrentino et al., 2002), and genome-wide expression analyses identified a number of proteases to be upregulated in response to parasitoid infection in Drosophila (Wertheim et al., 2005). It remains to be determined whether a release of sequestered Thisbe ligand follows the upregulation of protease expression and contributes to the increased lamellocyte differentiation in the lymph gland that occurs upon wasp parasitization.
Previous studies in the lymph gland have revealed signaling networks that actively maintain progenitors, uncovering niche-, progenitor-, and differentiated hemocyte-derived maintenance signals that serve this function (Mandal et al., 2007; Mondal et al., 2011; Sinenko et al., 2009). Our study highlights the role of a Heartless-Ras-Pointed signaling pathway that is both required and sufficient for progenitor differentiation and CZ formation. We uncover a novel mechanism of progenitor maintenance in the lymph gland, wherein sequestration of a differentiation signal developmentally also supports progenitor maintenance and prevents their precocious differentiation. This mechanism of ligand sequestration may be relevant in vertebrate hematopoiesis, wherein FGFs have been implicated in myeloid-lineage restricted progenitors and cell types (Allouche and Bikfalvi, 1995; Berardi et al., 1995; Moroni et al., 2002). Furthermore, signaling through ETS, FOG and the target of rapamycin pathway may be important in conditions of dysregulated FGF signaling, such as acute myeloid leukemias (Bieker et al., 2003; Karajannis et al., 2006).
Supplementary Material
Acknowledgments
This work was supported by an institutional Ruth L Kirschstein National Research Service Award (NRSA) [(GM007185) to M.D.-M.] from the National Institutes of Health, and the David Geffen School of Medicine to J.A.M.-A. We are very grateful for the kind contributions from Angela Stathopoulos (CalTech) and members of her laboratory, especially Va Si, for generously supplying the unpublished FRT42B pyramusEx36 and FRT82B heartlessAB42 alleles (funding source: CIRM Bridges to Stem Cell Internship Program at Pasadena City College). We also thank Young Bae for designing the PCR primers for Thisbe and Pyramus and providing reagents used for generating amplified genomic fragments. We are grateful for reagents provided by Kathy Ngo and Steven Klein which were used for in situ hybridization. We also thank all the research laboratories for their generosity in providing reagents, in particular J. Fessler, I. Ando and M. Kanost for providing antibodies essential for this study. M.D.M. designed and performed the experiments, data analysis, and wrote the manuscript; J.A.M.-A. designed and supervised the experiments and wrote the manuscript.
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at http://dx.doi.Org/10.1016/j.ydbio.2013.04.004.
Footnotes
The authors declare no conflict of interest.
References
- Allouche M, Bikfalvi A. The role of fibroblast growth factor-2 (FGF-2) in hematopoiesis. Prog. Growth Factor Res. 1995;6:35–48. doi: 10.1016/0955-2235(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. NY Acad. Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–215. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Rochex A, Boyer K, Polesello C, Gobert V, Osman D, Roch F, Auge B, Zanet J, Haenlin M, Waltzer L. An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC 10, 65Dev. Biol. 2010;10:65. doi: 10.1186/1471-213X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Rochex A, Kaul AK, Gatt AP, McNeill H, Bateman JM. Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain. Development. 2012 doi: 10.1242/dev.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/ TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- Berardi A, Wang A, Abraham J, Scadden D. Basic fibroblast growth factor mediates its effects on committed myeloid progenitors by direct action and has no effect on hematopoietic stem cells. Blood. 1995;86:2123–2129. [PubMed] [Google Scholar]
- Bieker R, Padro T, Kramer J, Steins M, Kessler T, Retzlaff S, Herrera F, Kienast J, Berdel WE, Mesters RM. Overexpression of basic fibroblast growth factor and autocrine stimulation in acute myeloid leukemia. Cancer Res. 2003;63:7241–7246. [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholzi H, Klambt C. The ETS domain protein Pointed-P2 is a target of MAP kinase in the Sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Neumann M, Affolter M. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J. Appl. Physiol. 2004;97:2347–2353. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- Dowd CJ, Cooney CL, Nugent MA. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. J. Biol. Chem. 1999;274:5236–5244. doi: 10.1074/jbc.274.8.5236. [DOI] [PubMed] [Google Scholar]
- Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Faloon P, Zhang WJ, Hirashima M, Reid T, Stanford WL, Orkin S, Choi K, Rossant J. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Sinenko SA, Mandal L, Martinez-Agosto JA, Hartenstein V, Banerjee U. Genetic dissection of hematopoiesis using Drosophila as a model system. In: Wassarman P, editor. Advances in developmental biology. New York: Elsevier Science; 2007. pp. 259–299. [Google Scholar]
- Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do M-T, Yackle K, Cespedes A, Hartenstein V, Call GB, Banerjee U. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Meth. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N, Zhang Q, Gajewski K, Choi CY, Kim Y, Schulz RA. The multitype zinc-finger protein U-shaped functions in heart cell specification in the Drosophila embryo. Proc. Natl. Acad. Sci. 2000;97:7348–7353. doi: 10.1073/pnas.97.13.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Upregulation of the Drosophila Friend of GATA gene u-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol. Cell. Biol. 2009;29:6086–6096. doi: 10.1128/MCB.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hakimi M, Ortiz I, Hartenstein V. The convergence of Notch and MAPK signaling specifies the blood progenitor fate in the Drosophila mesoderm. Dev. Biol. 2011a;353:105–118. doi: 10.1016/j.ydbio.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev. Genes Evol. 2011b;221:121–131. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M, Liu T, Banerjee U, Hartenstein V. The proteoglycan Trol controls the architecture of the extracellular matrix and balances proliferation and differentiation of blood progenitors in the Drosophila lymph gland. Dev. Biol, this issue. doi: 10.1016/j.ydbio.