Background: SHP2 regulates cancer development and progression.
Results: SHP2 promotes TGFβ1-induced EMT requiring its phosphatase activity. PTP and N-SH2 domains of SHP2 interact with Hook1, which negatively regulates TGFβ1-induced EMT.
Conclusion: SHP2-Hook1 complex regulates TGFβ1-induced EMT.
Significance: SHP2 plays important roles in tumor metastases due to promoting EMT in cancer cells.
Keywords: Epithelial to Mesenchymal Transition, Epithelial-Mesenchymal Transition (EMT), Metastasis, Protein Phosphatase, Protein-Protein Interaction, Hook1, Mesenchymal, Protein-tyrosine Phosphatase, shp2
Abstract
The epithelial-mesenchymal transition (EMT) is an essential process for embryogenesis. It also plays a critical role in the initiation of tumor metastasis. Src homology 2 (SH2)-domain containing protein-tyrosine phosphatase-2 (SHP2) is a ubiquitously expressed protein-tyrosine phosphatase and is mutated in many tumors. However, its functional role in tumor metastasis remains largely unknown. We found that TGFβ1-induced EMT in lung epithelial A549 cells was partially blocked when SHP2 was decreased by transfected siRNA. The constitutively active form (E76V) promoted EMT while the phosphatase-dead mutation (C459S) and the SHP2 inhibitor PHPS1 blocked EMT, which further demonstrated that the phosphatase activity of SHP2 was required for promoting TGFβ1-induced EMT. Using the protein-tyrosine phosphatase domain of SHP2 as bait, we identified a novel SHP2-interacting protein Hook1. Hook1 was down-regulated during EMT in A549 cells. Overexpression of Hook1 inhibited EMT while knockdown of Hook1 promoted EMT. Moreover, both the protein-tyrosine phosphatase domain and N-terminal SH2 domain of SHP2 directly interacted with Hook1. Down-regulation of Hook1 increased SHP2 activity. These results suggested that Hook1 was an endogenous negative regulator of SHP2 phosphatase activity. Our data showed that the protein-tyrosine phosphatase SHP2 was involved in the process of EMT and Hook1 repressed EMT by regulating the activation of SHP2. SHP2-Hook1 complex may play important roles in tumor metastases by regulating EMT in cancer cells.
Introduction
Metastasis is the leading cause of mortality in human cancer (1). There are several stages during metastasis, including local invasion, hematogenous spread, and colonization. The epithelial-mesenchymal transition (EMT)3 participates in the initiation stage of metastasis, and endows the cancer cells with migratory and invasive properties (2). EMT has also been found to participate in the processes of development, fibrosis, and wound-healing (3).
EMT is a form of cell differentiation. Previous reports have shown that the Src homology 2 (SH2)-domain containing protein-tyrosine phosphatase-2 (SHP2) plays important roles in cell differentiation, indicating that it might participate in the regulation of EMT (4–6). SHP2, a member of the non-receptor protein-tyrosine phosphatase family, participates in many signal transduction and activation of the Ras-ERK signaling pathway, mostly beginning by EGF binding to the EGF receptor (7, 8). This also suggests that SHP2 may play regulatory roles in the EMT process (9, 10). SHP2 also plays important roles in development, cancer, inflammation, transcription regulation, and cell migration (11–15).
SHP2 contains two SH2 domains, N-SH2 and C-SH2 domain, at its N terminus. In the basal state, the N-SH2 domain binds to the PTP domain and blocks the active site. Thus, the activity of SHP2 is inhibited. With stimulation, the N-terminal SH2 domain binds to a phosphorylated tyrosine residue, detaches from the PTP domain, and SHP2 is activated (16, 17). Germline mutations in SHP2 encoding gene PTPN11 are associated with Noonan syndrome, LEOPARD syndrome, and metachondromatosis (18–21). Somatic activated SHP2 mutations have also been detected in acute myeloid leukemia, neuroblastoma, melanoma, breast cancer, lung cancer, and colorectal cancer (22, 23). These data suggest that PTPN11 functions as a proto-oncogene. However, it has been recently reported that SHP2 can act as either a tumor promoter or suppressor (24–26). Depending on the tissues and disease stages, SHP2 plays different roles in different tumors. The exact role of SHP2 in cancer, especially metastasis, is not clear.
It has been reported that several growth factor and cytokine factor signaling pathways (TGFβ1, FGF, EGF, HGF, Wnt/β-catenin, and Notch) participate in EMT (27). In A549 cells (human lung adenocarcinoma), TGFβ1 has almost no effect on cell growth, but induces a remarkable EMT phenotype (28). Here, we used the TGFβ1-induced EMT in A549 cells to investigate the role of SHP2 in EMT. We found that SHP2 promoted EMT requiring its protein phosphatase activity. The novel interacting protein Hook1, interacting with SHP2 N-terminal SH2 and PTP domains, negatively regulated EMT.
EXPERIMENTAL PROCEDURES
Cell Culture
A549 and HEK293T cells were cultured in RPMI 1640 and DMEM, respectively (Hyclone, Logan, UT), supplemented with 10% FBS (Hyclone), penicillin (100 units/ml), and streptomycin (100 mg/ml) (Hyclone).
Materials
Recombinant human TGFβ1 (mammalian) was from PeproTech (Rocky Hill, NJ). Phenylhydrazonopyrazolone sulfonate 1 (PHPS1) was from Sigma-Aldrich. HOOK1 siRNA (target sequence: GUUGAGAUAUAUCGUCAGA) was from Thermo Fisher Scientific (Dharmacon Products, Lafayette, CO). SHP2 siRNA and all siRNA negative controls were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies for E-cadherin, vimentin, SHP2 and Hook1 were from Santa Cruz Biotechnology. Antibodies for pERK, ERK, pSmad2 (Ser467), pSmad3 (Ser423/425), Smad2, Smad3, COL1A1, and Snail1 were from Cell Signaling Technologies (Beverly, MA). Anti-Myc monoclonal antibody was from OriGene (Rockville, MD). Anti-Flag® antibody produced in rabbit was from Sigma-Aldrich. IRDye 680LT/IRDye 800CW secondary antibodies were from LI-COR Biosciences (Lincoln, NE). Primer synthesis and DNA sequencing were performed by Invitrogen.
