Abstract
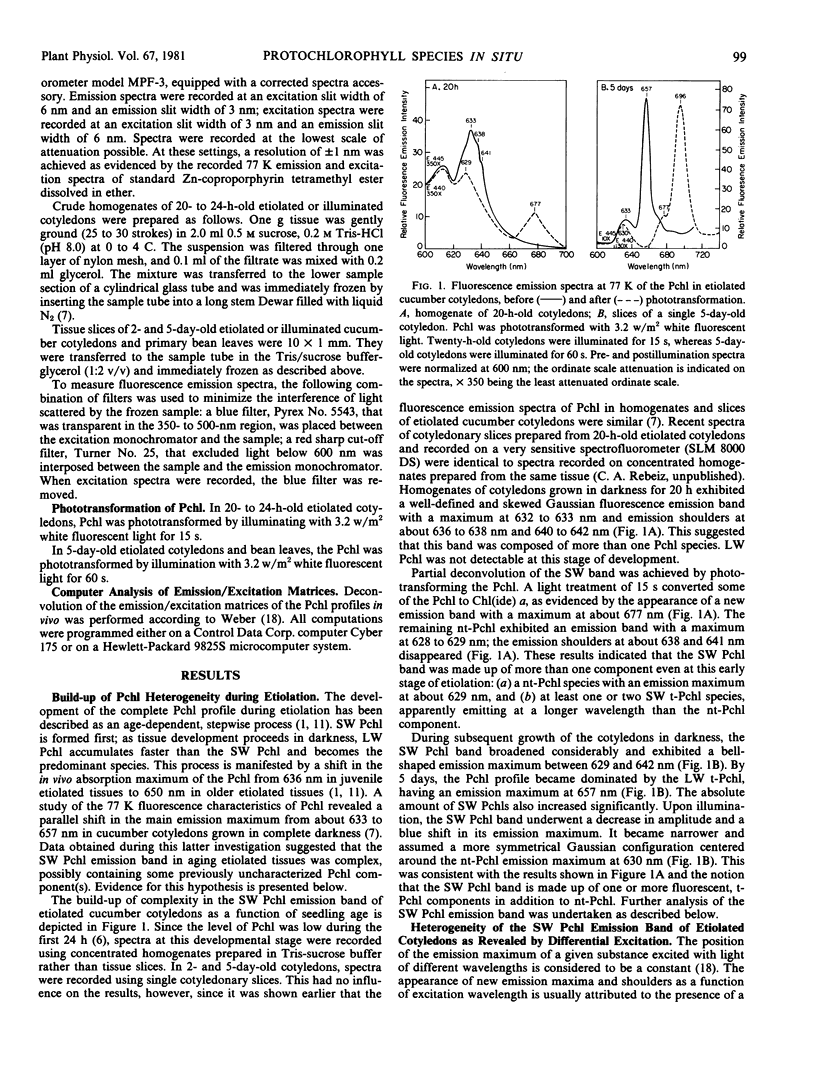
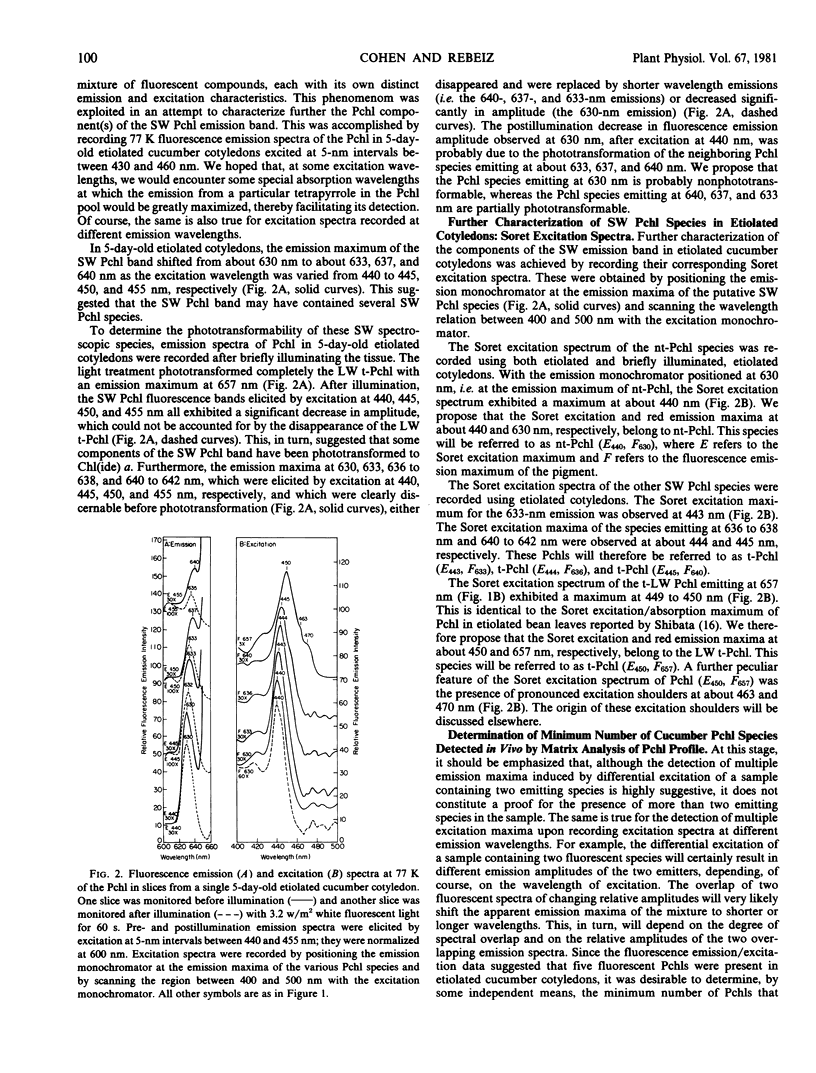
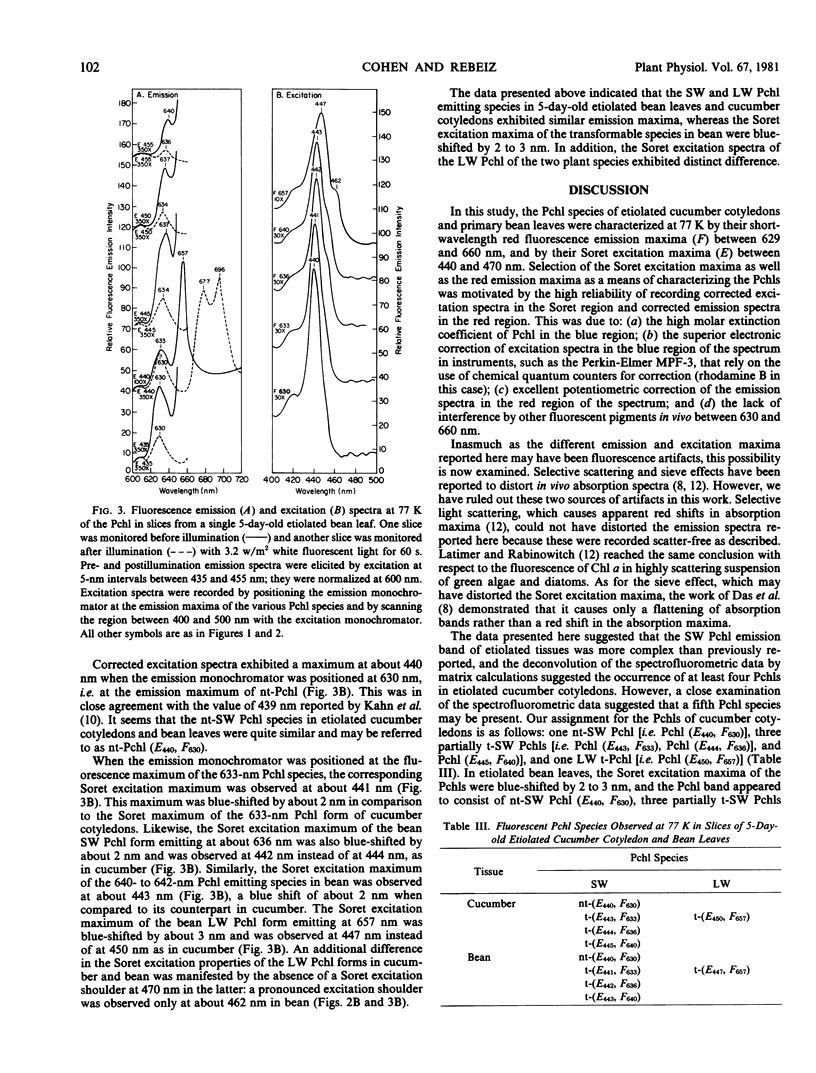
The fluorescence emission and excitation properties of protochlorophyll in etiolated cucumber (Cucumis sativus L.) cotyledons and primary bean (var. Red Kidney) leaves were characterized at 77 K. Contrary to previous studies, it appears that the short-wavelength protochlorophyll emission band consists of four fluorescent components, instead of only one nonphototransformable protochlorophyll. It was demonstrated that etiolated cucumber cotyledons synthesize and accumulate nontransformable protochlorophyll (E440, F630) as well as short-wavelength phototransformable protochlorophyll (E433, F633), (E444, F636), and (E445, F640). Long-wavelength phototransformable protochlorophyll (E450, F657) is also formed. In this context, E refers to the Soret excitation maxima and F refers to the red emission maxima of the protochlorophylls.
In etiolated bean leaves, the corresponding species were: nontransformable protochlorophyll (E440, F630), short-wavelength phototransformable protochlorophylls (E441, F633), (E442, F636), and (E443, F640), and long-wavelength phototransformable protochlorophyll (E447, F657).