Abstract
Chlamydophila psittaci is found worldwide, but is particularly common among psittacine birds in tropical and subtropical regions. While investigating a human psittacosis outbreak that was associated with avian chlamydiosis in Hong Kong, we identified a novel adenovirus in epidemiologically linked Mealy Parrots, which was not present in healthy birds unrelated to the outbreak or in other animals. The novel adenovirus (tentatively named Psittacine adenovirus HKU1) was most closely related to Duck adenovirus A in the Atadenovirus genus. Sequencing showed that the Psittacine adenovirus HKU1 genome consists of 31,735 nucleotides. Comparative genome analysis showed that the Psittacine adenovirus HKU1 genome contains 23 open reading frames (ORFs) with sequence similarity to known adenoviral genes, and six additional ORFs at the 3′ end of the genome. Similar to Duck adenovirus A, the novel adenovirus lacks LH1, LH2 and LH3, which distinguishes it from other viruses in the Atadenovirus genus. Notably, fiber-2 protein, which is present in Aviadenovirus but not Atadenovirus, is also present in Psittacine adenovirus HKU1. Psittacine adenovirus HKU1 had pairwise amino acid sequence identities of 50.3–54.0% for the DNA polymerase, 64.6–70.7% for the penton protein, and 66.1–74.0% for the hexon protein with other Atadenovirus. The C. psittaci bacterial load was positively correlated with adenovirus viral load in the lung. Immunostaining for fiber protein expression was positive in lung and liver tissue cells of affected parrots, confirming active viral replication. No other viruses were found. This is the first documentation of an adenovirus-C. psittaci co-infection in an avian species that was associated with a human outbreak of psittacosis. Viral-bacterial co-infection often increases disease severity in both humans and animals. The role of viral-bacterial co-infection in animal-to-human transmission of infectious agents has not received sufficient attention and should be emphasized in the investigation of disease outbreaks in human and animals.
Author Summary
Chlamydophila psittaci is a bacterial pathogen which can cause avian chlamydiosis and human psittacosis. Although C. psittaci is frequently detected in birds from the tropical and subtropical regions, large outbreaks of human infections rarely occur. During the investigation of a human psittacosis outbreak that was associated with avian chlamydiosis in Hong Kong, we identified a novel adenovirus in epidemiologically linked Mealy Parrots, which was not present in healthy birds unrelated to the outbreak or in other animals. Since there was a positive correlation between the adenovirus viral load and C. psittaci bacterial load in the lungs of the infected Mealy parrots and because adenoviruses are known to cause immunosuppression in birds, we speculate that our novel Psittacine adenovirus may have caused immunosuppression among infected parrots resulting in a larger number of Mealy Parrots being infected by C. psittaci with a higher bacterial load, leading to a greater chance of zoonotic transmission to humans.
Introduction
About 70% of microbial agents causing outbreaks of emerging infectious diseases in humans originate directly from animals [1]. Among respiratory virus infections, the influenza A viruses H5N1 and H7N9 from avian species, and the severe acute respiratory syndrome coronavirus from bats have caused large epidemics [1]–[3]. Atypical bacterial pathogens causing community-acquired pneumonia include Chlamydophila psittaci from psittacine birds and Coxiella burnetti from livestock and other animals [4]. However, human outbreaks due to zoonotic bacteria associated with the emergence of a novel animal virus in the animal host were not previously documented.
In November 2012, an outbreak of human psittacosis affecting six staff members occurred at the New Territories North Animal Management Centre (NTNAMC) in Hong Kong [5]. The human outbreak was preceded by an outbreak of avian chlamydiosis among the detained Mealy Parrots (Amazona farinose). Although birds in the tropical and sub-tropical areas are commonly infected with C. psittaci, most infected birds are asymptomatic [6], [7]. Large human outbreaks are rare even among bird handlers [8]. Although co-infection of C. psittaci and viruses has been reported in outbreaks of avian species [9]–[12], no virus-bacterium co-infection of implicated avian species has ever been reported in outbreaks of human psittacosis. In this study, we sought to investigate viruses that cause avian co-infection, which may have led to this outbreak of psittacosis.
Methods
Outbreak investigation
A case was defined as a staff member working at the NTNAMC who was hospitalized for respiratory tract infection between November 1 and November 30, 2012, and confirmed to have C. psittaci infection by polymerase chain reaction (PCR) and/or a four-fold rise in serum microimmunofluorescent antibody titer against C. psittaci (Focus Diagnostics, Cypress, California, USA).
Post-mortem examination of Mealy Parrots and animal samples obtained for microbiological testing
For the investigation of the suspected outbreak of avian chlamydiosis, four Mealy Parrot carcasses from the batch were sent to Tai Lung Veterinary Laboratory of the Agriculture, Fisheries and Conservation Department for postmortem examination and disease diagnosis. Various tissues were collected and tested for C. psittaci PCR, bacterial culture and histopathological examination. Postmortem examination was performed with tissue blocks taken for sectioning and staining by hematoxylin and eosin stain. For microbiological testing, tissue (approximately 5 mm×5 mm block) or cloacal swab samples were immersed in viral transport medium [13]. Precautions were taken to avoid cross contamination as described previously [14]. Cloacal swabs, pharyngeal swabs or EDTA blood samples were also obtained from healthy parrots in NTNAMC as controls. Fecal samples were collected from other animals in NTNAMC by cloacal/rectal swabs.
Total nucleic acid extraction
Total nucleic acid extraction was performed by EZ1 virus Mini Kit v2.0 (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Avian lung, liver, spleen, kidney, and muscle tissues were disrupted mechanically before proceeding to nucleic acid extraction.
PCR detection of C. psittaci
The bacterial load of C. psittaci was detected using real-time PCR with LightCycler 96 system (Roche Applied Science, Mannheim, Germany). Primer and probe sequences are presented in Table 1. The lower detection limit was 100 copies per reaction (12,000 copies/ml of viral transport medium).
Table 1. Sequences of C. psittaci and adenovirus primers and probes used in the study.
| Pathogen | Type of PCR | Target gene | Target length (base pairs) | Primer/probe sequences |
| Chlamydophila psittaci | Real-time qPCR | ITS | 145 | Forward 5′-TTGGTCTGTAAATTATTGATCC-3′ |
| Reverse 5′-CATTTAGTTTACGATCAAGTATG-3′ | ||||
| Probe 5′-FAM-ATGCAAGTTAACWTCACCTAAAGACAT-BHQ1-3′ | ||||
| Adenovirus* | Conventional PCR | pol | 324 | Forward 5′-GTCTACGAYATHTGYGGMATGTA-3′ |
| Reverse 5′-TCTCCATCCTCDRTTRTGVA-3′ | ||||
| Psittacine adenovirus HKU1 | Conventional PCR | pol | 175 | Forward 5′-GGGTGACTATGTCTATCGACG-3′ |
| Reverse 5′-GATTTCATACTTCGACAGCG-3′ | ||||
| Psittacine adenovirus HKU1 | Real-time qPCR | pol | 175 | Forward 5′-GGGTGACTATGTCTATCGACG-3′ |
| Reverse 5′-GATTTCATACTTCGACAGCG-3′ | ||||
| Probe 5′-FAM-AGGTAGTGTGTCGAGGCGCT-BHQ1-3′ |
qPCR, quantitative polymerase chain reaction.
