Background: Ssu72 is a RNAPII CTD phosphatase; its function in the transcription cycle has not been established.
Results: Ssu72 dephosphorylates Ser(P)5 at the initiation-elongation transition and regulates Ser2 phosphorylation status.
Conclusion: Ssu72 functions at multiple stages of the RNAPII transcription cycle.
Significance: Our results correct two previous misconceptions: (i) that Rtr1 is a Ser(P)5 phosphatase and (ii) that Ssu72 dephosphorylates Ser(P)5 only during transcription termination.
Keywords: Phosphatase, RNA Polymerase II, Transcription, Transcription Initiation Factor, Transcription Regulation, Transcription Termination
Abstract
Transitions between the different stages of the RNAPII transcription cycle involve the recruitment and exchange of factors, including mRNA capping enzymes, elongation factors, splicing factors, 3′-end-processing complexes, and termination factors. These transitions are coordinated by the dynamic phosphorylation of the C-terminal domain (CTD) of the largest subunit of RNAPII (Rpb1). The CTD is composed of reiterated heptapeptide repeats (Y1S2P3T4S5P6S7) that undergo phosphorylation and dephosphorylation as RNAPII transitions through the transcription cycle. An essential phosphatase in this process is Ssu72, which exhibits catalytic specificity for Ser(P)5 and Ser(P)7. Ssu72 is unique in that it is specific for Ser(P)5 in one orientation of the CTD and for Ser(P)7 when bound in the opposite orientation. Moreover, Ssu72 interacts with components of the initiation machinery and affects start site selection yet is an integral component of the CPF 3′-end-processing complex. Here we provide a comprehensive view of the effects of Ssu72 with respect to its Ser(P)5 phosphatase activity. We demonstrate that Ssu72 dephosphorylates Ser(P)5 at the initiation-elongation transition. Furthermore, Ssu72 indirectly affects the levels of Ser(P)2 during the elongation stage of transcription but does so independent of its catalytic activity.
Introduction
Transcription by RNAPII3 occurs in distinct stages that include assembly of the preinitiation complex, promoter melting, initiation, promoter clearance, elongation, mRNA 3′-end processing, and termination (1). As RNAPII progresses through the transcription cycle, an array of complexes are recruited to RNAPII, including capping enzymes, elongation factors, splicing factors, 3′-end-processing complexes, and termination factors (2–4). Recruitment of these factors to RNAPII is coordinated by the dynamic phosphorylation of the C-terminal domain (CTD) of the Rpb1 subunit of RNAPII (5–12).
The CTD is composed of multiple heptad repeats of the consensus sequence Y1S2P3T4S5P6S7. The CTD is conserved among eukaryotic RNAPIIs, although the number of repeats varies among species. In budding yeast, the CTD is composed of 26 repeats of which 18 exactly match the consensus sequence. In mammalian cells, the CTD is longer. For example, the human CTD consists of 52 repeats, but only 21 match the consensus sequence (13). Every residue of the CTD is subject to post-translational modifications during different stages of the transcription cycle. All five of the hydroxylated amino acids (Ser2, Ser5, and Ser7 as well as Tyr1 and Thr4) are phosphorylated in mammalian cells (1, 14–18). Also, both prolines undergo cis-trans isomerization that affects the phosphorylation status of the other residues (19).
The RNAPII CTD needs to be in a hypophosphorylated state to be recruited to the promoter (20). Once assembled into the preinitiation complex, RNAPII Ser5 and Ser7 are phosphorylated (Ser(P)5 and Ser(P)7) by the Kin28 (yeast) or Cdk7 (mammalian) kinase subunit of TFIIH (16). As RNAPII clears the promoter and enters the elongation phase, Ser(P)5 undergoes dephosphorylation, Ser(P)7 levels remain relatively constant (21, 22), and Ser2 gradually becomes phosphorylated (Ser(P)2) (16). In mammalian cells, Tyr(P)1 levels rise downstream of the transcription start site, similar to Ser(P)2, and then decrease before the polyadenylation site (1). A pattern for the phosphorylation status of Thr4 remains to be elucidated (12). All of these modifications alter the structure of the CTD, which in turn regulates the recruitment and exchange of processing factors (22–26).
Ssu72 is a CTD phosphatase specific for Ser(P)5 and Ser(P)7 and is essential for cell viability (21, 22, 27, 28). Despite its essential role in CTD dephosphorylation, the specific role of Ssu72 in the transcription cycle remains unresolved. Intriguingly, the orientation of Ssu72 relative to the backbone polarity of the CTD is critical for substrate specificity: in one orientation, specificity is for Ser(P)5, whereas in the opposite orientation, specificity is for Ser(P)7, albeit with much lower activity (28). Ssu72 was first identified based on a genetic interaction with the general transcription factor TFIIB, an interaction that affects the accuracy of start site selection (29). Ssu72 physically associates with TFIIB; the Rpb2 subunit of RNAPII; the Taf2, Taf3, and Taf6 subunits of TFIID; the Kin28 subunit of TFIIH; and regulators of TFIIH activity (22, 30). These interactions implicate Ssu72 in initiation, yet Ssu72 is an integral component of the CPF mRNA 3′-end-processing complex (30–32). Consistent with its presence in the CPF complex, Ssu72 mutations adversely affect 3′-end processing and termination (33). Chromatin immunoprecipitation (ChIP) experiments reveal that Ssu72 localizes to the 3′-end of genes but also associates with the promoter (21, 22, 32). Ser(P)7 levels accumulate evenly throughout non-coding and protein coding genes in Ssu72 mutants (22). However, the levels of Ser(P)5 were reported to accumulate only at the 3′-ends of genes in ssu72 mutants, suggesting that the phosphatase activity of Ssu72 acts on Ser(P)5 specifically during the elongation-termination stage of the transcription cycle (21).
The phylogenetically conserved Rtr1 protein was also reported to have Ser(P)5 phosphatase activity, and this activity manifests early in the transcription cycle (34). However, the role of Rtr1 as a CTD phosphatase has been challenged because its structure lacks an apparent catalytic site, and extensive efforts to demonstrate CTD phosphatase activity were unsuccessful (35). A more recent report described Rtr1 as a dual specificity phosphatase that dephosphorylates Tyr(P)1 and Ser(P)5 (36). Nonetheless, the structure of Rtr1 lacks a well defined catalytic groove that would serve as an active site, and it is not active using monophosphorylated Tyr(P)1 or Ser(P)5 substrates (36). Rtr1 clearly affects CTD phosphorylation, but its specific function in the transcription cycle and its relationship to other CTD phosphatases remain to be resolved.
In this report, we investigated the role of Ssu72 in the transcription cycle. We report that Ssu72 dephosphorylates Ser(P)5 at the initiation-elongation transition. We also demonstrate an unanticipated function for Ssu72 in regulation of Ser2 phosphorylation status, a function that is independent of Ssu72 catalytic activity.
EXPERIMENTAL PROCEDURES
Yeast Strains
The S. cerevisiae strains used in this study are listed in Table 1. Strain YMH1111 is an rtr1Δ deletion mutant derived from BY4741 (37). Strain YMH650 (ssu72-2) is an isogenic derivative of H-51. The ssu72-2 allele encodes an alanine replacement of the conserved arginine at position 129 (R129A). This mutant is viable at 30 °C but fails to grow at 37 °C (38). Cell extracts of YMH650 exhibit ∼30% of the phosphatase activity of H-51 as determined by cleavage of the p-nitrophenyl phosphate substrate (39). Western blot analysis revealed that the Ser(P)5 form of RNAPII accumulates in the ssu72-2 mutant following a 60-min shift to the non-permissive temperature of 37 °C (39). Accumulation of Ser(P)5 is not due to Ssu72 instability because no effect of the temperature shift on the steady-state level of the Ssu72-R129A protein was observed (39). Strain XH-24 is an isogenic derivative of FY23 (31) in which the normal SSU72 gene has been replaced by the ssu72-td allele, which enables repression of SSU72 transcription and degron-mediated turnover of the Ssu72 protein following a 30-min shift to 37 °C (27, 31). Strains YMH1237 and YMH1238 are derivatives of XH-24 (ssu72-td) harboring plasmid pM712 [SSU72-CEN-TRP1] or pM698 [ssu72-4-CEN-TRP1], respectively. The ssu72-4 allele encodes a serine replacement of cysteine 15 (C15S) that lies within the PTPase domain (14VCX5RS22) of Ssu72. Cys15 is responsible for nucleophilic attack of the substrate phosphorus atom, leading to formation of a phosphoenzyme intermediate (27, 38, 40). Because the C15S replacement eliminates catalytic activity of the essential Ssu72 protein, plasmid-borne ssu72-4 was introduced into XH-24 (ssu72-td), followed by depletion of degron-tagged Ssu72 upon temperature shift to 37 °C. Accordingly, this study utilizes three isogenic sets of ssu72 mutants: one that eliminates the Ssu72 protein (ssu72-td), one that retains stable protein but eliminates its catalytic activity (ssu72-4), and one that is temperature-sensitive, expressing ∼30% of normal Ser(P)5 phosphatase activity in vivo.
