Background: Legionella governs pathogen-host interactions by translocating ∼300 “effector” proteins through a type IV secretion system.
Results: The hitherto unrecognized effector LppA is a phytase that counteracts intracellular bacterial growth restriction by phytate.
Conclusion: The chelator phytate is a bacteriostatic component of cell-autonomous immunity, which is degraded by a bacterial effector.
Significance: Legionella LppA represents the first translocated phytase and a potential therapeutic target.
Keywords: Bacterial Pathogenesis, Dictyostelium, Host-Pathogen Interaction, Phosphatidylinositol Phosphatase, Phosphoinositide, Legionella, Bacterial Effector Protein, myo-Inositol Hexakisphosphate, Pathogen Vacuole, Phytate
Abstract
The causative agent of Legionnaires' pneumonia, Legionella pneumophila, colonizes diverse environmental niches, including biofilms, plant material, and protozoa. In these habitats, myo-inositol hexakisphosphate (phytate) is prevalent and used as a phosphate storage compound or as a siderophore. L. pneumophila replicates in protozoa and mammalian phagocytes within a unique “Legionella-containing vacuole.” The bacteria govern host cell interactions through the Icm/Dot type IV secretion system (T4SS) and ∼300 different “effector” proteins. Here we characterize a hitherto unrecognized Icm/Dot substrate, LppA, as a phytate phosphatase (phytase). Phytase activity of recombinant LppA required catalytically essential cysteine (Cys231) and arginine (Arg237) residues. The structure of LppA at 1.4 Å resolution revealed a mainly α-helical globular protein stabilized by four antiparallel β-sheets that binds two phosphate moieties. The phosphates localize to a P-loop active site characteristic of dual specificity phosphatases or to a non-catalytic site, respectively. Phytate reversibly abolished growth of L. pneumophila in broth, and growth inhibition was relieved by overproduction of LppA or by metal ion titration. L. pneumophila lacking lppA replicated less efficiently in phytate-loaded Acanthamoeba castellanii or Dictyostelium discoideum, and the intracellular growth defect was complemented by the phytase gene. These findings identify the chelator phytate as an intracellular bacteriostatic component of cell-autonomous host immunity and reveal a T4SS-translocated L. pneumophila phytase that counteracts intracellular bacterial growth restriction by phytate. Thus, bacterial phytases might represent therapeutic targets to combat intracellular pathogens.
Introduction
Legionella spp. are ubiquitous water-borne bacteria that colonize diverse environmental niches, including biofilms, plant material, and protozoa (1–4). In free living amoebae and mammalian phagocytes, Legionella pneumophila replicates within a “Legionella-containing vacuole” (LCV),4 employing the Icm/Dot type IV secretion system (T4SS) and ∼300 different “effector” proteins (5–7). Some L. pneumophila Icm/Dot substrates target and subvert pivotal regulators of eukaryotic signal transduction and vesicle trafficking, such as small GTPases (8–13) or phosphoinositide (PI) lipids (14–16). Several Legionella effectors anchor to the LCV membrane by specifically binding to the phosphorylated phosphatidylinositol (PtdIns) headgroup of PI lipids, namely PtdIns(4)P (17–22) and PtdIns(3)P (23–25), which are implicated in secretory and endosomal vesicle trafficking, respectively. Moreover, L. pneumophila produces two non-homologous Icm/Dot-translocated PI 3-phosphatases, SidF and SidP, which might modulate the LCV PI pattern (26, 27). SidF localizes to the LCV membrane and hydrolyzes in vitro the phagosomal/endosomal PIs PtdIns(3,4)P2 and PtdIns(3,4,5)P3, possibly yielding PtdIns(4)P on LCVs directly or through the activity of the host PI 5-phosphatase OCRL1 (23). SidP hydrolyzes in vitro the endosomal PI lipids PtdIns(3)P and PtdIns(3,5)P2, thereby possibly contributing to the evasion of the endocytic pathway by the LCV. By virtue of their phosphorylated inositol headgroups, PIs bear high resemblance to phytate (myo-inositol hexakisphosphate) and lower inositol phosphates.
Phytate is used as the major phosphorus storage compound by plants and is the most abundant organic phosphorus compound in the environment (28–30). Protozoa also synthesize phytate, and in particular, the social soil amoeba Dictyostelium discoideum produces the compound in millimolar quantities (31–33). Phytate is rather inert, yet phosphorus mineralization is catalyzed in a stepwise manner by specific phosphohydrolases (phytases), which are produced by a wide range of bacteria (29). The plant pathogen Xanthomonas oryzae uses a secreted phytase as a virulence factor for growth on phytate as the sole phosphate source (34). In addition to serving as a source of phosphorus, carbon, and energy, phytate may also play a role in bacterial iron acquisition by chelating metal ions. Accordingly, Pseudomonas aeruginosa employs phytate as an iron siderophore (35, 36). Phytate is also a prominent anti-nutrient able to complex several metal ion micronutrients, thus restricting their bioavailability (37).