*Consensus primers for adenovirus were designed by performing multiple alignments of pol genes of adenovirus and related sequences available in NCBI GenBank.
Screening and quantification of adenovirus in Mealy Parrots
Tissue and cloacal swab samples of the Mealy Parrots were screened by consensus primers for common respiratory viral agents including influenza virus, coronavirus, infectious bursal disease virus, beak and feather disease virus, hepatitis E virus and the virus families Paramyxoviridae, Picornaviridae, Caliciviridae, Astroviridae, Arteriviridae, and Herpesviridae as previously reported [15]–[18]. For adenovirus, we used a nested PCR as reported previously [19] and a single-step consensus PCR using primers designed by performing multiple alignments of pol genes of adenovirus and related sequences available in NCBI GenBank (Table 1). The PCR products were gel purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3130xl DNA analyzer (Applied Biosystems, Foster City, California), using the PCR primers. The sequences of the PCR products were compared with known sequences by BLAST analysis against the NCBI database. For quantification of Psittacine adenovirus HKU1, real-time quantitative PCR (qPCR) with specific primers was performed using LightCycler 96 system (Roche Applied Science, Mannheim, Germany) (Table 1). Standards were included for quantification and no false-positive results were observed in the negative controls. The lower limit of detection was 10 copies per reaction (1200 copies/ml of viral transport medium).
Screening of Psittacine adenovirus HKU1 in other animals and humans
Conventional PCR using specific primers were used to screen for the novel adenovirus (Psittacine adenovirus HKU1) in other animals and humans (Table 1).
Virus genome sequencing
Full-length genome sequences spanning the entire protein-coding regions were determined for three strains of Psittacine adenovirus HKU1 identified in the present study. Briefly, complete genome sequences of the three strains of Psittacine adenovirus HKU1 were amplified and sequenced directly from the Mealy Parrot samples. Degenerate primers in regions of IVa, polymerase, pIIIa, hexon, 100K and fiber were designed by multiple alignment of genomes of related adenoviruses, including Bovine adenovirus 6 (GenBank accession no. NC_020074), Duck adenovirus A (GenBank accession no. NC_001813) and Ovine adenovirus D (GenBank accession no. NC_004037). Additional primers for subsequent rounds of PCR were designed based on the results of earlier rounds of genome sequencing. These primer sequences are available on request. Sequences for the 5′ and 3′ ends of the viral genome were obtained by random amplification of cDNA ends strategies using the SMARTer RACE cDNA Amplification kit (Clontech). The sequence has been submitted to NCBI GenBank and is available under the accession number KJ675568.
Phylogenetic tree and sequence analysis
Open reading frames were located using the ORF Finder tool at NCBI (http://www.ncbi.nlm.nih.gov/projects/gorf/), the gene finding program fgenesV0 (http://www.softberry.com/berry.phtml?topic=index&group=programs&subgroup=gfindv), and by comparison with genome annotations of other adenoviruses [20], [21]. Functional annotation of predicted proteins was performed by BLAST similarity search against annotations in RefSeq database and by InterProScan search tool [22]. Multiple alignments of sequences were constructed using MUSCLE [23], and phylogenetic informative regions were extracted using GBLOCKS [24]. Maximum-likelihood phylogenetic trees were constructed using PHYML version 3 [25], under the best-fit protein evolution model as selected by ProtTest 3 [26]. Recombination detection was performed using bootscan analysis in SimPlot [27], with window size of 1000 bp and step size of 100 bp. Whole genome DNA-DNA hybridization values were predicted using Genome-to-Genome Distance Calculator (GGDC) 2.0 [28].
Viral culture
Viral culture was performed using chick embryo, the avian cell line DF-1, and five other cell lines (HEp-2, Vero E6, human embryonic lung fibroblast, Caco-2 and BSC-1) as previously reported [29], [30]. All infected cell lines were incubated at 37°C for seven days, passaged once and then incubated for another seven days. The cell lines were examined for cytopathic effect using inverted light microscopy on days 1, 3, 5, and 7 of incubation.
Detection of adenovirus positive cells in tissue specimens
Adenovirus in tissue specimens was detected by immunofluorescence staining. The Psittacine adenovirus HKU1 fiber protein was cloned and expressed to generate specific antibodies. The gene was amplified by primers 5′-GCGAGCTAGCATGTGGCATTTTACTGCAGCCAGT-3′ and 5′-CACTAAGCTTTTATTGTAGTGAAATAAAAGCAGAA-3′. The fiber fragment was then cloned into the NheI and HindIII sites of expression vector pET-28b(+) (Novagen, Madison, Wisconsin) in frame and downstream of a series of six histidine residues. The His6-tagged recombinant fiber protein was expressed and purified by Ni2+-loaded HiTrap chelating system (GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer's instructions. Specific guinea pig antiserum was produced by injecting 200 µg of purified His6-tagged recombinant fiber protein, along with an equal volume of complete Freund's adjuvant, into the thighs of guinea pigs intramuscularly. Incomplete Freund's adjuvant was used in subsequent immunizations. Three inoculations were completed in six weeks, with one injection every two weeks. Serum samples were collected two weeks after the last injection. Immunofluorescence staining for adenovirus fiber protein antigen was performed as described previously [30]. The tissue sections were deparaffinized and rehydrated, followed by blocking with 1% bovine serum albumin in PBS to minimize non-specific binding. The sections were incubated overnight with immune serum from guinea pigs at 4°C. After washing three times with PBS, the sections were incubated with FITC-conjugated rabbit anti-guinea pig IgG (Life technology, Invitrogen, Carlsbad, California) at 1∶50 dilution for 30 min at room temperature. The images were captured by Nikon 80i imaging system with Spot Advanced Software. The animal experiment has been approved by the Committee on the Use of Live Animals in Teaching and Research, The University of Hong Kong, in accordance with the Guidelines laid down by the NIH in the USA regarding the care and use of animals for experimental procedures (CULATR 2489-11).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0. Correlation of C. psittaci bacterial load and adenovirus viral load was determined using linear regression analysis. For statistical calculation, 50% of the lower detection limit (6,000 copies/ml for C. psittaci and 600 copies/ml for adenovirus) was assigned to specimens that tested negative. Log-transformed viral loads were used for statistical analysis.
