Abstract
Two methods of measuring protein breakdown resulting from self-digestion during incubation in extracts of soybean leaves were examined. The release of free α-amino-nitrogen was measured with ninhydrin, and the disappearance of the large subunit of ribulose bisphosphate carboxylase (RuBPcase) was followed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Rates of protein breakdown were measured as a function of temperature, pH, and leaf developmental stage and in the presence of various proteinase inhibitors. These treatments had differential effects on apparent proteolysis, depending on the method used. Determination of the ratio of α-amino-nitrogen plus peptide bond-nitrogen to α-amino-nitrogen indicated that the ninhydrin method detected the activity of exopeptidases preferentially. The disappearance of the large subunit of RuBPCase as shown on gels was due primarily to the activity of endopeptidases. The sensitivity of the two types of proteolytic degradation to proteinase inhibitors differed.
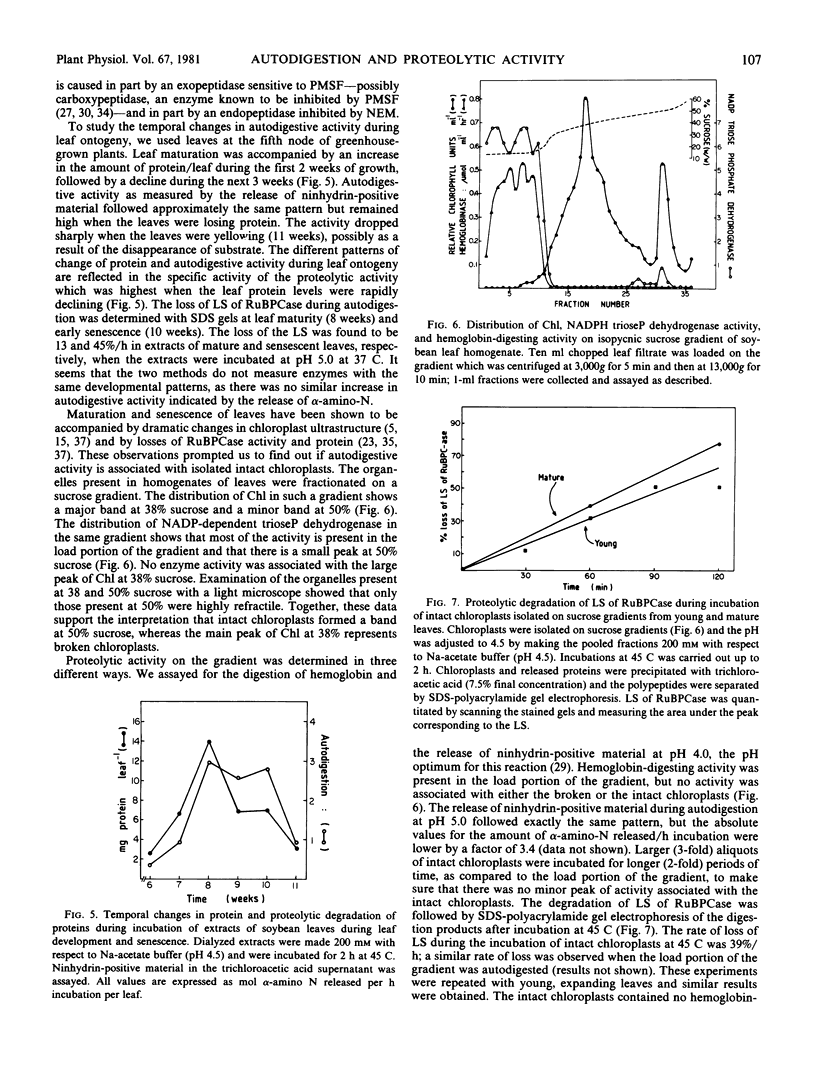
Determination of temporal changes in proteolytic activity during leaf development showed that total proteolytic activity, measured by either method, increased during leaf expansion and maturation and decreased during senescence. Incubation of intact isolated chloroplasts at 37 C resulted in the breakdown of the large subunit of RuBPcase, although the chloroplasts contained no measurable proteinase activity as determined by the release of α-amino-nitrogen during the incubation. No acid proteinase (pH 4.5) activity was detected in the chloroplasts when hemoglobin was used as a substrate. These results indicate that the proteinases which break down RuBPCase in isolated chloroplasts may not be detectable by conventional assay procedures.
