Abstract
Our previous studies indicated that transcription factor Brn-4 is upregulated in the surgically denervated hippocampus in vivo, promoting neuronal differentiation of hippocampal neural stem cells (NSCs) in vitro. The molecules mediating Brn-4 upregulation in the denervated hippocampus remain unknown. In this study we examined the levels of insulin-like growth factor-1 (IGF-1) in hippocampus following denervation. Surgical denervation led to a significant increase in IGF-1 expression in vivo. We also report that IGF-1 treatment on NSCs in vitro led to a marked acceleration of Brn-4 expression and cell differentiation down neuronal pathways. The promotion effects were blocked by PI3K-specific inhibitor (LY294002), but not MAPK inhibitor (PD98059); levels of phospho-Akt were increased by IGF-1 treatment. In addition, inhibition of IGF-1 receptor (AG1024) and mTOR (rapamycin) both attenuated the increased expression of Brn-4 induced by IGF-1. Together, the results demonstrated that upregulation of IGF-1 induced by hippocampal denervation injury leads to activation of the PI3K/Akt signaling pathway, which in turn gives rise to upregulation of the Brn-4 and subsequent stem cell differentiation down neuronal pathways.
Introduction
Degeneration, necrosis, or loss of neurons is pathological characteristics of many nervous system diseases. Replacement of the lost neurons by transplantation of exogenous neurons, or by activation of endogenous neurons or their precursors, may provide a treatment for nervous system diseases. Previous studies have shown that neural stem cells (NSCs) are present not only in embryonic brain tissue but also in the adult dentate gyrus of the hippocampus and subventricular zone [1], [2]. These cells possess stem cell properties including self-renewal, proliferation, and multipotent differentiation. NSCs are therefore generally considered to be a potential source of cells for cell replacement therapy. However, NSCs only produce a small number of neurons under normal conditions. Some external factors such as NG2 and Mash1 [3], [4], [5] can promote NSC differentiation into neurons, but the numbers of differentiated neurons remain too low to meet treatment demands. It is therefore important to identify the factors and mechanisms involved in neuronal differentiation of NSCs to guide the production of NSCs for clinical needs.
We previously reported that the environment of the denervated hippocampus following fimbria fornix (FiFx) transection significantly improved the survival, migration, and neuronal differentiation of both grafted and endogenous newborn NSCs compared with normal hippocampus [6], [7]. These results indicated that the denervated hippocampus provides a microenvironment suitable for the survival and differentiation of NSCs. It is therefore important to determine the cues in the denervated hippocampus that are responsible for this phenomenon. We previously reported that Brn-4, a member of the POU-III class of transcription factors [8], is upregulated in the hippocampus after denervation surgery [9]. Previous studies showed that POU genes display cell type-specific gene expression in mammals [8], [10], [11], [12], [13]. Transcription in NSCs is regulated by a combination of POU-domain factors [14] and we previously presented evidence that upregulation of Brn-4 is involved in the differentiation of NSCs into neurons [9], [15]. Shimazaki et al. [16] confirmed that exposure of NSCs derived from embryonic (E) day E14 mouse striatum to either insulin-like growth factor-1 (IGF-1) or brain-derived neurotrophic factor (BDNF) resulted in rapid upregulation of Brn-4 mRNA and protein levels; upregulation was accompanied by increased neuronal differentiation which could be attenuated by Brn-4 antisense oligonucleotides.
These results suggest that Brn-4 plays an important role in driving neuronal differentiation of NSCs, and that its expression is subject to growth factor control. However, the molecular mechanisms underlying Brn-4 upregulation and/or Brn-4-mediated neuronal differentiation of NSCs remain unknown. In the present study we wished to determine whether IGF-1 is involved in the upregulation of Brn-4 expression and neuronal differentiation taking place in the hippocampus following denervation. We therefore investigated potential changes in IGF-1 levels in the denervated hippocampus in vivo, and also whether IGF-1 influences Brn-4 expression and neuronal differentiation of hippocampus-derived NSCs in vitro. This work led to the identification of a molecular pathway controlling Brn-4 expression. The results of this study provide a theoretical basis for inducing neuronal differentiation of hippocampal NSCs and facilitating the development of NSCs for clinic use.
Materials and Methods
1. Reagents
Dulbecco’s modified Eagle’s medium/F12 (1∶1, DMEM/F12,) and B27 were from Gibco (Grand Island, NY, USA). Epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), trypsin, and dimethyl sulfoxide (DMSO) were purchased from Sigma (St Louis, MO, USA). IGF-1, PD98059, and LY294002 were from Invitrogen (Carlsbad, CA, USA). Both PD98059 and LY294002 were dissolved in DMSO and stored at 50 mM. Rapamycin was from Beyotime (Nantong, CHN). AG1024 was form Selleck (Housten, TX, US). Other reagents are described below.
2. Animals and surgery
All animal experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Care and Use committee of Laboratory Animal Research Center of Nantong University. All efforts were made to minimize the number and suffering of animals used in this study. Adult female Sprague-Dawley rats weighing 200–250 g and pregnant Sprague-Dawley rats were purchased from the experimental animal center of Nantong University. Transection of the right FiFx was performed as described previously [7]. After transection, rats were caged in an approved facility with ad libitum access to food and water. Seven days later, the rats were anesthetized with chloral hydrate (2 ml/kg body weight, delivered intraperitoneally), and perfused with 0.9% (w/v) NaCl and 4% (w/v) paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Coronal sections (20 µm) of the lesioned site or hippocampus were prepared using a Leica cryostat (Leica CM1900, Solms, Germany). Nissl staining (0.1% cresyl violet) [54] and immunofluorescence assays were used to examine the success of the FiFx lesion model. For IGF-1 administration, IGF-1 (0.5 µg/100 g body weitht) was injected to the right side hippocampus of the rat (coordinates: 3.0 mm caudal and 2.0 mm right from bregma; 3.0 mm deep). The injection was completed in 5 min. Then the needle was kept in the position for additional 3 min and retrieved slowly out of the brain. Three days later, coronal sections (20 µm) of the hippocampus were prepared as described previously.
3. NSC culture
NSCs were derived from the hippocampus of E14 rats as described previously [6], [9], [55]. In brief, after anesthesia with chloral hydrate (2 ml/kg body weight), the hippocampus was quickly dissected, digested with 0.125% trypsin, and then dissociated mechanically into single-cell suspensions. The cell suspensions were plated into flasks at a density of 1×104 cells/ml with NSC culture medium (1∶1, DMEM/F12) containing 2% B27, 10 ng/ml EGF, 10 ng/ml bFGF, 100 U/ml penicillin/streptomycin, and maintained in a humidified 95% air 5% (v/v) CO2 incubator at 37°C. After 5 days, neurospheres were dissociated into single-cell suspensions and seeded in 96-well plates at 1–2 cells per well. The subclonal neurospheres were then digested and passaged again as before. Cells were passaged three times to obtain neurospheres originating from a single primary cell. After digestion of the neurospheres, the NSCs were seeded into multi-well plates at a density of 5×105 cells/ml for subsequent experimentation. For neuronal differentiation, NSC culture medium was replaced by differentiation medium (DMEM +2% fetal bovine serum, FBS) and incubation continued as before.