Results
The index case
The patient was a 55-year-old male artisan working at the NTNAMC. He presented after four days of dyspnea and two days of hemoptysis. Chest radiograph showed bilateral patchy consolidation. Blood test showed neutrophilia and elevated liver enzymes with alanine transaminase of 51 U/L. He was put on non-invasive positive pressure ventilation for respiratory failure. Bronchoalveolar lavage tested positive for C. psittaci and rhinovirus by PCR and reverse transcriptase-PCR respectively. Direct fluorescent antigen detection and viral culture did not reveal other viral co-pathogens. Paired serology, collected 18 days apart (on days 2 and 20 after hospitalization), showed a rise of C. psittaci IgG titer from <32 to 128 by microimmunofluorescence assay, but there was no increase in adenovirus or other respiratory virus antibody titer. The patient recovered with oral doxycycline. He had contact with birds, monkeys, iguanas and snakes at the NTNAMC within one month of symptom onset.
The human outbreak
Epidemiological investigation by the Centre for Health Protection of Hong Kong showed that five other members of staff at the NTNAMC were hospitalized for respiratory tract infection, with onset of symptoms from November 6–24, 2012. The details of the five other patients have been reported previously [5].
Avian chlamydiosis in Mealy Parrots
Sixteen Mealy Parrots imported from Guyana were detained at NTNAMC for observation since October 20, 2012. NTNAMC is a center where animal case-exhibits are detained while pending legal processing. Two Mealy Parrots died naturally, while 14 developed disease and were euthanized. Of the 16 Mealy Parrots, 4 were sent for postmortem examination and 8 were sent for microbiological testing. The 4 remaining Mealy Parrots were not available for further testing.
Postmortem examination of four Mealy Parrots that were euthanized showed necrotising splenitis in three birds (bird 1, 3, and 4), and necrotising hepatitis in two birds (bird 1 and 4) (Fig. 1A and 1B, Table 2). C. psittaci PCR was positive for all four birds, and Giemsa and Gimenez stains for C. psittaci were positive in the liver tissue of bird 1 (Fig. 1C).
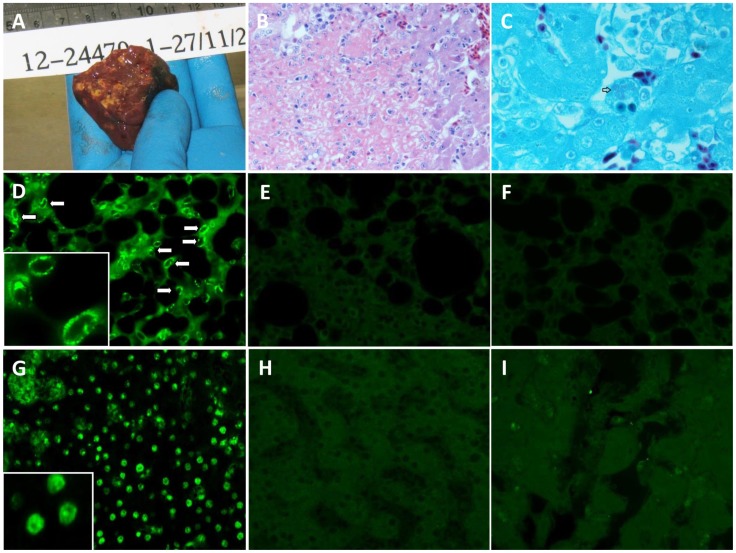
Figure 1. Illustrative histopathological changes of infected birds.
Panels A–C: Macroscopic and microscopic changes in the liver of bird 1. Panel A: cut surface of liver showing numerous 1–2 mm coalescing necrosis. Panel B: areas of acute coagulative necrosis, which are randomly distributed throughout the liver (hematoxylin and eosin staining; original magnification, ×400). Panel C: Intracytoplasmic accumulations of Chlamydial organisms (arrow) are commonly found throughout the liver parenchyma (gimenez staining; original magnification, ×1000). Panels D–I: immunochemical staining for Psittacine adenovirus HKU1 fiber protein (Original magnification, ×400). The tissue sections were deparaffinized and rehydrated, followed by blocking with 1% bovine serum albumin in PBS to minimize non-specific binding. The sections were incubated with immune serum (panels D, F, G, I) or with non-immune serum (panels E and H) of guinea pigs. After washing three times with PBS, the sections were incubated with FITC-conjugated rabbit anti-guinea pig IgG. For bird 2, apple green fluorescent foci were seen in the cytoplasm of pneumocytes (D) and in the nucleus of hepatocytes (G) with immune serum. As controls, fluorescent foci were not detected in the lung (E) and liver (H) tissue of bird 2 with non-immune serum. For bird 3, fiber protein-positive cells were not detected in the lung (F) and liver (I) tissue.
Table 2. Postmortem findings of four parrots obtained during the avian chlamydiosis outbreak.
| Bird no. | Gross appearance | Pathology | Specimens tested positive by Chlamydophila psittaci PCR |
| 1 | Both abdominal air sacs: multiple yellowish plaques of fibrin | Necrotising splenitis | Liver tissue, lung tissue, esophagus tissue, pharyngeal swab, conjunctival swab, cloacal swab |
| Liver: numerous pale yellowish foci | Necrotising hepatitis | ||
| Pericardium: thickened, granular and tightly adherent to the heart | Necrotising nephropathy | ||
| Spleen: congested and swollen | No significant findings in the lung | ||
| 2 | Abdominal air sacs: multiple yellowish plaques of fibrin | No significant findings in heart, lung, liver, spleen, small intestine, large intestine, muscle, gizzard and air sac | Esophagus tissue and tracheal swab |
| Liver: numerous pale yellowish foci | |||
| Pericardium: thickened, granular | |||
| 3 | Left abdominal air sac: cloudy and thickened | Necrotising splenitis | Liver tissue, esophagus tissue, pharyngeal swab and cloacal swab |
| Both lungs: greyish pink in color and rubbery to feel | No significant findings in heart, lung, liver, kidney, muscle and trachea | ||
| Trachea: small amount of yellow caseous material | |||
| Liver: swollen | |||
| Pericardium: thickened, granular | |||
| Peritoneum over the cranial pole of the left kidney: thickened and granular | |||
| Spleen: enlarged and swollen | |||
| 4 | Both lungs: greyish pink in color and rubbery to feel | Necrotising splenitis | Liver tissue, pharyngeal swab, tracheal swab, conjunctival swab and cloacal swab |
| Liver: swollen | Necrotising hepatitis | ||
| Spleen: enlarged and swollen | No significant findings in the heart, lung, kidney, trachea, muscle |
Eight other parrots detained during the outbreak were retrieved for microbiological testing (Table 3), in which seven were euthanized because they developed disease and one died. Seven out of eight parrots (87.5%) were positive for C. psittaci. All eight parrots were positive for adenovirus by one-step consensus PCR but not the nested PCR. All other viruses were negative by consensus PCR screening. Blood samples were not available from infected parrots for testing. Forty-one additional specimens from 18 healthy parrots unrelated to the outbreak were negative for C. psittaci or adenovirus (Table 3). Among parrots, there was a significant association between the presence of adenovirus and C. psittaci (C. psittaci was present in 7/8 adenovirus-positive parrots vs. 0/18 adenovirus-negative parrots, P<0.0001 by Fisher exact test). Moreover, fecal samples taken from 25 other detained mammals, reptiles and avians in NTNAMC during the outbreak were negative for both adenovirus and C. psittaci.