TABLE 1.
List of yeast strains
| Straina | Genotype | Source/Reference |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems (Huntsville, AL) |
| YMH1111 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rtr1::KanMX | Open Biosystems (Huntsville, AL) |
| H-51 | MATa his3Δ200 leu2-3,112 ura3-52 | Ref. 38 |
| YMH650 | MATa his3Δ200 leu2-3,112 ura3-52 ssu72–2 | Ref. 38 |
| FY23 | MATa ura3-52 trp1Δ63 leu2Δ1 | Ref. 42 |
| XH-24 | MATa ura3-52 trp1Δ63 leu2Δ1 ssu72-td | Ref. 31 |
| YMH1237 | MATa ura3-52 trp1Δ63 leu2Δ1 ssu72-td (pM712: SSU72-CEN-TRP1) | This study |
| YMH1238 | MATa ura3-52 trp1Δ63 leu2Δ1 ssu72-td (pM698: ssu72-4-CEN-TRP1) | This study |
a Strain H-51 is identical to LRB535 (38).
Chromatin Immunoprecipitation
ChIP experiments were performed using isogenic strain pairs H-51 (SSU72) and YMH650 (ssu72-2); FY23 (SSU72) and XH-24 (ssu72-td); YMH1237 (ssu72-td [pM712: ssu72-4-CEN-TRP1]) and YMH1238 (ssu72-td [pM698: SSU72-CEN-TRP1]); and BY4741 (RTR1) and YMH1111 (rtr1Δ). Cells were grown under permissive (30 °C) or restrictive (37 °C) conditions, as indicated. RNAPII occupancy was assessed for (i) RNAPII, independently of CTD phosphorylation status, using antibody to the Rpb3 subunit (Neoclone); (ii) RNAPII hypophosphorylation using the 8WG16 antibody (Covance); (iii) RNAPII Ser(P)5, using the 3E8 monoclonal antibody; and (iv) RNAPII Ser(P)2, using the 3E10 monoclonal antibody. Rpb3 and 8WG16 antibodies are commercially available from the indicated vendors and are used routinely to probe RNAPII by ChIP. The specificity of the 8WG16 antibody is directed against the CTD when Ser2 is unphosphorylated (41). The 3E8 and 3E10 antibodies where generated in Dirk Eick's laboratory and exhibit specificity for Ser(P)5 and Ser(P)2, respectively; these antibodies have also been used successfully to probe RNAPII by ChIP (16, 21, 22).
Yeast cells were grown at 30 °C in either YPD medium, or −Trp medium for strains YMH1237 and YMH1238 to maintain selection for the ssu72 plasmids. Cells were grown to a density of A600 = 0.6, shifted either to 30 °C or to prewarmed medium at 37 °C, incubated for 1 h, cross-linked with 1% formaldehyde for 15 min at either 30 or 37 °C, and harvested. The reaction was stopped by the addition of glycine to 125 mm, and cultures were incubated for an additional 5 min at either 30 or 37 °C. The cell pellet obtained from the 100-ml culture was washed twice with 10 ml of 1× TBS buffer (10 mm Tris-HCl (pH 7.5), 200 mm NaCl, 1% Triton X-100) and resuspended in 500 μl of FA lysis buffer (50 mm HEPES-KOH (pH 7.9), 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mm PMSF). Approximately 500 μl of acid-washed glass beads were added, and cells were lysed by vigorous shaking in a mini-bead beater (MiniBeadBeater-16, model 607) for 4 min at 4 °C. Eppendorf tubes were punctured with a 22-gauge needle, and filtrates were collected in a 15-ml tube, transferred to a 1.5-ml tube, and spun for 15 min at 4 °C in a microcentrifuge. The crude chromatin pellet was washed with 500 μl of FA lysis buffer twice and resuspended in 800 μl of FA lysis buffer. Chromatin was sonicated 10 times for 15 s with 30-s intervals in ice-cold FA lysis buffer. Samples were spun at 13,000 rpm in a refrigerated microcentrifuge. The supernatant was mixed with 10 μl of anti-RPB3 monoclonal antibody, 10 μl of 8WG16 monoclonal antibody, 10 μl of 3E8 (anti-Ser(P)5) antibody or 10 μl of 3E10 (anti-Ser(P)2) antibody, followed by incubation for 4 h (in the case of the 8WG16 antibody) or overnight (in the case of the anti-Rpb3, 3E10 and 3E8 antibodies) at 4 °C with gentle shaking. Differences in incubation periods reflect different binding affinities for each antibody. The antigen-antibody complex was adsorbed on 50 μl of protein A-Sepharose beads (Invitrogen) in the case of the 8WG16 antibody, anti-rat IgG beads (Sigma) in the case of the 3E10 and 3E8 antibodies, or anti-mouse IgG beads (Sigma) for the Rpb3 antibody and then washed successively with 1 ml each of FA lysis buffer containing 500 mm NaCl, ChIP wash buffer (10 mm Tris-HCl (pH 8.0), 250 mm LiCl, 0.5% Nonidet-P40, 0.5% sodium deoxycholate, 1 mm EDTA), and 1× TE buffer (10 mm Tris-HCl (pH 8.0), 1 mm EDTA). The beads were incubated with 10 μg of DNase-free RNase (Qiagen) for 30 min at 37 °C followed by 20 μg of Proteinase K (Invitrogen) for 1 h at 42 °C. Cross-links were reversed by overnight incubation at 65 °C in the same buffer. Samples were extracted with phenol-chloroform and precipitated with ethanol in the presence of glycogen. DNA pellets were resuspended in 100 μl of TE and used as templates for PCR amplification. PCRs were performed using 1 μl of immunoprecipitated DNA and the primer pairs defined in Table 2. PCR products were fractionated in 1.5% agarose gels containing ethidium bromide. Band intensity was quantified using an AlphaImager 2200 (Alpha Innotech).
TABLE 2.