4. Immunofluorescence assay
Cells seeded in 24-well plates were washed twice with ice-cold PBS, fixed with 100% methanol for 7 min at –20°C, and permeated with fresh 4% paraformaldehyde for 20 min at room temperature. These cells and the coronal cryostat sections through the hippocampi were blocked with blocking buffer (10% goat serum in PBS containing 0.3% Triton X-100 and 0.03% NaN3) overnight at 4°C. The cells and sections were then incubated with the primary antibody diluted in blocking buffer at 4°C for 24 h, followed by incubation with the secondary antibody overnight at 4°C. After further washing with PBS, the cells and sections were stained with Hoechst (1∶1,000; Pierce, Rockford, IL, USA) for 0.5 h at room temperature, and then viewed under a fluorescence microscope (Leica DMIRB, Germany). Primary antibodies were as follows: mouse monoclonal anti-choline acetyltransferase (ChAT) (1∶500, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-Brn-4 (1∶200, Santa Cruz, CA, USA), mouse monoclonal anti-microtubule-associated protein (MAP)-2 (1∶1,000; Millipore, Billerica, MA, USA) and rabbit polyclonal anti-Brn4 (1∶500; Santa Cruz, CA, USA). Secondary antibodies were: Alexa Fluor 568-conjugated (red) goat anti-mouse IgG (1∶500; Invitrogen, Carlsbad, CA, USA), and Alexa Fluor 488-conjugated (green) goat anti-rabbit IgG (1∶200; Invitrogen, Carlsbad, CA, USA).
5. Western blotting analysis
Seven days after right FiFx transection, rats were anesthetized, killed by cervical dislocation, and hippocampi removed. Proteins from hippocampal tissue proteins and from NSCs seeded in the 6-well plates were extracted using a mammalian Protein Extraction Reagent (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. The protein contents were determined using an Enhanced BCA (bicinchoninic acid) Protein Assay kit (Beyotime, Jiangsu, CHN). Equivalent amounts of protein (30 µg) were loaded into each well and separated on 20% polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS) to detect IGF-1, or on 10% gels to detect other proteins, then transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). Membranes were subsequently blocked with 5% non-fat milk in TBS and incubated overnight at 4°C with mouse monoclonal anti-IGF-1 (1∶1,000, Sigma, St Louis, MO, USA), rabbit polyclonal anti-Brn-4 (1∶1,000, Santa Cruz, CA, USA), mouse monoclonal anti-β-actin (1∶10,000, Sigma, St Louis, MO, USA), rabbit polyclonal anti-Akt or anti-phospho-Akt (1∶1,000, Beyotime, Jiangsu, CHN), rabbit polyclonal anti-mitogen-activated protein kinase (MAPK) and phospho-MAPK (1∶1,000, Beyotime, Jiangsu, CHN). Membranes were developed by incubation with horseradish peroxidase-conjugated goat anti-mouse or rabbit IgG (1∶3,000, Pierce, Rockford, IL, USA) for 2 h at room temperature. After washing, the complexes were visualized by enhanced chemiluminescence (Santa Cruz, CA, USA) and exposed to X-ray film (Kodak, Rochester, NY, USA). The intensity of each band was quantified using the Shine-tech Image System (Shanghai, CHN).
6. Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the treated cells and the hippocampi of FiFx transected rats using a Trizol reagent kit (BBI, CAN). In each case 2 µg of total RNA were reverse transcribed into cDNA using oligo(dT) primers and Omniscript reverse transcriptase (Qiagen, Hilden, GER) according to the manufacturer’s instructions. PCR was performed using the following primers (synthesized by Sangon, Shanghai, CHN): IGF-1: forward, 5′-CAG TTC GTG TGT GGA CCA AG-3′, reverse, 5′-GTC TTG GGC ATG TCA GTG TG-3′; Brn-4: forward, 5′-GGG TGA CCA GTC TTA GCG AC-3′, reverse, 5′-GCG AGT ACA CAT TGA GGG GT-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward, 5′-ACCACAGTCCATGCCATCAC-3′, reverse, 5′-TCCACCACCCTGTTGCTGTA-3′; β-actin: forward, 5′-CCCTAAGGCCAACCGTGAAAAGATG-3′, reverse, 5′-GAACCGCTCATTGCCGATAGTGATG-3′.
PCR products were separated by agarose gel electrophoresis and band densities of IGF-1 and Brn-4, relative to β-actin or GAPDH, was quantified using an image analysis system (Leica Q550I W, Solms, GER).
7. IGF-1 enzyme-linked immunosorbent assay (ELISA)
Seven days after FiFx transection, normal and denervated hippocampi were dissociated using an aseptic glass homogenizer in cold DMEM (1 ml/100 mg hippocampus) and homogenized for 10 min. The homogenate was then centrifuged at 4°C at 250 g for 5 min and the supernatant was harvested and tested using a rat IGF-1 ELISA kit (Jianglai, Shanghai, CHN) according to the manufacturer’s recommendations.
8. Statistical analysis
Statistical analysis was carried out using GraphPad software (GraphPad Prism v4.0, GraphPad Software, San Diego, CA, USA). Data are expressed as means ± SEM and submitted to one/two-way ANOVA followed by either, Newman-Keuls or Bonferroni’s multiple comparison test (as a post hoc test). P<0.05 was considered to indicate statistical significance.
Results
1. FiFx transection induced loss of cholinergic fibers in the hippocampus
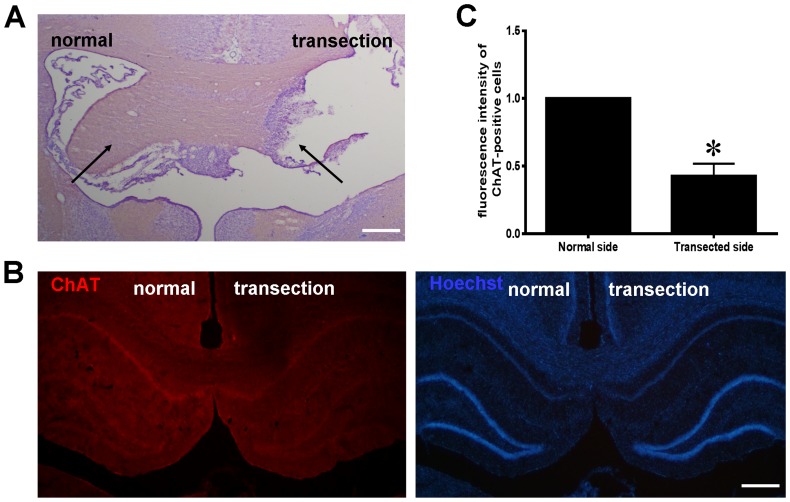
Hippocampal denervation was performed through unilateral FiFx transection, severing afferent cholinergic fibers from the septal area to the hippocampus. To assess the efficacy of denervation, sections through the FiFx and hippocampus were stained using either Nissl or ChAT immunofluorescence. Nissl staining showed that the right FiFx was completely transected whereas the left remained intact (Fig. 1A). ChAT-positive fluorescence intensity in the denervated hippocampus was significantly weaker than in the control contralateral hippocampus (Fig. 1B, C), confirming successful FiFx transection.
Figure 1. Confirmation of the FiFx transection model.
(A) Nissl staining of a coronal section through the FiFx at day 7 after right FiFx transection. Arrows show loss of the right FiFx whereas the left FiFx remains intact (n = 10; a representative panel is shown). (B) ChAT immunofluorescence staining of coronal section through hippocampus at day 7 after right FiFx transection. (C) Quantification of fluorescence intensity of ChAT-positive cells in the transected or normal side. The normal side set to 1. *, P<0.05. Scale bar, 300 µm.