Table 3. Detection of Chlamydophila psittaci and Psittacine adenovirus HKU1 among eight affected parrots and control parrots and animals detained during the outbreak.
| Total no. of specimens available | No. of specimens positive for C. psittaci | No. of specimens positive for Psittacine adenovirus HKU1 | ||
| Affected Mealy Parrots n = 8 | Lung | 8 | 4 | 7 |
| Kidney | 7 | 6 | 6 | |
| Liver | 8 | 4 | 5 | |
| Spleen | 5 | 4 | 5 | |
| Cloacal swab | 8 | 8 | 8 | |
| Unaffected control parrots* n = 18 | All specimens | 41 | 0 | 0‡ |
| Other detained animals† n = 25 | Cloacal/rectal samples | 25 | 0 | 0‡ |
*18 healthy parrots, including three Lesser Sulphur-crested Cockatoos (Cacatua sulphurea), two Cockatiels (Nymphicus hollandicus), two Grey Parrots (Psittacus erithacus), two Rose-ringed Parakeets (Psittacula krameri), one Greater Sulphur-crested Cockatoo (Cacatua galerita galerita), one Red-shouldered Macaw (Diopsittaca nobilis), one Monk Parakeet (Myiopsitta monachus), one Eclectus Parrot (Eclectus roratus), one Salmon-crested Cockatoo (Cacatua moluccensis), one Eastern Rosella (Platycercus eximius), one Rosy-faced Lovebird (Agapornis roseicollis), one Sun Conure (Aratinga solstitialis), and one Blue-and-yellow Macaw (Ara ararauna). Cloacal swabs (n = 18), pharyngeal swabs (n = 7) and whole blood samples (n = 16).
14 mammals (seven dogs, four cats, three monkeys), seven reptiles (three ball pythons [Python regius], two African Spurred Tortoises [Geochelone sulcata], two Common Iguanas [Iguana iguana]) and four birds (two Grey Parrots, one green peafowl [Pavo muticus], one swan).
Consensus nested PCR for adenovirus DNA polymerase was also performed on these specimens, but all were negative.
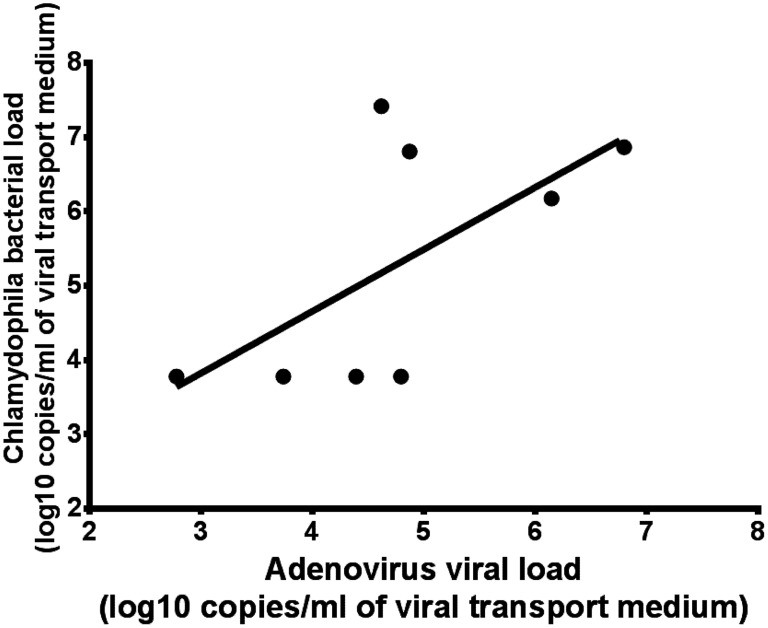
Adenovirus viral load testing was performed on tissue and cloacal swab specimens from the infected birds. The C. psittaci bacterial load was positively correlated with adenovirus viral load in the lung (r2 = 0.4053; P = 0.0897) (Fig. 2). Immunofluorescence staining targeting the Psittacine adenovirus HKU1 fiber protein was performed for bird 2 and bird 3. Psittacine adenovirus HKU1 antigen was detected in the lung and liver tissue cells of bird 2 but not bird 3 (Fig. 1D–1I). Viral culture was negative for seven cloacal swabs, three lung samples, two liver samples and two kidney samples, which were positive for adenovirus PCR.
Figure 2. Correlation of adenovirus viral loads and C. psittaci bacterial loads in lung specimens from Mealy Parrots.
Screening of the Psittacine adenovirus HKU1 among infected human cases
PCR for the Psittacine adenovirus HKU1 was performed on specimens from four patients, including the whole blood and bronchoalveolar lavage of the index patient, the nasopharyngeal aspirate of two patients, and the sputum of one patient. None of the specimens were positive for this novel avian adenovirus.
Genome sequencing and analysis of novel Psittacine adenovirus HKU1
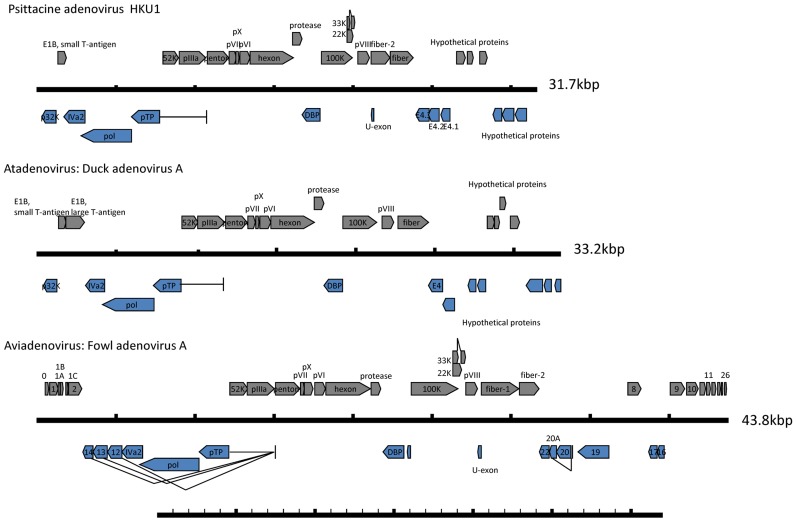
Whole viral genome sequencing was performed on three samples positive for adenovirus. Nucleotide sequences of the adenovirus genomes in the three samples were fully identical. The full-length genomic sequence is 31,735 bp in length, with a G+C content of 53.5%. Inverted terminal repeat (ITR) regions of 44 bp at both ends of the genome are complementary to each other. The viral genome contains 23 ORFs with sequence similarity to known adenoviral genes (Fig. 3 and Table 4). Additionally, six ORFs, ranging from 381 to 741 bp were identified at the 3′ end of the genome. While lacking significant sequence similarity to known adenoviral sequences, they were predicted to have coding potential by fgenesV0. The genome organization of this novel virus most closely resembles that of Atadenovirus, with minor variations. Similar to Duck adenovirus A, the novel adenovirus lacks LH1, LH2 and LH3, which distinguishes it from the bovine adenovirus E, bovine adenovirus D and ovine adenovirus D. Similar to other Atadenovirus, the novel virus possesses a DNA polymerase, terminal protein and DNA-binding protein for DNA replication; the E1B small T-antigen, IVa2 and 52K proteins for DNA encapsidation; and the IIIa, penton protein/III, core protein 1/pVII, core protein 2/pX, pVI protein, hexon protein, endopeptidase/protease, 100K, pVIII protein and fiber protein for the formation and structure of the virion [31]. The novel adenovirus has two fiber proteins. Notably, the fiber-2 protein (or short fiber) is not found in any Atadenovirus, but can be found in Aviadenovirus. As in other adenoviruses, a putative splicing site was identified in the pTP gene. Recombination analysis by bootscanning showed possible phylogenetic incongruence in small regions of the viral genome (Fig. 4), but further examination by phylogenetic analysis of the corresponding regions did not reveal convincing evidence of recombination.
