Abstract
Interleukin-8 (IL-8) gene expression is highly up-regulated in canine hemangiosarcoma (HSA); however, its role in the pathogenesis of this disease is unknown. We investigated the expression of IL-8 in canine HSA tissues and cell lines, as well and the effects of IL-8 on canine HSA in vitro, and in vivo using a mouse xenograft model for the latter. Constitutive expression of IL-8 mRNA, IL-8 protein, and IL-8 receptor were variable among different tumor samples and cell lines, but they showed stable steady states in each cell line. Upon the addition of IL-8, HSA cells showed transient intracellular calcium fluxes, suggesting that their IL-8 receptors are functional and that IL-8 binding activates relevant signaling pathways. Yet, neither addition of exogenous IL-8 nor blockade of endogenous IL-8 by neutralizing anti-IL-8 antibody (α-IL-8 Ab) affected HSA cell proliferation or survival in vitro. To assess potential effects of IL-8 in other tumor constituents, we stratified HSA cell lines and whole tumor samples into “IL-8 high” and “IL-8 low” groups. Genome-wide gene expression profiling showed that samples in the “IL-8 high” tumor group were enriched for genes associated with a “reactive microenvironment,” including activation of coagulation, inflammation, and fibrosis networks. Based on these findings, we hypothesized that the effects of IL-8 on these tumors were mostly indirect, regulating interactions with the microenvironment. This hypothesis was supported by in vivo xenograft experiments where survival and engraftment of tumor cells was inhibited by administration of neutralizing α-IL-8 Ab. Together, our results suggest that IL-8 contributes to establishing a permissive microenvironment during the early stages of tumorigenesis in HSA.
Keywords: canine, hemangiosarcoma, interleukin-8, tumor microenvironment, gene expression profiling
Introduction
Hemangiosarcoma (HSA), which occurs commonly in dogs, is a highly metastatic and almost always incurable cancer with extensive vascular networks where cells form abnormal, distorted blood vessels, capillaries, and sinusoids [1, 2]. This cancer is a heterogeneous disease, and its precise origins still remain to be elucidated [3]. Treatment options for this disease are limited and outcomes are generally unrewarding [4], as over 50% of dogs affected with this disease still die within 6 months of diagnosis, and clinical trials using new combinations of old drugs [5] and different treatment modalities [6] have shown no benefit over the standard of care. The lack of effective treatments is at least partly due to the fact that the pathogenesis and biological features of canine HSA are incompletely understood.
IL-8, also known as CXCL8, is a pro-inflammatory and pro-angiogenic CXC chemokine, which is mainly known to promote neutrophil chemotaxis and degranulation [7]. IL-8 is produced by fibroblasts, endothelial cells, and monocytes during stimulation, but constitutive expression in these normal cells is negligible [8]. In cancer, high expression of IL-8 is frequently observed in a range of tumor types, and its effects on cell proliferation and survival have been demonstrated in human cancers such as breast, gastric, colon, pancreatic, ovarian, bladder, prostate, and melanoma [7]. The cancer cells secreting IL-8 proliferate and survive through autocrine IL-8 signaling pathways, including activation of Akt [9] and through regulation of the activity of the mitogen-activated protein kinase signaling cascade [10, 11]. In addition, cancer-derived IL-8 promotes not only cell invasion and migration but also metastasis by inducing neutrophil infiltration and tumor-associated macrophage production of growth factors at the tumor site [12–14]. Furthermore, IL-8 is associated with angiogenesis in various human tumors, suggesting this cytokine might be an important regulatory factor in the tumor microenvironment [7, 15, 16].
Previously, we used genome-wide profiling to discover that inflammation and angiogenesis are critical features in the pathogenesis of canine HSA [17]. More specifically, we showed that IL-8 was highly up-regulated in canine HSA cells compared to non-malignant endothelial cells derived from splenic hematomas, suggesting that IL-8 has a crucial role in the malignancy of these tumors [17]. Recent studies also have shown that the levels of IL-8 were higher in blood obtained from dogs with mammary cancer than in blood from healthy dogs, suggesting that IL-8 may be an important prognostic serum marker [18, 19]. However, a functional role of IL-8 in spontaneous canine cancers has not been reported.
The aims of this study were to examine the constitutive expression of IL-8 and to explore its functional roles in canine HSA in terms of direct effects on cells in vitro and/or modulation of the tumor microenvironment in vivo. We analyzed genome-wide gene expression profiling in canine HSA samples, and then showed that IL-8 promotes HSA growth by modulating the tumor microenvironment in vivo.
