Abstract
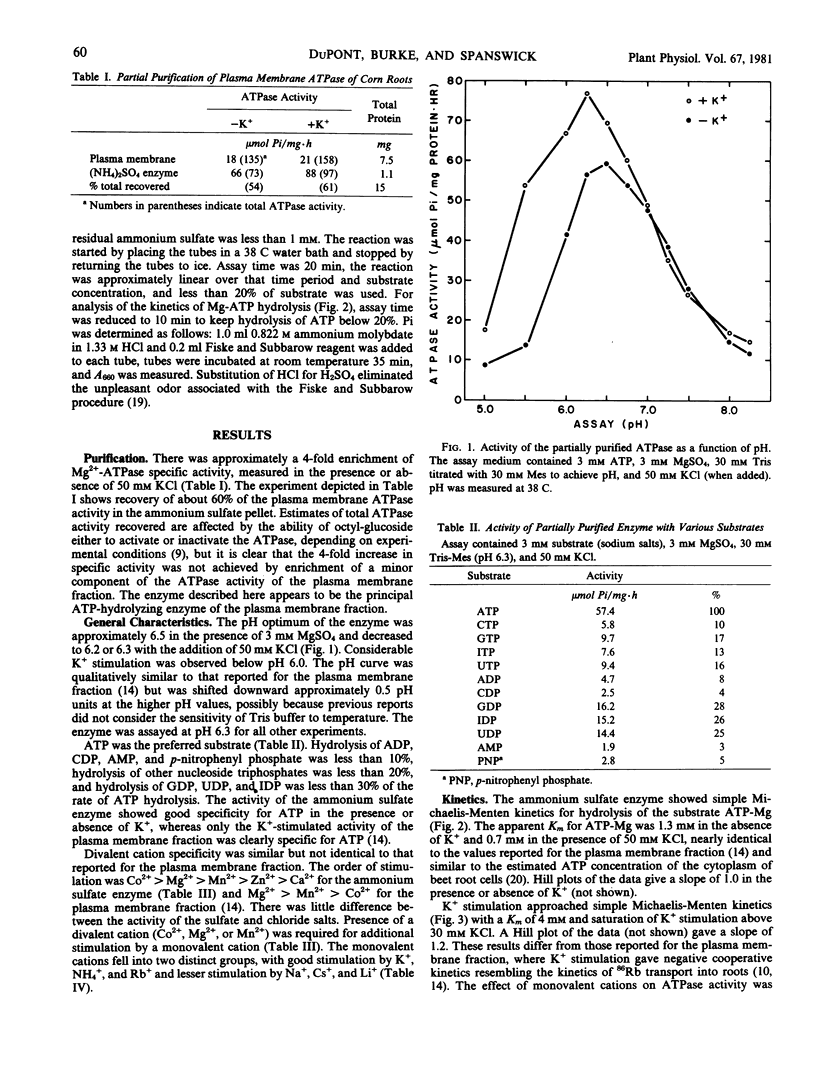
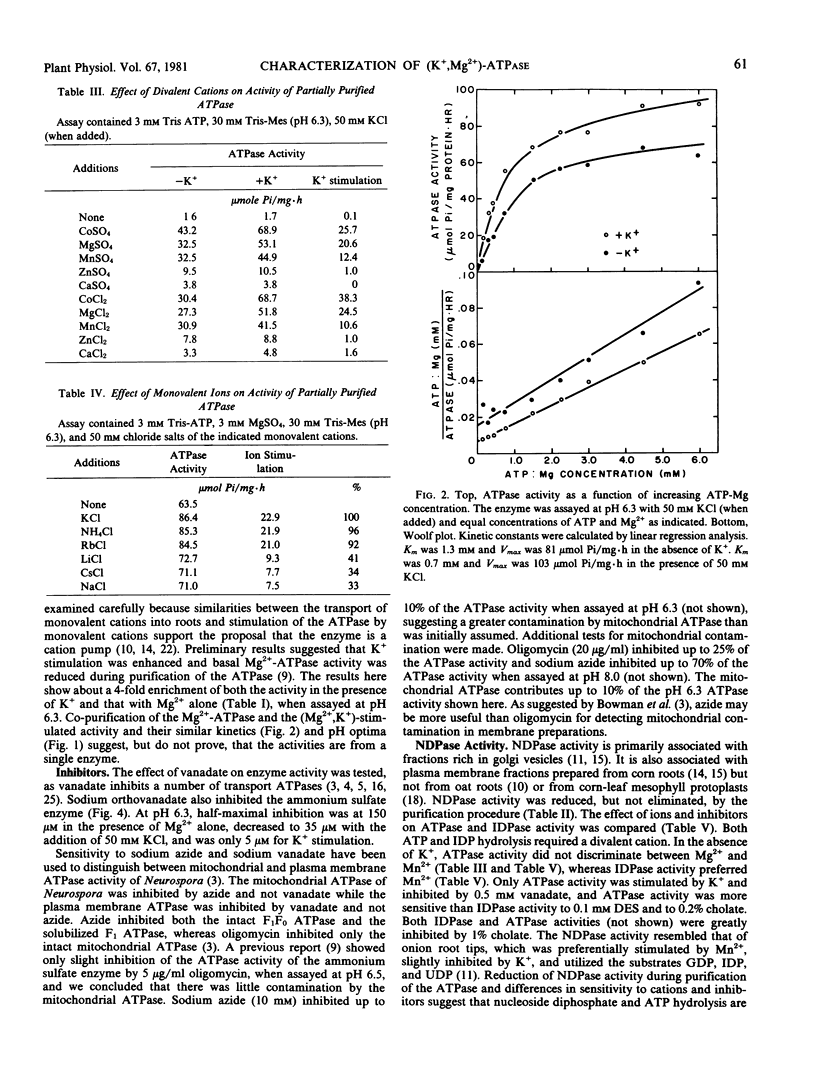
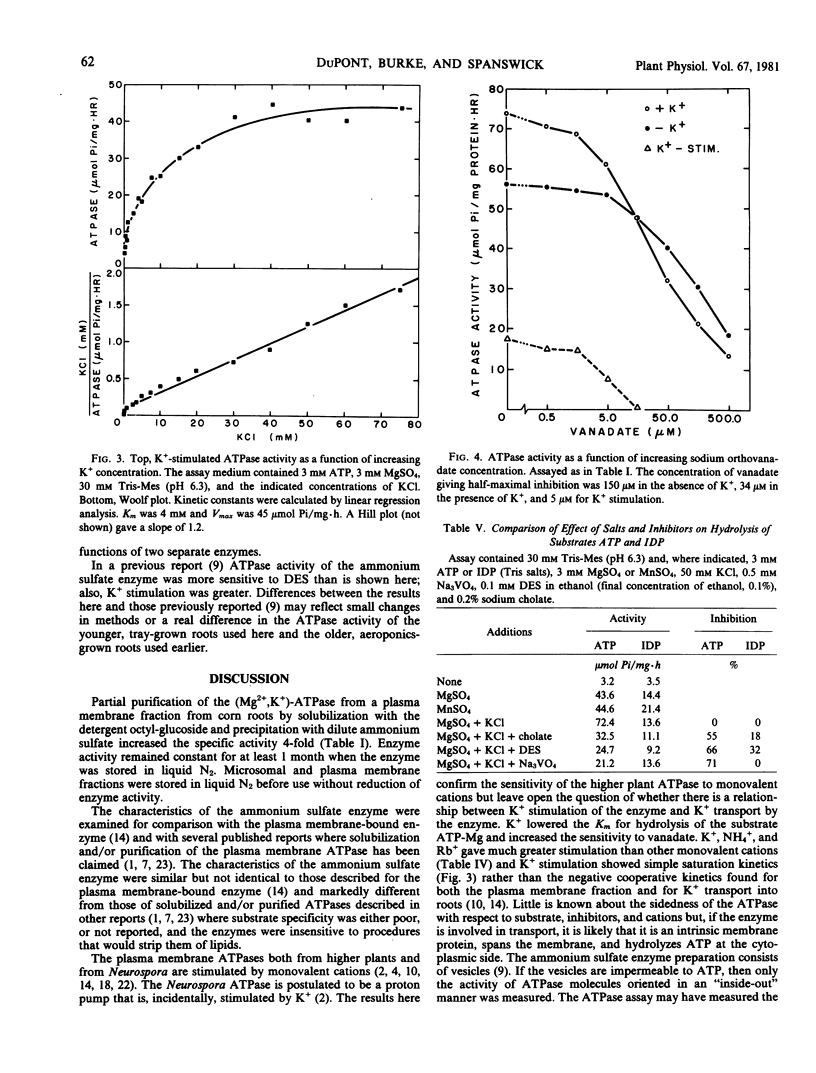
The (K+,Mg2+)-ATPase was partially purified from a plasma membrane fraction from corn roots (WF9 × Mol7) and stored in liquid N2 without loss of activity. Specific activity was increased 4-fold over that of the plasma membrane fraction. ATPase activity resembled that of the plasma membrane fraction with certain alterations in cation sensitivity. The enzyme required a divalent cation for activity (Co2+ > Mg2+ > Mn2+ > Zn2+ > Ca2+) when assayed at 3 millimolar ATP and 3 millimolar divalent cation at pH 6.3. When assayed in the presence of 3 millimolar Mg2+, the enzyme was further activated by monovalent cations (K+, NH4+, Rb+ ≫ Na+, Cs+, Li+). The pH optima were 6.5 and 6.3 in the absence and presence of 50 millimolar KCl, respectively. The enzyme showed simple Michaelis-Menten kinetics for the substrate ATP-Mg, with a Km of 1.3 millimolar in the absence and 0.7 millimolar in the presence of 50 millimolar KCl. Stimulation by K+ approached simple Michaelis-Menten kinetics, with a Km of approximately 4 millimolar KCl. ATPase activity was inhibited by sodium orthovanadate. Half-maximal inhibition was at 150 and 35 micromolar in the absence and presence of 50 millimolar KCl. The enzyme required the substrate ATP. The rate of hydrolysis of other substrates, except UDP, IDP, and GDP, was less than 20% of ATP hydrolysis. Nucleoside diphosphatase activity was