Figure 3. Genome organization of the Psittacine adenovirus HKU1, comparison with duck adenovirus A and fowl adenovirus A.
Table 4. Predicted proteins in the Psittacine adenovirus HKU1.
| Open reading frame | Gene name | Coding sequence positions | Frame | Size (aa) | |
| Start | Stop | ||||
| 1 | p32K | 1210 | 293 | −3 | 305 |
| 2 | E1B, small T-antigen | 1177 | 1713 | +1 | 178 |
| 3 | IVa2 protein | 3033 | 1702 | −1 | 443 |
| 4 | DNA polymerase | 6005 | 2772 | −2 | 1077 |
| 5 | Terminal protein precursor | 7777 | 5978 | −3 | 606 |
| 10540 | 10520 | −1 | |||
| 6 | 52K protein | 7863 | 8837 | +3 | 324 |
| 7 | IIIa protein | 8840 | 10519 | +2 | 559 |
| 8 | Penton protein/III | 10550 | 11902 | +2 | 450 |
| 9 | Core protein 1/pVII | 11945 | 12406 | +2 | 153 |
| 10 | Core protein 2 (mu protein)/pX | 12426 | 12623 | +3 | 65 |
| 11 | pVI protein | 12671 | 13300 | +2 | 209 |
| 12 | Hexon protein | 13321 | 16062 | +1 | 913 |
| 13 | Endopeptidase/protease | 16059 | 16667 | +3 | 202 |
| 14 | DNA-binding protein | 17808 | 16672 | −1 | 378 |
| 15 | 100K protein | 17845 | 19812 | +1 | 655 |
| 16 | 22K protein | 19616 | 19819 | +2 | 67 |
| 17 | 33K protein | 19616 | 19811 | +2 | 147 |
| 19888 | 20135 | +3 | |||
| 18 | pVIII protein | 20144 | 20896 | +2 | 250 |
| 19 | U-exon | 21073 | 20909 | −3 | 54 |
| 20 | Fiber-2 protein | 21143 | 22345 | +2 | 400 |
| 21 | Fiber protein | 22356 | 23816 | +3 | 486 |
| 22 | E4.3 protein | 24648 | 23818 | −1 | 276 |
| 23 | E4.2 protein | 25331 | 24648 | −2 | 227 |
| 24 | E4.1 protein | 25922 | 25347 | −2 | 191 |
| 25 | Hypothetical protein | 26401 | 26970 | +1 | 189 |
| 26 | Hypothetical protein | 27242 | 27622 | +2 | 126 |
| 27 | Hypothetical protein | 28029 | 28505 | +3 | 158 |
| 28 | Hypothetical protein | 29419 | 28859 | −3 | 186 |
| 29 | Hypothetical protein | 30185 | 29445 | −2 | 246 |
| 30 | Hypothetical protein | 30983 | 30255 | −2 | 242 |
Figure 4. Bootscan analysis using SimPlot did not show strong phylogenetic signal of recombination in the present adenovirus.
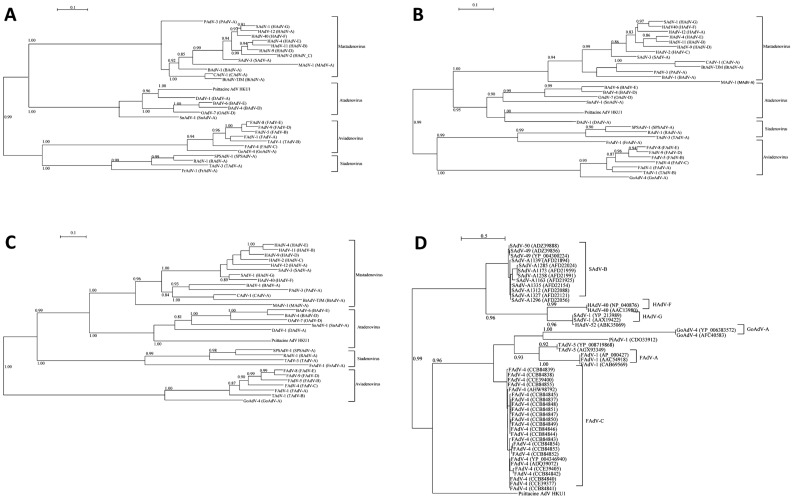
Pairwise amino acid sequence comparison of the major conserved adenoviral genes of this novel adenovirus showed that they were most similar to Atadenovirus, with pairwise amino acid identities of 50.3–54.0% for the DNA polymerase, 64.6–70.7% for the penton protein, and 66.1–74.0% for the hexon protein (Table 5). Phylogenetic analysis of the hexon, penton and DNA polymerase amino acid sequences consistently showed clustering of the novel adenovirus with viruses in the genus Atadenovirus, most closely related to sequences from Duck adenovirus A (Fig. 5A–5C). The fiber-2 protein amino acid sequence of the novel adenovirus was most closely related to that of Fowl adenovirus C (Fig. 5D). In silico DNA-DNA hybridization (DDH) using GGDC 2.0 was also performed to estimate genomic DNA similarity between the novel adenovirus and other adenoviruses. However, the estimated DDH values were at the lower limit of analysis (12.5% for formula 1, and 0% for formulae 2 and 3), which suggested that the genomic DNA sequences are too dissimilar for DDH analysis.