Materials and Methods
Samples and histopathological examination
Samples were obtained from client-owned dogs diagnosed with spontaneous HSA as described [20]. Demographic and clinicopathologic data including age, breed, sex, and affected site for each dog are shown in Supplementary Table 1. The metastatic status for every dog in this group was either confirmed positive (n = 14) or uncertain (n = 11), where “uncertain” means the dog was euthanized at diagnosis, a necropsy was not done, or the case was lost to follow up. Samples from twenty-four primary or metastatic tumor tissues were formalin-fixed and paraffin embedded. Sections (4 μm) were stained with hematoxylin and eosin (H&E) for histopathological evaluation to confirm the diagnosis. Semi-quantitative inflammation scores were assigned to each sample based on cellular infiltrates (myeloid and mononuclear cells), necrosis, and fibrosis. Samples with no inflammation were scored as 0, and those with mild, moderate, and severe inflammation were scored respectively as 1, 2, and 3 as previously described [21]. Twelve cell lines were used for this study. The characteristics of the DD-1, Dal-4, SB, FH, GM, and EFS/EFB cell lines were described previously [3, 20, 22]. The JHE, JSP, JLI, and JLU cell lines were established from tissues of one dog with disseminated HSA (heart, spleen, liver, and lung), and the SH cell line was established from a splenic HSA using previously described methods [17, 22]. Newly acquired samples were obtained with owner consent and with approval of the University of Minnesota Institutional Animal Care and Use Committees (protocols 0802A27363 and 1101A94713).
Cell culture
Seven of the HSA cell lines (JHE, JLI, JLU, JSP, COSB, Dal-4, and DD-1) were derived from the tumors of four dogs diagnosed with HSA. Four of these cell lines (JHE, JLI, JLU, and JSP) were obtained from tumor tissues in different distant organs from the same dog. These HSA cells were cultured as described previously [3, 20, 22] and used for the in vitro experiments in this study. COSB was a low passage derivative of the SB cell line isolated from a mouse xenograft.
Genome-wide gene expression profiling
Twenty-four tumor tissue samples (n = 24) and twelve cell lines (n = 12) were used for genome-wide gene expression profiling (Supplementary Table 1). RNA was isolated using the TriPure Isolation Reagent (Roche Applied Science, Indianapolis, IN, USA) and the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA from the samples was quantified and assessed for quality. Briefly, samples determined to be suitable for analysis based on RNA quality (ratio of absorbance at 260 nm over 280 nm between 1.95 and 2.1 and Bioanalyzer RNA Integrity Number > 6.1) were labeled using Agilent’s Microarray One-Color Low-Input Quick Amp Labeling kit, hybridized to Agilent canine 4 × 44,000 feature gene chips according to Agilent’s Protocol Version 6, and read using an Agilent array scanner (Agilent, Santa Clara, CA, USA). Bioanalyzer quality control, RNA labeling, and microarray hybridization were performed by the BioMedical Genomics Core of the University of Minnesota.
After microarray hybridization, Agilent quality control algorithms in Expressionist Refiner Module (v. 7.5; Genedata, Basel, Switzerland,) were used to confirm that data from each chip met the manufacturer’s standards for quality control and quality assurance. Of 45,220 features on each array, 35,676 that had annotation to known genes were used for analysis. Annotated probe signal levels were quantile-normalized and summarized using the GeneChip-Robust Multichip Averaging algorithm in the Expressionist Analyst Module (v. 7.1), and these normalized data were then mean-centered and log2-transformed.
The tumor tissue and the cell line samples were stratified into “IL-8 high” and “IL-8 low” groups, separated by the median (and by the mean) value of IL-8 gene expression. Supervised hierarchical clustering was based on complete linkage using Gene Cluster 3.0 for Mac OS X. Gene Cluster 3.0 data were visualized in Java TreeView version 1.1.6. Two group T-tests were performed to determine genes that were differentially expressed between the two groups. Differential expressed genes in the two groups with a P-value < 0.05 (in cell lines) or < 0.01 (in tumor tissues) and average fold-change > 2 were identified.
Biological functions and canonical pathways of significantly differently expressed genes between the two sample groups were defined by Ingenuity Pathway Analysis (IPA) software v8.6 (Ingenuity Systems, Redwood City, CA, USA) using BH multiple testing corrections to evaluate significance.
Quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR)
RNA isolation and qRT-PCR to validate mRNA expression of IL-8 and IL-8 receptor (IL-8R) in canine HSA cell lines were performed as previously described [23]. Briefly, RNA was isolated from cell lines cultured for this study using the RNeasy Mini Kit (Qiagen). RNA concentration was examined using NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
cDNA synthesis was performed using a QuantiTect Reverse Transcription Kit (Qiagen), and qPCR was carried out on an Eppendorf Mastercycler ep realplex with FastStart SYBR Green Master Mix Protocol (Roche, Indianapolis, IN, USA). Primers were designed to amplify fragments of IL-8 and IL-8R, and GAPDH was used for normalization. Relative values of mRNA were calculated using the comparative [delta][delta] Ct method. [23, 24] The primer pairs were: IL-8, forward 5′-TGG CAG CTT TTG TCC TTT CT-3′, reverse 5′-GGG CCA CTG TCA ATC ACT CT-3′; IL-8R, forward 5′-CAC GGA GAT GCC CAT AAT TC -3′, reverse 5′-CAG CAG ATA GAC GTC GGT GA -3′; GAPDH, forward 5′-GGA GTC CAC TGG CGT CTT CAC -3′, reverse 5′-R: 5′-GAG GCA TTG CTG ATG ATC TTG AGG -3′.