2. IGF-1 expression is increased in FiFx transected hippocampus
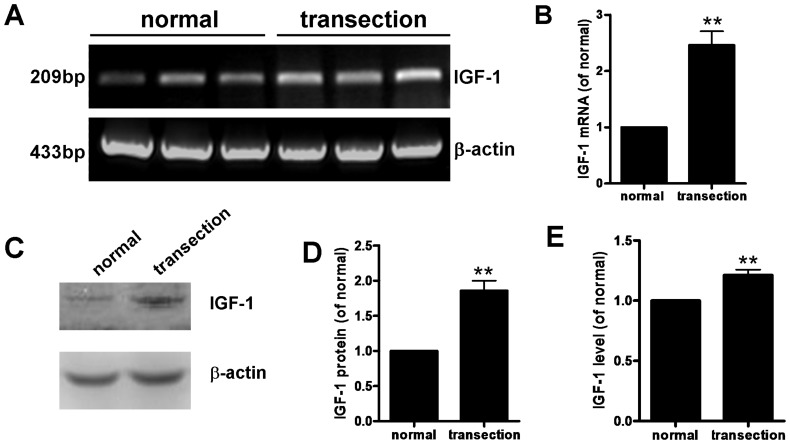
On day 7 after FiFx transection, total RNA and protein were extracted from normal and denervated hippocampi; levels of IGF-1 mRNA and protein in the hippocampus after FiFx transection were determined by RT-PCR (Fig. 2A, B), western blotting analysis (Fig. 2C, D), and ELISA (Fig. 2E). RT-PCR revealed that IGF-1 mRNA levels in the denervated hippocampus were significantly greater than in the contralateral (normal) tissue (P<0.01, Fig. 2A, B). Western blotting and ELISA (Fig. 2C–E) both confirmed that denervation significantly increased IGF-1 in the hippocampus, and IGF-1 protein levels in denervated hippocampus determined by western blotting were more than twofold increased versus control hippocampus (P<0.01). Although the extent of the increase was less marked when determined by ELISA, the increase remained statistically significant (P<0.05). These results indicate that hippocampal denervation is accompanied by increased IGF-1 expression at both the mRNA and protein levels.
Figure 2. IGF-1 mRNA and protein in normal and denervated hippocampi at day 7 after right FiFx transection.
(A) RT-PCR determination of IGF-1 and β-actin (reference) mRNA levels. (B) Quantification of IGF-1 mRNA levels in (A). (C) Levels of IGF-1 protein (7 kDa) detected by western blotting (β-actin reference, 42 kDa). (D) Quantification of IGF-1 protein levels in (C). (E) ELISA showing IGF-1 protein levels in normal and denervated hippocampi. Data are means ± s.e.m.; **, P<0.01 versus normal hippocampus.
3. IGF-1 increases Brn-4 expression in vivo and in vitro
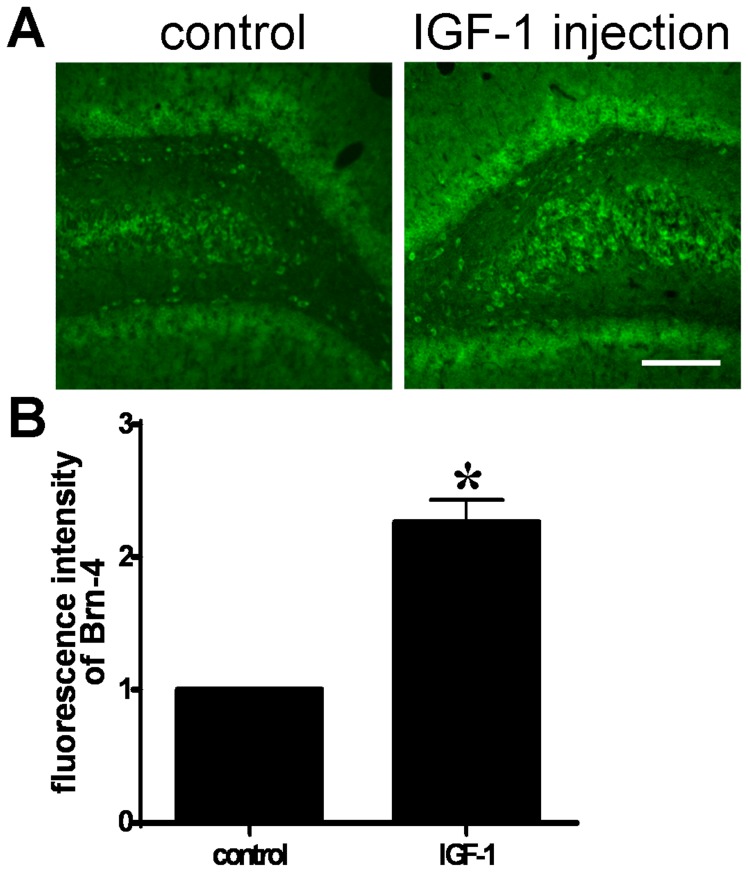
The increase in the levels of IGF-1 following denervation raised the question of whether IGF-1 might be responsible for the increased expression of Brn-4 seen after FiFx transection. We therefore investigated the effect of IGF-1 on Brn-4 expression. Firstly, IGF-1 was injected to the right side hippocampus of normal adult rat. Three days later, we compared the Brn-4 levels in the injected hippocampus with the contraisolateral side. The coronal sections through hippocampus were stained with Brn-4 antibody. Analysis of the immunofluorescence intensity showed that IGF-1 increased significantly Brn-4 expression in hippocampus (Fig. 3A and B).
Figure 3. IGF-1 increases Brn-4 expression in adult hippocampus in vivo.
IGF-1 (0.5 µg/100 g body weight) was injected to the right side hippocampus of normal adult rat. Three days later, coronal sections (20 µm) of the hippocampus were prepared. (A) Immunofluorescence staining with Brn-4 antibody of a coronal section through hippocampus. Scale bar, 300 µm. (B) Quantification of immunofluroscence intensity in (A). The left side (control) set to 1. Data are expressed as means ± SEM (n = 3). *, P<0.05 compared with control.
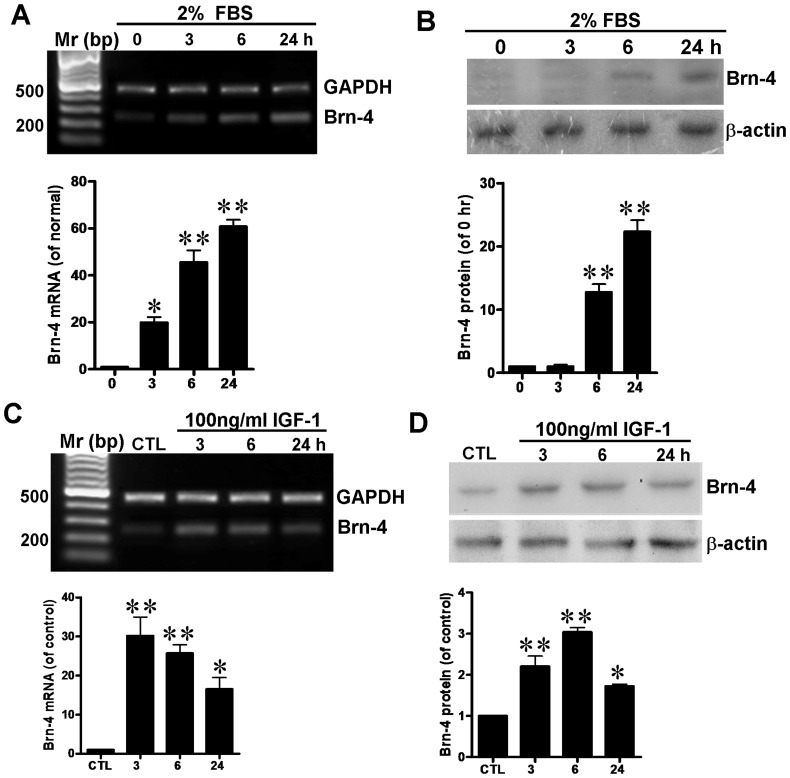
In vitro, hippocampus-derived NSCs were transferred to differentiation medium (DMEM containing 2% FBS). At 0, 3, 6 or 24 h after culture, levels of Brn-4 were determined using RT-PCR and western blotting analysis. Exposure of hippocampal NSCs to differentiation medium increased Brn-4 transcription in a time-dependent manner (Fig. 4A), and there was a parallel increase in levels of Brn-4 protein (Fig. 4B). We then examined whether supplementation of differentiation medium with IGF-1 would affect the extent or the kinetics of Brn-4 expression. Hippocampal NSCs were transferred to differentiation medium containing IGF-1 (100 ng/ml) as described by others [16], [17], [18] for 3, 6 or 24 h. Control hippocampal NSCs were cultured only with differentiation medium for 24 h. As shown in Fig. 4C, and D, supplementation with IGF-1 brought a rapid increase in levels of Brn-4 mRNA determined by RT-PCR, with a marked upregulation at 3 h versus differentiation medium alone (compare Fig. 4C with Fig. 4A). At later time-points there was a fall in the levels of Brn-4 levels in IGF-1-treated NSCs versus cells treated with differentiation medium alone (Fig. 4C versus Fig. 4A). At the protein level there was an even more marked acceleration of Brn-4 expression. Western blotting revealed that Brn-4 protein in hippocampal NSCs was significantly increased and reached a peak 6 h after treatment with IGF-1, and decreased again at 24 h (Fig. 4D), whereas peak protein levels in cells treated with differentiation medium alone were only seen at 24 h (compare Fig. 4D with Fig. 4B).