Based on their sequence similarities, phytases are classified as four different groups comprising histidine acid phosphatases, purple acid phosphatases, β-propeller phosphatases, and cysteine phytases (29, 38, 39). The conserved amino acid motif CX5R is a hallmark of the “P-loop” (phosphate-binding) catalytic site of the prototypic cysteine phytase PhyA in Selenomonas ruminantium (39) as well as of eukaryotic and prokaryotic PI phosphatases, such as PTEN (phosphatase and tensin homologue deleted on chromosome 10), L. pneumophila SidF and SidP (26, 27, 40), dual specificity Ser/Thr and Tyr protein phosphatases (DSP) (41), and the triple specificity DSP/PI phosphatase MptpB from Mycobacterium tuberculosis (42, 43).
By using the phosphatase consensus sequence HCXXGXXRT as a search motif, we identified an L. pneumophila cysteine phytase. The phytase, termed LppA, hydrolyzes phytate and PIs in vitro and is translocated by the Icm/Dot T4SS into host cells, where it counteracts intracellular bacterial growth restriction by the chelator phytate.
EXPERIMENTAL PROCEDURES
Bacteria, Cells, and Growth Conditions
L. pneumophila strains (Table 1) were grown for 3 days on charcoal yeast extract agar plates buffered with N-(2-acetamido)-2-aminoethane-sulfonic acid. Liquid cultures were inoculated in AYE medium at an A600 of 0.1 and grown at 37 °C to an A600 of 3.0–3.4 (∼21–22 h) (17). Chloramphenicol was added at 5 μg/ml to select for pMMB207-C-derived plasmids. Murine RAW 264.7 macrophages were cultivated in RPMI 1640 medium supplemented with 10% FCS and 2 mm l-glutamine. Acanthamoeba castellanii (ATCC 30234) and D. discoideum amoebae were propagated as described (44).
TABLE 1.
Strains and plasmids
| Strain/plasmid | Relevant propertiesa | Source/Reference |
|---|---|---|
| E. coli | ||
| TOP10 | Invitrogen | |
| BL21 | Novagen | |
| L. pneumophila | ||
| GS3011 | L. pneumophila JR32 icmT3011::KanR (ΔicmT) | Ref. 58 |
| JR32 | Virulent L. pneumophila serogroup 1 strain Philadelphia-1 | Ref. 59 |
| RM01 | L. pneumophila JR32 lpg2819::KanR (ΔlppA) | This work |
| Plasmids | ||
| pAK7 | Expression of N-terminal His tag fusions, pET-28a(+)-based | This work |
| pCR33 | Legionella expression vector, ΔmobA, RBS, M45-(Gly)5, CamR (=pMMB207-C-RBS-M45) | Ref. 17 |
| pCR76 | pMMB207-C-Ptac-RBS-gfp-RBS-MCS | Ref. 25 |
| pET-28a(+) | Expression of N-terminal His tag fusions; PT7; KanR | Novagen |
| pGEX-6P-1 | GST expression vector | GE Healthcare |
| pGEX-4T-1 | GST expression vector | GE Healthcare |
| pGEM-T-easy | Cloning vector | Promega |
| pLAW344 | L. pneumophila suicide vector | Ref. 60 |
| pMMB207-C | Legionella expression vector, ΔmobA, w/o RBS, CamR | Ref. 46 |
| pNT28 | pMMB207-C-RBS-gfp (constitutive gfp) | Ref. 44 |
| pPhyA | Synthetic construct of S. ruminantium phyA gene | This work |
| pRM1 | pMMB207-C-RBS-M45-lppA | This work |
| pRM2 | pGEM-T-easy-lppAdown_KanR_lppAup | This work |
| pRM3 | pLAW344-lppAdown_KanR_lppAup | This work |
| pRM4 | pGEX-4T-1-lppA | This work |
| pRM9 | pGEX-6P-1-lppAΔ1–16 | This work |
| pSE7 | pGEX-6P-1-lppAΔ1–16, C231A | This work |
| pSE8 | pGEX-6P-1-lppAΔ1–16, K235A | This work |
| pSE9 | pGEX-6P-1-lppAΔ1–16, R237A | This work |
| pSE10 | pGEX-6P-1-lppAΔ1–16, G236D | This work |
| pSH97 | pMMB207-C-RBS-cyaA (including polylinker) | Ref. 25 |
| pSH100 | pMMB207-C-cyaA-ralF | Ref. 25 |
| pSH108 | pMMB207-C-cyaA-lppA | This work |
| pSU4 | GFP-SidCP4C in pDXA, G418R | Ref. 18 |
| pSW001 | pMMB207-C-Ptac-dsred ΔlacIq (constitutive dsred) | Ref. 61 |
| pSW013 | pGEX-4T-1-lpnE | Ref. 23 |
| pWS11 | pET-28a(+)-lppA21–314 | This work |
| pWS25 | pGEX-6P-1-phyAΔ1–16 | This work |
| pWS31 | pMMB207-C-RBS-gfp-RBS-lppA | This work |
a Cam, chloramphenicol; G418, Geneticin.
Chromosomal Deletion of lppA, Plasmid Construction, and Site-directed Mutagenesis
All plasmids used are listed in Table 1. DNA manipulations were performed according to standard protocols, and plasmids were isolated using kits from Qiagen or Macherey-Nagel. All PCR fragments were sequenced.