Enzyme linked immunosorbent assay (ELISA)
2×105 HSA cells were plated per well in 6-well plate and incubated for 18 hours. IL-8 was quantified in the culture medium using a canine CXCL8/IL-8 DuoSet ELISA Development kit (R&D Biosystems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All measurements were performed in triplicate.
Flow cytometry
IL-8 binding to its receptor was determined using human CXCL8/IL-8 Biotinylated Fluorokine Kit (R&D Biosystems) according to the manufacturer’s instructions. Briefly, HSA cells were harvested and resuspended (concentrations of 4×106 cells/ml) in 10 mM phosphate-buffered saline (PBS). Fc-mediated interactions were blocked with purified goat IgG, and 10 μL of biotinylated IL-8 were added to 25 μL of the cell suspensions. In separate tubes, a biotinylated negative control reagent or goat 3-human IL-8 Ab (provided in kit) were used as controls for the assay. Cells were incubated for 60 minutes at 4°C, and 10 μL of avidin-FITC reagent were added to each tube. The reaction mixture was incubated for 30 minutes at 4° C in the dark, and the cells were washed twice with 2 mL of 1× RDF1 wash buffer (provided in kit) to remove unreacted avidin-FITC and resuspended in 0.2 mL of 1× RDF1 for final flow cytometric analysis. 5×104 cells per sample were evaluated on a BD FACS Calibur (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software 8.8.7 (Tree Star Inc., Ashland, OR, USA).
Cytosolic free calcium mobilization assay
Cells (107 cells/ml) were loaded with Indo-1 AM (3 μM) in Hanks Balanced Salt Solution containing 1 mM calcium, 1 mM magnesium, and 0.5% Bovine Serum Albumin by incubation for 1 hour at 37°C. The cells were washed twice and resuspended in the same buffer. Changes in Indo-1 AM fluorescence to determine intracellular calcium concentration were measured using a BD LSR II Flow Cytometer (BD Biosciences). The cells were stimulated by recombinant canine IL-8 (1 μg/ml) at 37°C. Ionomycin (1.4 μM) and acetylcholine (5 μM) were used as positive controls. Ethylene glycol tetraacetic acid (EGTA; 8 mM) was to chelate ionized calcium for negative controls.
Cell proliferation and viability assay
Proliferation and viability of HSA cells in culture were evaluated using the colorimetric Cell Titer 96® Aqueous Non-Radioactive Cell Proliferation Assay (MTS assay, Promega, Madison, WI, USA). 5×103 cells in 100 μL of culture media were plated per well in triplicate in 96-well plates. The effects of IL-8 on proliferation and survival were examined over a 7 day time course, and as a function of dose in different culture conditions; i.e.: in the presence or absence of recombinant human IL-8 (rhIL-8, R&D Biosystems) or α-canine IL-8 Ab (R&D Biosystems); growth factor-deprived (serum-free) media; doxorubicin (Bedford Laboratories, Bedford, OH, USA) treatment. Absorbance (A490) was measured using a Wallac Victor2 1420 Multilabel Counter (Perkin Elmer, Waltham, MA, USA). Statistics were determined using the Prism 5 for Mac OS X program (GraphPad Software, Inc., San Diego, CA, USA). A p value of less than 0.05 was considered statistically significant. Proliferation assays were also performed using CellTrace™ structure of carboxyfluorescein diacetate, succinimidyl ester (CFSE) Cell Proliferation Kit (Invitrogen, Eugene, OR, USA) by flow cytometry and according to the manufacturer’s instructions.
IL-8 blockade in mouse xenografts
The canine HSA xenograft model was adapted from Akhtar et al. [25], to assess engraftment and survival of tumor cells using in vivo imaging (Supplementary Materials and Methods). The effect of IL-8 blockade on tumor development was evaluated in this HSA xenograft mouse model using COSB cells that were modified to express GFP and firefly luciferase (COSB-g/L). Seven million COSB-g/L cells were injected intraperitoneally (I.P.) in 150 μl of PBS into male immunodeficient SCID/Beige mice (n = 8). The mice were divided into two groups of four mice each, where the control group received IgG and the experimental group received mouse α-canine IL-8 Abs (100 μg per mouse) twice a week for six weeks. Animals were imaged once a week for 6 weeks to detect luciferase activity using a Xenogen IVIS 100 imaging system (Caliper Life Sciences, Hopkinton, MA, USA). Images were taken within 10 minutes after injection of D-luciferin following anesthesia by isoflurane inhalation and then analyzed with Living Image software (Caliper Life Sciences). All mice were housed, treated, and handled with approval from and according to guidelines of the University of Minnesota Institutional Animal Care and Use Committee (protocol 1006A84813).