Figure 4. Brn-4 expression in hippocampus-derived NSCs cultured in differentiation medium with (C, D) or without (A, B) IGF-2.
(A) Brn-4 mRNA levels in hippocampal NSCs determined by RT-PCR at different time-points (0, 3, 6, and 24 h) following transfer to differentiation medium (DMEM, 2% FBS). GAPDH was used as the reference. Lower panel is quantification of Brn-4 mRNA. (B) Brn-4 protein (39 kDa) levels detected by western blotting different time-points (0, 3, 6, and 24 h) following transfer to differentiation medium; β-actin (42 kDa) provided the reference. Lower panel is quantification of Brn-4 protein. Data are means ± s.e.m.; *, P<0.05; **, P<0.01 versus 0 h. (C, D) Effect of IGF-1 on Brn-4 mRNA and protein expression in hippocampal NSCs in vitro. Hippocampal NSCs were incubated in differentiation medium containing IGF-1 (100 ng/ml) for 3, 6 or 24 h; control cells were incubated in differentiation medium alone for 24 h. Brn-4 mRNA and protein were determined by RT-PCR (C) and western blotting (D) analysis, demonstrating upregulation of Brn-4 after IGF-1 treatment in time-course manner. Data are means ± s.e.m.; *, P<0.05; **, P<0.01 versus control (CTL).
Together, these results demonstrate that IGF-1 markedly accelerates the expression of Brn-4, at both the mRNA and protein levels, versus expression in differentiation medium alone. This finding is broadly consistent with a report that IGF-1 increased Brn-4 expression in striatal NSCs [16].
4. IGF-1 induces neuronal differentiation of hippocampal NSCs in vitro
Because IGF-1 accelerates the expression of Brn-4, we determined whether IGF-1 might similarly expedite the differentiation of NSCs into neurons. Hippocampal NSCs were seeded onto poly-L-lysine-coated coverslips and transferred to differentiation medium with or without IGF-1 (100 ng/ml) for 6 h, followed withdraw of the factor treatment. After further 5-day culture, the neuronal differentiation of hippocampal NSCs was examined by immunofluorescence assay using antibody against MAP2, a marker of neuronal differentiation (Fig. 5A).
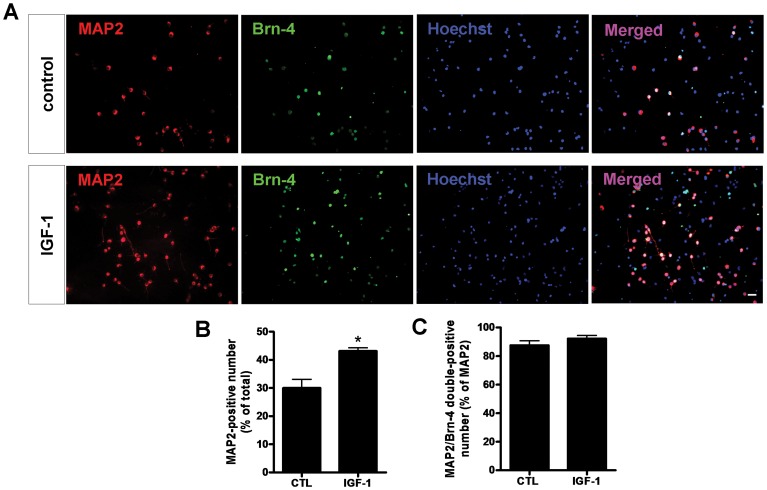
Figure 5. Immunofluorescence analysis of Brn-4 expression and neuronal differentiation (MAP2 expression) of hippocampal NSCs after treatment with (IGF-1) or without (control) 100 ng/ml IGF-1.
(A) Cells were stained separately for MAP2, Brn-4 and the total cells were indicated by Hoechst staining. Scale bar, 25 µm. (B) Quantification of MAP2 positive neurons as a percentage of total cells. (C) Quantification of MAP2/Brn-4 double-positive neurons as a percentage of MAP2-positive neurons. Data are means ± s.e.m of 10 randomly selected microscopic fields in each experiment (n = 3). *, P<0.05 versus control.
In the control group, 29.99±5.39% of total cells were MAP2-positive, indicative of neuronal differentiation (Fig. 5A, B). By contrast, in the IGF-1-treated cultures 43.10±1.99% of cells were MAP2-positive (P<0.05) (Fig. 5A, B). To confirm the association between Brn-4 expression and neuronal differentiation under both conditions, cultures were examined for fluorescence with an anti-Brn-4 antibody. As shown in Fig. 5A, in control cultures 87.61±5.38% of MAP2-positive cells were also Brn-4-positive in control cells, whereas in IGF-1-treated cultures 92.23±3.81% of MAP2 positive cells were also positive for Brn-4 (P>0.05). These results indicate that neuronal differentiation is accompanied by Brn-4 expression under both conditions, but that IGF-1 treatment promotes both Brn-4 expression and neuronal differentiation of hippocampal NSCs.
5. PI3K/Akt mediates IGF-1 induced Brn-4 expression in vitro
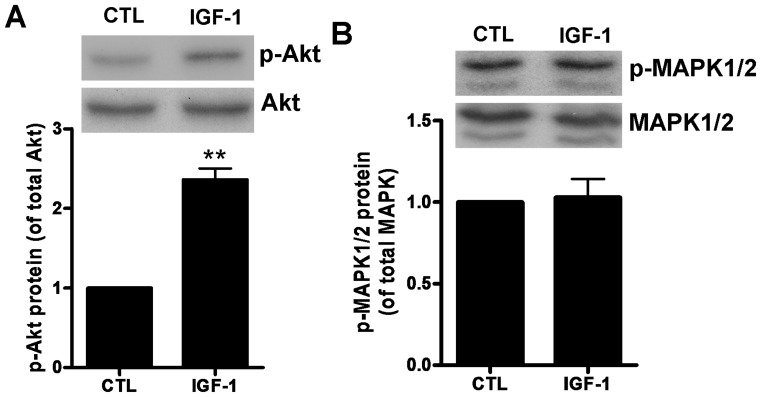
IGF-1, acting via the IGF-1 receptor (IGF1R), is known to regulate diverse aspects of cellular physiology via the phosphoinositide 3-kinase (PI3K)–Akt pathway; however, other pathways such as via the MAP kinase (MAPK/ERK) kinase MEK have also been implicated in some aspects of IGF-1 signaling. PI3K is thought to act via phosphorylation and activation of Akt. To determine the signaling pathway underlying IGF-1-induced Brn-4 expression in NSCs, we examined the levels of phospho-Akt (p-Akt) and phospho-MAPK (p-MAPK) in hippocampal NSCs treated with IGF-1 (100 ng/ml, 6 h). Western blotting analysis revealed that total Akt protein levels were unaffected by IGF-1 treatment, whereas p-Akt levels were increased by over twofold (Fig. 6A). Confirming the specificity of the phosphorylation, IGF-1 treatment was without any effect on either phosphorylation or protein levels of MAPK (Fig. 6B).