The chromosomal deletion of lppA was performed by double homologous recombination as described (44); 500 bp downstream and upstream fragments were amplified by PCR using the primer pairs oRM1/oRM2 and oRM3/oRM4, respectively (Table 2). Both fragments were inserted by a four-way ligation into a pGEM-T-easy vector with a kanamycin resistance (KanR) cassette in between using BamHI sites and adenosine overhangs, yielding plasmid pRM2. Clones were analyzed by restriction digestion and sequencing. The KanR cassette flanked by upstream and downstream fragments was transferred into the pLAW344 suicide plasmid using NotI, yielding plasmid pRM3. L. pneumophila JR32 was transformed by electroporation with pRM3 and selected for KanR and SucS colonies. Positive clones were tested by PCR using the primer pairs oRM7/oKan3′ and oRM8/oKan3′.
TABLE 2.
Oligonucleotides used in this study
Restriction sites are underlined.
| Oligonucleotide | Sequence (5′–3′) | Comments |
|---|---|---|
| oCR149 | GCTGTTGACAATTAATCATCGG | pMMB207 fo (sequencing) |
| oCR150 | CGTTCTGATTTAATCTGTATCAGGC | pMMB207 re (sequencing) |
| oCR161 | AAAAACGCGGATCCATGAGCTTTAAAGGATTTAAAGTG | 5′ of lppA, BamHI |
| oCR162 | AAAAACGCTCTAGACTATTACTAGATATTGAGCTTTTTCCATTC | 3′ of lppA, XbaI |
| oAK7fo | GGACGCTCATGAAAAAAAATCACCACCATCATCACCACCTAGTTCCGCGTGGATCCGAATGG | BspHI/BamHI |
| oAK7re | CCATTCGGATCCACGCGGAACTAGGTGGTGATGATGGTGGTGATTTTTTTTCATGAGCGTCC | BamHI/BspHI |
| oKan3′ | TCACTTTCTGGCTGGATGATGG | 5′ out of KanR (deletion) |
| oKan5′ | GAATATGGCTCATAACACCCC | 3′ out of KanR (deletion) |
| oRM1 | AAAAACGCGGATCCCATCTAGTGCCATTTGCAAG | 5′ of lppA downstream region (deletion), BamHI |
| oRM2 | TTAGCGATACCTGGAAATAAG | 3′ of lppA downstream region (deletion) |
| oRM3 | GGTCATAACGATGCAAATAGTAG | 5′ of lppA upstream region (deletion) |
| oRM4 | AAAAACGCGGATCCAATAAATCCTTTTTAAATCCCTAAAGATG | 3′ of lppA upstream region (deletion), BamHI |
| oRM5 | AAAAACGCGGATCCATGAGCTTTAAAGGATTTAAAG | 5′ of lppA, BamHI |
| oRM6 | AAAAACGCGTCGACCTAGATATTGAGCTTTTTCCATTC | 3′ of lppA, SalI |
| oRM7 | GGCTGTCTGATTTTCCTCAAG | 3′ of lppA downstream region |
| oRM8 | CTGCCCGAATGGTATCAG | 5′ of lppA upstream region |
| oRM13 | AAAAACGCGGATCCCAAAGTTATGCCTCTAAATTG | 5′ of lppAΔ1–16, BamHI |
| oSE7 | GGTATCATGTCCACGCCCGTGGCGGGAAAGGC | 5′ of lppA C231A |
| oSE8 | GCCTTTCCCGCCACGGGCGTGGACATGATACC | 3′ of lppA C231A |
| oSE9 | GCCGTGGCGGGGCAGGCCGTACGACG | 5′ of lppA K235A |
| oSE10 | CGTCGTACGGCCTGCCCCGCCACGGC | 3′ of lppA K235A |
| oSE11 | CGTGGCGGGAAAGGCGCTACGACGACTGTTTTTGC | 5′ of lppA R237A |
| oSE12 | GCAAAAACAGTCGTCGTAGCGCCTTTCCCGCCACG | 3′ of lppA R237A |
| oSE13 | CCGTGGCGGGAAAGACCGTACGACGACTG | 5′ of lppA G236D |
| oSE14 | CAGTCGTCGTACGGTCTTTCCCGCCACGG | 3′ of lppA G236D |
| oWS25 | AGTTATGGATCCAAATTGGCATCCTCC | 5′ of lppA21–314, BamHI (crystallization) |
| oWS26 | CTAGATGTCGACTTATTACCATTCTGACCA | 3′ of lppA21–314, SalI (crystallization) |
| oWS73 | AAAAAAGGATCCGCCAAGGCGCCGGAGCAGACGGTGACGG | 5′ of phyA, BamHI |
| oWS91 | AAAAAAGTCGACCTACTTCGCCGGATGGCTCTTGAGCCAG | 3′ of phyA, SalI |
Translational CyaA and M45 fusion proteins of lppA were constructed by PCR amplification of the corresponding DNA. The fragments were cut with appropriate restriction enzymes and inserted into pSH97, pMMB207-C-RBS-M45, or pMMB207-C-RBS-gfp-RBS, using the oligonucleotides oCR161/oCR162 or oRM5/oRM6, yielding plasmids pSH108, pRM1, and pWS31, respectively. Translational GST fusion proteins of lppA were constructed as above with the exception that the first 16 amino acids of the LppA protein were deleted by PCR amplification of the lppA template. The lppA fragment was cloned into pGEX-6P-1 using the oligonucleotides oRM13 and oRM6, yielding plasmid pRM9.