Results
IL-8 and IL-8R are expressed constitutively in canine HSA
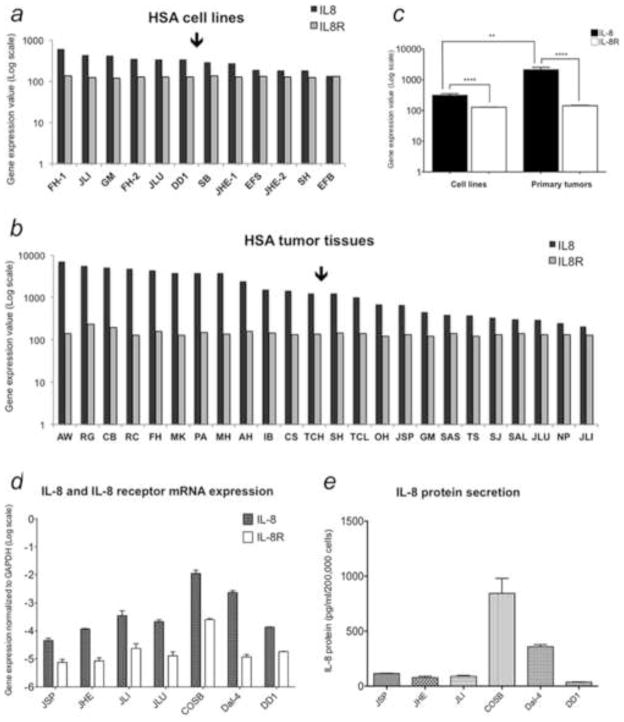
Levels of IL-8 and IL-8R gene expression were compared in tissues and cell lines using data from microarray gene expression profiles. IL-8 gene expression was readily detectable in cell lines and tumor tissues with moderate variance among samples. In contrast, IL-8R mRNA expression was relatively consistent across all samples (Figures 1a and 1b). The mean expression of IL-8 was greater than that of IL-8R gene in both HSA cells and tumor tissues, and IL-8 was expressed at higher levels in tissues than in cell lines (Figure 1c), consistent with our previous observations [17]. Higher expression of IL-8 in tissue could result from production by cancer cells or by other cell types such as fibroblasts and inflammatory cells in the tissue microenvironment.
Figure 1.
IL-8 and IL-8 receptor (IL-8R) gene expression in canine HSA (a – c). Bar graphs show relative levels of IL-8 and IL-8R expression in HSA cell lines (a, n = 12) and tumor tissues (b, n = 24) from microarray data (Agilent Platform). Values are derived from quantile-normalized data using GeneChip-Robust Multichip Averaging. (c) Mean (± SEM) levels of IL-8 and IL-8R gene expression in HSA cell lines and tissues from panels a and b. Quantification of IL-8 and IL-8R mRNA and IL-8 protein in representative canine HSA cell lines (d and e). (d) IL-8 and IL-8R mRNA expression were quantified by qRT-PCR, and normalized to expression of the housekeeping gene GAPDH using the ΔΔCt method. (e) IL-8 secretion into the cell culture medium was measured using a canine-specific ELISA.
We next used qRT-PCR to validate the microarray data. These experiments confirmed that IL-8 mRNA expression was moderately variable among the cell lines (Figure 1d), and this variability also was reflected in the levels of IL-8 protein secreted into the media as measured by ELISA (Figure 1e). However, the steady state levels of IL-8 production were relatively stable in each cell line tested, with COSB and Dal-4 cell lines showing higher levels of IL-8 mRNA and protein expression than JSP, JHE, JLI, and DD-1 cells. We then quantified IL-8R expression in HSA cell lines using flow cytometry to measure binding of biotinylated IL-8, detected with avidin-FITC (Figure 2). The relatively small variance in IL-8R expression and the rank order of IL-8R expression in the cell lines was consistent when analyzed at the level of mRNA and protein. Taken together, these results demonstrate that IL-8 production and IL-8R expression are common features of canine HSA.
Figure 2.
Quantification of IL-8R expression in HSA cell lines. (a – e) IL-8R expression was quantified flow cytometrically using biotinylated recombinant IL-8 and avidin FITC. Gray areas represent background (negative controls). Black lines represent binding to biotinylated IL-8. (f) Bar graphs show the mean fluorescence intensity (MFI) for IL-8 binding by each cell line compared to negative controls.