Oligonucleotide sequences used for ChIP
| Primer | Location | Orientation | Sequencea |
|---|---|---|---|
| PMA1 | −304 | Forward | CAAATGTCCTATCATTATCGTCTAAC |
| PP1 | −47 | Reverse | CTTTTCAATGATTTTCTTTAACTAGCTGG |
| PMA1 | +168 | Forward | CGACGACGAAGACAGTGATAACG |
| PP2 | +376 | Reverse | ATTGAATTGGACCGACGAAAAACATAAC |
| PMA1 | +584 | Forward | AAGTCGTCCCAGGTGATATTTTGCA |
| PP3 | +807 | Reverse | AACGAAAGTGTTGTCACCGGTAGC |
| PMA1 | +2018 | Forward | CTATTATTGATGCTTTGAAGACCTCCAG |
| PP5 | +2290 | Reverse | TGCCCAAAATAATAGACATACCCCATAA |
| PMA1 | +2841 | Forward | GATACACTAAAAAGAATTAGGAGCCAAC |
| PP6 | +3073 | Reverse | CAAGAAAGAAAAAGTACCATCCAGAG |
| PMA1 | +3448 | Forward | GCGCCCATACAGACACTCAAGATAC |
| PP8 | +3662 | Reverse | GGCCTGGCGATTTGTTTGCTTTCTTG |
| PMA1 | +3619 | Forward | CTTCATCACAAGAAAGCAAACAAATCG |
| PP9 | +3905 | Reverse | CGTGATGAGTGAGTTAAGTTCTGCTG |
| PYK1 | −142 | Forward | CAT GGT CCC CTT TCA AAG TTA |
| PP1 | −23 | Reverse | TGA TTG GTG TCT TGT AAA TAG AAA CA |
| PYK1 | +32 | Forward | CGT TGT TGC TGG TTC TGA CTT G |
| PP2 | +193 | Reverse | CAA TGA CAG ACT TGT GGT ATT CG |
| PYK1 | +194 | Forward | ACA ACG CCA GAA AGT CCG AAG A |
| PP3 | +367 | Reverse | CGT CAC AAG CCT TAG CGT ACT TG |
| PYK1 | +917 | Forward | CAACCCAAGACCAACCAGAG |
| PP6 | +1058 | Reverse | ACAGCGGTTTCAGCCATAGT |
| PYK1 | +1263 | Forward | CTT GGT TAC CAG ATG CCC AAG A |
| PP7 | +1416 | Reverse | AAG ATA CCG AAT TCC TTA GCC |
| PYK1 | +1569 | Forward | GAC ATG GTT TTT CTT TTC AAC TC |
| PP8 | +1710 | Reverse | GCA ACA CCT CAT CGT TAT GAC G |
| ADH1 | +165 | Forward | GCCATTGCCAGTTAAGCTAC |
| PP1 | +365 | Reverse | TGGGTGTAACCAGACAAGTCA |
| ADH1 | +844 | Forward | TTCAACCAAGTCGTCAAGTCCATCTC |
| PP2 | +1013 | Reverse | ATTTGACCCTTTTCCATCTTTTCGTAA |
| ADH1 | +1204 | Forward | GAGGTCGCTCTTATTGACCA |
| PP3 | +1374 | Reverse | CGTGTGGAAGAACGATTACA |
| HMR | Chr III | Forward | CCTACCACATTATCAATCCTTGC |
| Reverse | ACATGTCACCAACATTTTCGTAT |
a All DNA sequences are indicated in 5′–3′ polarity.
All values used for quantification were established to be within linear range of the PCR. For all ChIP experiments, factor association was quantified by normalizing the IP/input ratio of each probed region to the IP/input ratio of the HMR region ((IPx/INPUTx)/(IPHMR/INPUTHMR)). Values represent the mean of three independent biological replicates; error bars indicate S.E.
RESULTS
This study is focused on the function of the Ssu72 RNAPII CTD phosphatase in the RNAPII transcription cycle. We have assayed the presence of RNAPII and the phosphorylation status of its CTD using three sets of isogenic ssu72 mutants: (i) one depleted of Ssu72 (ssu72-td), which correlates with depletion of the hypophosphorylated form of RNAPII and a marked increase in the Ser(P)5 form of RNAPII in whole cell extracts; one expressing stable but catalytically inactive Ssu72 (ssu72-4); and one expressing Ssu72 with diminished catalytic activity (ssu72-2) (see “Experimental Procedures” and Table 1). Protein-DNA interactions were monitored by ChIP using a set of genes (PMA1, PYK1, and ADH1) that has been used extensively to assay recruitment and exchange of proteins and chromatin marks during the transcription cycle. Chromatin was immunoprecipitated using well defined, specific antibodies. We have organized the presentation of our data according to the antibody used in each ChIP experiment.
Loss of Ssu72 Phosphatase Activity Does Not Impair Progression of RNAPII through the Transcription Cycle
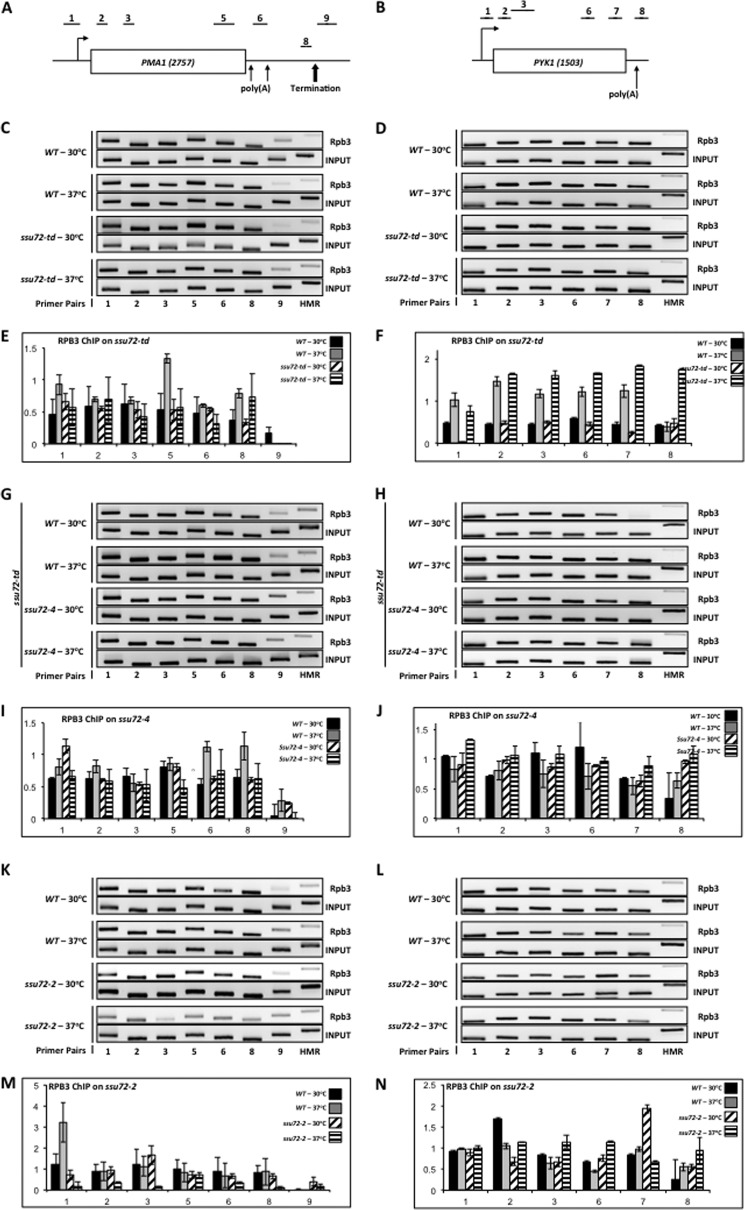
Previous attempts to monitor the progression of RNAPII through the transcription cycle in ssu72 mutants were performed by ChIP using the 8WG16 antibody, which binds preferentially to the hypophosphorylated form of RNAPII rather than total RNAPII, or by using a catalytically inactive mutant (ssu72-C15S) (21, 22). To obtain a more comprehensive depiction of how Ssu72 affects the progression of RNAPII through the transcription cycle, we performed ChIP experiments using a monoclonal antibody directed against the Rpb3 subunit of RNAPII with our three sets of ssu72 mutants. When Ssu72 was depleted in the ssu72-td strain, we found no effect on the levels of RNAPII cross-linked to PMA1 and PYK1 (Fig. 1, C and D). Similar results were observed when the catalytically inactive form of Ssu72 (ssu72-4) was introduced into this strain, followed by depletion of WT Ssu72; RNAPII levels remained the same across both PMA1 and PYK1 (Fig. 1, G and H). Thus, degradation of Ssu72 (ssu72-td) or the complete absence of its phosphatase activity (ssu72-4) did not prevent RNAPII elongation in vivo.
FIGURE 1.