Table 5. Pairwise amino acid sequence identities between the novel Psittacine adenovirus HKU1 and other adenoviruses.
| Pairwise amino acid sequence identities (%) to novel Psittacine adenovirus HKU1 | |||||||||||||
| IVa2 | Pol | pTP | 52K | IIIa | Penton | pVII | pVI | Hexon | Protease | DBP | 100K | pVIII | |
| Atadenovirus | |||||||||||||
| BAdV-4 (BAdV-D) | 47.7 | 51.4 | 42.1 | 43.9 | 46.7 | 65.2 | 33.3 | 47.5 | 66.1 | 52.9 | 40.9 | 48.7 | 35.3 |
| DAdV-1 (DAdV-A) | 54 | 54 | 44.8 | 55.8 | 57.6 | 70.7 | 59.3 | 52.1 | 74 | 60.3 | 42.4 | 48.5 | 45.9 |
| OAdV-7 (OAdV-D) | 47.5 | 51.9 | 41.4 | 41.5 | 44.1 | 66.5 | 29.9 | 47.1 | 69.9 | 51.4 | 40.8 | 46.4 | 35.1 |
| SnAdV-1 (SnAdV-A) | 55.4 | 50.3 | 39.7 | 42.3 | 46.4 | 66.8 | 31.2 | 54 | 68.8 | 57.4 | 39.1 | 45.4 | 42.2 |
| BAdV-6 (BAdV-E) | 46.6 | 50.3 | 42.4 | 43.3 | 41.8 | 64.6 | 32.2 | 46.1 | 68.5 | 53.9 | 41 | 47.5 | 35.7 |
| Aviadenovirus | |||||||||||||
| FAdV-1 (FAdV-A) | 25.7 | 32.4 | 26.3 | 24.8 | 19.7 | 45.5 | 15.4 | 30 | 46 | 40 | 22 | 21 | 15.8 |
| FAdV-5 (FAdV-B) | 25 | 30.6 | 23.9 | 22.2 | 20.4 | 44.7 | 16.4 | 29.9 | 45.6 | 40.3 | 18.8 | 19.5 | 15.5 |
| FAdV-4 (FAdV-C) | 25.6 | 31.8 | 27.5 | 21.8 | 19.9 | 45.2 | 12 | 32.4 | 45.4 | 36.6 | 18.6 | 23.6 | 14.7 |
| FAdV-9 (FAdV-D) | 25.5 | 31.7 | 27.3 | 21.7 | 18.7 | 41.7 | 11.2 | 31 | 44.5 | 38.8 | 21.6 | 20.6 | 15.6 |
| FAdV-8 (FAdV-E) | 26.3 | 32 | 23.9 | 21.6 | 18.9 | 43.6 | 14.6 | 18 | 45.4 | 40.4 | 20.4 | 19.5 | 16 |
| GoAdV-4 (GoAdV-A) | 25.1 | 31.5 | 24.9 | 22.2 | 19.7 | 44.6 | 16.1 | 31 | 46.3 | 40 | 16.9 | 20 | 15.7 |
| TAdV-1 (TAdV-B) | 26.5 | 30.9 | 27.3 | 23 | 20.2 | 44.5 | 15.6 | 29.7 | 45.4 | 37.6 | 18.2 | 20.1 | 15.7 |
| Siadenovirus | |||||||||||||
| FrAdV-1 (FrAdV-A) | 24.5 | 34.6 | 26.5 | 22.4 | 21.3 | 52.4 | 10.4 | 28.6 | 49.7 | 40.5 | 22.1 | 25.3 | 17.5 |
| RAdV-1 (RAdV-A) | 24.3 | 37 | 26.8 | 18.9 | 22.3 | 51.7 | 13.1 | 30 | 49.7 | 41 | 20.6 | 26.9 | 18.6 |
| SPSAdV-1 (SPSAdV-A) | 24.5 | 37 | 25.2 | 17.9 | 22.4 | 51.5 | 11.6 | 30 | 47.5 | 42.5 | 22.1 | 27.8 | 18.4 |
| TAdV-3 (TAdV-A) | 24.1 | 36.3 | 26.3 | 18.6 | 21.4 | 49.4 | 11.1 | 29.8 | 49.5 | 38 | 21.1 | 27.6 | 15.7 |
| Mastadenovirus | |||||||||||||
| BtAdV-TJM (BtAdV-A) | 32.4 | 35.7 | 25.8 | 18.9 | 27.2 | 45.1 | 22.8 | 15.8 | 52 | 39.2 | 19.1 | 24.8 | 18.9 |
| BAdV-1 (BAdV-A) | 33.9 | 38.4 | 25.5 | 21.9 | 26.4 | 52.9 | 18.1 | 12.3 | 52.5 | 37.9 | 24.2 | 25.5 | 18.5 |
| CAdV-1 (CAdV-A) | 32.8 | 39.5 | 25.2 | 20.2 | 27.1 | 48.4 | 22.8 | 16 | 52.3 | 38.2 | 20.8 | 25.4 | 18.4 |
| HAdV-12 (HAdV-A) | 33.1 | 37.4 | 25.9 | 19.8 | 27.5 | 48.1 | 25.2 | 15.2 | 49.4 | 40 | 17.6 | 25.1 | 19.7 |
| HAdV-11 (HAdV-B) | 32.6 | 37.6 | 25.9 | 19.7 | 28 | 43.9 | 24.2 | 16.6 | 47.7 | 38.5 | 17.9 | 24.1 | 20 |
| HAdV-2 (HAdV-C) | 32.8 | 37.4 | 25 | 18.7 | 26.6 | 42.8 | 23.5 | 16.1 | 47.9 | 38.8 | 17.7 | 23.9 | 20 |
| HAdV-9 (HAdV-D) | 32.6 | 37.7 | 25.9 | 20.3 | 27.8 | 47.4 | 23.5 | 16 | 48.3 | 38.5 | 19.9 | 26 | 19.3 |
| HAdV-4 (HAdV-E) | 32.6 | 37.3 | 26.2 | 20.2 | 27.2 | 46.2 | 25.2 | 16 | 48.6 | 39.6 | 18.7 | 25.6 | 20 |
| HAdV-40 (HAdV-F) | 32.4 | 37.4 | 26.2 | 21.3 | 27.5 | 48.4 | 23.9 | 15.7 | 49.5 | 39.8 | 20.5 | 25.4 | 19.7 |
| SAdV-1 (HAdV-G) | 32.6 | 37.7 | 26.5 | 21 | 27 | 48.7 | 23.1 | 15.2 | 49 | 40.7 | 18.6 | 26.6 | 20.4 |
| MAdV-1 (MAdV-A) | 35.6 | 36.8 | 23.4 | 21.5 | 24 | 45.8 | 23.1 | 17.2 | 50.9 | 43.6 | 20.6 | 25.7 | 19.4 |
| PAdV-3 (PAdV-A) | 32.4 | 37.5 | 26.9 | 19.8 | 24.3 | 51.7 | 21.6 | 18.1 | 50.9 | 38.3 | 21.3 | 23.1 | 18.1 |
| SAdV-3 (SAdV-A) | 32.6 | 38 | 26.5 | 19.7 | 26.3 | 48.5 | 23.8 | 13.9 | 49 | 39.3 | 20.4 | 25.3 | 21.5 |
Figure 5. Maximum-likelihood phylogenetic tree showing the relationship of Psittacine adenovirus HKU1 to other adenoviruses inferred from A) hexon protein, B) penton protein and C) polymerase protein, D) fiber-2 protein.