The S. ruminantium phytase gene phyA (39) was synthesized by GenScript USA Inc. and delivered in commercial vector pUC57-Kan with flanking BamHI and SalI restriction sites. In the design, the intrinsic SalI site of the gene was removed by a silent single base substitution, maintaining the original amino acid sequence. The phyA gene was cloned with BamHI and SalI into pGEX-6P-1, yielding pWS25.
Site-directed mutagenesis of the P-loop residue C231A, K235A, G236D, or R237A of lppAΔ1–16 was performed with the QuikChange Lightning multisite-directed mutagenesis kit (Agilent Technologies) according to the manufacturer's instructions. PCR amplification was carried out using primers carrying the corresponding point mutations to yield the plasmids pSE7 to pSE10. The methylated template pRM9 was digested with DpnI.
Vector pAK7 was constructed by PCR amplification of the complementary primers pAK7fo/pAK7re, followed by restriction digestion with BamHI/BspHI, and insertion into pET-28a(+) cut with BamHI/NcoI (compatible with BspHI). The plasmid pWS11 was cloned by BamHI/SalI insertion of the PCR product of oWS25 and oWS26 into pAK7.
Phosphate Release and Protein-Lipid Overlay Assay
GST fusion proteins were purified as described previously (25). Phytate (phytic acid sodium salt hydrate) was obtained from Sigma-Aldrich, and synthetic dioctyl PI and inositol phosphates were purchased from Echelon Biosciences Inc. (Salt Lake City, UT). Phosphate release from phytate or PI substrates was assayed by a malachite green assay (protein-tyrosine phosphatase (PTP) assay kit, Sigma-Aldrich) in a total volume of 50 μl/well in a 384-well plate (Greiner) at 25 °C. Substrates were prepared in 25 μl of assay buffer (100 mm Tris-HCl, 120 mm NaCl, pH 7.4). To initiate the reaction, 25 μl of enzyme solution in assay buffer (0.05–5 μg of protein) was added and mixed three times rapidly without blowing out. 25 μl of the malachite green acidic dye and vanadate ion complex (mixed 100:1 at least 30 min before use) were added to terminate reactions. The color was developed for 20 min, and absorbance was measured at 620 nm with a FLUOstar plate reader (BMG Labtech). All values for a series were standardized against the zero reading (enzyme solution and malachite green mixed, followed by the addition of substrate solution), and 2–4 samples were used for each time point. A standard reference curve for phosphate release was generated using the 1 mm phosphate standard supplied with the kit.
The products of LppA from various PI lipids were assessed with PIP-strips (Echelon Biosciences Inc.) treated with 0.5 μg/ml LppA for 10 min. Membranes were washed three times for 10 min with PBS. Subsequent binding of GST-SidCP4C or GST-LpnE probes and anti-GST Western blots were carried out as described previously (17, 18). Peroxidase-labeled secondary antibodies were visualized by ECL (Amersham Biosciences).
Detection of LppA by Western Blot
30-ml AYE cultures of L. pneumophila wild type, ΔlppA, or wild type harboring vector pRM1 were grown overnight at 37 °C to early stationary phase. Cultures were standardized to an A600 of 3.0 with AYE and pelleted at 12,000 × g for 15 min. Supernatants were decanted to new tubes and centrifuged again; this was repeated once. 5 ml of cell-free supernatants of each strain were loaded with a dot blot apparatus onto a nitrocellulose membrane under vacuum suction. The bacterial pellets were suspended in 30 ml of Tris-buffered saline (TBS), and a 1.5-ml aliquot of each was boiled for 10 min at 95 °C. Cell debris was pelleted, and 500 μl of lysate for each strain was loaded onto a nitrocellulose membrane under vacuum suction. The membrane was blocked for 1 h in TBS containing 4% milk powder and stained for 1 h with an affinity-purified polyclonal anti-LppA antibody (1:500; GenScript), followed by an anti-rabbit HRP-tagged antibody (1:5,000) for 30 min.