Figure 6. IGF-1 promotes Brn-4 expression through PI3K/Akt pathway.
(A, B): Hippocampal NSCs were transferred to differentiation medium with (IGF-1) or without (control) 100 ng/ml IGF-1 for 6 h. Cell lysates were analyzed by western blotting using antibodies specific for Akt, phospho-Akt (p-Akt), MAPK1/2, or phospho-MAPK (p-MAPK1/2). (A) Upper: Western blotting assay for p-Akt and total Akt protein showing in increase in p-Akt levels but no change in Akt protein levels; Lower: Quantification of the upper image in (A) expressed as p-Akt/Akt ratios. (B) Upper: Western blotting for p-MAPK1/2 and total MAPK1/2 protein; Lower: Quantification of the upper image in (B) expressed as p-MAPK/MAPK ratios. Data are means ± s.e.m. **, P<0.01 compared to control (CTL).
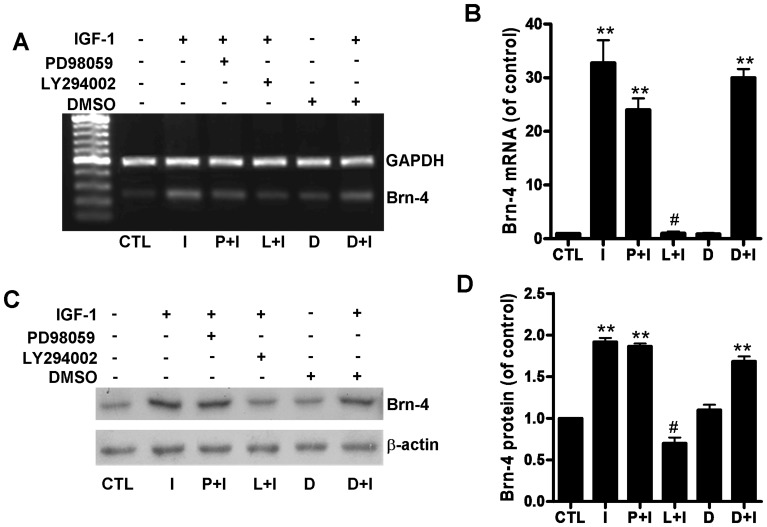
We therefore employed selective inhibitors of PI3K (LY294002) or MEK (PD98059) to determine if these pathways are involved in IGF-1-mediated upregulation of Brn-4 and neuronal differentiation. NSCs were transferred to differentiation medium containing the following supplements: (1) no addition, (2) IGF-1 (100 ng/ml), (3) IGF-1 (100 ng/ml) plus PD98059 (50 µM), (4) IGF-1 (100 ng/ml) plus LY294002 (50 µM) (5) DMSO (1%), and (6) DMSO (1%)+IGF-1 (100 ng/ml). In all cases treatment with either inhibitor (PD98059 and LY294002) or vehicle (DMSO) was commenced 40 min [17], [18] before addition, where appropriate, of IFG-1. After 6 h of IGF-1 treatment, total protein and RNA were extracted and examined for Brn-4 expression using RT-PCR and western blotting. As shown in Fig. 7, IGF-1 produced a significant increase in Brn-4 mRNA levels assessed by RT-PCR. Pretreatment with either the MEK inhibitor PD98059 or DMSO vehicle did not affect Brn-4 expression. By contrast, treatment with the PI3K inhibitor LY294002 markedly attenuated the IGF-1-mediated Brn-4 upregulation of NSCs (Fig. 7A, B). A similar profile was seen at the protein level, where IGF-1 treatment led to a twofold increase in Brn-4 polypeptide levels. The increase was unaffected by MEK inhibitor PD98059 or DMSO, whereas the IGF-1-mediated increase in Brn-4 protein levels was abrogated by PI3K inhibition with LY294002 (Fig. 7C, D).
Figure 7. PI3K inhibition attenuates IGF-1-induced Brn-4 upregulation in hippocampal NSCs.
Cells were pretreated with PD98059, LY294002, or DMSO vehicle for 40 min and then stimulated by IGF-1 for 6 h. (A, B) Brn-4 mRNA levels determined by RT-PCR, GAPDH was used as a reference. (C, D) Western blotting analysis using Brn-4 and β-actin (reference) antibodies. Data are expressed as means ± SEM. **, P<0.01 compared with control; #, P<0.01 versus the IGF-1 group. Abbreviations: D, DMSO. I, IGF-1; L, LY294002; P, PD98059.
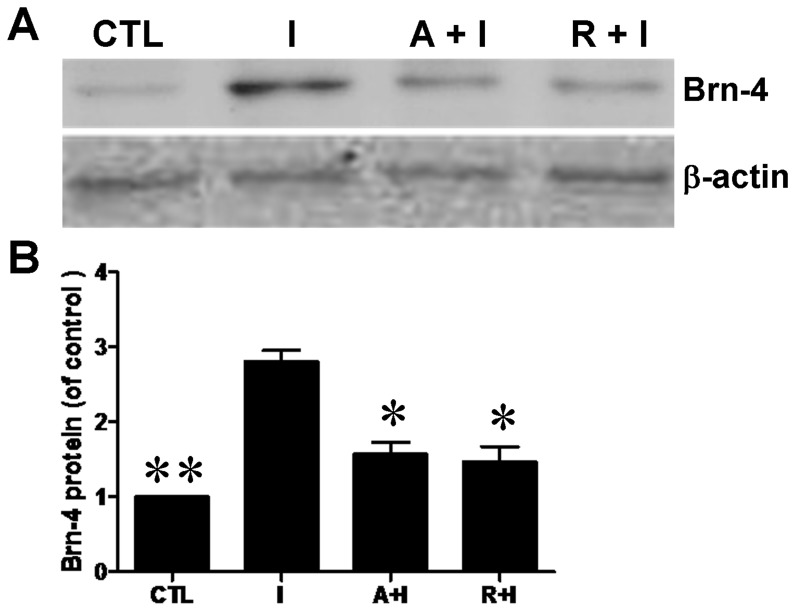
Mammalian target of rapamycin (mTOR) is reported to be a downstream signal molecule of PI3K/Akt and play an important role in the differentiation of several types of cells [19], [20]. In order to further clarify the pathway through which IGF-1 induces the differentiation of hippocampal NSCs, we used AG1024, inhibitor of IGF-1 receptor and rapamycin, inhibitor of mTOR. Hippocampal NSCs were pretreated with AG1024 (10 µM) or rapamycin (5 nM) for 1 h, and then IGF-1 (100 ng/ml) was added to the medium for 6 h. The lysates were collected for western blotting analysis. As shown in Fig. 8, both rapamycin and AG1024 could attenuate the increased expression of Brn-4 induced by IGF-1. Thus, we conclude that IGF-1 is likely to promote Brn-4 expression (and by inference neuronal differentiation) of hippocampal NSCs by acting via the PI3K/Akt signaling pathway in vitro.