less than 30% of ATPase activity, was not inhibited by vanadate, was not stimulated by K+, and preferred Mn2+ to Mg2+. The results demonstrate that the (K+,Mg2+)-ATPase can be clearly distinguished from nonspecific phosphohydrolase and nucleoside diphosphatase activities of plasma membrane fractions prepared from corn roots.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. J., Tipton C. L. Purification and Characterization of a Cation-stimulated Adenosine Triphosphatase from Corn Roots. Plant Physiol. 1978 Aug;62(2):165–172. doi: 10.1104/pp.62.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Auxin receptors of maize coleoptile membranes do not have ATPase activity. Plant Physiol. 1978 Apr;61(4):581–584. doi: 10.1104/pp.61.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J. P., Goffeau A. Molecular and kinetic properties of the purified plasma membrane ATPase of the yeast Schizosaccharomyces pombe. Eur J Biochem. 1980 Mar;105(1):145–154. doi: 10.1111/j.1432-1033.1980.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Leonard R. T. Solubilization and partial purification of the adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1980 May;65(5):931–938. doi: 10.1104/pp.65.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klohs W. D., Goff C. W. A characterization of nucleoside diphosphatase in the onion root tip. J Histochem Cytochem. 1973 May;21(5):417–425. doi: 10.1177/21.5.417. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G., Kustin K., Cantley L. C., Jr Glutathione reduces cytoplasmic vanadate. Mechanism and physiological implications. Biochim Biophys Acta. 1980 Apr 17;629(1):95–106. doi: 10.1016/0304-4165(80)90268-8. [DOI] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Labeling and isolation of plasma membranes from corn leaf protoplasts. Plant Physiol. 1980 Jun;65(6):1053–1057. doi: 10.1104/pp.65.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplified method for analysis of inorganic phosphate in the presence of interfering substances. Anal Biochem. 1978 Jan;84(1):164–172. doi: 10.1016/0003-2697(78)90495-5. [DOI] [PubMed] [Google Scholar]
- Petraglia T., Poole R. J. ATP Levels and their Effects on Plasmalemma Influxes of Potassium Chloride in Red Beet. Plant Physiol. 1980 May;65(5):969–972. doi: 10.1104/pp.65.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe H., Satter R. L. Effect of vanadate on rhythmic leaflet movement in albizzia julibrissin. Plant Physiol. 1979 Nov;64(5):905–907. doi: 10.1104/pp.64.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Selectivity of alkali cation influx across the plasma membrane of oat roots: cation specificity of the plasma membrane ATPase. Plant Physiol. 1977 Apr;59(4):641–646. doi: 10.1104/pp.59.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Berkowitz R. L. Characterization of Soybean Plasma Membrane during Development: FREE STEROL COMPOSITION AND CONCANAVALIN A BINDING STUDIES. Plant Physiol. 1980 May;65(5):871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]