Full text
PDF
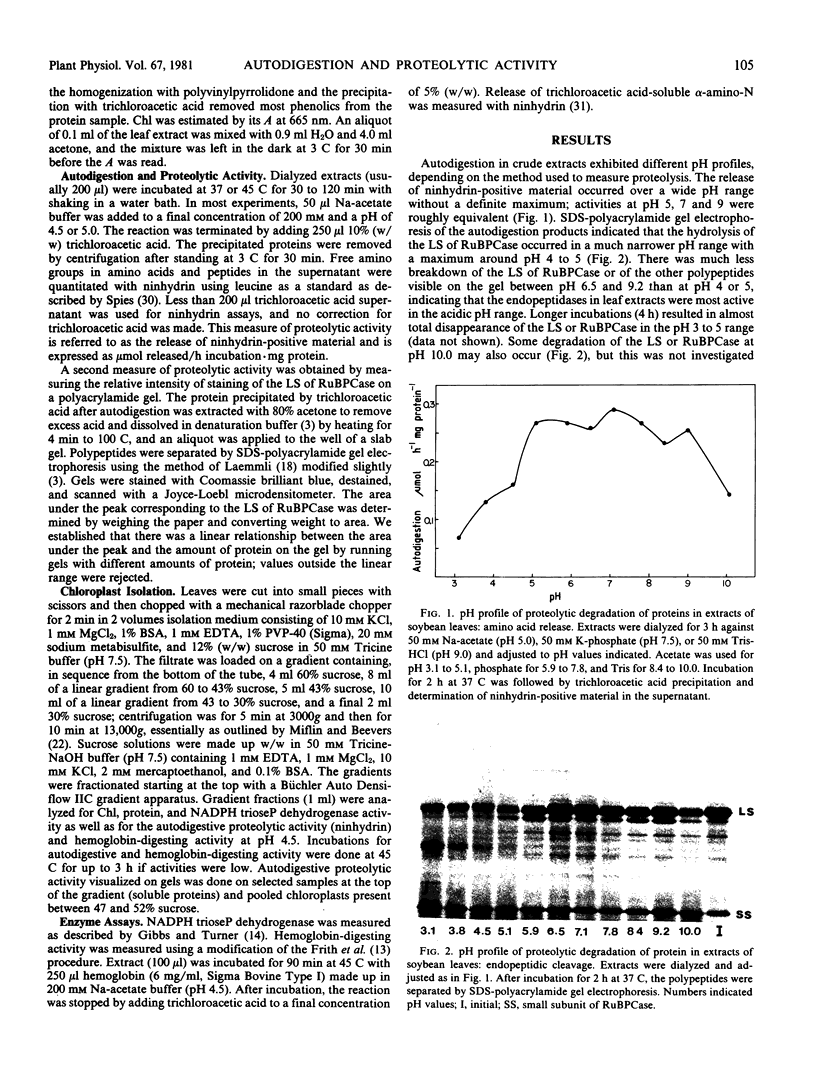
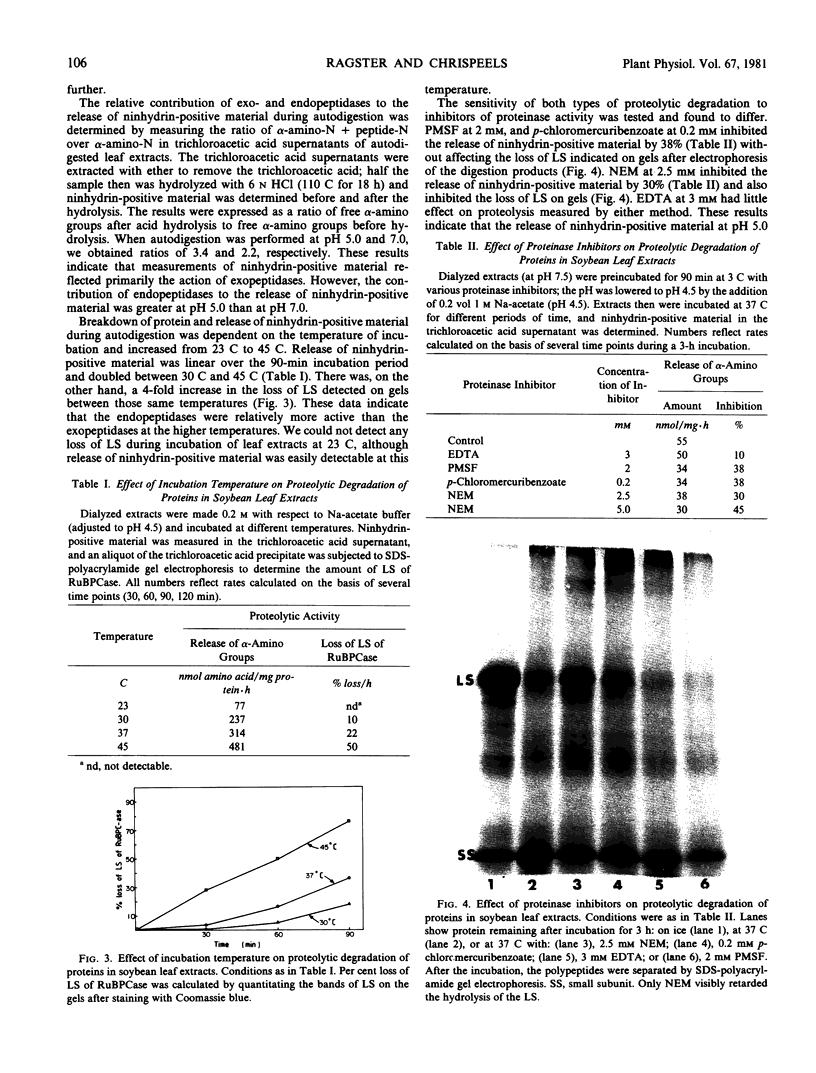


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Rowan K. S. Activity of peptidase in tobacco-leaf tissue in relation to senescence. Biochem J. 1965 Dec;97(3):741–746. doi: 10.1042/bj0970741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H. T., Thimann K. V. The Metabolism of Oat Leaves during Senescence: III. The Senescence of Isolated Chloroplasts. Plant Physiol. 1975 May;55(5):828–834. doi: 10.1104/pp.55.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of senescing oat leaves: I. Purification and general properties. Plant Physiol. 1977 Jun;59(6):1059–1063. doi: 10.1104/pp.59.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U. K., Soong T. S., Hageman R. H. Leaf Proteolytic Activities and Senescence during Grain Development of Field-grown Corn (Zea mays L.). Plant Physiol. 1977 Feb;59(2):290–294. doi: 10.1104/pp.59.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung S. Tobacco fraction 1 protein: a unique genetic marker. Science. 1976 Feb 6;191(4226):429–434. doi: 10.1126/science.1108201. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K. R., Kruger J. E. Purification and properties of two proteolytic enzymes with carboxypeptidase activity in germinated wheat. Plant Physiol. 1976 Oct;58(4):516–520. doi: 10.1104/pp.58.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. C., Wells J. R. Radiochemical determination of a unique sequence around the reactive serine residue of a di-isopropyl phosphorofluoridate-sensitive plant carboxypeptidase and a yeast peptidase. Biochem J. 1972 Jun;128(2):229–235. doi: 10.1042/bj1280229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. R. Characterisation of three proteolytic enzymes from French beans. Biochim Biophys Acta. 1968 Oct 8;167(2):388–398. doi: 10.1016/0005-2744(68)90218-0. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Ribulose Bisphosphate Carboxylase and Proteolytic Activity in Wheat Leaves from Anthesis through Senescence. Plant Physiol. 1979 Nov;64(5):884–887. doi: 10.1104/pp.64.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]