The trees were constructed using PHYML version 3 under the best-fit protein evolution model as selected by ProtTest 3. The bootstrap values were calculated from 1,000 trees. (AdV, adenovirus; BAdV, bovine adenovirus; BtAdV, bat adenovirus; CAdV, canine adenovirus; DAdV, duck adenovirus; FAdV, fowl adenovirus; FrAdV, frog adenovirus; GoAdV, goose adenovirus; MAdV, murine adenovirus; OAdV, ovine adenovirus; PAdV; porcine adenovirus; PiAdV, pigeon adenovirus; RAdV, raptor adenovirus; SAdV, simian adenovirus; SnAdV, snake adenovirus; SPSAdV; south polar skua adenovirus; TAdV; turkey adenovirus).
Discussion
To the best of our knowledge, this is the first report of a co-infection of psittacine birds with avian adenovirus and C. psittaci associated with an outbreak of human psittacosis. In this study, we have identified a novel adenovirus that was most closely related to Duck adenovirus A of the Atadenovirus genus in the epidemiologically linked Mealy Parrots. In contrast, this novel adenovirus was not identified in any of the healthy parrots and other detained animals without C. psittaci infection. Psittacine adenovirus HKU1 antigen was detected in lung and liver tissue cells using immunostaining, which indicated active viral replication instead of latent infection. A positive correlation between adenovirus viral loads with C. psittaci bacterial loads was observed in lung specimens, which suggested a possible synergistic interaction between adenovirus and C. psittaci in disease pathogenesis.
In birds, many adenoviral infections are subclinical, but some infections can lead to severe disease [32], [33]. Previous studies have suggested that avian adenoviruses may cause immunosuppression in birds. Aviadenovirus can lead to immunosuppressive disease, such as the hydropericardium syndrome. Fowl adenovirus and chicken anemia virus co-infection causes much more severe disease than either virus alone [34]. Avian adenoviruses have been shown to directly infect lymphocytes and dendritic cells in the spleen [35] and causes depletion of lymphocytes [36], [37]. Fowl adenovirus 1 has been shown to affect antibody response of chicks to Brucella abortus [38]. We speculate that our novel adenovirus may have caused immunosuppression among the infected parrots, and therefore a larger number of Mealy Parrots were infected by C. psittaci with a higher bacterial load, leading to a higher chance of zoonotic transmission to humans. Other studies have also proposed that reovirus and avian pneumovirus infection may cause immunosuppression leading to avian chlamydiosis [11], [12]. Experimental infection showed that avian pneumovirus could exacerbate acute C. psittaci infection in turkeys [12]. Alternatively, in a report demonstrating adenovirus-C. psittaci co-infection in a parakeet using electron microscopy and antigen detection without molecular confirmation, Desmidt M et al proposed that C. psittaci could cause immunosuppression, which can lead to reactivation of latent adenovirus infection [10]. However, no further studies have been performed to verify this hypothesis.
In psittacine birds, adenovirus infection manifests as depression, anorexia, diarrhea and cloacal hemorrhage [39]. Gross examination may show hepatomegaly, splenomegaly, dilatation of the duodenum and proventriculus, swollen kidneys, and edema, congestion, and hemorrhage of the lungs. Histological changes may include necrosis in the liver and the spleen. A case of inclusion body hepatitis has been described [40]. The pathological features in our infected parrots were typical of C. psittaci infection, but some of these are actually indistinguishable from adenovirus infection as one Mealy Parrot was also positive for the Psittacine adenovirus HKU1 on immunofluorescence staining.
Current ICTV guidance on adenovirus taxonomy specifies species designation by phylogenetic distance, host range, DNA hybridization, nucleotide composition, cross-neutralization and gene organization at the right end of the viral genome [41]. With the exception of nucleotide composition and cross-neutralization, for which no data are available, our data and analysis of the Psittacine adenovirus HKU1 are consistent with it being a novel member of the genus Atadenovirus. Notably, the genome of the Psittacine adenovirus HKU1 has a higher G+C content than other Atadenovirus genomes, which might be viewed as a feature against its designation as a novel Atadenovirus. Indeed, the high A+T content of the first few identified Atadenoviruses was considered characteristic among adenoviruses and was the basis for naming the genus. Nonetheless, the snake adenovirus 1, a member of the Atadenovirus, has a genomic G+C content of 50.2%, which is lower than but comparable to the G+C content of 53.5% for the Psittacine adenovirus HKU1. Hence, we consider that the high genomic G+C content of the present Psittacine adenovirus HKU1 is not a major contraindication to its inclusion in the genus Atadenovirus.
Besides the genomic G+C content, there are several unusual genomic features in the Psittacine adenovirus HKU1. Firstly, a second viral protein, fiber-2, is present in our novel adenovirus, but not in any other Atadenovirus. The fiber-2 protein has also been found in the Aviadenovirus, like the fowl adenovirus. Since fiber proteins are responsible for binding to cellular receptor, it has been postulated that the presence of different fiber proteins may determine the tropism of the adenovirus [42]. It remains to be determined whether the fiber-2 protein is important for the virus to infect psittacine hosts. Another interesting feature is the predicted CCU start codon of the IVa2 protein. The annotation is made on the basis of sequence conservation among other adenoviral IVa2 proteins, and the lack of an in-frame ATG in its vicinity. The function of the non-canonical CCU start codon may be augmented by the presence of an upstream Kozak sequence ACCACC. The use of CCU as the start codon is rare in other organisms, but has been reported in psittacine viruses. The other notable genomic feature in the Psittacine adenovirus HKU1 is the intron between the two exons of 33K, which usually overlaps with coding sequences in other adenoviruses, but not in the present case.
Virus and bacteria often act synergistically in causing diseases in humans or animals. C. psittaci-avian pneumovirus co-infection has been associated with an outbreak in turkeys [12], C. psittaci-fowlpox virus co-infection with an outbreak in hens [9] and C. psittaci-reovirus with an outbreak in budgerigars [11]. Infectious bursal disease virus and chicken anaemia virus can cause immunosuppression, leading to secondary bacterial infection such as bacterial chondronecrosis with osteomyelitis [43]. However, it is unclear whether co-infection in birds can increase the risk of transmission of avian pathogens to humans. Our investigation suggested that the novel Psittacine adenovirus may have been associated with immunosuppression among infected birds, leading to higher C. psittaci bacterial loads in the lungs of psittacine birds, and hence increasing the risk of infection in exposed humans.