Plasmids
The Hook1 (NM_015888) human cDNA clone (cat. no. SC114574) was from OriGene (Rockville, MD). The plasmids were generated in the PXJ40 vector as previously described (29). The plasmids used in the yeast two-hybrid system were constructed according to the Clontech protocol (TaKaRa Inc., Otsu, Japan). The mutant plasmids were constructed with the Fast Mutagenesis System (Transgen Biotech, Beijing, China) according to the manufacturer's instructions. Plasmids: pXJ40-FLAG-Hook1 (full-length), -Hook1C (AA573–728), pXJ40-MYC-SHP2 (full-length), -NSH2 (AA2–109), -CSH2 (AA110–220), -PTP (AA240–525), pXJ40-MYC-SHP2 mutant (C459S, E76V); pGADT7-SHP2-PTP (AA240–525), pGADT7-Hook1N (AA2–353), -Hook1M (AA353–573), -Hook1C (AA573–728), pGBKT7-SHP2 (full-length), -NSH2 (AA2–109), -CSH2 (AA110–220), -SHP2-PTP (AA240–525). All primers used for construction are listed in supplemental Table S1.
Transwell Migration Assay
For cell migration assays, 1 × 105 A549 cells were plated on 8-μl transwell filters (Corning, Corning, NY). The cells were induced to migrate toward medium containing 10% FBS with or without TGFβ1 (5 ng/ml) for 16 h in a 5% CO2 incubator. Non-invading cells were removed with a swab. The remaining cells were fixed in 4% paraformaldehyde, stained with crystal violet, and analyzed under a bright-field microscope. The number of migrating cells that had infiltrated the filter was measured by counting 3 random fields per filter. Mean values were obtained from at least three separate experiments.
Protein Extraction and Western Blot Analysis
Protein extracts from cultured cells were prepared in RIPA buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) according to the instructions (Beyotime, Beijing, China). Protein concentrations were measured with a protein assay kit (Bio-Rad). Routine Western blot analysis was performed. In brief, cell lysates were separated by SDS-PAGE on 10% or 14% polyacrylamide gels and transferred to a nitrocellulose membrane (Pall, Port Washington, NY). Then, the membrane was blocked with 5% dry milk in TBST (50 mm Tris, 150 mm NaCl, 0.05% Tween 20, pH 7.6) for 1 h at room temperature. The membrane was then incubated with primary antibodies at 4 °C overnight. Afterward, the membrane was washed with TBST and probed with IRDye 680LT/IRDye 800CW secondary antibodies (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature. Signals were visualized on an Odyssey two-color infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Immunoprecipitation
The cells in 100-mm dishes were lysed in 1 ml of RIPA buffer (Beyotime, Beijing, China). The lysate was pre-cleaned with protein A/G-Sepharose beads (Santa Cruz Biotechnology) by rotation at 4 °C for 1 h. After centrifugation, the supernatant was incubated with antibody for 1 h at 4 °C. Then, protein A/G-Sepharose (Santa Cruz Biotechnology) was added and incubated at 4 °C overnight with rotation. Beads were centrifuged and washed three times with RIPA buffer. Then, the beads were suspended with 2× Laemmli sample buffer (Bio-Rad). After boiling for 5 min, proteins were separated by SDS-PAGE, and Western blotting was performed as described above.
Real-time PCR Analysis
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was reversed to cDNA using the ReverTra Ace qPCR RT Kit (Toyobo Inc., Osaka, Japan). Real-time PCR was performed on a Light-Cycler Roche480 (Roche Molecular Systems) using the Light-Cycler Roche480 master kit. All real-time PCR assays were performed in duplicate, and data were from at least three independent experiments. All values are shown as ratios to gapdh levels. The primers used are listed in supplemental Table S2.
Yeast Two-hybrid Assay
The yeast two-hybrid screen was performed using the Matchmaker Gold Yeast Two-hybrid System (TaKaRa Inc., Otsu, Japan) and followed the manufacturer's instructions. The screening library was a Mate and Plate library human universal normalized cDNA library (TaKaRa Inc., Otsu, Japan). The bait was constructed by ligating full-length SHP2 or an SHP2-PTP fragment (AA240-525, NM 002834.3) into the BamH I and EcoR I sites of the pGBKT7 vector (TaKaRa Inc., Otsu, Japan). The screen was performed under high-stringency growth conditions as recommended by the manufacturer. Transformations were performed according to the Clontech protocol (TaKaRa Inc., Otsu, Japan).
Monolayer Wound-healing Assay
Cells were grown to 100% confluence, wounded by scratching with a pipette tip, and incubated for another 40 h. Phase-contrast images were captured at 0, 16, and 40 h.
SHP2 PTP Activity Assay
Cells were harvested in PTP lysis buffer (25 mm Hepes, pH7.4, 150 mm NaCl, 1 mm DTT, 2 mm EDTA, 0.5% Triton X-100) supplemented with protease inhibitor mixture. The supernatants (0.3 mg each) were incubated with anti-SHP2 antibody for 1 h, then protein A/G-Sepharose was added and incubated for 3 h at 4 °C. The immuno-precipitates were washed in the PTP lysis buffer twice and then washed twice with phosphatase reaction buffer (25 mm Hepes, pH7.4, 50 mm NaCl, 1 mm DTT, 0.05% Triton X-100). The immunocomplexes were resuspended in 250 μl of reaction buffer with 50 μm 6,8 difluoro-4-methylumbelliferyl phosphate (DiFUMP) (Invitrogen) and incubated at 37 °C for 30 min. DiFUMP fluorescence signal was measured at an excitation of 355 nm and an emission of 460 nm using SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). Levels of immunoprecipitated SHP2 were analyzed by Western blotting. All values were normalized to the value of control.
SBE4-Luc Activity Assay
SBE4-Luc was from Addgene, and pRL-TK was from Promega Corporation (Madison, WI). A549 cells were cotransfected with SBE4-Luc, pRL-TK and control siRNA/SHP2 siRNA. Luciferase activity was detected according to the protocols for Dual-Luciferase Reporter Assay System (Promega) and measured by SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA).
Statistical Analysis
Quantitative data are expressed as mean ± S.E. Statistical significance was determined by Student's t test. p value of <0.05 was considered to be statistically significant.