Ssu72 phosphatase activity is not required for progression of RNAPII through the transcription cycle. A, schematic depiction of the PMA1 gene showing the position of the promoter (bent arrow), the two 3′-end processing/polyadenylation sites (light arrows), and the termination site (heavy arrow). The regions probed by ChIP are denoted 1–3, 5–6, and 8–9. The PMA1 PCR primers are defined in Table 2 and are identical to the primer pairs described previously (43). B, schematic depiction of the PYK1 gene. The regions probed by ChIP are denoted 1–3 and 6–8. The PYK1 ChIP primers are defined in Table 2 and are identical to the primer pairs described previously (34). C, ChIP analysis of Rpb3 cross-linked to PMA1 using isogenic strains H-51 (WT) and YMH650 (ssu72-2) that had been incubated at either the permissive (30 °C) or restrictive (37 °C) temperature for 1 h prior to cross-linking. Chromatin was immunoprecipitated using monoclonal α-Rpb3 antibody. Lanes correspond to the regions depicted in A; HMR denotes a transcriptionally silent domain on chromosome III. The input signal represents DNA prior to immunoprecipitation. D, ChIP analysis of Rpb3 cross-linked to PYK1 using the same strains and conditions as in C. E, quantification of the data shown in C. For all ChIP experiments, factor association was quantified by normalizing the IP/input ratio of each probed region to the IP/input ratio of the HMR region ((IPx/INPUTx)/(IPHMR/INPUTHMR)). Values represent the mean of three independent biological replicates; error bars, S.E. In all graphs, the y axis represents -fold enrichment, and the x axis represents the region probed on the gene. F, quantification of the data shown in D. G, identical to B, except chromatin was immunoprecipitated from isogenic strains FY23 (WT) and XH-24 (ssu72-td) that had been incubated at either the permissive (30 °C) or restrictive (37 °C) temperature for 1 h prior to cross-linking. Incubation of XH-24 at 37 °C results in depletion of the Ssu72 CTD phosphatase (31). H, identical to D, except chromatin was immunoprecipitated from isogenic strains FY23 (WT) and XH-24 (ssu72-td). I, quantification of the data shown in G. J, quantification of the data shown in H. K, ChIP analysis of Rpb3 cross-linking to PMA1 using strain YMH1237 (ssu72-td [SSU72-CEN-TRP1]) (labeled WT) or YMH1238 (ssu72-td [ssu72-4-CEN-TRP1]) (Table 1). Strains were incubated at either the permissive (30 °C) or restrictive (37 °C) temperature for 1 h. prior to cross-linking, as indicated. L, ChIP analysis of Rpb3 cross-linked to PYK1 using the same strains and conditions as in K. M, quantification of the data shown in K. N, quantification of the data shown in L.
Interestingly, the ssu72-2 temperature-sensitive mutant showed decreased levels of RNAPII across the PMA1 gene when incubated at the restrictive temperature of 37 °C (Fig. 1K). This mutant, however, exhibited no defect in the RNAPII profile across PYK1 under the same conditions (Fig. 1L). From these results, it appears that Ssu72 with partial catalytic activity (ssu72-2) can cause different effects on RNAPII elongation. We do not yet understand why the ssu72-2 mutant displays low levels of RNAPII across the PMA1 gene or how many genes exhibit a similar effect. Nonetheless, we conclude that neither Ssu72 (ssu72-td) nor its catalytic activity (ssu72-4) is essential for progression of RNAPII through the transcription cycle, although partial catalytic activity can exert different effects at different genes.
Ssu72 Dephosphorylates Ser(P)5 during the Early Stages of the Transcription Cycle
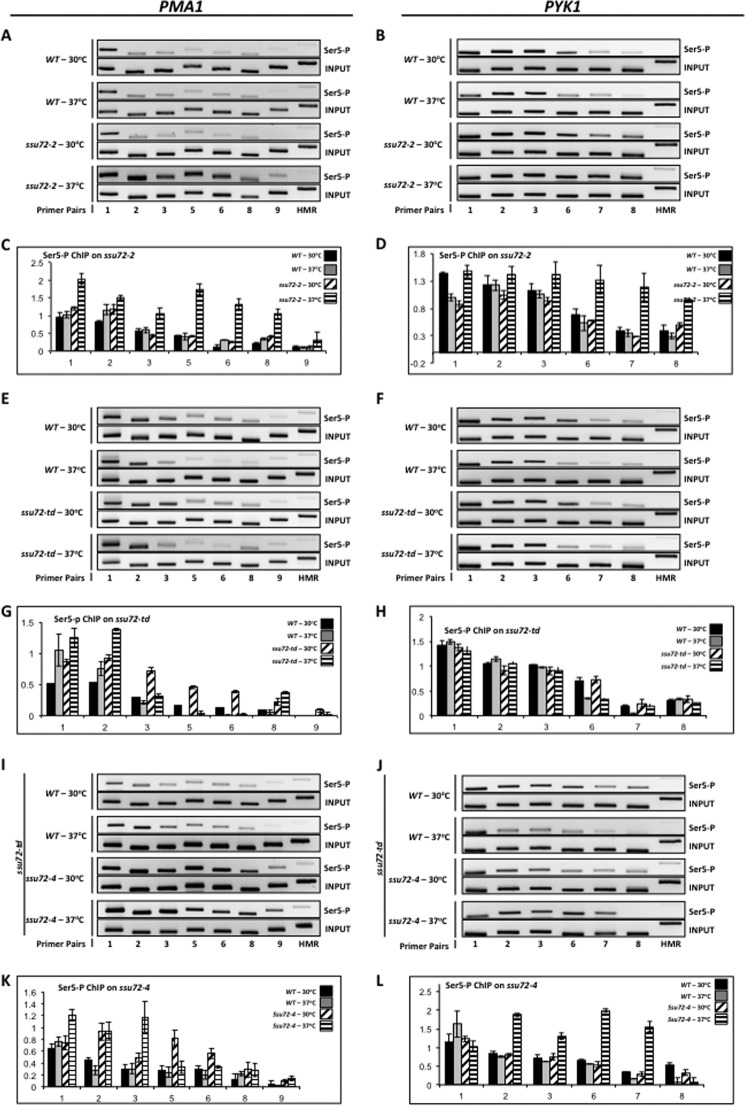
Bataille et al. (21) reported that the Ssu72 phosphatase activity acts specifically during transcription termination, a conclusion that would seem to be consistent with Ssu72 being an integral component of the CPF mRNA 3′-end-processing complex. However, the SSU72 gene was initially discovered based on genetic interaction with the transcription initiation factor TFIIB, and the ssu72-1 allele affects transcription start site selection (29). To determine whether the function of the Ssu72 phosphatase is specific to termination or also affects initiation, we carried out ChIP experiments using the 3E8 (Ser(P)5) monoclonal antibody and our set of ssu72 mutants. Incubation of the ssu72-2 mutant at the restrictive temperature resulted in accumulation of RNAPII Ser(P)5 across the PMA1 and PYK1 genes, indicating that Ssu72 dephosphorylates Ser(P)5 from actively transcribing RNAPII (Fig. 2, A and B). Moreover, accumulation of RNAPII Ser(P)5 occurred close to the 5′-ends of PMA1 and PYK1, indicating that Ssu72 acts early in the transcription cycle rather than exclusively at the 3′-ends of genes (21). A different result, however, was observed using the ssu72-td degron mutant. In this strain, which results in total depletion of Ssu72, no effect on RNAPII Ser(P)5 levels was observed (Fig. 2, E and F). However, introduction of the catalytically inactive form of Ssu72 into the ssu72-td degron strain led to elevated levels of RNAPII Ser(P)5 upon depletion of WT Ssu72 (Fig. 2, I and J). These results indicate that in the absence of normal Ssu72 activity (ssu72-2 or ssu72-4), the cell is unable to dephosphorylate Ser(P)5. However, in the complete absence of the Ssu72 protein (ssu72-td), the ability to dephosphorylate Ser(P)5 is restored. Taken together, these results demonstrate that the Ssu72 phosphatase affects the initiation-elongation transition but also suggest that another CTD phosphatase is able to substitute for Ssu72 and dephosphorylate Ser(P)5 but does so only in the absence of Ssu72. We suggest that Ssu72 bound to the CTD prevents another phosphatase, perhaps Rtr1, from interacting with the CTD (see “Discussion”).
FIGURE 2.
Loss of Ssu72 phosphatase activity results in accumulation of the Ser(P)5 form of RNAPII across the PMA1 and PYK1 genes. A, ChIP analysis of Ser(P)5 cross-linking to PMA1 using isogenic strains H-51 (WT) and YMH650 (ssu72-2). Chromatin was immunoprecipitated using monoclonal antibody 3E8 (α-Ser(P)5). B, ChIP analysis of Ser(P)5 cross-linking to PYK1 using the same strains and conditions as in A. C, quantification of the data shown in A. D, quantification of the data shown in B. E, identical to A, except chromatin was immunoprecipitated from isogenic strains FY23 (WT) and XH-24 (ssu72-td). F, identical to B, except chromatin was immunoprecipitated from isogenic strains FY23 (WT) and XH-24 (ssu72-td). G, quantification of the data shown in E. H, quantification of the data shown in F. I, ChIP analysis of Ser(P)5 cross-linking to PMA1 using the XH-24 strain transformed with plasmid DNA carrying either wild type SSU72 (pM712, labeled as WT), or catalytically inactive ssu72-C15S (pM698, labeled as ssu72-4) (Table 1). Strains were incubated at either the permissive (30 °C) or restrictive (37 °C) temperature for 1 h prior to cross-linking. J, ChIP analysis of Ser(P)5 cross-linked to PYK1 using the same strains and conditions as in I. K, quantification of the data shown in I. L, quantification of the data shown in J.