IL-8 transduces biologically relevant signals through the IL-8R in HSA cells
We sought to confirm that IL-8Rs expressed by HSA cells were functional by evaluating intracellular calcium mobilization upon addition of exogenous IL-8. IL-8 induced transient increases in intracellular calcium levels in each of two cell lines tested. Figure 3 shows data from one of the cell lines (COSB), where the magnitude of change in the intracellular calcium concentration induced by IL-8 was comparable to that induced by acetylcholine, These results indicate that the binding of IL-8 to IL-8Rs on HSA cells transduces a relevant biological signal, and suggest that this ligand-receptor pair has a functional role in these tumors.
Figure 3.
Calcium mobilization induced by IL-8 in HSA cells. COSB cells were loaded with Indo-1 AM calcium sensor dye, and agonist-induced calcium mobilization was measured over time using flow cytometry. The top panel shows calcium mobilization in response to ionomycin (1.4 μM), the middle panel shows calcium mobilization in response to acetylcholine (5 μM), and the bottom panel shows calcium mobilization in response to IL-8 (1 μg/ml). The results are representative of two independent experiments. Similar data were obtained in the JHE cell line.
IL-8 does not affect cell proliferation or survival of HSA cells in vitro
To determine if IL-8 contributes to cell proliferation and survival, we performed MTS and CFSE proliferation assays by treating HSA cell lines with either rhIL-8 or α-IL-8 Ab. Neither addition of exogenous IL-8, nor blockade of IL-8 using neutralizing Abs affected proliferation of cells grown using conventional culture conditions (Supplementary Figure 1). In fact, IL-8 did not alter HSA cell proliferation rate over a 7-day time course (data not shown). The viability of HSA cells was similarly unaffected by addition of rhIL-8 or neutralizing α-IL-8 Ab: IL-8 did not rescue HSA cells from death induced by growth factor deprivation or by doxorubicin (Supplementary Figure 2).
Gene expression profiling shows that an IL-8 gene signature is associated with a reactive tumor microenvironment
We used genome-wide gene expression profiles to stratify HSA cell lines and tumor tissue samples into “IL-8 high” and “IL-8 low” groups separated by the median and mean values as described in the Methods section. Both methods generated comparable results. Supervised analysis grouped according to the median value revealed 34 and 58 transcripts that were differentially expressed between groups in the cell lines and in the tumor tissues, respectively (Figures 4a and 4b, Supplementary Tables 2 and 3). When we restricted the analysis to the top and the bottom quartile samples, the groups were more clearly separated, and additional differentially expressed genes were identified using the same parameters for significance (40 and 149 in cell lines and tissues, respectively).
Figure 4.
Stratification of HSA cells by genome-wide gene expression according to IL-8 production. Cell lines (a) and tissue samples (b) were stratified into “IL-8 high” and “IL-8 low” groups based on the median value of IL-8 gene expression as depicted in Figure 1. Heatmaps show differential expression using data from 15,555 Entrez Gene IDs. Specifically, the heat maps show 34 (P < 0.05) and 58 (P < 0.01) differentially expressed transcripts in the cell lines and tumor tissues, respectively, with a mean average fold-change > 2. Heat map colors represent mean-centered fold change expression following log2 transformation (a quantitative representation of the colors is provided in the scale). Up-regulated genes are shown in red and down-regulated genes are shown in green.
IPA analysis showed that the “IL-8 high” group in cell lines had enrichment of genes in canonical pathways including fatty acid metabolism, fibrosis, IL-17A signaling, and inflammation-dependent inhibition of RXR function, among others (Figure 5a). These molecular functions were generally conserved in the intact tissue samples, where the “IL-8 high” group had enrichment of genes in pathways related to tissue factor in cancer, coagulation, fibrosis, and inflammation (Figure 5b). Overall, the data reflect both cell-intrinsic characteristics that would modulate the local microenvironment (in the cell lines) and the presence of a reactive microenvironment (in the tissues) that is consistent with a direct association between elevated levels of IL-8 production with coagulation, fibrosis, and inflammation (Supplementary Table 4). This interpretation also is supported by the direct correlation between differentially expressed transcripts and IPA-based biological functions of inflammation, tissue development, and angiogenesis in both cell lines and tissues (Supplementary Tables 5 and 6).
Figure 5.
Enrichment of Gene Pathways in “IL-8 high” cells defined by Ingenuity Pathway Analysis (IPA). Horizontal bar graphs show statistically significant canonical pathways identified by enrichment of differentially expressed genes in the “IL-8 high” HSA group, as compared to the “IL-8 low” HSA group. Data represent cell lines (a) and tumor tissues (b) in descending rank order based on their respective P values.
To assess if the different signatures defined by IL-8 expression in tumor tissue samples were affected by the inflammation status of the tumor cells in tissues, we reviewed the microscopic features of 20 tumors. There were no appreciable differences in inflammation score (granulocytic or mononuclear infiltrates), hemorrhage, tumor necrosis, or tumor-associated fibrosis between the IL-8 high and IL-8 low samples (Supplementary Table 1).