RESULTS
SHP2 Was Required for EMT
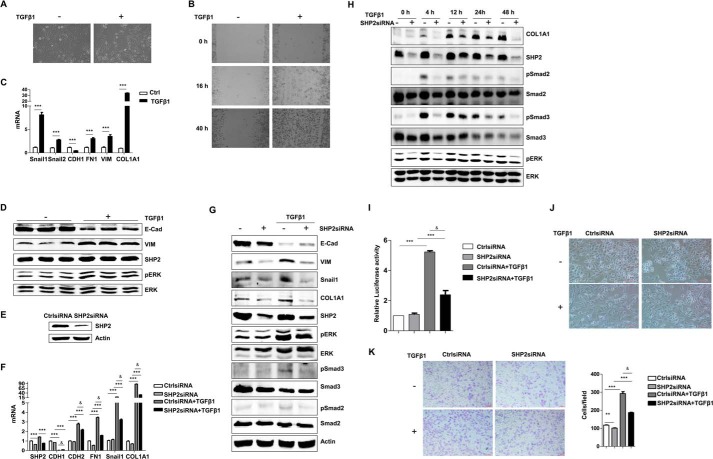
Non-small cell lung cancer cells (A549) were stimulated with TGFβ1 (5 ng/ml) for 24 h. The cells had notable morphological changes, from slab-like to an elongated spindle shape (Fig. 1A). The migration capacity was enhanced as shown by the wound-healing assay (Fig. 1B). Quantitative PCR demonstrated decreased transcription of the epithelial gene E-cadherin (CDH1) and increased mRNA levels of vimentin (VIM), Snail1, Snail2, fibronectin1 (FN1), and collagen1 A1 (COL1A1) (Fig. 1C), demonstrating that the TGFβ1-treated A549 cells lost their epithelial characteristics and gained mesenchymal phenotype.
FIGURE 1.
SHP2 positively regulated EMT. A, morphology of A549 cells with or without TGFβ1 treatment (5 ng/ml) for 24 h. B, cell wound assay was used to test the migration of A549 cells, with or without TGFβ1 treatment (5 ng/ml). Images were captured at 0, 16, and 40 h after wounding. C, relative mRNA levels of EMT-related transcription factors (Snail1 and Snail2) and markers (CDH1, FN1, VIM, COL1A1) detected by real-time PCR in A549 cells with or without TGFβ1 treatment (5 ng/ml) for 24 h (***, p < 0.005). D, Western blot analysis of the changes in EMT markers (E-Cad, VIM) and the activation of ERK in A549 cells with or without TGFβ1 treatment (5 ng/ml) for 48 h. The assay was performed in three independent experiments under the same conditions. E, SHP2 in A549 cells was successfully reduced after SHP2siRNA transfection. F, quantification PCR of EMT-related markers in control siRNA (CtrlsiRNA) and SHP2siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. ***, p < 0.005 between with and without TGFβ1; &, p < 0.005 between CtrlsiRNA and SHP2siRNA. G, Western blot analysis of the EMT markers (E-Cad, VIM, Snail, COL1A1) and the activation of ERK, Smad2, Smad3 in control siRNA and SHP2siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 48 h. H, Western blot analysis of the activation of ERK, Smad2, and Smad3 in SHP2siRNA-transfected A549 cells, with TGFβ1 treatment (5 ng/ml) for 0, 4, 12, 24, 48 h. I, SBE4-Luc activity assay to detect the activation of Smad pathway in SBE4-Luc, pRL-TK, control siRNA or SHP2siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 8 h. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.005 between CtrlsiRNA and SHP2siRNA. J, morphological changes of control siRNA and SHP2siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. Scale bar, 50 μm. K, transwell assays to assess the migration of control siRNA and SHP2siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. Scale bar, 100 μm. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.005 between CtrlsiRNA and SHP2siRNA.
In the process of EMT in A549 cells induced by TGFβ1, the expression of SHP2 did not change, the activation of ERK pathway was dramatically induced after TGFβ1 stimulation for 48 h (Fig. 1D). Then we investigated the effect of knockdown of SHP2 by siRNA on the EMT. SHP2 was successfully decreased by transfection with siRNA (Fig. 1, E and F). TGFβ1 greatly decreased the mRNA level of the epithelial gene CDH1, and this was partially blocked by the decreased expression of SHP2. Accordingly, down-regulation of SHP2 repressed the mRNA levels of mesenchymal genes (CDH2, FN1, Snail1, and Col1A1) (Fig. 1F). We further confirmed these results by Western blotting (Fig. 1G). The increased protein levels of VIM, Snail1, COL1A1 and activation of ERK, Smad pathway were partially attenuated by transfection with SHP2siRNA. We also detected the activation of ERK and Smad pathways at different time points (0, 4, 12, 24, 48 h) with TGFβ1 stimulation when SHP2 was or was not knockdown. The results showed that SHP2 knockdown inhibited TGFβ1 induced Smad and ERK pathway (Fig. 1H). TGFβ1 non-Smad pathway-ERK pathway might be important and essential for EMT in A549 cells. Furthermore, we used SBE4-Luc reporter system to test the activation of Smad pathway with or without SHP2 siRNA transfection (30). The activation of Smad pathway was repressed when SHP2 was knockdown (Fig. 1I). And the TGFβ1-induced alterations of cell morphology and cell motility were also interrupted by down-regulation of SHP2 (Fig. 1, J and K). Therefore, SHP2 positively regulated the transition of epithelial to mesenchymal characteristics of A549 cells.