Effect of Ssu72 on CTD Ser2 Phosphorylation
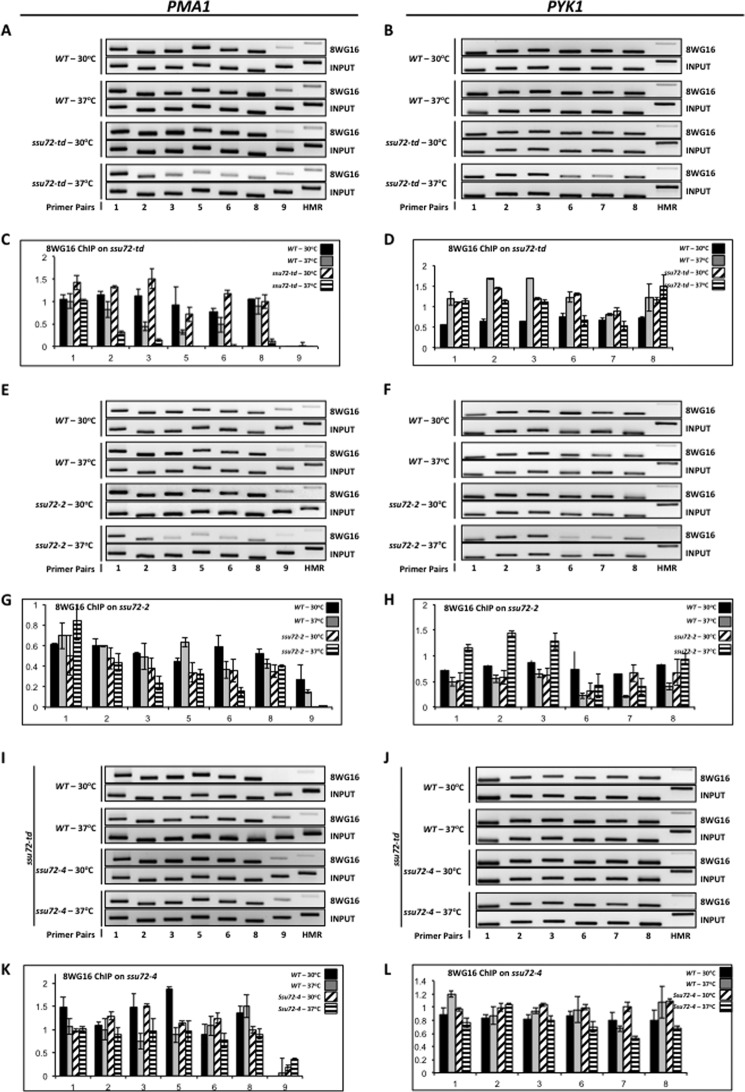
Another reagent to monitor CTD phosphorylation status is the 8WG16 antibody. 8WG16 binds hypophosphorylated CTD that is specifically unphosphorylated at Ser2 (41). Degradation of Ssu72 in the ssu72-td mutant (XH-24) led to diminished levels of hypophosphorylated CTD as detected by Western blot (27), suggesting that Ssu72 either has catalytic activity toward Ser(P)2 (in addition to Ser(P)5 and Ser(P)7) or that Ssu72 has an indirect effect on Ser(P)2 dephosphorylation.
To assess whether the effect of Ssu72 on Ser(P)2 levels was occurring on the CTD of actively transcribing RNAPII, we performed ChIP using the 8WG16 antibody and our set of ssu72 mutants. Consistent with Western blot results (27), the 8WG16 ChIP signal decreased when Ssu72 is degraded in the ssu72-td strain (Fig. 3, A and B). This result implies that Ser(P)2 levels increase when Ssu72 activity is diminished or lost. Similar results were observed when the ssu72-2 mutant was incubated at the restrictive temperature (Fig. 3, E and F). However, when the only source of Ssu72 in the cell is the catalytically inactive C15S mutant, no reduction in the 8WG16 ChIP signal is observed (Fig. 3, I and J). This result suggests that the increase in Ser(P)2 in the other ssu72 mutants (Fig. 3, A–H), detected as a drop in the 8WG16 signal, is not due to an increase in Ser(P)5.
FIGURE 3.
Ssu72 affects the levels of hypophosphorylated RNAPII across the PMA1 and PYK1 genes. A, ChIP analysis of hypophosphorylated CTD cross-linked to PMA1 using isogenic strains FY23 (WT) and XH-24 (ssu72-td). Chromatin was immunoprecipitated using the monoclonal antibody 8WG16. B, ChIP analysis of hypophosphorylated CTD cross-linking to PYK1 using the same strains and conditions as in A. C, quantification of the data shown in A. D, quantification of the data shown in B. E, identical to A, except chromatin was immunoprecipitated from isogenic strains H-51 (WT) and YMH650 (ssu72-2). F, identical to B, except chromatin was immunoprecipitated from isogenic strains H51 (WT) and YMH650 (ssu72-2). G, quantification of the data shown in E. H, quantification of the data shown in F. I, ChIP analysis of hypophosphorylated CTD cross-linking to PMA1 using the XH-24 strain transformed with plasmid DNA carrying either wild type SSU72 or catalytically inactive ssu72-C15S. J, ChIP analysis of hypophosphorylated CTD cross-linked to PYK1 using the same strains and conditions as in I. K, quantification of the data shown in I. L, quantification of the data shown in J. Error bars, S.E.
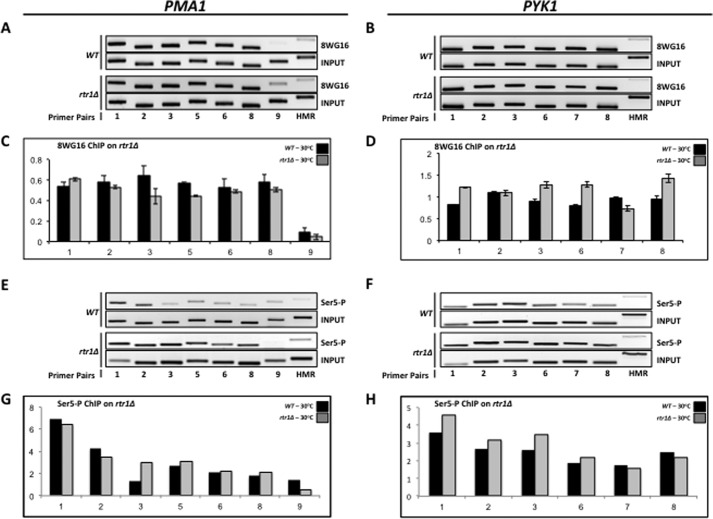
To confirm this conclusion, we performed 8WG16 ChIP analysis using an rtr1Δ deletion mutant (YMH1111), which was shown previously to exhibit an increase in Ser(P)5 levels (14, 16). If our conclusion that elevated Ser(P)5 levels are not affecting 8WG16 epitope levels is correct, we should observe a strong 8WG16 signal even when Ser(P)5 levels are elevated. Our results reveal a slight, uniform decrease in the 8WG16 signal (Fig. 4, A and B), which is probably due to the loss of RNAPII occupancy on PMA1 and PYK1, as reported previously (34). As a control, we confirmed that Ser(P)5 levels are indeed elevated in the rtr1Δ mutant by ChIP analysis of PMA1 and PYK1 (Fig. 4, E and F), thereby proving that elevated Ser(P)5 levels are not affecting 8WG16 epitope (Ser2) levels. Furthermore, the diminished 8WG16 signal is not due to an accumulation of Ser(P)7 because the catalytically inactive ssu72-4 mutant shows elevated levels of Ser(P)7 on actively transcribing RNAPII (22) as well as a strong 8WG16 ChIP signal (14, 16, 34). Taken together, our results demonstrate that Ssu72 not only catalyzes Ser(P)5 dephosphorylation but also regulates the phosphorylation status of the CTD in a manner independent of its Ser(P)5 phosphatase activity.