Protein Purification and Crystallization
To produce LppA21–314 for crystallization, plasmid pWS11 was transformed into chemically competent E. coli NiCo[DE3] (New England Biolabs) and plated on LB-Kan agar plates containing 50 μg/ml Kan. Several colonies were picked, transferred into 100 ml of LB medium containing 50 μg/ml Kan, and incubated overnight at 37 °C in an orbital shaker. 4 liters of ZYM5052-Kan autoinduction medium (for protein production by autoinduction in high density shaking cultures (45)) containing 50 μg/ml Kan were inoculated with 25 ml (1:40) of the overnight culture and grown at 37 °C for 7 h in a large orbital shaker. The temperature was subsequently lowered to 20 °C, and incubation was continued overnight. The cells were harvested by centrifugation at 5,000 rpm. Cell pellets were suspended in 100 ml of lysis buffer (50 mm Tris, pH 7.5, 500 mm NaCl, 10 mm imidazole, 10 mm β-mercapthoethanol (β-ME), 1 protease inhibitor mixture tablet (Complete EDTA-free, Roche Applied Science)) and lysed on ice using three 1-min 100% amplitude sonication pulses (Vibra-Cell sonication device (Sonics) equipped with a large preparative sonication tip). Soluble proteins were separated from cell debris by centrifugation (25,000 × g, 30 min), followed by filtration of the supernatant (0.45 μm).
Recombinant LppA was purified by immobilized metal affinity chromatography, followed by size exclusion chromatography, using an ÄKTA Xpress chromatography machine. The supernatant was loaded onto a 5-ml HisTrap crude FF column. The column was washed with 20 column volumes of wash buffer 1 (50 mm Tris, pH 7.5, 500 mm NaCl, 10 mm imidazole, 10 mm β-ME) and 20 column volumes of wash buffer 2 (50 mm Tris, pH 7.5, 500 mm NaCl, 60 mm imidazole, 10 mm β-ME). Recombinant LppA was eluted with 5 column volumes of elution buffer (50 mm Tris, pH 7.5, 500 mm NaCl, 500 mm imidazole, 10 mm β-ME). The eluted protein was injected to a 26/60 HiLoad Superdex200 size exclusion column (buffer: 50 mm Tris, pH 7.5, 500 mm NaCl, 10 mm β-ME). The total yield was 155 mg of pure LppA. The protein was concentrated to 5 mg/ml using a 10,000 molecular weight cut-off ultrafiltration concentrator (Millipore) and used for crystallization trials.
Crystallization experiments were carried out at the crystallization facility of the Swiss Light Source. Crystals were obtained with the sitting drop vapor diffusion method at 293 K, using a well solution containing 40 mm KH2PO4, pH 4.1, 16% PEG 8000, and 20% glycerol. Drops consisted of 1 μl of protein solution and 1 μl of well solution.
Data Collection and Structure Determination
Diffraction data from a single LppA crystal were collected at 100 K on a PILATUS 6M pixel detector at the Swiss Light Source beamline X10SA using an x-ray wavelength of 1.0000 Å. The data were processed using XDS (62) to a resolution of 1.4 Å. The structure was solved by molecular replacement and autorebuilt using PHENIX (63); four molecules per asymmetric unit were found using the monomeric structure of protein tyrosine phosphatase-like phytase from Mitsuokella multacida (Protein Data Bank code 3F41) as a search model. Refinement and manual model rebuilding were carried out with PHENIX and Coot (64), respectively. The model was validated using MolProbity (65); analysis with EPPIC (66) did not find any biologically relevant interfaces in the crystal lattice. Data collection and refinement statistics are shown in Table 3.
TABLE 3.
Data collection and refinement statistics for LppA
| Space group | P 1 21 1 |
| Cell dimensions a, b, c (Å), β (degrees) | 98.65 55.49 131.42 99.61 |
| Resolution (Å) | 50–1.4 (1.5–1.4)a |
| Rmeas (%) | 5.4 (82.8) |
| I/σI | 15.9 (2.3) |
| CC(1/2) (%) | 99.9 (81.7) |
| Completeness (%) | 96.0 (90.5) |
| Redundancy | 4.5 (4.6) |
| Refinement | |
| Resolution (Å) | 50.0–1.4 |
| No. of reflections (test reflections) | 264665 (1060) |
| Rwork, Rfree (%) | 14.4, 16.9 |
| No. of atoms | 12052 |
| Protein | 10529 |
| Ligand/ion | 59 |
| Water | 1464 |
| B-factors (A2) | |
| Protein | 23.8 |
| Ligand/ion | 19.5 |
| Water | 35.6 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.007 |
| Bond angles (degrees) | 1.13 |
| Ramachandran plot (Molprobity) | |
| Allowed (%) | 1.9 |
| Favored (%) | 98.1 |
a Outermost resolution shell.
Translocation Assay
To determine translocation of LppA, adenylate cyclase fusion proteins were generated and used to quantify the production of cAMP in the host cell as described (46). Briefly, RAW 264.7 macrophages were seeded at 5 × 105/ml into 96-well plates in a final volume of 100 μl/well and incubated at 37 °C overnight. The macrophages were infected (MOI 50) with L. pneumophila wild type or ΔicmT harboring plasmid pSH108 grown for 21 h in AYE supplemented with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside. After 30 min of infection, cells were washed with PBS and lysed with 200 μl of sterile water for 10 min. Lysis was enhanced by shaking the plate on a microplate shaker. Intracellular cAMP was measured by using the cAMP Biotrak enzyme immunoassay system (Amersham Biosciences).