Phosphatase Activity of SHP2 Was Required for Regulating EMT
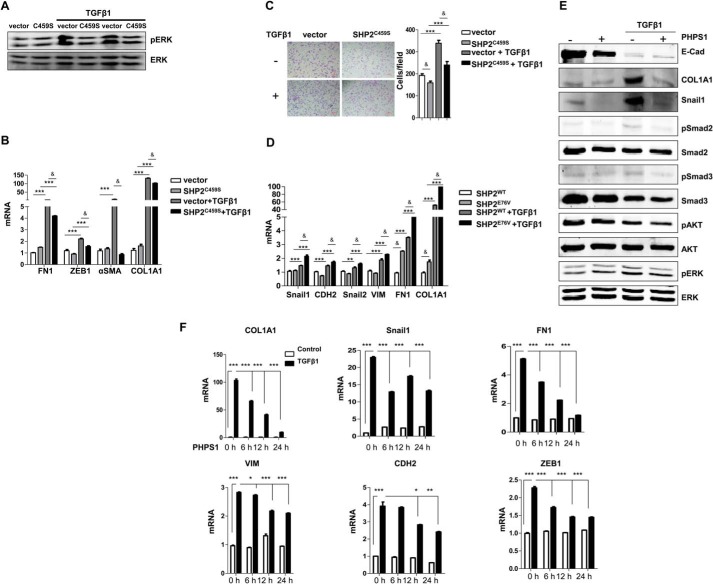
To further investigate whether the phosphatase activity of SHP2 is involved in EMT, the phosphatase active center of SHP2 was mutated by substitution of Cys-459 to Ser. This phosphatase-dead mutation (SHP2C459S) markedly repressed the TGFβ1-induced ERK activation in A549 cells (Fig. 2A). Similar to SHP2 siRNA, the SHP2C459S mutation also partially blocked the TGFβ1-induced EMT in A549 cells as indicated by repressed mRNA levels of transcriptor and mesenchymal genes (ZEB1, FN1, αSMA, and Col1A1) (Fig. 2B) and cell migration (Fig. 2C). Another mutation, E76V, that releases SHP2 from auto-inhibition, was also used. The results demonstrated that the constitutively activated SHP2 mutant (SHP2E76V) promoted the mesenchymal phenotype in A549 cells as indicated by increased mRNA levels of transcriptor and mesenchymal genes (Snail1, Snail2, CDH2, FN1, VIM, and COL1A1) (Fig. 2D). Overexpression of wild-type SHP2 also slightly enhanced the mesenchymal gene transcription. To further confirm that phosphatase enzymatic activity was required for its role in promoting EMT, SHP2 inhibitor PHPS1 was introduced. We examined the effect of PHPS1 on the protein levels of EMT-associated markers. The results showed that the increased levels of Snail1, COL1A1 and activation of ERK and Smad pathways with TGFβ1 stimulation were interrupted by the PHPS1 treatment (Fig. 2E). Our results showed that PHPS1 attenuated the EMT phenotype in a time-dependent manner (Fig. 2F). Our data strongly suggested that SHP2 was positively involved in the process of TGFβ1-induced EMT and its enzymatic activity was required.
FIGURE 2.
Phosphatase activity of SHP2 was required for EMT. A, Western analysis of effects of SHP2 C459S overexpression on ERK activation with or without TGFβ1 treatment (5 ng/ml) for 48 h. B, quantification of EMT-associated markers (FN1, ZEB1, αSMA, COL1A1) in empty vector and SHP2C459S-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.01 between vector and SHP2C459S. C, transwell assay was used to detect the migration of empty vector and SHP2C459S-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 16 h. Scale bar, 100 μm. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.01 between vector and SHP2C459S. D, QPCR analysis of EMT-related markers (Snail1, Snail2, CDH2, VIM, FN1, COL1A1) in A549 cells transfected with SHP2WT and SHP2E76V, with or without TGFβ1 treatment (5 ng/ml) for 24 h. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.05 between SHP2WT and SHP2E76V. E, Western blot analysis of changes in EMT markers (E-Cad, COL1A1, Snail1) and the activation of ERK, Smad2, Smad3 in control and PHPS1 (20 μm)-treated A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 48 h. F, QPCR analysis of EMT-related markers (COL1A1, Snail1, FN1, VIM, CDH2, ZEB1) in A549 cells treated with PHPS1 (20 μm) for 0, 6, 12, and 24 h in the absence or presence of TGFβ1 (5 ng/ml) for 24 h. (*, p < 0.05; **, p < 0.01; ***, p < 0.005).
Hook1 Interacted with SHP2
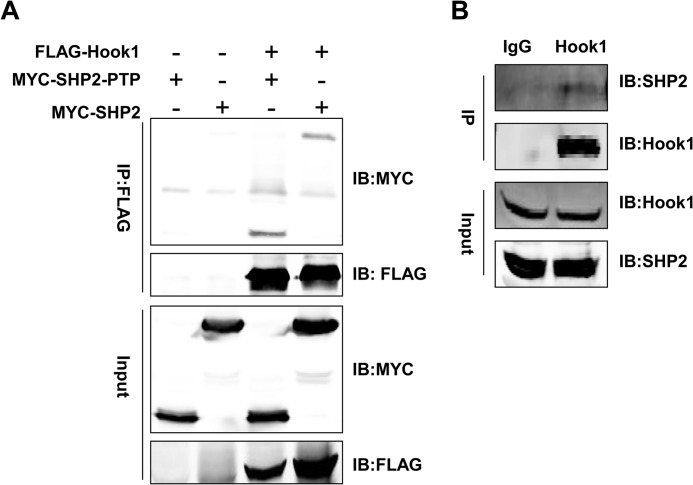
To discover the molecular mechanisms that by which SHP2 promotes EMT and how SHP2 is activated in EMT, yeast two-hybrid assays were performed. Both full-length and the protein-tyrosine phosphatase domain of SHP2 were constructed in pGBKT7 as baits. When full-length SHP2 was used, there were no positive clones. When the PTP domain of SHP2 was used, there were several positive clones (supplemental Fig. 1A). DNA sequencing showed that Hook1 was one of the positive clones with the correct reading frame (supplemental Fig. 1B). To further confirm the interaction, either full-length SHP2 or its PTP was co-transformed with plasmid containing Hook1 into yeast cells, the results showed that SHP2-PTP but not full-length SHP2 interacted with Hook1 (supplemental Fig. 1, C and D). Further, co-immuno-precipitation assay in HEK293T cells showed that both MYC-tagged SHP2 and its PTP domain interacted with FLAG-tagged Hook1 (Fig. 3A). We further showed that endogenous SHP2 was co-immunoprecipitated with Hook1 from the lysate of HEK293T cells (Fig. 3B). We thus demonstrated that Hook1, an endogenous SHP2-interacting protein, bound to its PTP domain.
FIGURE 3.
Hook1 was a new binding protein of SHP2. A, co-immunoprecipitation assay to verify the interaction of overexpressed full-length SHP2 (MYC-SHP2) or PTP domain of SHP2 (MYC-SHP2-PTP) with FLAG-Hook1 in HEK293T cells. B, co-immunoprecipitation assay to verify the endogenous interaction of SHP2 with Hook1 in HEK293T cells.