Figure 8. Increase of Brn-4 induced by IGF-1 is attenuated by both AG1024 and rapamycin.
Hippocampal NSCs were pretreated with AG1024 or rapamycin for 1 h and subsequently stimulated with IGF-1 for 6 h, and then cell lysates were analyzed by western blotting using antibody for Brn-4. Upper: Western blotting assay for Brn-4 and β-actin. Lower: Quantification of the upper image expressed as Brn-4/β-actin ratio. Data are means ± s.e.m. *, P<0.05 and **, P<0.01 compared to IGF-1 group. Abbreviations: I, IGF-1; A, AG1024; R, rapamycin.
6. PI3K/Akt mediates IGF-1 induced neural differentiation
To make sure whether PI3K/Akt pathway is involved in the IGF-1 induced neural differentiation of hippocampus-derived NSCs, the cells were pretreated with inhibitors of PI3K, LY294002 (20 µM) for 2 hours, followed with IGF-1 treatment. 5 days later, neural differentiation was assessed using MAP2 immunostaining. The result showed that IGF-1 induced neural differentiation was significantly reduced by LY294002 pretreatment (Fig. 9), suggesting that PI3K/Akt signal pathway is involved in the neural differentiation of the hipppocampal NSCs when induced by IGF-1.
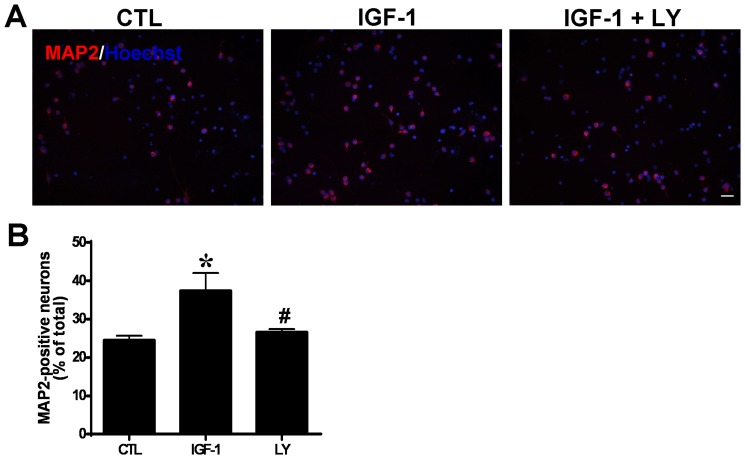
Figure 9. PI3K inhibition decreases IGF-1-induced neuronal differentiation of hippocampal NSCs.
Cells were pretreated with LY294002 or DMSO for 40 min and then stimulated by IGF-1. (A) MAP-2 immunofluorescence analysis of neuronal differentiation of hippocampal NSCs. Nuclei were counterstained with Hoechst (blue). Scale bar, 25 µm. (B) Quantification of the MAP2-positive neurons in (A), Data are expressed as means ± SEM. **, P<0.01 compared with control; #, P<0.01 versus the IGF-1 group. LY, LY294002.
Discussion
Because of their neuronal differentiation ability, NSCs provide a potential source of cells for replacement therapy in neurodegenerative disease [21], [22]. However, the low efficiency of in vitro neuronal differentiation of NSCs is so far inadequate to meet therapeutic demands [3], [4], [5]. There is thus an urgent need to explore the pathways and mechanisms involved in neuronal differentiation to produce sufficient neurons/neural precursors to meet the needs of clinical treatment.
In our previous studies we reported that FiFx transection robustly induced the proliferation, migration, and neuronal differentiation of engrafted or endogenous NSCs in the hippocampal dentate gyrus [6], [7]. Neuronal differentiation was accompanied by upregulation of hippocampal Brn-4 expression, and gain or loss of function experiments have argued that Brn-4 plays a positive role in the neuronal differentiation of hippocampus-derived NSCs [9], [15]. However, the underlying pathways or mechanisms remain unclear. Shimazaki et al. [16] reported that exposure of NSCs derived from the E14 mouse striatum to IGF-1 and BDNF resulted in rapid upregulation of Brn-4 mRNA and protein. IGF-1 is predominantly produced by the liver, but is also expressed in neurons and glia [23], [24] where it is known to play an important role in normal brain development, promoting neuronal growth, cellular proliferation and differentiation [25], [26], [27], [28], [29]. Learning and memory in adult rats are promoted by an IGF-1-dependent mechanism related to hippocampal neurogenesis [30], [31]. BDNF stimulates the formation and increases of dendritic spines in hippocampal neurons [32], [33], [34] and BDNF can induce neuronal differentiation, synaptic plasticity [35], [36], and neuroprotection [37], [38]. We thus hypothesized that IGF-1, and perhaps also BDNF, might be involved in hippocampal neurogenesis following denervation damage, and might therefore promote the survival and differentiation of hippocampal NSCs.
In the present study we demonstrated that in vivo levels of IGF-1 mRNA and protein in the hippocampus (but not BDNF; data not shown), were upregulated at day 7 following surgical FiFx transection. Treatment in vitro of NSCs derived from E14 rat hippocampus with IGF-1 (100 ng/ml) caused significant increases in Brn-4 mRNA and protein expression. Moreover, the number of cells positive for the neuronal marker MAP2 was also increased by IGF-1 treatment.
Overall, our previous and present results suggest a cause/effect cascade in which hippocampal denervation damage induces IGF-1 expression in hippocampus which, in turn, promotes local Brn-4 expression that is thought to drive neuronal differentiation of NSCs. The increase of Brn-4 expression after IGF-1 treatment may be due to the more neuronal differentiation induced by IGF-1. We also found that a small number MAP2-negative cells expressed Brn-4; because Shimazaki et al. [16] observed low levels of Brn-4 expression in some, but not all, astroglial cells, we speculate that these MAP2-negative Brn-4-positive cells may be glial cells. Other members of POU class III genes are expressed in both neuronal and glial cells in the developing central nervous system [39], [40]. Therefore, it is possible that Brn-4 also plays a role in glial cell development, although the results of our experiments suggest that this role may be minor.
Depending on cell type and context, IGF-1 is known to promote neuronal survival, maturation [41], [42], [43], [44], [45], and cell-cycle progression [46] by activating the PI3K/Akt and/or Ras/MAPK pathways. Using previously established protocols [16], [17], [18], we investigated whether inhibition of either the PI3K or MAPK pathways would interfere with IGF-1-induced upregulation of Brn-4. We clarified that cells treated with the PI3K inhibitor LY294002 failed to upregulate Brn-4 in response to IGF-1, whereas Brn-4 upregulation was unaffected by treatment with the MAPK inhibitor PD98059. In addition, we found that both AG1024 and rapamycin could attenuate the increased expression of Brn-4 induced by IGF-1. Thus, as shown in Fig. 10, we speculate that extracellular IGF-1 binds to the IGF-1 receptor in the membrane of hippocampal NSCs, then intracellular PI3K/Akt and their downstream signal molecule, mTOR, are activated in sequence. Combined with our previous findings, we think these changes will lead to increased expression of Brn-4, and ultimately neuronal differentiation of NSCs. However, the reactions in nucleus caused by these changes remain to be addressed. Interestingly, various inhibitors used in our experiments did not completely inhibit the expression of Brn-4. These phenomena may be related to time and dose of inhibitors. Of course, we can not deny that except for PI3K/Akt pathway, other pathways may also participate in the process of Brn-4 expression induced by IGF-1. Additionally, we clarified that exogenous IGF-1 can stimulate Brn-4 expression in the adult rat hippocampus. But we didn’t test whether IGF-1 effect Brn-4 expression via PI3K/Akt pathway in vivo. All of these need to be further demonstrated. In a word, we report that Brn-4 expression induced by IGF-1 is dependent on the PI3K/Akt pathway in vitro, but not on the Ras/MAPK pathway.