Extracellular Growth Assays
L. pneumophila wild type was inoculated with an A600 of 0.1 in 200 μl of AYE in 96-well plate format. Three wells were used per sample per test condition. After 12 h of growth at 37 °C and 600 rpm on a temperature-controlled plate shaker (Eppendorf), 10 mm phytate was added for 6 h to one growth set. After an additional 6 h, the bacteria were pelleted for 10 min at 1000 × g and carefully suspended in fresh 37 °C AYE medium. Growth on the plate shaker proceeded for another 12 h. A600 measurements were taken with a FLUOstar plate reader and subtracted from input values.
Alternatively, L. pneumophila wild type, ΔlppA mutant, or wild type harboring pRM1 (M45-LppA) was inoculated at an A600 of 0.1 in 200 μl of AYE in 96-well plate format. Three wells were used per sample per test condition (0–5 mm phytate). Unused wells were filled with 200 μl of sterile water. Cultures were incubated on a temperature-controlled plate shaker at 24 °C and 600 rpm. Growth was measured after 5 days (A600). Wild type L. pneumophila was also grown in 3-ml AYE cultures at 37 °C. Micronutrient supplementation was carried out with FeN3O9 × 9H2O (Sigma-Aldrich), ZnCl2 (Fluka), CaCl2 (Fluka), or MgCl2 × 6H2O (Fluka). Phytate (final concentration 10 mm) was added to cultures at a ratio of 1:1 with each micronutrient. Cultures were inoculated in triplicate at an A600 of 0.1 and allowed to grow for 21 h.
Intracellular Growth Assays and Fluorescence Microscopy
Intracellular growth assays under phytate load were performed with A. castellanii or D. discoideum amoebae cultured with increasing concentrations of phytate. The compound was added at 2.5 mm, and the concentration was increased every 2 days until the cells were maintained in 10 or 5 mm phytate for A. castellanii or D. discoideum, respectively. L. pneumophila wild type, ΔicmT, ΔlppA harboring pNT28 (GFP), or ΔlppA harboring pWS31 (GFP, LppA) was inoculated in AYE with chloramphenicol (5 μg/ml) and isopropyl 1-thio-β-d-galactopyranoside (1 mm) and grown to early stationary phase. A. castellanii or D. discoideum was seeded at 5 × 104/well in 200 μl of LoFlo (ForMedium) in a 96-well plate and allowed to adhere for 1 h. Each measured time point of the experiment required one 96-well plate with three allocated wells per strain. L. pneumophila cultures were diluted in LoFlo to 2.5 × 104/ml. Medium was removed from adhered cells and replaced with 200 μl of L. pneumophila dilutions. Plates were centrifuged at 1,000 × g for 5 min and incubated at 37 °C for A. castellanii or 23 °C for D. discoideum. As an input control, 20 μl of a 1:100 dilution of each strain used for infection was plated onto charcoal yeast extract agar, and colonies were counted after a 3-day incubation at 37 °C.
After centrifugation, one plate for A. castellanii (t = 0) was imaged by confocal microscopy using a Nikon Eclipse TE300 microscope with a PerkinElmer Life Sciences UltraVIEW spinning disk system and a Hamamatsu Orca Flash 4.0 C-MOS camera. A Nikon 20× Plan Fluor objective was used in combination with filters 488-10BP/525-50BP and bright field. Image evaluation was carried out with Velocity version 6.0.1 software (PerkinElmer Life Sciences). Confocal images of the GFP channel and bright field were captured for each time point.
Quantitative plating assays were performed to complement microscope images because GFP expression could vary between strains. To this end, the infected cells were lysed after microscopic imaging at a given time point, and bacterial dilutions were plated onto charcoal yeast extract agar following the method described previously for colony counting (47). D. discoideum amoebae were lysed and plated without imaging. Colony counts for L. pneumophila growth in cells not cultured under phytate load followed the same procedure in the absence of phytate supplementation.
Competitive growth in A. castellanii between wild type and lppA deletion strains was performed as described (47). Finally, the observation of PtdIns(4)P in D. discoideum by GFP-SidCP4C was carried out as described previously (21).
Statistical Methods
Differences between L. pneumophila strains were evaluated by two-tailed unpaired Student's t test assuming unequal variances. Statistical error of the mean is presented as ± one S.D. with 95% confidence intervals.
RESULTS
The Type IV Translocated Cysteine Phosphatase LppA Hydrolyzes Phytate and PIs in Vitro
A PSI-BLAST search using the phosphatase consensus sequence HCXXGXXRT identified in the genome of L. pneumophila strain Philadelphia-1 (taxid: 272624) a predicted 36.3-kDa protein, Lpg2819, annotated as a putative protein-tyrosine phosphatase II of the DSP superfamily. The only other protein thus identified is annotated as a lysophospholipid acyltransferase. The same search with generic L. pneumophila (taxid: 446) yielded results for “protein tyrosine phosphatase” in all sequenced strains. Finally, a search of all available genomes of the family Legionellaceae (taxid: 118969) yielded a hit for at least nine different Legionella spp. Lpg2819 is conserved with shared synteny among all L. pneumophila strains sequenced thus far (including Philadelphia-1, Paris, Lens, Corby, Alcoy, 130b/AA100, and Lorraine) as well as in Legionella longbeachae (64% identity), L. shakespearei (64% identity), and L. dumoffii (62% identity).