IL-8 potentiates tumor cell survival and engraftment into host tissues
Our in vitro data and our gene expression data suggested that IL-8 did not affect HSA cells directly; thus we surmised it might have an effect in the microenvironment. To test this directly, we assessed the effect of IL-8 blockade on survival and engraftment of HSA xenografts. Growth of HSA cells in this model followed a predictable course, with 10/12 mice showing erosion of cells over the first 40 days followed by stabilization and recovery indicative of engraftment. The other two mice died 14 and 21 days after tumor cell injection (Supplementary Figure 3). Luminescent emission at the onset of recovery was directly proportional to the initial dose of cells injected (Supplementary Figure 4). And hemangiosarcomas showed comparable morphology to canine tumors, with the proliferative (Ki-67+) pool consisting of both canine tumor cells and supporting murine stroma (Supplementary Figures 5). Here, we evaluated the importance of IL-8 in the initial stages of HSA cell survival and engraftment. COSB-g/L cells showed erosion with subsequent stabilization 40–50 days after injection in mice that received control mouse IgG, whereas luciferase activity was blunted with failure to achieve stabilization and recovery after 20 days in mice treated with α-IL-8 Abs. Specifically, tumor cell engraftment was observed in four of four control mice, which was comparable to what was observed in mice receiving HSA cells alone (Group 1), whereas only two of four mice injected with α-IL-8 (Group 2) showed tumor cell engraftment by 6 weeks (Figure 6). This suggests that HSA cells have impaired survival and/or cannot engraft efficiently in an environment depleted of IL-8.
Figure 6.

Effect of IL-8 blockade on tumor development in an HSA xenograft model. Seven million HSA cells expressing GFP-Luciferase were injected intraperitoneally (I.P.) in 150 μL of PBS into immunodeficient SCID/Beige mice (n = 8). The mice were divided in two groups of four mice each, where the control group (Group 1, panel a) received IgG and the experimental group (Group 2, panel b) received mouse α-canine IL-8 Ab (100 μg per mouse) twice a week for six weeks. Live animal imaging was used to detect luciferase activity once a week for six weeks. Data points represent luminescent signal intensity (total flux measured as photons/sec/cm2/steradian).
Discussion
Canine HSA is regarded as a counterpart of human vascular cancers such as angiosarcoma and Kaposi’s sarcoma. It occurs with high incidence, and there are currently no effective treatments for these tumors [17, 25]. A better understanding of the biology and associated pathogenesis of both canine and human vascular cancers should lead to improved treatment approaches. Canine HSA includes distinct features of inflammation and angiogenesis, and the IL-8 gene is highly upregulated in this malignancy [17]. Here, we show that IL-8 also contributes to the development of canine HSA by modifying its environment and creating a favorable growth niche.
As we showed previously [17], robust IL-8 production is a recurrent feature of HSA cells. HSA cells also express functional IL-8Rs that initiate biologically relevant signals upon binding of IL-8. Curiously, IL-8 did not promote HSA cell proliferation or survival. Unlike some tumor cell types that can undergo growth arrest in the early G1 phase of the cell cycle and survive days to weeks under conditions of serum-deprivation [26, 27], serum-starved HSA cells died within 72 hours, and their survival was not improved by addition of exogenous IL-8. Conversely, IL-8 potentiates doxorubicin cytotoxicity in human breast cancer cells [28], but we also saw no association between IL-8 and doxorubicin-mediated cell death in canine HSA cells. These results were consistent in each HSA cell line tested, despite the variable levels of constitutive IL-8 produced by each cell line.
The gene expression profiles from canine HSA samples were informative. These data identified a direct correlation between IL-8 expression and biological pathways and networks reflecting reactive tumor microenvironments that included inflammation, fibrosis, coagulation, and tissue activation. These signatures were observed in both tumor tissues and cell lines. While genes that mediate inflammation, fibrosis, coagulation, and tissue activation in tissues could be associated with stromal elements in the tumor, the presence of genes that modulate these pathways in cell lines suggests that IL-8 production is part of an adaptive mechanism used by HSA cells to modulate their microenvironment.
It is known that IL-8 may alter cancer progression by affecting angiogenesis and other growth and survival pathways [7, 29, 30], yet the pathway by which IL-8 alters the microenvironment niche to promote tumor growth is unknown. Intriguingly, IL-17 related signaling pathways were enriched as part of the IL-8 gene signature. IL-17, which is mainly produced by T helper 17 (Th17) cells, is a cytokine that induces matrix destruction and mediates pro-inflammatory responses, including IL-8 production [31, 32]. IL-17 is though to play a role in various immune-mediated diseases [32] and might regulate the inflammatory niche in the tumor microenvironment, although its role in cancer remains incompletely understood [32, 33]. In this regard, our data provide clues to IL-8 related-mechanisms that may alter the microenvironment, such as tissue factor, coagulation, fatty acid activation, and macrophage, fibroblast, and endothelial cell activation.