Hook1 Negatively Regulated EMT
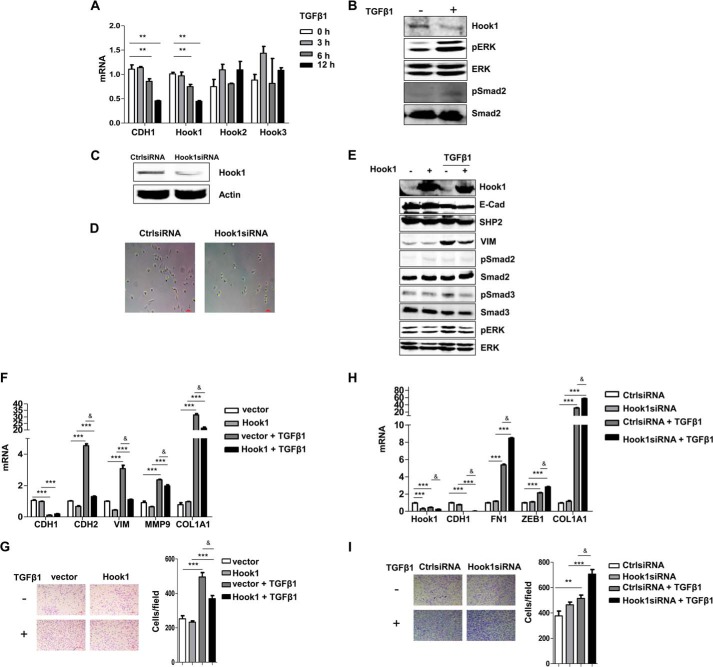
It has been reported that Hook1 is a microtubule-binding protein and participates in cell skeleton reorganization. To explore the possibility that Hook1 plays a role in EMT, we first found that Hook1 was down-regulated at both the mRNA and protein levels in TGFβ1-stimulated A549 cells (Fig. 4, A and B). Three members of the Hook family have been reported (31–33). Expression of the other two members, Hook2 and Hook3, did not change in the process of TGFβ1 induced-EMT (Fig. 4A). Knockdown of Hook1 reduced cell adhesion (Fig. 4, C and D). Overexpression of Hook1 inhibited the activation of ERK induced by TGFβ1 as well as the EMT phenotype indicated by the blockade of TGFβ1-induced expression of VIM (Fig. 4E). This was further confirmed by the transcriptional levels of the epithelial gene CDH1 and mesenchymal genes (CDH2, VIM, MMP9, and COL1A1) (Fig. 4F). Cell migration was also repressed by overexpression of Hook1 (Fig. 4G). In line with these observations, knockdown of Hook1 by siRNA promoted the expression of mesenchymal genes and cell migration (Fig. 4, H and I). These results indicated that Hook1 was a negative regulator of EMT.
FIGURE 4.
Hook1 negatively regulated EMT. A, quantification of the mRNA levels of CDH1, Hook1, Hook2, and Hook3 in A549 cells with TGFβ1 treatment (5 ng/ml) for 0, 3, 6, and 12 h (**, p < 0.01). B, Western blot analysis of the expression levels of Hook1 after treatment with TGFβ1 (5 ng/ml) for 48 h. C, Hook1 was knockdown after Hook1siRNA transfection in A549 cells. D, cell adhesion in control siRNA and Hook1siRNA-treated A549 cells. E, Western blot analysis of the effects of Hook1 overexpression on EMT markers (E-Cad and VIM) and ERK, Smad2, Smad3 activation in A549 cells with or without TGFβ1 treatment (5 ng/ml) for 24 h. F, QPCR analysis of EMT markers (CDH1, CDH2, VIM, MMP9, and COL1A1) under the same conditions as E. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.01 between vector and Hook1. G, transwell assays to assess the migration of control and Hook1-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 16 h. Scale bar, 100 μm. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.01 between vector and Hook1. H, QPCR analysis of EMT-related markers (CDH1, FN1, ZEB1, and COL1A1) in control siRNA and Hook1siRNA-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.05 between CtrlsiRNA and Hook1siRNA. I, transwell assays to assess the migration of control and Hook1siRNA-treated A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 16 h. Scale bar, 100 μm. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.05 between CtrlsiRNA and Hook1siRNA.
Implications of Hook1 and SHP2 Interaction in EMT
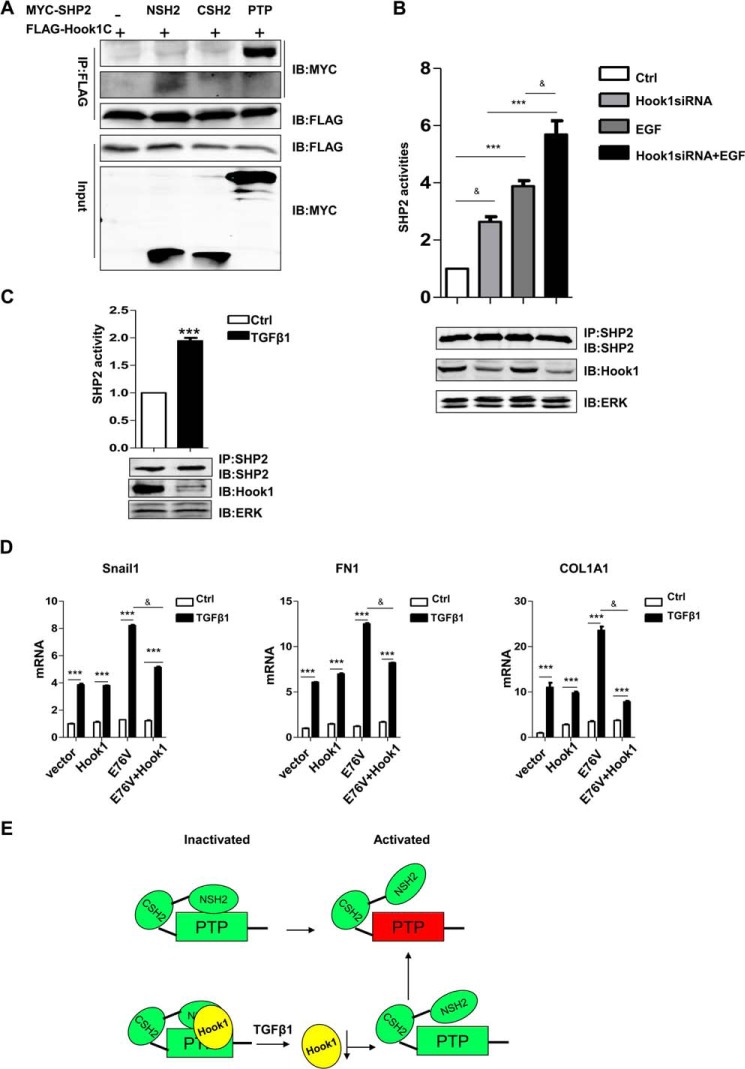
The results showed that both SHP2 and Hook1 were involved in EMT but acted reversely. Therefore, we investigated the interaction between SHP2 and Hook1 in more detail. We found that the C terminus of Hook1 (Hook1-C) bound not only to the PTP domain of SHP2, similar to the yeast two-hybrid screening results, but also to the N terminus SH2 domain (N-SH2) (supplemental Table S3 and Fig. 1, E and F). Next, the interactions were verified by in vitro co-immuno-precipitation experiment (Fig. 5A). Our data demonstrated that both the N-SH2 and PTP domains of SHP2 interacted directly with the Hook1 C-terminal. We further tested SHP2 PTP activity in the SHP2 immuno-complex, and found that when HEK293T cells were transfected with Hook1 siRNA, SHP2 activity was up-regulated with or without EGF stimulation for 15 min (Fig. 5B). And further, SHP2 activity was also up-regulated after TGFβ1 stimulation for 24 h in A549 (Fig. 5C). Moreover, co-expression of Hook1 and SHP2E76V inhibited the promotional effect of SHP2E76V on EMT (Fig. 5D). So we supposed that the direct interactions of Hook1 with SHP2 NSH2 and PTP domains inhibited the activation of SHP2. With TGFβ1 stimulation, the expression of Hook1 was down-regulated, and SHP2 was easy to be activated (Fig. 5E).