A closer bioinformatic inspection of Lpg2819 revealed an overall similarity to cysteine phytases of Clostridium (39% identity), Stigmatella (36% identity), Pseudomonas (34% identity), and Xanthomonas spp. (32% identity) as well as to PhyA from S. ruminantium (30% identity) (29, 39, 48) (Fig. 1A). The P-loop consensus sequence of Legionella spp. is strictly conserved in the phytases from Clostridium acetobutylicum and Stigmatella aurantiaca. Based on these similarities and the catalytic activities of recombinant Lpg2819 (see below), we termed the protein Lpg2819 “LppA” (Legionella pneumophila phytase A).
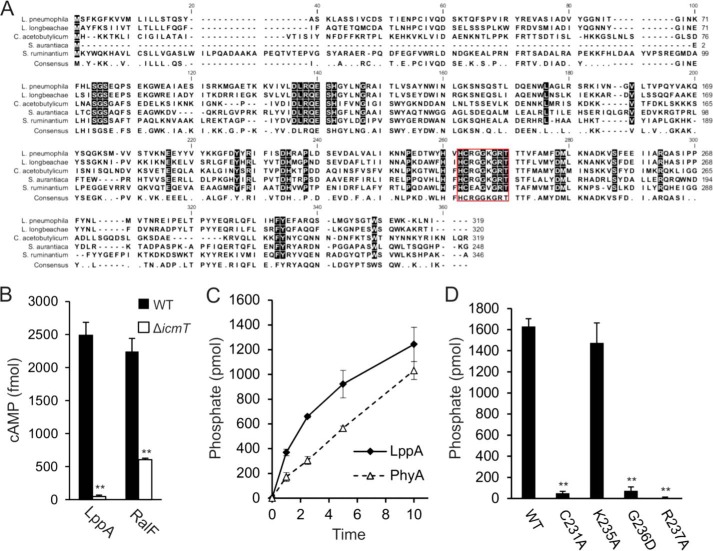
FIGURE 1.
The type IV translocated cysteine phosphatase LppA hydrolyzes phytate in vitro. A, alignment of (predicted) cysteine phytases of L. pneumophila (LppA), L. longbeachae, S. aurantica, C. acetobutylicum, and S. ruminantium (PhyA) in order of decreasing homology to LppA. The red box highlights the established or predicted P-loop catalytic active sites for LppA and PhyA as well as for the other putative phytases. B, LppA is a substrate of the Icm/Dot T4SS. RAW 264.7 macrophages were infected with wild type or ΔicmT L. pneumophila harboring pSH108 (CyaA-LppA) or pSH100 (CyaA-RalF). Levels of cAMP (mean ± S.D. (error bars), four duplicate experiments) were measured 30 min postinfection (MOI 50). C and D, hydrolysis of phytate by GST-LppAΔ1–16, GST-PhyAΔ1–16, or GST-LppAΔ1–16 point mutations of putative catalytically essential residues was measured by phosphate release as a malachite green vanadate dye complex. Reactions proceeded at 25 °C with 2 nmol of phytate and 0.5 μg of protein for up to 10 min (C) or 15 min (D). Absorbance at 620 nm was measured 20 min after termination of each reaction. The data shown are means and S.D. of triplicates (B–D) and are representative of three independent experiments (**, p < 0.005).
LppA is not predicted to be translocated and has not been identified as an Icm/Dot T4SS substrate using bioinformatics or experimental approaches (49–52). To determine whether LppA is translocated by the Icm/Dot T4SS, we constructed an N-terminal fusion with the calmodulin-dependent adenylate cyclase CyaA that allows assessment of the production of cAMP upon translocation of the fusion protein to host cell cytoplasm. To this end, RAW 246.7 macrophages were infected with either wild type L. pneumophila or the translocation-defective ΔicmT mutant strain producing the CyaA-LppA fusion protein (Fig. 1B). Calmodulin-dependent production of cAMP was only observed upon infection with wild type L. pneumophila; therefore, LppA represents a hitherto unrecognized substrate of the Icm/Dot T4SS. Similarly, the positive control CyaA-RalF was translocated into the host cells in an Icm/Dot-dependent manner. These findings imply that LppA has access to the host cell cytoplasm and performs an intracellular function.
To test potential phytase activity of LppA, we performed a phosphate release assay and compared its activity with that of S. ruminantium PhyA. Under the conditions tested, purified GST-LppA hydrolyzed phytate at an initial rate of 5 pmol/s/μg of protein, confirming its activity as an efficient phytase (Fig. 1C). The rate of GST-LppA was approximately twice as fast as that of GST-PhyA tested under these conditions. In order to determine the amino acids essential for enzymatic activity, we constructed point mutations in the putative catalytic motif. Mutations of the catalytic residue Cys231 or Arg237 to Ala resulted in loss of phytase activity (Fig. 1D). Moreover, replacing Gly236 with a more bulky and charged amino acid, Asp, also abolished phytate hydrolysis, whereas changing Lys235 to Ala did not alter the activity. Taken together, L. pneumophila produces a translocated cysteine phosphatase that in vitro shows a 2-fold higher phytase activity than PhyA and harbors the conserved amino acids Cys215 and Arg221 implicated in catalysis.