Acknowledgments
We thank Patrick Lau, Geraldine Luk, Russell Graydon, Alan Chi-Kong Wong and Siu-Fai Leung (Agriculture, Fisheries, and Conservation Department, HKSAR) for microbiological sampling, data provision, postmortem and histopathological examination.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
Carol Yu, Richard Yu, Hui Hoy, and Hui Ming supported the genomic sequencing platform. This work was partly supported by the Commissioned research grant from the Research Fund for the Control of Infectious Diseases of the Food and Health Bureau of the Hong Kong SAR and the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chan JF, To KK, Tse H, Jin DY, Yuen KY (2013) Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol 21: 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. To KK, Chan JF, Chen H, Li L, Yuen KY (2013) The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect Dis 13: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng VC, Lau SK, Woo PC, Yuen KY (2007) Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 20: 660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cunha BA (2006) The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect 12 Suppl 3: 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centre for Health Protection (2013) Psittacosis outbreak in an animal management centre, 2012. Communicable Diseases Watch 10: 9–10. [Google Scholar]
- 6. Piasecki T, Chrzastek K, Wieliczko A (2012) Detection and identification of Chlamydophila psittaci in asymptomatic parrots in Poland. BMC Vet Res 8: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spickler AR, Roth JA, Galyon J, Lofstedt J (2009) Emerging and exotic diseases of animals: The Center for Food Security and Public Health, Iowa. [Google Scholar]
- 8. Stewardson AJ, Grayson ML (2010) Psittacosis. Infect Dis Clin North Am 24: 7–25. [DOI] [PubMed] [Google Scholar]
- 9. Karpinska TA, Kozaczynski W, Niemczuk K, Jasik A, Kycko A, et al. (2014) Mixed infection by fowlpox virus and Chlamydophila psittaci in a commercial laying hen flock. Acta Vet Hung 62: 42–51. [DOI] [PubMed] [Google Scholar]
- 10. Desmidt M, Ducatelle R, Uyttebroek E, Charlier G, Hoorens J (1991) Respiratory adenovirus-like infection in a rose-ringed parakeet (Psittacula krameri). Avian Dis 35: 1001–1006. [PubMed] [Google Scholar]
- 11. Perpinan D, Garner MM, Wellehan JF, Armstrong DL (2010) Mixed infection with reovirus and Chlamydophila in a flock of budgerigars (Melopsittacus undulatus). J Avian Med Surg 24: 316–321. [DOI] [PubMed] [Google Scholar]
- 12. Loock MV, Loots K, Zande SV, Heerden MV, Nauwynck H, et al. (2006) Pathogenic interactions between Chlamydophila psittaci and avian pneumovirus infections in turkeys. Vet Microbiol 112: 53–63. [DOI] [PubMed] [Google Scholar]
- 13. Chan KH, To KK, Chan BW, Li CP, Chiu SS, et al. (2013) Comparison of pyrosequencing, Sanger sequencing, and melting curve analysis for detection of low-frequency macrolide-resistant mycoplasma pneumoniae quasispecies in respiratory specimens. J Clin Microbiol 51: 2592–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tse H, Tsoi HW, Teng JL, Chen XC, Liu H, et al. (2011) Discovery and genomic characterization of a novel ovine partetravirus and a new genotype of bovine partetravirus. PLoS One 6: e25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tse H, Chan WM, Tsoi HW, Fan RY, Lau CC, et al. (2011) Rediscovery and genomic characterization of bovine astroviruses. J Gen Virol 92: 1888–1898. [DOI] [PubMed] [Google Scholar]
- 16. Katoh H, Ohya K, Fukushi H (2008) Development of novel real-time PCR assays for detecting DNA virus infections in psittaciform birds. J Virol Methods 154: 92–98. [DOI] [PubMed] [Google Scholar]
- 17. Lau SK, Woo PC, Yip CC, Choi GK, Wu Y, et al. (2012) Identification of a novel feline picornavirus from the domestic cat. J Virol 86: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. To KK, Chan KH, Li IW, Tsang TY, Tse H, et al. (2010) Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol 82: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wellehan JF, Johnson AJ, Harrach B, Benko M, Pessier AP, et al. (2004) Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J Virol 78: 13366–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hess M, Blocker H, Brandt P (1997) The complete nucleotide sequence of the egg drop syndrome virus: an intermediate between mastadenoviruses and aviadenoviruses. Virology 238: 145–156. [DOI] [PubMed] [Google Scholar]
- 21. Chiocca S, Kurzbauer R, Schaffner G, Baker A, Mautner V, et al. (1996) The complete DNA sequence and genomic organization of the avian adenovirus CELO. J Virol 70: 2939–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zdobnov EM, Apweiler R (2001) InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17: 847–848. [DOI] [PubMed] [Google Scholar]
- 23. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552. [DOI] [PubMed] [Google Scholar]
- 25. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 26. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- 27. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, et al. (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Auch AF, von Jan M, Klenk HP, Goker M (2010) Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2: 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan JF, Chan KH, Choi GK, To KK, Tse H, et al. (2013) Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis 207: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo PC, Lau SK, Wong BH, Fan RY, Wong AY, et al. (2012) Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc Natl Acad Sci U S A 109: 5435–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davison AJ, Benko M, Harrach B (2003) Genetic content and evolution of adenoviruses. J Gen Virol 84: 2895–2908. [DOI] [PubMed] [Google Scholar]
- 32. Schade B, Schmitt F, Bohm B, Alex M, Fux R, et al. (2013) Adenoviral gizzard erosion in broiler chickens in Germany. Avian Dis 57: 159–163. [DOI] [PubMed] [Google Scholar]
- 33. McFerran JB, Smyth JA (2000) Avian adenoviruses. Rev Sci Tech 19: 589–601. [PubMed] [Google Scholar]
- 34. Toro H, Gonzalez C, Cerda L, Hess M, Reyes E, et al. (2000) Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Dis 44: 51–58. [PubMed] [Google Scholar]
- 35. Gomez-Villamandos JC, Bautista MJ, Carrasco L, Hervas J, Sierra MA (1995) Electron microscopic evidence for infection of splenic dendritic cells by adenovirus in psittacine birds. Res Virol 146: 389–395. [DOI] [PubMed] [Google Scholar]
- 36. Schonewille E, Singh A, Gobel TW, Gerner W, Saalmuller A, et al. (2008) Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Vet Immunol Immunopathol 121: 130–139. [DOI] [PubMed] [Google Scholar]
- 37. Saifuddin M, Wilks CR (1992) Effects of fowl adenovirus infection on the immune system of chickens. J Comp Pathol 107: 285–294. [DOI] [PubMed] [Google Scholar]
- 38. Singh A, Grewal GS, Maiti NK, Oberoi MS (2006) Effect of fowl adenovirus-1 (IBH isolate) on humoral and cellular immune competency of broiler chicks. Comp Immunol Microbiol Infect Dis 29: 315–321. [DOI] [PubMed] [Google Scholar]
- 39. Katoh H, Ogawa H, Ohya K, Fukushi H (2010) A review of DNA viral infections in psittacine birds. J Vet Med Sci 72: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 40. Gomez-Villamandos JC, Mozos E, Sierra MA, Perez J, Mendez A (1992) Inclusion bodies containing adenovirus-like particles in the intestine of a psittacine bird affected by inclusion body hepatitis. J Wildl Dis 28: 319–322. [DOI] [PubMed] [Google Scholar]
- 41.International Committee on Taxonomy of Viruses Virus Taxonomy: 2013 Release. Available at http://www.ictvonline.org/virusTaxonomy.asp. Accessed on 22 March 2014.
- 42. Roelvink PW, Kovesdi I, Wickham TJ (1996) Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol 70: 7614–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNamee PT, Smyth JA (2000) Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian Pathol 29: 477–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.