FIGURE 5.
Interaction of SHP2-NSH2 and PTP domains with Hook1. A, co-immunoprecipitation assay to verify the direct interaction of FLAG-Hook1C with either MYC-SHP2-NSH2 or MYC-SHP2-PTP in HEK293T cells. B, PTP activity assay to test SHP2 activity in immunocomplex from control siRNA or Hook1siRNA-transfected HEK293T cells, with or without EGF (50 ng/ml) stimulation for 15 min. ***, p < 0.005 between Ctrl and EGF; &, p < 0.05 between CtrlsiRNA and Hook1siRNA. C, PTP activity assay to test SHP2 activity in immunocomplex from A549 cells with or without TGFβ1 (5 ng/ml) stimulation for 24 h (***, p < 0.005). D, QPCR analysis of mRNA levels of EMT markers (Snail1, COL1A1, FN1) in vector, Hook1, SHP2E76V-transfected, Hook1 and E76V co-transfected A549 cells, with or without TGFβ1 treatment (5 ng/ml) for 24 h. ***, p < 0.005 between with and without TGFβ1 treatment; &, p < 0.05 between SHP2E76V only and SHP2E76V plus Hook1. E, schematic model of the interaction between SHP2 and Hook1 in EMT. Hook1 locks the auto-inhibition caused by the intramolecular interaction between N-SH2 and PTP. Upon TGFβ1 treatment, Hook1 is reduced, and this weakens the interaction between N-SH2 and PTP, leading to the full activation of SHP2 by other molecules, including substrates.
DISCUSSION
A widely expressed and important phosphatase, SHP2 plays essential roles in various cell functions, including cell proliferation, metabolism, migration, and transformation (4, 34, 36–39). EMT is a form of transformation, which is important for cancer cells to obtain the capacities of migration and invasion. Our data showed that SHP2 positively regulated TGFβ1-induced EMT in A549 cells (Fig. 1, F–J). SHP2 was required for TGFβ1-induced Smad and ERK pathway. This indicated that SHP2 may also participate in other cell function and signal pathways that TGFβ1 regulated, including extracellular matrix synthesis and deposition. Furthermore, the activated mutant SHP2E76V promoted the expression of mesenchymal genes (Fig. 2D). Dominant negative mutation of SHP2 or the SHP2 inhibitor PHPS1 partially inhibited the EMT (Fig. 2, B, E, F). It has been reported that inhibition of SHP2 promotes mesenchymal-to-epithelial transformation in breast cancer cells (40), which was consistent with our current finding that SHP2 promoted EMT in lung epithelial cells. Somatic activated SHP2 mutations have also been detected in neuroblastoma, melanoma, acute myeloid leukemia, breast cancer, lung cancer, and colorectal cancer. Our findings strongly suggest that SHP2 is a key regulator in tumor metastasis by promoting EMT.
We have identified a novel interacting protein Hook1, the function of which is opposite to that of SHP2 in the process of EMT. Hook1 is a microtubule-binding protein that participates in spermatogenesis, cell differentiation, and endocytosis (31, 41–45). Hook1 has been mentioned in previous EMT studies (46, 47). The claudin-low and metaplastic subtypes of breast cancer display a significant expression pattern of EMT transcriptional factors, which correlates negatively with a pathological complete response (48). Gene expression extracted from microarray data showed that either overexpression of one of the EMT transcription factors TGFβ, Twist, Gsc, or Snaill or knockdown of E-cadherin significantly down-regulates the expression of Hook1 (48). Hook1 is down-regulated in colon cancer cells by inducible expression of hSnaill (46, 47, 49). Bioinformatics analysis showed that there is an E-BOX motif (CAGGTG) in the promoter region of Hook1, which might be recognized by the important EMT-related transcriptors Snaill, Slug, and ZEB1 (46). This supports the idea that Hook1 is a target gene in EMT. In the A549 cells, with TGFβ1 stimulation, the expression of EMT-related transcriptors was increased, which suppressed the expression of Hook1. Our results provided strong evidence that microtubule-associated Hook1 was negatively associated with EMT. Recently, it was reported that EMT-associated cell skeleton changes were microtubule-dependent, indicating that Hook1, as a microtubule-binding protein, participated the regulation of cytoskeletal reorganization in EMT (50). Confirmation that Hook1 is negatively involved in the process of EMT suggests that it might be a potential target for interrupting EMT in cancer cell, which is important for metastasis.
Hook1 was identified to be a SHP2-interacting protein when the SHP2 PTP domain was used as bait. Further studies showed that both the N-SH2 and PTP domains of SHP2 interacted with the Hook1 C terminus. Interestingly, in the inactive state, SHP2 is auto-inhibited by N-SH2 binding to the PTP domain. SHP2 activation requires detachment of the NSH2 domain from the PTP domain. SHP2 activity was increased by TGF treatment through down-regulation of Hook1. Co-expression of Hook1 and SHP2E76V could partially offset the promotional role of SHP2E76V in TGFβ1-induced EMT. EGF also activated SHP2 without affected the protein levels of Hook1. We proposed that SHP2 enzymatic activity is temporally and spatially regulated through scaffolding proteins, including Hook1. It is likely that SHP2 is activated rapidly by EGF stimulation, generating a faster response without affecting the Hook1 protein level. Alternatively, TGFβ1-induced EMT may require the persistent activation of SHP2 where Hook1 transcriptional regulation is initiated. The expression of Hook1 is reduced by transcription factors such as Snaill and ZEB1, and further loosens the interaction between SHP2-NSH2 and PTP, so SHP2 becomes easier to be activated and promotes EMT (35, 51–53). These results suggested that Hook1 was a negative regulator of SHP2 activation (Fig. 5E). In line with this finding, Hook1, in contrast to SHP2, negatively regulated the TGFβ1-induced EMT.