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryzik T, Müller HAJ. FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila Gastrula. Curr. Biol.: CB. 2004;14:659–667. doi: 10.1016/j.cub.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Haenlin M, Cubadda Y, Blondeau E, Heitzler P, Lutz Y, Simpson P, Ramain P. Transcriptional activity of Pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 1997;11:3096–3108. doi: 10.1101/gad.11.22.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Murakami H, Okawa A, Okimoto N, Hiraoka S, Nakahara T, Akasaka R, Shiraishi Y-I, Futatsugi N, Mizutani-Koseki Y, Kuroiwa A, Shirouzu M, Yokoyama S, Taiji M, Iseki S, Ornitz DM, Koseki H. FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat. Genet. 2009;41:289–298. doi: 10.1038/ng.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Evans C, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–747. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karajannis MA, Vincent L, DiRenzo R, Shmelkov SV, Zhang F, Feldman EJ, Bohlen P, Zhu Z, Sun H, Kussie P, Rafii S. Activation of FGFR1[beta] signaling pathway promotes survival, migration and resistance to chemotherapy in acute myeloid leukemia cells. Leukemia. 2006;20:979–986. doi: 10.1038/sj.leu.2404203. [DOI] [PubMed] [Google Scholar]
- Klambt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development. 1993;117:163–176. doi: 10.1242/dev.117.1.163. [DOI] [PubMed] [Google Scholar]
- Klingseisen A, Clark IBN, Gryzik T, Muller HAJ. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development. 2009;136:2393–2402. doi: 10.1242/dev.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev. Biol. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Kumar Madhu S, Hancock David C, Molina-Areas M, Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E, Lassailly F, Matthews N, Nye E, Stamp G, Behrens A, Downward J. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Jung S-H, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki R, Meister M, Pennetier D, Ubeda J-M, Braun A, Daburon V, Krzemien J, Bourbon H-M, Zhou R, Vincent A, Crozatier M. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8:e1000441. doi: 10.1371/journal.pbio.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto J, Evans C, Hartenstein V, Banerjee U. A Hedgehog-and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Albert Basson M, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK//MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- McMahon A, Reeves GT, Supatto W, Stathopoulos A. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development. 2010;137:2167–2175. doi: 10.1242/dev.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Tan W, Steward R. JAK/STAT and the GATA factor Pannier control hemocyte maturation and differentiation in Drosophila. Dev. Biol. 2011;352:308–316. doi: 10.1016/j.ydbio.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni E, Dell’era P, Rusnati M, Presta M. Fibroblast growth factors and their receptors in hematopoiesis and hematological tumors. J. Hematother. Stem Cell Res. 2002;11:19–32. doi: 10.1089/152581602753448513. [DOI] [PubMed] [Google Scholar]
- Munier A-I, Doucet D, Perrodou E, Zachary D, Meister M, Hoffman JA, Janeway CA, Jr1, Lagueux M. PVF2, a PDGF/VEGF-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 2002;3:1195–1200. doi: 10.1093/embo-reports/kvf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Gururaja-Rao S, Jones KT, Slattery M, Negre N, Braas D, Christofk H, White KP, Mann R, Banerjee U. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes Dev. 2012;26:2027–2037. doi: 10.1101/gad.183061.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson RE, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJR, McDermott S, Datta S. Drosophila Perlecan modulates FGF and Hedgehog signals to activate neural stem cell division. Dev. Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Rizki RM, Rizki TM. Selective destruction of a host blood cell type by a parasitoid wasp. Proc. Natl. Acad. Sci. 1984;81:6154–6158. doi: 10.1073/pnas.81.19.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Deatrick J, Klaes A, Klambt C. Genetic dissection of pointed, a Drosophila gene encoding two ETS-related proteins. Genetics. 1993;135:455–468. doi: 10.1093/genetics/135.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdisciplinary Rev.: Syst. Biol. Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido E, Ono N, Kojima T, Saigo K. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–2128. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of Wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Shim J, Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 2012;13:83–89. doi: 10.1038/embor.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R, Tokusumi T, Schulz R. The Friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev. Biol. 2007;311:311–323. doi: 10.1016/j.ydbio.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A, Tarn B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–699. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitell MA, Mikkola HKA. Transcriptional activators, repressors, and epigenetic modifiers controlling hematopoietic stem cell development. Pediatr. Res. 2006;59:33R–39R. doi: 10.1203/01.pdr.0000205155.26315.c7. [DOI] [PubMed] [Google Scholar]
- Tepass U, Fessler L, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Tokusumi Y, Tokusumi T, Stoller-Conrad J, Schulz RA. Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development. 2010;137:3561–3568. doi: 10.1242/dev.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Bataille L, Peyrefitte S, Haenlin M. Two isoforms of Serpent containing either one or two GATA zinc fingers have different roles in Drosophila haematopoiesis. EMBO J. 2002;21:5477–5486. doi: 10.1093/emboj/cdf545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Crispino JD, Letting DL, Nakazawa M, Poncz M, Blobel GA. Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: role of Ets transcription factors. EMBO J. 2002;21:5225–5234. doi: 10.1093/emboj/cdf527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim B, Kraaijeveld A, Schuster E, Blanc E, Hopkins M, Pletcher S, Strand M, Partridge L, Godfray HC. Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 2005;6:R94. doi: 10.1186/gb-2005-6-11-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall C-J, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.