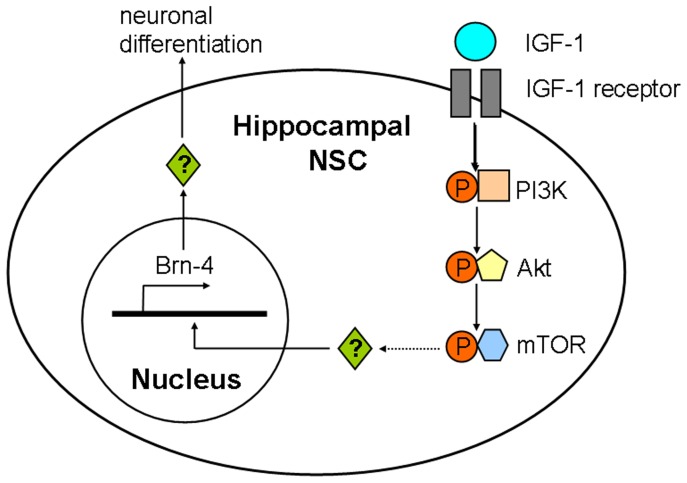
Figure 10. Schematic diagram of the proposed pathway through which IGF-1 induces expression of Brn-4 and neuronal differentiation of hippocampal NSCs.
IGF-1 binds to its receptor in the membrane of NSCs. Then PI3K/Akt are phosphorylated. Subsequently, mTOR, the downstream signal molecule of PI3K/Akt, is activated. As found in this study, mTOR mediated the expression of Brn-4 and neurogenesis function of hippocampal NSCs induced by IGF-1, whereas its downstream effectors are needed to be clarified further.
It has previously been reported that PI3K/Akt signaling pathway plays an important role in neural cell proliferation and differentiation [47], [48], [49]. Moreover, this pathway is required in the process of IGF-1-induced neural progenitor proliferation and differentiation [50], [51], [52]. In addition, Lin et al. [53] reported that administration of IGF-1 could attenuate hypoxic–ischemic brain injury in neonatal rats acting via the PI3K/Akt pathway leading to inhibition of apoptotic cell death. However, these results contrast with those of Shimazaki [16] who have demonstrated that either IGF-I or BDNF resulted in a rapid upregulation of Brn-4 that mediates differentiation of striatal neuron-precursor, although it is possible that this apparent discrepancy may be explained by the use of different cell species.
The overall picture emerging is that, following brain injury such as FiFx lesion, damage leads to upregulation of IGF-1 which, acting via the PI3K/Akt signaling pathway, in turn leads to upregulation of the key transcription factor Brn-4, thereby promoting NSCs differentiation along neuronal pathways. By this mechanism, IGF-1 expression in the lesioned hippocampus would provide a microenviroment for the survival and differentiation of NSCs in vivo. We surmise that these changes are likely to underlie the processes of hippocampal repair and neurogenesis after injury. Although the mechanisms underlying this process have not yet been fully elucidated in vivo, our results provide a theoretical basis for promoting neuronal differentiation of hippocampal NSCs in vitro. These findings have potential in the development of NSCs for the clinical treatment of neurological disease.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Grant 31171038), Jiangsu Natural Science Foundation (BK2011385), Jiangsu Natural College Foundation (11KJB180008, 13KJB310010), the Grant of Nantong University for Innovation Talent, National Training Programs of Innovation and Entrepreneurship for Undergraduates (201210304031), and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gage FH (2002) Neurogenesis in the Adult Brain. The Journal of Neuroscience 22:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colucci-D’Amato L, Bonavita V, Porzio U (2006) The end of the central dogma of neurobiology: stem cells and neurogenesis in adult CNS. Neurological Sciences 27:266–270. [DOI] [PubMed] [Google Scholar]
- 3. Donato R, Miljan EA, Hines SJ, Aouabdi S, Pollock K, et al. (2007) Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieto M, Schuurmans C, Britz O, Guillemot F (2001) Neural bHLH Genes Control the Neuronal versus Glial Fate Decision in Cortical Progenitors. Neuron 29:401–413. [DOI] [PubMed] [Google Scholar]
- 5. Yi S-H, Jo AY, Park C-H, Koh H-C, Park R-H, et al. (2008) Mash1 and Neurogenin 2 Enhance Survival and Differentiation of Neural Precursor Cells After Transplantation to Rat Brains via Distinct Modes of Action. Mol Ther 16:1873–1882. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Jin G, Tian M, Qin J, Huang Z (2007) The denervated hippocampus provides proper microenvironment for the survival and differentiation of neural progenitors. Neuroscience Letters 414:115–120. [DOI] [PubMed] [Google Scholar]
- 7. Zou L, Jin G, Zhang X, Qin J, Zhu H, et al. (2010) Proliferation, Migration, and Neuronal Differentiation of the Endogenous Neural Progenitors in Hippocampus after Fimbria Fornix Transection. International Journal of Neuroscience 120:192–200. [DOI] [PubMed] [Google Scholar]
- 8. Scheidereit C, Cromlish JA, Gerster T, Kawakami K, Balmaceda CG, et al. (1988) A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature 336:551–557. [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Jin G, Wang L, Hu W, Tian M, et al. (2009) Brn-4 is upregulated in the deafferented hippocampus and promotes neuronal differentiation of neural progenitors in vitro. Hippocampus 19:176–186. [DOI] [PubMed] [Google Scholar]
- 10. Bodner M, Castriilo J-L, Theill LE, Deerinck T, Ellisman M, et al. (1988) The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55:505–518. [DOI] [PubMed] [Google Scholar]
- 11. Clerc RG, Corcoran LM, LeBowitz JH, Baltimore D, Sharp PA (1988) The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes & Development 2:1570–1581. [DOI] [PubMed] [Google Scholar]
- 12. Ingraham HA, Chen R, Mangalam HJ, Elsholtz HP, Flynn SE, et al. (1988) A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell 55:519–529. [DOI] [PubMed] [Google Scholar]
- 13. Ko H-S, Fast P, McBride W, Staudt LM (1988) A human protein specific for the immunoglobulin octamer DNA motif contains a functional homeobox domain. Cell 55:135–144. [DOI] [PubMed] [Google Scholar]
- 14. Josephson R, Muller T, Pickel J, Okabe S, Reynolds K, et al. (1998) POU transcription factors control expression of CNS stem cell-specific genes. Development 125:3087–3100. [DOI] [PubMed] [Google Scholar]
- 15. Shi J, Jin G, Zhu H, Tian M, Zhang X, et al. (2010) The role of Brn-4 in the regulation of neural stem cell differentiation into neurons. Neuroscience Research 67:8–17. [DOI] [PubMed] [Google Scholar]
- 16. Shimazaki T, Arsenijevic Y, Ryan AK, Rosenfeld MG, Weiss S (1999) A role for the POU-III transcription factor Brn-4 in the regulation of striatal neuron precursor differentiation. EMBO J 18:444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Gao Y, Dai Z, Meng T, Tu S, et al. (2011) IGF-1 Reduces BACE-1 Expression in PC12 Cells via Activation of PI3-K/Akt and MAPK/ERK1/2 Signaling Pathways. Neurochemical Research 36:49–57. [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Zhang Q, Zhang L, Little PJ, Xie X, et al. (2012) Insulin-like growth factor-1 induces the phosphorylation of PRAS40 via the PI3K/Akt signaling pathway in PC12 cells. Neuroscience Letters 516:105–109. [DOI] [PubMed] [Google Scholar]
- 19. Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr. (1998) Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci U S A 95:7772–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, et al. (2000) A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 60:3504–3513. [PubMed] [Google Scholar]
- 21. Diamandis P, Wildenhain J, Clarke ID, Sacher AG, Graham J, et al. (2007) Chemical genetics reveals a complex functional ground state of neural stem cells. Nat Chem Biol 3:268–273. [DOI] [PubMed] [Google Scholar]