FIGURE 4.
Loss of Rtr1 function does not affect the levels of hypophosphorylated RNAPII across the PMA1 and PYK1 genes. A, identical to Fig. 3A, except chromatin was immunoprecipitated from isogenic strains BY4741 (WT) and YMH1111 (rtr1Δ). B, identical to Fig. 3B, except chromatin was immunoprecipitated from isogenic strains BY4741 (WT) and YMH1111 (rtr1Δ). C, quantification of the data shown in A. D, quantification of the data shown in B. E, identical to A, except chromatin was immunoprecipitated using monoclonal antibody 3E8 (α-Ser(P)5). F, identical to B, except chromatin was immunoprecipitated using monoclonal antibody 3E8 (α-Ser(P)5). G, quantification of the data shown in E. H, quantification of the data shown in F. Error bars, S.E.
Loss of Ssu72 Leads to Deregulation of Ser2 Phosphorylation
As noted above, a decrease in the 8WG16 epitope signal implies an increase in Ser(P)2 on actively transcribing RNAPII in the ssu72 mutants. To test this premise, we performed ChIP experiments using the 3E10 monoclonal antibody against Ser(P)2 with the ssu72-td strain (XH-24). Here we assayed the ADH1 gene because the Ser(P)2 profile of this gene follows a clear linear increase in the ChIP signal from promoter to terminator, making it easier to recognize pattern changes when comparing WT and mutant strains (43). Our results indicate that degradation of Ssu72 results in a clear increase in Ser(P)2 (Fig. 5, A and B). Furthermore, this effect is evident from the promoter region of ADH1, as opposed to the gradual increase in the signal characteristic of Ser(P)2 ChIPs in a WT strain (43). Because Ssu72 is specific for dephosphorylation of Ser(P)5 and Ser(P)7 (21, 22, 28), with no activity toward Ser(P)2 (27, 44), our results imply that Ssu72 indirectly regulates Ser2 phosphorylation. Ssu72 could exert this effect by facilitating the recruitment and/or activation of the Ser(P)2 phosphatase, Fcp1, at promoters. Perhaps depletion of Ssu72 inactivates the phosphatase activity of Fcp1 and allows the principal Ser(P)2 kinase, Ctk1, to phosphorylate Ser2 earlier than usual in the transcription cycle.
FIGURE 5.
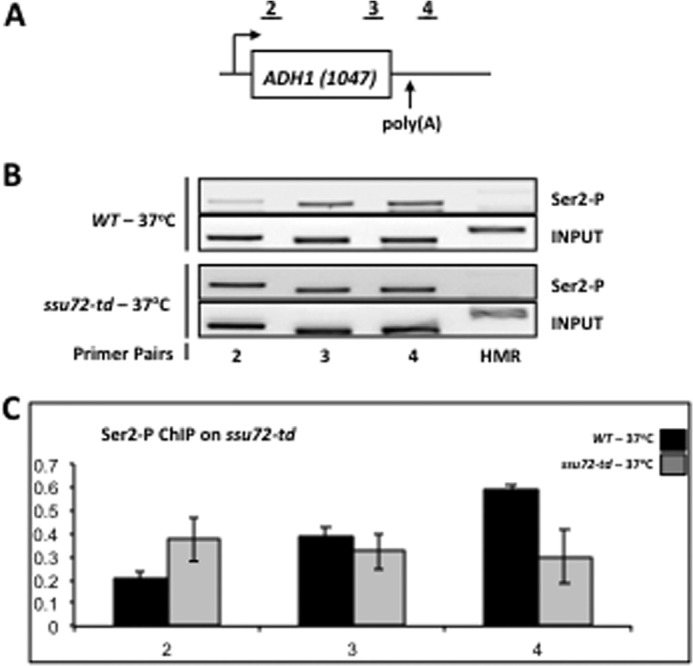
Loss of Ssu72 leads to deregulation of Ser2 phosphorylation. A, schematic depiction of the ADH1 gene. The regions probed by ChIP are denoted 1–3. The ADH1 ChIP primers are defined in Table 2 and are identical to the primer pairs described previously (43). B, ChIP analysis of Ser(P)2 cross-linking to ADH1 using isogenic strains FY23 (WT) and XH-24 (ssu72-td) that had been incubated at either the permissive (30 °C) or restrictive (37 °C) temperature for 1 h prior to cross-linking. Chromatin was immunoprecipitated using the monoclonal antibody 3E10 (α-Ser(P)2). C, quantification of the data shown in B. Error bars, S.E.
DISCUSSION
Using a novel set of ssu72 mutants, we demonstrate that Ssu72 is a RNAPII CTD Ser(P)5 phosphatase that acts during the initiation to elongation transition of the transcription cycle. This conclusion is in contrast to the previous report that Ssu72 acts specifically at termination (21). Both the ssu72-2 temperature-sensitive mutant and ssu72-4 null mutant show clear accumulation of the Ser(P)5 signal, which appears to be uniformly high across the genes examined here rather than diminishing with increasing distance from the promoter. Thus, the Ssu72 phosphatase acts early in the transcription cycle, consistent with the initial identification of Ssu72 as a protein that interacts with TFIIB to affect start site selection (29). The significance of this result lies in two previous misconceptions: (i) that Rtr1 is the sole Ser(P)5 phosphatase acting early in the transcription cycle and (ii) that Ssu72 dephosphorylates Ser(P)5 only during transcription termination (21, 34, 35).
A striking result from this study is that degradation of Ssu72 in the ssu72-td strain did not result in accumulation of Ser(P)5 on transcribing RNAPII, although Western blot experiments show a clear accumulation of bulk, steady-state Ser(P)5. A possible explanation for this result is that in the absence of the Ssu72 protein, as opposed to the presence of catalytically inactive Ssu72, transcribing RNAPII undergoes Ser(P)5 dephosphorylation by another CTD phosphatase. We suggest that catalytically inactive Ssu72 (ssu72-2 or ssu72-4) retains the ability to associate with the CTD and render it inaccessible to the other Ser(P)5 phosphatase, perhaps Rtr1 (36). This conclusion is reminiscent of catalytically inactive mutants of Fcp1, the Ser(P)2 phosphatase, which dwell on the CTD (45).
Failure to dephosphorylate Ser(P)5 did not affect RNAPII progression through the transcription cycle. This result, although novel, is not entirely unexpected because previous studies showed that inactivation of the CTD Ser5 kinase, Kin28, had little effect on promoter clearance (16, 46, 47). Also, the rtr1Δ deletion, which results in high levels of Ser(P)5, allows for RNAPII progression through genes as well (34). Although RNAPII CTD phosphorylation/dephosphorylation plays a crucial role in coupling transcription with RNA processing, phosphorylation status does not seem to affect the ability of RNAPII to transcribe a gene. It is important to recognize that our studies addressed only the effects of ssu72 mutations on the phosphorylation status of RNAPII and its progression across genes and not on RNA processing.
Why RNAPII occupancy across the PMA1 gene is diminished when the ssu72-2 strain is incubated at the restrictive temperature is not clear. Ssu72 affects the expression of a small set of genes in different ways. For example, the Faye laboratory showed that expression of <200 genes was at least 2-fold higher and that of <150 genes was at least 2-fold lower in their ssu72-ts69 strain (48). It is possible that transcription of PMA1 is diminished in the ssu72-2 mutant, leading to less Rpb3 cross-linking over its open reading frame. This possibility is unlikely, however, because Rpb3 occupancy over PMA1 in the ssu72-td and ssu72-4 strains is unaffected. Our set of ssu72 mutants is likely to be important to resolve this issue by analyzing RNAPII occupancy on a genome-wide scale.
We had noticed previously in whole-cell extracts that inactivation of Ssu72 resulted in diminished levels of hypophosphorylated RNAPII detected by the 8WG16 antibody (27). Here we showed, however, that the decrease in the 8WG16 ChIP signal is not related to elevated levels of Ser(P)5 on transcribing RNAPII. This result is evident in the ssu72-td background, where we did not see an accumulation of Ser(P)5 on either PMA1 or PYK1 but did see a marked decrease in the hypophosphorylated form of RNAPII as detected by 8WG16. Remarkably, the opposite is true in the ssu72-4 background, where, although high levels of Ser(P)5 are detected across PMA1 and PYK1 at the restrictive temperature, we did not see a drop in the 8WG16 ChIP signal under the same conditions. In agreement with these results, we found that the rtr1Δ deletion, which results in the accumulation of RNAPII Ser(P)5 across genes (34), does not affect the pattern of the 8WG16 ChIP. This information suggests that Ssu72 has a function that affects the phosphorylation status of the CTD that is independent of its role in catalyzing Ser(P)5 dephosphorylation.