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis, XXVII. Detection of novel chlorophyll and chlorophyll precursors in higher plants. Biochem Biophys Res Commun. 1979 May 28;88(2):365–371. doi: 10.1016/0006-291x(79)92057-6. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis. Detection of divinyl protochlorophyllide in higher plants. J Biol Chem. 1980 Feb 25;255(4):1266–1272. [PubMed] [Google Scholar]
- Cohen C. E., Bazzaz M. B., Fullett S. H., Rebeiz C. A. Chloroplast Biogenesis: XX. Accumulation of Porphyrin and Phorbin Pigments in Cucumber Cotyledons during Photoperiodic Greening. Plant Physiol. 1977 Nov;60(5):743–746. doi: 10.1104/pp.60.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. E., Rebeiz C. A. Chloroplast Biogenesis: XXII. Contribution of Short Wavelength and Long Wavelength Protochlorophyll Species to the Greening of Higher Plants. Plant Physiol. 1978 May;61(5):824–829. doi: 10.1104/pp.61.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., Boardman N. K., Thorne S. W. Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex vivo and in vitro. J Mol Biol. 1970 Feb 28;48(1):85–101. doi: 10.1016/0022-2836(70)90220-2. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A. The Correlated Appearance of Prolamellar Bodies, Protochlorophyll(ide) Species, and the Shibata Shift during Development of Bean Etioplasts in the Dark. Plant Physiol. 1972 Apr;49(4):619–626. doi: 10.1104/pp.49.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATIMER P., RABINOWITCH E. Selective scattering of light by pigments in vivo. Arch Biochem Biophys. 1959 Oct;84:428–441. doi: 10.1016/0003-9861(59)90605-8. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Belanger F. C., Freyssinet G., Saab D. G. Chloroplast biogenesis. XXIX. The occurrence of several novel chlorophyll a and b chromophores in higher plants. Biochim Biophys Acta. 1980 Apr 2;590(2):234–247. doi: 10.1016/0005-2728(80)90028-6. [DOI] [PubMed] [Google Scholar]
- Redlinger T. E., Apel K. The effect of light on four protochlorophyllide-binding polypeptides of barley (Hordeum vulgare). Arch Biochem Biophys. 1980 Mar;200(1):253–260. doi: 10.1016/0003-9861(80)90352-5. [DOI] [PubMed] [Google Scholar]
- WEBER G. Enumeration of components in complex systems by fluorescence spectrophotometry. Nature. 1961 Apr 1;190:27–29. doi: 10.1038/190027a0. [DOI] [PubMed] [Google Scholar]