- 22. Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, et al. (2007) Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proceedings of the National Academy of Sciences 104:12506–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bondy C, Werner H, Roberts Jr CT, LeRoith D (1992) Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: Comparison with insulin-like growth factors I and II. Neuroscience 46:909–923. [DOI] [PubMed] [Google Scholar]
- 24. D’Ercole AJ, Stiles AD, Underwood LE (1984) Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proceedings of the National Academy of Sciences 81:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Åberg MAI, Åberg ND, Hedbäcker H, Oscarsson J, Eriksson PS (2000) Peripheral Infusion of IGF-I Selectively Induces Neurogenesis in the Adult Rat Hippocampus. The Journal of Neuroscience 20:2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, et al. (2003) IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Molecular and cellular neurosciences 24:23–40. [DOI] [PubMed] [Google Scholar]
- 27. Arsenijevic Y, Weiss S, Schneider B, Aebischer P (2001) Insulin-Like Growth Factor-I Is Necessary for Neural Stem Cell Proliferation and Demonstrates Distinct Actions of Epidermal Growth Factor and Fibroblast Growth Factor-2. The Journal of Neuroscience 21:7194–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Ercole AJ, Ye P, Calikoglu A, Gutierrez-Ospina G (1996) The role of the insulin-like growth factors in the central nervous system. Molecular Neurobiology 13:227–255. [DOI] [PubMed] [Google Scholar]
- 29. Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, et al. (2004) In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. European Journal of Neuroscience 19:2056–2068. [DOI] [PubMed] [Google Scholar]
- 30. Lupien SB, Bluhm EJ, Ishii DN (2003) Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. Journal of Neuroscience Research 74:512–523. [DOI] [PubMed] [Google Scholar]
- 31. Trejo JL, Llorens-Martín MV, Torres-Alemán I (2008) The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Molecular and Cellular Neuroscience 37:402–411. [DOI] [PubMed] [Google Scholar]
- 32. Ji Y, Pang PT, Feng L, Lu B (2005) Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci 8:164–172. [DOI] [PubMed] [Google Scholar]
- 33. An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, et al. (2008) Distinct Role of Long 3′ UTR BDNF mRNA in Spine Morphology and Synaptic Plasticity in Hippocampal Neurons. Cell 134:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwon M, Fernández JR, Zegarek GF, Lo SB, Firestein BL (2011) BDNF-Promoted Increases in Proximal Dendrites Occur via CREB-Dependent Transcriptional Regulation of Cypin. The Journal of Neuroscience 31:9735–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poo M-m (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32. [DOI] [PubMed] [Google Scholar]
- 36. Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Developmental Neurobiology 70:271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, et al. (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12:1329–1343. [DOI] [PubMed] [Google Scholar]
- 38.Murray PS, Holmes PV (2011) An Overview of Brain-Derived Neurotrophic Factor and Implications for Excitotoxic Vulnerability in the Hippocampus. International Journal of Peptides 2011. [DOI] [PMC free article] [PubMed]
- 39. Ryan AK, Rosenfeld MG (1997) POU domain family values: flexibility, partnerships, and developmental codes. Genes & Development 11:1207–1225. [DOI] [PubMed] [Google Scholar]
- 40. Schreiber J, Enderich J, Sock E, Schmidt C, Richter-Landsberg C, et al. (1997) Redundancy of Class III POU Proteins in the Oligodendrocyte Lineage. Journal of Biological Chemistry 272:32286–32293. [DOI] [PubMed] [Google Scholar]
- 41. Marsh H, Scholz W, Lamballe F, Klein R, Nanduri V, et al. (1993) Signal transduction events mediated by the BDNF receptor gp 145trkB in primary hippocampal pyramidal cell culture. The Journal of Neuroscience 13:4281–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heumann R (1994) Neurotrophin signalling. Current Opinion in Neurobiology 4:668–679. [DOI] [PubMed] [Google Scholar]
- 43. de Pablo F, de la Rosa EJ (1995) The developing CNS: a scenario for the action of proinsulin, insulin and insulin-like growth factors. Trends in Neurosciences 18:143–150. [DOI] [PubMed] [Google Scholar]
- 44. Segal RA, Greenberg ME (1996) Intracellular Signaling Pathways Activated by Neuropathic Factors. Annual Review of Neuroscience 19:463–489. [DOI] [PubMed] [Google Scholar]
- 45. Kaplan DR, Miller FD (1997) Signal transduction by the neutrophin receptors. Current Opinion in Cell Biology 9:213–221. [DOI] [PubMed] [Google Scholar]
- 46. Mairet-Coello G, Tury A, DiCicco-Bloom E (2009) Insulin-Like Growth Factor-1 Promotes G1/S Cell Cycle Progression through Bidirectional Regulation of Cyclins and Cyclin-Dependent Kinase Inhibitors via the Phosphatidylinositol 3-Kinase/Akt Pathway in Developing Rat Cerebral Cortex. The Journal of Neuroscience 29:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peng Y, Jiang B-H, Yang P-H, Cao Z, Shi X, et al. (2004) Phosphatidylinositol 3-Kinase Signaling Is Involved in Neurogenesis during Xenopus Embryonic Development. Journal of Biological Chemistry 279:28509–28514. [DOI] [PubMed] [Google Scholar]
- 48. Otaegi G, Yusta-Boyo MJ, Vergaño-Vera E, Méndez-Gómez HR, Carrera AC, et al. (2006) Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. Journal of Cell Science 119:2739–2748. [DOI] [PubMed] [Google Scholar]
- 49. Sung S, Jung D, Kwon C, Park J, Kang S, et al. (2007) Hypoxia/Reoxygenation Stimulates Proliferation Through PKC-Dependent Activation of ERK and Akt in Mouse Neural Progenitor Cells. Neurochemical Research 32:1932–1939. [DOI] [PubMed] [Google Scholar]
- 50. Kalluri HSG, Vemuganti R, Dempsey RJ (2007) Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. European Journal of Neuroscience 25:1041–1048. [DOI] [PubMed] [Google Scholar]
- 51. Peltier J, O’Neill A, Schaffer DV (2007) PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Developmental Neurobiology 67:1348–1361. [DOI] [PubMed] [Google Scholar]
- 52. Cui Q-L, Almazan G (2007) IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. Journal of Neurochemistry 100:1480–1493. [DOI] [PubMed] [Google Scholar]
- 53. Lin S, Fan L-W, Rhodes PG, Cai Z (2009) Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Experimental Neurology 217:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parent JM, Valentin VV, Lowenstein DH (2002) Prolonged Seizures Increase Proliferating Neuroblasts in the Adult Rat Subventricular Zone-Olfactory Bulb Pathway. The Journal of Neuroscience 22:3174–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luskin MB, Zigova T, Soteres BJ, Stewart RR (1997) Neuronal Progenitor Cells Derived from the Anterior Subventricular Zone of the Neonatal Rat Forebrain Continue to Proliferatein Vitroand Express a Neuronal Phenotype. Molecular and Cellular Neuroscience 8:351–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.