Our results showing that IL-8 blockade inhibited tumor cell survival or engraftment in the mouse xenografts support these conclusions. Treatment with α-IL-8 Ab was previously shown to decrease tumor growth of human bladder cancer [34] and melanoma [35] in xenograft models, and other recent studies have emphasized the effects of IL-8 on tumor microenvironment [16, 29]. Conversely, Lee et al. used immunodeficient transgenic mice expressing skin-specific IL-8 to document that high levels of IL-8 in the tumor microenvironment accelerated both growth and metastasis of colon cancer [16], and Asfaha et al. reported that IL-8 aggravated inflammation and promoted colon carcinogenesis by mobilizing immature myeloid cells [29]. By combining a xenograft model with in situ gene signatures from dog “patient” tumors and from cell lines, we can establish testable predictions about mechanisms that are operative during the early stages of tumorigenesis in HSA.
Human angiosarcoma is less common than HSA in a dog, but the two diseases share clinical features of aggressiveness, lethality, and high level of metastasis [25, 36, 37]. HSAs are dynamic, heterogeneous, disseminated neoplasms comprised by various biological molecules, nutrients, and blood cells including endothelial, and inflammatory cells and fibroblasts, each employing potentially distinct metabolic pathways of biomolecular synthesis and secretion, as well as altered immune response [38, 39].
In conclusion, the results of the current study show that HSA-derived IL-8 may play a role in tumor development by modulating the tumor microenvironment, providing a unique feature that adds to the comparative value of this disease.
Supplementary Material
Highlights.
IL-8 is expressed in canine hemangiosarcoma tumor samples and cell lines.
IL-8 transduces a relevant biological signal in canine hemangiosarcoma cells.
IL-8 gene signature is associated with reactive tumor microenvironments.
IL-8 potentiates tumor cell survival and engraftment into host tissues.
Canine hemangiosarcoma provides a unique comparative model for IL-8 studies.
Acknowledgments
The authors thank Drs. Chris Pennel and Nicola Mason for review of the manuscript and insightful suggestions. This work was supported by grants CHF 422, CHF 1131, and CHF 1429 from the AKC Canine Health Foundation, DM06CO-002 from the National Canine Cancer Foundation, D10CA-501 from Morris Animal Foundation, and by funds from the Animal Cancer Care and Research Program, University of Minnesota. JHK was supported by a Fellowship from Morris Animal Foundation (D13CA-400). KLA was supported by grant T35 RR032321 from the NIH. AMF was supported by the DVM/PhD combined degree program of the College of Veterinary Medicine, University of Minnesota, by a pre-doctoral fellowship from Morris Animal Foundation (D09CA-405) and by a doctoral dissertation fellowship from the Graduate School, University of Minnesota. SR was supported by NIH T32 Comparative Medicine and Pathology training grant T32 RR018719. The NIH Comprehensive Cancer Center Support Grant to the Masonic Cancer Center (P30 CA077598) provided support for flow cytometry and imaging services.
Abbreviations
- HSA
hemangiosarcoma
- IL-8
interleukin-8
- IL-8R
interleukin-8 receptor
- α-IL-8 Ab
anti-IL-8 antibody
- IPA
Ingenuity Pathway Analysis
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldschmidt MH, Hendrick MJ. Skin and soft tissue. In: Meuten DJ, editor. Tumours in Domestic Animals. Iowa State Press; Ames: 2002. pp. 45–117. [Google Scholar]
- 2.Goritz M, Muller K, Krastel D, Staudacher G, Schmidt P, Kuhn M, Nickel R, Schoon HA. Canine Splenic Haemangiosarcoma: Influence of Metastases, Chemotherapy and Growth Pattern on Post-splenectomy Survival and Expression of Angiogenic Factors. J Comp Pathol. 2012 doi: 10.1016/j.jcpa.2012.11.234. [DOI] [PubMed] [Google Scholar]
- 3.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006;34:870–878. doi: 10.1016/j.exphem.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Clifford CA, Mackin AJ, Henry CJ. Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern Med. 2000;14:479–485. doi: 10.1892/0891-6640(2000)014<0479:tochab>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Lana S, U’Ren L, Plaza S, Elmslie R, Gustafson D, Morley P, Dow S. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007;21:764–769. doi: 10.1892/0891-6640(2007)21[764:clocfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.U’Ren LW, Biller BJ, Elmslie RE, Thamm DH, Dow SW. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. 2007;21:113–120. doi: 10.1892/0891-6640(2007)21[113:eoantv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 8.Tobler A, Moser B, Dewald B, Geiser T, Studer H, Baggiolini M, Fey MF. Constitutive expression of interleukin-8 and its receptor in human myeloid and lymphoid leukemia. Blood. 1993;82:2517–2525. [PubMed] [Google Scholar]
- 9.Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, Yuan Z, Wang LH, Cheng JQ. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8:2–6. [PubMed] [Google Scholar]