The increase in Ser(P)2 levels that we detected in the ssu72-td strain is likely to be an indirect effect because it is known from in vitro experiments that Ssu72 does not dephosphorylate Ser(P)2 (27, 44). In yeast, the three enzymes that affect Ser2 phosphorylation status are the two kinases, Ctk1 and Bur1, and the phosphatase Fcp1. All three proteins genetically interact with Ssu72 (48, 49). Therefore, it is possible that Ssu72 regulates the activity or recruitment of one or more of these proteins. The most likely explanation is that Fcp1 is aberrantly regulated in the absence of Ssu72. ChIP experiments with the fcp1-1 and fcp1-2 mutants exhibit a drop in the 8WG16 signal across the PMA1 and ADH1 genes starting from the promoters in a manner similar to our results for the ssu72-td and ssu72-2 mutants (21, 41). Also, overexpression of Fcp1 results in suppression of the temperature-sensitive phenotype of the ssu72-ts52 allele (48). This would explain how Ssu72 mutants alter the normal pattern of Ser2 phosphorylation without actually being a Ser(P)2 phosphatase. The Fcp1 phosphatase and the Ctk1 kinase are both recruited to RNAPII at promoters and travel with RNAPII throughout the transcription cycle (41). However, the gradual phosphorylation of Ser2 does not start until after RNAPII escapes the promoter (14, 16). It is possible that Ssu72 recruits or activates the phosphatase activity of Fcp1 at promoters, thereby preventing accumulation of Ser(P)2 at promoter proximal regions. As RNAPII escapes the promoter and transitions into the elongation phase, Ssu72 dissociates from RNAPII and the phosphatase activity of Fcp1 is diminished. The Ser(P)2 mark can now accumulate due to the kinase activity of Ctk1. A mutation in Ssu72 that inhibits the interaction between Ssu72 and Fcp1 would account for high levels of Ser(P)2 at the promoter. Whether Ssu72 regulates the recruitment or enzymatic activity of Fcp1, Ctk1, and/or Bur1 remains to be established.
Ssu72 is required for gene looping, which juxtaposes the promoter and terminator regions of genes (50–52). Looping defects have been observed in the ssu72-td strain as well as in the catalytically dead ssu72-4 mutant (50). The latter result suggests that during the initiation to elongation transition, the phosphatase activity of Ssu72 is critical for gene looping. However, Ssu72 is also required to recruit TFIIB to terminators, and failure to do so disrupts gene looping (51). Thus, the function of Ssu72 in gene looping is likely to be dependent upon its roles during both transcription initiation and 3′-end processing.
Acknowledgments
We are especially grateful to Dirk Eick (Munich Center for Integrated Protein Science) for his generous gift of CTD monoclonal antibodies. We thank Drew Vershon (Rutgers), Lucy Robinson (Louisiana State University Health Sciences Center), Fred Winston (Harvard Medical School), Xiaoyuan He and Claire Moore (Tufts Medical School), and Amber Mosley (Indiana University School of Medicine) for strains. We are grateful to Claire Moore and members of her laboratory and to Krishnamurthy Shankarling and B. N. Singh from our laboratory for valuable insight throughout the course of this work.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 GM39484 (to M. H.) and R01 GM068887 (to Claire Moore and M. H.).
- RNAPII
- RNA polymerase II
- CTD
- RNAPII C-terminal domain
- IP
- immunoprecipitation.
REFERENCES
- 1. Mayer A., Heidemann M., Lidschreiber M., Schreieck A., Sun M., Hintermair C., Kremmer E., Eick D., Cramer P. (2012) CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336, 1723–1725 [DOI] [PubMed] [Google Scholar]
- 2. Shatkin A. J., Manley J. L. (2000) The ends of the affair: capping and polyadenylation. Nat. Struct. Biol. 7, 838–842 [DOI] [PubMed] [Google Scholar]
- 3. Rino J., Carmo-Fonseca M. (2009) The spliceosome: a self-organized macromolecular machine in the nucleus? Trends Cell Biol. 19, 375–384 [DOI] [PubMed] [Google Scholar]
- 4. Zhou Q., Li T., Price D. H. (2012) RNA polymerase II elongation control. Annu. Rev. Biochem. 81, 119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho E. J., Rodriguez C. R., Takagi T., Buratowski S. (1998) Allosteric interactions between capping enzyme subunits and the RNA polymerase II carboxy-terminal domain. Genes Dev. 12, 3482–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E. D., Sevier C. S., Ding H., Koh J. L., Toufighi K., Mostafavi S., Prinz J., St Onge R. P., VanderSluis B., Makhnevych T., Vizeacoumar F. J., Alizadeh S., Bahr S., Brost R. L., Chen Y., Cokol M., Deshpande R., Li Z., Lin Z. Y., Liang W., Marback M., Paw J., San Luis B. J., Shuteriqi E., Tong A. H., van Dyk N., Wallace I. M., Whitney J. A., Weirauch M. T., Zhong G., Zhu H., Houry W. A., Brudno M., Ragibizadeh S., Papp B., Pal C., Roth F. P., Giaever G., Nislow C., Troyanskaya O. G., Bussey H., Bader G. D., Gingras A. C., Morris Q. D., Kim P. M., Kaiser C. A., Myers C. L., Andrews B. J., Boone C. The genetic landscape of a cell. Science 327, 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCracken S., Fong N., Rosonina E., Yankulov K., Brothers G., Siderovski D., Hessel A., Foster S., Shuman S., Bentley D. L. (1997) 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 11, 3306–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mueller C. L., Jaehning J. A. (2002) Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol. Cell. Biol. 22, 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ni Z., Schwartz B. E., Werner J., Suarez J. R., Lis J. T. (2004) Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol. Cell 13, 55–65 [DOI] [PubMed] [Google Scholar]
- 10. Phatnani H. P., Jones J. C., Greenleaf A. L. (2004) Expanding the functional repertoire of CTD kinase I and RNA polymerase II: novel phosphoCTD-associating proteins in the yeast proteome. Biochemistry 43, 15702–15719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilmes G. M., Bergkessel M., Bandyopadhyay S., Shales M., Braberg H., Cagney G., Collins S. R., Whitworth G. B., Kress T. L., Weissman J. S., Ideker T., Guthrie C., Krogan N. J. (2008) A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol. Cell 32, 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidemann M., Hintermair C., Voss K., Eick D. (2013) Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim. Biophys. Acta 1829, 55–62 [DOI] [PubMed] [Google Scholar]
- 13. Prelich G. (2002) RNA polymerase II carboxy-terminal domain kinases: emerging clues to their function. Eukaryot. Cell 1, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman R. D., Heidemann M., Albert T. K., Mailhammer R., Flatley A., Meisterernst M., Kremmer E., Eick D. (2007) Transcribing RNA polymerase II is phosphorylated at CTD residue serine-7. Science 318, 1780–1782 [DOI] [PubMed] [Google Scholar]
- 15. Hsin J. P., Manley J. L. (2012) The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim M., Suh H., Cho E. J., Buratowski S. (2009) Phosphorylation of the yeast Rpb1 C-terminal domain at serines 2, 5, and 7. J. Biol. Chem. 284, 26421–26426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsin J. P., Sheth A., Manley J. L. (2011) RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science 334, 683–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hintermair C., Heidemann M., Koch F., Descostes N., Gut M., Gut I., Fenouil R., Ferrier P., Flatley A., Kremmer E., Chapman R. D., Andrau J. C., Eick D. (2012) Threonine-4 of mammalian RNA polymerase II CTD is targeted by Polo-like kinase 3 and required for transcriptional elongation. EMBO J. 31, 2784–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanes S. D. (2014) The Ess1 prolyl isomerase: traffic cop of the RNA polymerase II transcription cycle. Biochim. Biophys. Acta 1839, 316–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu H., Flores O., Weinmann R., Reinberg D. (1991) The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc. Natl. Acad. Sci. U.S.A. 88, 10004–10008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bataille A. R., Jeronimo C., Jacques P. É., Laramée L., Fortin M. È., Forest A., Bergeron M., Hanes S. D., Robert F. (2012) A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol. Cell 45, 158–170 [DOI] [PubMed] [Google Scholar]