Supplementary Material
Acknowledgment
We thank Dr. I. C. Bruce for reading the manuscript.
This work was supported by the Major Research Program of the State Ministry of Science and Technology of China (2010CB912004, to Y. K.) and the National Natural Science Foundation of China (81101662, to Q. Z.; 81090421, to Y. K.).

This article contains supplemental Tables S1–S3 and Fig. 1.
- EMT
- epithelial-mesenchymal transition
- SH2
- Src homology 2
- SHP
- SH2-domain containing protein-tyrosine phosphatase
- DiFUMP
- 6,8 difluoro-4-methylumbelliferyl phosphate
- PHPS
- phenylhydrazonopyrazolone sulfonate.
REFERENCES
- 1. Mehlen P., Puisieux A. (2006) Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458 [DOI] [PubMed] [Google Scholar]
- 2. Tsai J. H., Yang J. (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 27, 2192–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qu C. K., Feng G. S. (1998) Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 17, 433–439 [DOI] [PubMed] [Google Scholar]
- 5. Chan R. J., Johnson S. A., Li Y., Yoder M. C., Feng G. S. (2003) A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood 102, 2074–2080 [DOI] [PubMed] [Google Scholar]
- 6. Wright J. H., Drueckes P., Bartoe J., Zhao Z., Shen S. H., Krebs E. G. (1997) A role for the SHP-2 tyrosine phosphatase in nerve growth-induced PC12 cell differentiation. Mol. Biol. Cell 8, 1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu C. K. (2002) Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim. Biophys. Acta 1592, 297–301 [DOI] [PubMed] [Google Scholar]
- 8. Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. (2002) Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 277, 9498–9504 [DOI] [PubMed] [Google Scholar]
- 9. Janda E., Lehmann K., Killisch I., Jechlinger M., Herzig M., Downward J., Beug H., Grünert S. (2002) Ras and TGF[β] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oft M., Peli J., Rudaz C., Schwarz H., Beug H., Reichmann E. (1996) TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477 [DOI] [PubMed] [Google Scholar]
- 11. Grossmann K. S., Rosário M., Birchmeier C., Birchmeier W. (2010) The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 106, 53–89 [DOI] [PubMed] [Google Scholar]
- 12. Ke Y., Zhang E. E., Hagihara K., Wu D., Pang Y., Klein R., Curran T., Ranscht B., Feng G. S. (2007) Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol. Cell Biol. 27, 6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li F. F., Shen J., Shen H. J., Zhang X., Cao R., Zhang Y., Qui Q., Lin X. X., Xie Y. C., Zhang L. H., Jia Y. L., Dong X. W., Jiang J. X., Bao M. J., Zhang S., Ma W. J., Wu X. M., Shen H., Xie Q. M., Ke Y. (2012) Shp2 plays an important role in acute cigarette smoke-mediated lung inflammation. J. Immunol. 189, 3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ke Y., Lesperance J., Zhang E. E., Bard-Chapeau E. A., Oshima R. G., Muller W. J., Feng G. S. (2006) Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J. Biol. Chem. 281, 34374–34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin X. J., Zhang G. S., Zhang X., Qiu Z. W., Wang P. L., Li Y. W., Li W., Xie Q. M., Ke Y. H., Lee J. J., Shen H. H. (2012) Protein tyrosine phosphatase SHP2 regulates TGF-beta1 production in airway epithelia and asthmatic airway remodeling in mice. Allergy 67, 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hof P., Pluskey S., Dhe-Paganon S., Eck M. J., Shoelson S. E. (1998) Crystal structure of the tyrosine phosphatase SHP-2. Cell 92, 441–450 [DOI] [PubMed] [Google Scholar]
- 17. Yang J., Liu L., He D., Song X., Liang X., Zhao Z. J., Zhou G. W. (2003) Crystal structure of human protein-tyrosine phosphatase SHP-1. J. Biol. Chem. 278, 6516–6520 [DOI] [PubMed] [Google Scholar]
- 18. Tartaglia M., Mehler E. L., Goldberg R., Zampino G., Brunner H. G., Kremer H., van der Burgt I., Crosby A. H., Ion A., Jeffery S., Kalidas K., Patton M. A., Kucherlapati R. S., Gelb B. D. (2001) Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29, 465–468 [DOI] [PubMed] [Google Scholar]
- 19. Legius E., Schrander-Stumpel C., Schollen E., Pulles-Heintzberger C., Gewillig M., Fryns J. P. (2002) PTPN11 mutations in LEOPARD syndrome. J. Med. Genet. 39, 571–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sobreira N. L., Cirulli E. T., Avramopoulos D., Wohler E., Oswald G. L., Stevens E. L., Ge D., Shianna K. V., Smith J. P., Maia J. M., Gumbs C. E., Pevsner J., Thomas G., Valle D., Hoover-Fong J. E., Goldstein D. B. (2010) Whole-genome sequencing of a single proband together with linkage analysis identifies a Mendelian disease gene. PLoS Genet. 6, e1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowen M. E., Boyden E. D., Holm I. A., Campos-Xavier B., Bonafe L., Superti-Furga A., Ikegawa S., Cormier-Daire V., Bovee J. V., Pansuriya T. C., de Sousa S. B., Savarirayan R., Andreucci E., Vikkula M., Garavelli L., Pottinger C., Ogino T., Sakai A., Regazzoni B. M., Wuyts W., Sangiorgi L., Pedrini E., Zhu M., Kozakewich H. P., Kasser J. R., Seidman J. G., Kurek K. C., Warman M. L. (2011) Loss-of-function mutations in PTPN11 cause metachondromatosis, but not Ollier disease or Maffucci syndrome. PLoS Genet. 7, e1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bentires-Alj M., Paez J. G., David F. S., Keilhack H., Halmos B., Naoki K., Maris J. M., Richardson A., Bardelli A., Sugarbaker D. J., Richards W. G., Du J, Girard L., Minna J. D., Loh M. L., Fisher D. E., Velculescu V. E., Vogelstein B., Meyerson M., Sellers W. R., Neel B. G. (2004) Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 64, 8816–8820 [DOI] [PubMed] [Google Scholar]
- 23. Chan G., Kalaitzidis D., Neel B. G. (2008) The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 27, 179–192 [DOI] [PubMed] [Google Scholar]
- 24. Bard-Chapeau E. A., Li S., Ding J., Zhang S. S., Zhu H. H., Princen F., Fang D. D., Han T., Bailly-Maitre B., Poli V., Varki N. M., Wang H., Feng G. S. (2011) Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 19, 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li S., Hsu D. D., Wang H., Feng G. S. (2012) Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Front. Med. 6, 275–279 [DOI] [PubMed] [Google Scholar]