- 10.MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 11.Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275:6868–6875. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 12.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–1957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 13.Lang K, Niggemann B, Zanker KS, Entschladen F. Signal processing in migrating T24 human bladder carcinoma cells: role of the autocrine interleukin-8 loop. Int J Cancer. 2002;99:673–680. doi: 10.1002/ijc.10424. [DOI] [PubMed] [Google Scholar]
- 14.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 15.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD, Nagulapalli Venkata KC, Rosenberg DO, Petasis NA, Lenz HJ, Hong YK. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamburini BA, Phang TL, Fosmire SP, Scott MC, Trapp SC, Duckett MM, Robinson SR, Slansky JE, Sharkey LC, Cutter GR, Wojcieszyn JW, Bellgrau D, Gemmill RM, Hunter LE, Modiano JF. Gene expression profiling identifies inflammation and angiogenesis as distinguishing features of canine hemangiosarcoma. BMC Cancer. 2010;10:619. doi: 10.1186/1471-2407-10-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelaleti GB, Jardim BV, Leonel C, Moschetta MG, Zuccari DA. Interleukin-8 as a prognostic serum marker in canine mammary gland neoplasias. Vet Immunol Immunopathol. 2012;146:106–112. doi: 10.1016/j.vetimm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 19.de Andres PJ, Illera JC, Caceres S, Diez L, Perez-Alenza MD, Pena L. Increased levels of interleukins 8 and 10 as findings of canine inflammatory mammary cancer. Vet Immunol Immunopathol. 2013;152:245–251. doi: 10.1016/j.vetimm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Fosmire SP, Dickerson EB, Scott AM, Bianco SR, Pettengill MJ, Meylemans H, Padilla M, Frazer-Abel AA, Akhtar N, Getzy DM, Wojcieszyn J, Breen M, Helfand SC, Modiano JF. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Invest. 2004;84:562–572. doi: 10.1038/labinvest.3700080. [DOI] [PubMed] [Google Scholar]
- 21.Modiano JF, Bellgrau D, Cutter GR, Lana SE, Ehrhart NP, Ehrhart E, Wilke VL, Charles JB, Munson S, Scott MC, Pozniak J, Carlson CS, Schaack J, Duke RC. Inflammation, apoptosis, and necrosis induced by neoadjuvant fas ligand gene therapy improves survival of dogs with spontaneous bone cancer. Mol Ther. 2012;20:2234–2243. doi: 10.1038/mt.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamburini BA, Trapp S, Phang TL, Schappa JT, Hunter LE, Modiano JF. Gene expression profiles of sporadic canine hemangiosarcoma are uniquely associated with breed. PLoS One. 2009;4:e5549. doi: 10.1371/journal.pone.0005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott MC, Sarver AL, Gavin KJ, Thayanithy V, Getzy DM, Newman RA, Cutter GR, Lindblad-Toh K, Kisseberth WC, Hunter LE, Subramanian S, Breen M, Modiano JF. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49:356–367. doi: 10.1016/j.bone.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar N, Padilla ML, Dickerson EB, Steinberg H, Breen M, Auerbach R, Helfand SC. Interleukin-12 inhibits tumor growth in a novel angiogenesis canine hemangiosarcoma xenograft model. Neoplasia. 2004;6:106–116. doi: 10.1593/neo.03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modiano JF, Ritt MG, Wojcieszyn J, Smith R., 3rd Growth arrest of melanoma cells is differentially regulated by contact inhibition and serum deprivation. DNA Cell Biol. 1999;18:357–367. doi: 10.1089/104454999315259. [DOI] [PubMed] [Google Scholar]
- 27.Ritt MG, Mayor J, Wojcieszyn J, Smith R, Barton CL, Modiano JF. Sustained nuclear localization of p21/WAF-1 upon growth arrest induced by contact inhibition. Cancer Lett. 2000;158:73–84. doi: 10.1016/s0304-3835(00)00507-3. [DOI] [PubMed] [Google Scholar]
- 28.Gangadharan C, Thoh M, Manna SK. Late phase activation of nuclear transcription factor kappaB by doxorubicin is mediated by interleukin-8 and induction of apoptosis via FasL. Breast Cancer Res Treat. 2010;120:671–683. doi: 10.1007/s10549-009-0493-z. [DOI] [PubMed] [Google Scholar]
- 29.Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, Ericksen R, Frucht H, Fox JG, Wang TC. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 33.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9:3167–3175. [PubMed] [Google Scholar]
- 35.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, BarEli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046–1057. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Morrison WH, Byers RM, Garden AS, Evans HL, Ang KK, Peters LJ. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319–327. doi: 10.1002/1097-0142(19950715)76:2<319::aid-cncr2820760224>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Fishman AP. Endothelium: a distributed organ of diverse capabilities. Ann N Y Acad Sci. 1982;401:1–8. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- 39.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.