- 22. Zhang D. W., Mosley A. L., Ramisetty S. R., Rodríguez-Molina J. B., Washburn M. P., Ansari A. Z. (2012) Ssu72 phosphatase-dependent erasure of phospho-Ser7 marks on the RNA polymerase II C-terminal domain is essential for viability and transcription termination. J. Biol. Chem. 287, 8541–8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egloff S., Murphy S. (2008) Cracking the RNA polymerase II CTD code. Trends Genet. 24, 280–288 [DOI] [PubMed] [Google Scholar]
- 24. Phatnani H. P., Greenleaf A. L. (2006) Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 [DOI] [PubMed] [Google Scholar]
- 25. Eick D., Geyer M. (2013) The RNA polymerase II carboxy-terminal domain (CTD) code. Chem. Rev. 113, 8456–8490 [DOI] [PubMed] [Google Scholar]
- 26. Schwer B., Bitton D. A., Sanchez A. M., Bähler J., Shuman S. (2014) Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. Proc. Natl. Acad. Sci. U.S.A. 111, 4185–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krishnamurthy S., He X., Reyes-Reyes M., Moore C., Hampsey M. (2004) Ssu72 Is an RNA polymerase II CTD phosphatase. Mol. Cell 14, 387–394 [DOI] [PubMed] [Google Scholar]
- 28. Xiang K., Manley J. L., Tong L. (2012) An unexpected binding mode for a Pol II CTD peptide phosphorylated at Ser7 in the active site of the CTD phosphatase Ssu72. Genes Dev. 26, 2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun Z.-W., Hampsey M. (1996) Synthetic enhancement of a TFIIB defect by a mutation in SSU72, an essential gene encoding a novel protein that affects transcription start site selection in vivo. Mol. Cell. Biol. 16, 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dichtl B., Blank D., Ohnacker M., Friedlein A., Roeder D., Langen H., Keller W. (2002) A role for SSU72 in balancing RNA polymerase II transcription elongation and termination. Mol. Cell 10, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 31. He X., Khan A. U., Cheng H., Pappas D. L., Jr., Hampsey M., Moore C. L. (2003) Functional interactions between the transcription and mRNA 3′ end processing machineries mediated by Ssu72 and Sub1. Genes Dev. 17, 1030–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nedea E., He X., Kim M., Pootoolal J., Zhong G., Canadien V., Hughes T., Buratowski S., Moore C. L., Greenblatt J. (2003) Organization and function of APT, a subcomplex of the yeast cleavage and polyadenylation factor involved in the formation of mRNA and small nucleolar RNA 3′-ends. J. Biol. Chem. 278, 33000–33010 [DOI] [PubMed] [Google Scholar]
- 33. Steinmetz E. J., Brow D. A. (2003) Ssu72 protein mediates both poly(A)-coupled and poly(A)-independent termination of RNA polymerase II transcription. Mol. Cell. Biol. 23, 6339–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosley A. L., Pattenden S. G., Carey M., Venkatesh S., Gilmore J. M., Florens L., Workman J. L., Washburn M. P. (2009) Rtr1 is a CTD phosphatase that regulates RNA polymerase II during the transition from serine 5 to serine 2 phosphorylation. Mol. Cell 34, 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiang K., Manley J. L., Tong L. (2012) The yeast regulator of transcription protein Rtr1 lacks an active site and phosphatase activity. Nat. Commun. 3, 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu P. L., Yang F., Smith-Kinnaman W., Yang W., Song J. E., Mosley A. L., Varani G. (2014) Rtr1 is a dual specificity phosphatase that dephosphorylates Tyr1 and Ser5 on the RNA polymerase II CTD. J. Mol. Biol. 426, 2970–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., Chu A. M., Connelly C., Davis K., Dietrich F., Dow S. W., El Bakkoury M., Foury F., Friend S. H., Gentalen E., Giaever G., Hegemann J. H., Jones T., Laub M., Liao H., Liebundguth N., Lockhart D. J., Lucau-Danila A., Lussier M., M'Rabet N., Menard P., Mittmann M., Pai C., Rebischung C., Revuelta J. L., Riles L., Roberts C. J., Ross-MacDonald P., Scherens B., Snyder M., Sookhai-Mahadeo S., Storms R. K., Véronneau S., Voet M., Volckaert G., Ward T. R., Wysocki R., Yen G. S., Yu K., Zimmermann K., Philippsen P., Johnston M., Davis R. W. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 [DOI] [PubMed] [Google Scholar]
- 38. Pappas D. L., Jr., Hampsey M. (2000) Functional interaction between Ssu72 and the Rpb2 subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol. Cell. Biol. 20, 8343–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reyes-Reyes M., Hampsey M. (2007) Role for the Ssu72 C-terminal domain phosphatase in RNA polymerase II transcription elongation. Mol. Cell. Biol. 27, 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meinhart A., Silberzahn T., Cramer P. (2003) The mRNA transcription/processing factor ssu72 is a potential tyrosine phosphatase. J. Biol. Chem. 278, 15917–15921 [DOI] [PubMed] [Google Scholar]
- 41. Cho E. J., Kobor M. S., Kim M., Greenblatt J., Buratowski S. (2001) Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C-terminal domain. Genes Dev. 15, 3319–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winston F., Dollard C., Ricupero-Hovasse S. L. (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11, 53–55 [DOI] [PubMed] [Google Scholar]
- 43. Ahn S. H., Kim M., Buratowski S. (2004) Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13, 67–76 [DOI] [PubMed] [Google Scholar]
- 44. Hausmann S., Koiwa H., Krishnamurthy S., Hampsey M., Shuman S. (2005) Different strategies for carboxyl-terminal domain (CTD) recognition by serine 5-specific CTD phosphatases. J. Biol. Chem. 280, 37681–37688 [DOI] [PubMed] [Google Scholar]
- 45. Suh M. H., Ye P., Zhang M., Hausmann S., Shuman S., Gnatt A. L., Fu J. (2005) Fcp1 directly recognizes the C-terminal domain (CTD) and interacts with a site on RNA polymerase II distinct from the CTD. Proc. Natl. Acad. Sci. U.S.A. 102, 17314–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong S. W., Hong S. M., Yoo J. W., Lee Y. C., Kim S., Lis J. T., Lee D. K. (2009) Phosphorylation of the RNA polymerase II C-terminal domain by TFIIH kinase is not essential for transcription of Saccharomyces cerevisiae genome. Proc. Natl. Acad. Sci. U.S.A. 106, 14276–14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanin E. I., Kipp R. T., Kung C., Slattery M., Viale A., Hahn S., Shokat K. M., Ansari A. Z. (2007) Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 104, 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ganem C., Devaux F., Torchet C., Jacq C., Quevillon-Cheruel S., Labesse G., Facca C., Faye G. (2003) Ssu72 is a phosphatase essential for transcription termination of snoRNAs and specific mRNAs in yeast. EMBO J. 22, 1588–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ganem C., Miled C., Facca C., Valay J. G., Labesse G., Ben Hassine S., Mann C., Faye G. (2006) Kinase Cak1 functionally interacts with the PAF1 complex and phosphatase Ssu72 via kinases Ctk1 and Bur1. Mol. Genet. Genomics 275, 136–147 [DOI] [PubMed] [Google Scholar]
- 50. Ansari A., Hampsey M. (2005) A role for the CPF 3′-end processing machinery in RNAP II-dependent gene looping. Genes Dev. 19, 2969–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh B. N., Hampsey M. (2007) A transcription-independent role for TFIIB in gene looping. Mol. Cell 27, 806–816 [DOI] [PubMed] [Google Scholar]
- 52. Tan-Wong S. M., Zaugg J. B., Camblong J., Xu Z., Zhang D. W., Mischo H. E., Ansari A. Z., Luscombe N. M., Steinmetz L. M., Proudfoot N. J. (2012) Gene loops enhance transcriptional directionality. Science 338, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]