- 26. Feng G. S. (2012) Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell 21, 150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 28. Tang Y., Shu G., Yuan X., Jing N., Song J. (2011) FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. 21, 316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng H., Kimura K., Peter A. K., Cui L., Ouyang K., Shen T., Liu Y., Gu Y., Dalton N. D., Evans S. M., Knowlton K. U., Peterson K. L., Chen J. (2010) Loss of enigma homolog protein results in dilated cardiomyopathy. Circ. Res. 107, 348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chou Y. T., Wang H., Chen Y., Danielpour D., Yang Y. C. (2006) Cited2 modulates TGF-β-mediated upregulation of MMP9. Oncogene. 25, 5547–5560 [DOI] [PubMed] [Google Scholar]
- 31. Krämer H., Phistry M. (1996) Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J. Cell Biol. 133, 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walenta J. H., Didier A. J., Liu X., Krämer H. (2001) The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J. Cell Biol. 152, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baron Gaillard C. L., Pallesi-Pocachard E., Massey-Harroche D., Richard F., Arsanto J. P., Chauvin J. P., Lecine P., Krämer H., Borg J. P., Le Bivic A. (2011) Hook2 is involved in the morphogenesis of the primary cilium. Mol. Biol. Cell 22, 4549–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noguchi T., Matozaki T., Horita K., Fujioka Y., Kasuga M. (1994) Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell Biol. 14, 6674–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinelli S., Torreri P., Tinti M., Stella L., Bocchinfuso G., Flex E., Grottesi A., Ceccarini M., Palleschi A., Cesareni G., Castagnoli L., Petrucci T. C., Gelb B. D., Tartaglia M. (2008) Diverse driving forces underlie the invariant occurrence of the T42A, E139D, I282V and T468M SHP2 amino acid substitutions causing Noonan and LEOPARD syndromes. Hum. Mol. Genet. 17, 2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu D. H., Qu C. K., Henegariu O., Lu X., Feng G. S. (1998) Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273, 21125–21131 [DOI] [PubMed] [Google Scholar]
- 37. Inagaki K., Noguchi T., Matozaki T., Horikawa T., Fukunaga K., Tsuda M., Ichihashi M., Kasuga M. (2000) Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene. 19, 75–84 [DOI] [PubMed] [Google Scholar]
- 38. Qu C. K. (2000) The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 10, 279–288 [DOI] [PubMed] [Google Scholar]
- 39. Qu C. K., Nguyen S., Chen J., Feng G. S. (2001) Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood 97, 911–914 [DOI] [PubMed] [Google Scholar]
- 40. Zhou X. D., Agazie Y. M. (2008) Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ. 15, 988–996 [DOI] [PubMed] [Google Scholar]
- 41. Meistrich M. L., Kasai K., Olds-Clarke P., MacGregor G. R., Berkowitz A. D., Tung K. S. (1994) Deficiency in fertilization by morphologically abnormal sperm produced by azh mutant mice. Mol. Reprod. Dev. 37, 69–77 [DOI] [PubMed] [Google Scholar]
- 42. Krämer H., Phistry M. (1999) Genetic analysis of hook, a gene required for endocytic trafficking in drosophila. Genetics. 151, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mendoza-Lujambio I., Burfeind P., Dixkens C., Meinhardt A., Hoyer-Fender S., Engel W., Neesen J. (2002) The Hook1 gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Hum. Mol. Genet. 11, 1647–1658 [DOI] [PubMed] [Google Scholar]
- 44. Meistrich M. L., Trostle-Weige P. K., Russell L. D. (1990) Abnormal manchette development in spermatids of azh/azh mutant mice. Am. J. Anat. 188, 74–86 [DOI] [PubMed] [Google Scholar]
- 45. Xu L., Sowa M. E., Chen J., Li X., Gygi S. P., Harper J. W. (2008) An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol. Biol. Cell 19, 5059–5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurrey N. K., Jalgaonkar S. P., Joglekar A. V., Ghanate A. D., Chaskar P. D., Doiphode R. Y., Bapat S. A. (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27, 2059–2068 [DOI] [PubMed] [Google Scholar]
- 47. De Craene B., Gilbert B., Stove C., Bruyneel E., van Roy F., Berx G. (2005) The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 65, 6237–6244 [DOI] [PubMed] [Google Scholar]
- 48. Taube J. H., Herschkowitz J. I., Komurov K., Zhou A. Y., Gupta S., Yang J., Hartwell K., Onder T. T., Gupta P. B., Evans K. W., Hollier B. G., Ram P. T., Lander E. S., Rosen J. M., Weinberg R. A., Mani S. A. (2010) Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl. Acad. Sci. U.S.A. 107, 15449–15454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aigner K., Dampier B., Descovich L., Mikula M., Sultan A., Schreiber M., Mikulits W., Brabletz T., Strand D., Obrist P., Sommergruber W., Schweifer N., Wernitznig A., Beug H., Foisner R., Eger A. (2007) The transcription factor ZEB1 (ΔEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 26, 6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oyanagi J., Ogawa T., Sato H., Higashi S., Miyazaki K. (2012) Epithelial-mesenchymal transition stimulates human cancer cells to extend microtubule-based invasive protrusions and suppresses cell growth in collagen gel. PLoS One. 7, e53209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fragale A., Tartaglia M., Wu J., Gelb B. D. (2004) Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum. Mutat. 23, 267–277 [DOI] [PubMed] [Google Scholar]
- 52. Keilhack H., David F. S., McGregor M., Cantley L. C., Neel B. G. (2005) Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J. Biol. Chem. 280, 30984–30993 [DOI] [PubMed] [Google Scholar]
- 53. Tartaglia M., Martinelli S., Stella L., Bocchinfuso G., Flex E., Cordeddu V., Zampino G., Burgt Iv., Palleschi A., Petrucci T. C., Sorcini M., Schoch C., Foa R., Emanuel P. D., Gelb B. D. (2006) Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am. J. Hum. Genet. 78, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.