LppA Hydrolyzes Phosphoinositides in Vitro and Produces PtdIns(4)P
Given that inositol phosphates make up the identity-defining headgroup of PI lipids, we tested whether these derivatives of phytate could be metabolized by LppA in vitro. The P-loop consensus sequence of the L. pneumophila phytase is similar to those of the mammalian and bacterial PI phosphatases (Fig. 2A). In fact, the catalytically active site of LppA (HCRGGKGRT) is almost identical to the human PI 3-phosphatase PTEN (HCR/KAGKGRT) and very similar to the mycobacterial PI phosphatase MptpB (HCFAGKDRT). Therefore, we also tested the putative PI phosphatase activity of the enzyme toward dioctyl-PI lipids. LppA dephosphorylated the diphosphorylated PIs PtdIns(3,4)P2 and PtdIns(4,5)P2 very efficiently, followed by PtdIns(3,4,5)P3 as a substrate and, with ∼20 times lower activity, PtdIns(3,5)P2 (Fig. 2B). A 100-fold higher amount of enzyme was required to observe poor activity toward monophosphorylated PIs (Fig. 2C).
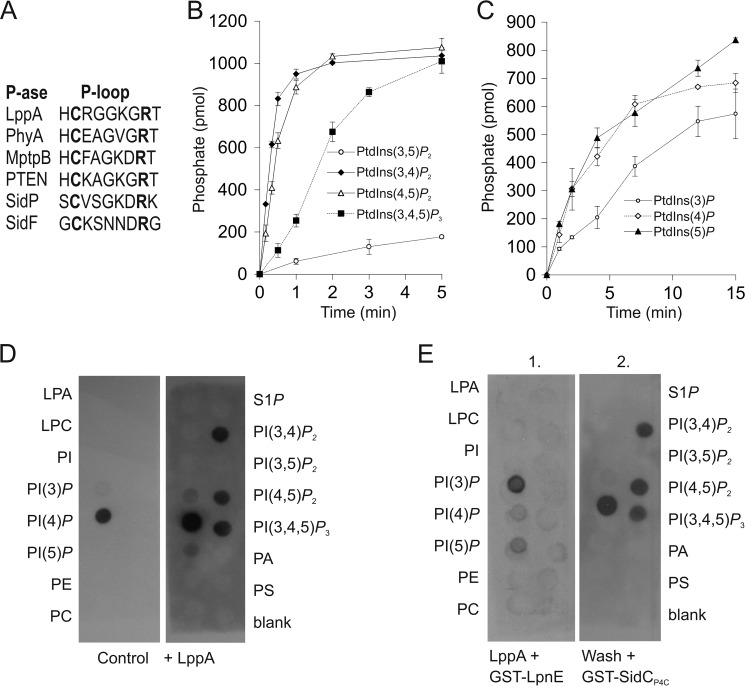
FIGURE 2.
LppA hydrolyzes phosphoinositides and produces PtdIns(4)P in vitro. A, alignment of P-loops of bacterial and eukaryotic cysteine phytases and phosphoinositide phosphatases: L. pneumophila (LppA, SidF, and SidP), S. ruminantium (PhyA), M. tuberculosis (MptpB), and Homo sapiens (PTEN). B and C, hydrolysis of PI lipids by LppAΔ1–16 was measured by phosphate release as a malachite green vanadate dye complex with 1 nmol of dioctyl PI lipids and 0.05 μg (B) or 5 μg (C) of protein, respectively. D and E, LppA hydrolyzes polyphosphorylated PIs and produces PtdIns(4)P but not PtdIns(3)P. Nitrocellulose membranes with PI and other lipids (100 pmol/spot) were treated (right) or not (left) with purified LppAΔ1–16 (0.5 μg/μl, 10 min) prior to binding of the PtdIns(4)P probe GST-SidCP4C (D) or treated with purified LppAΔ1–16 (0.5 μg/μl, 10 min) and overlaid with the PtdIns(3)P probe GST-LpnE (left), followed by washing and overlaying with GST-SidCP4C (right) (E). Binding was visualized using an anti-GST antibody. Left lanes (all membranes), lysophosphatidic acid (LPA), lysophosphocholine (LPC), PtdIns, phosphoinositide (PtdIns(x)P), phosphatidylethanolamine (PE), and phosphatidylcholine (PC). Right lanes (all membranes), sphingosine 1-phosphate (S1P), phosphoinositide, phosphatidic acid (PA), and phosphatidylserine (PS). The data shown are representative of three independent experiments. Error bars, S.D.
To analyze the PI product(s) generated by LppA in vitro, we treated nitrocellulose membranes spotted with all seven PIs and other lipids with the enzyme and subsequently performed a protein-lipid overlay using the PtdIns(4)P-specific probe GST-SidCP4C (Fig. 2D). GST-SidCP4C bound on untreated control membranes exclusively to the PtdIns(4)P spot as described previously (18). In contrast, on membranes pretreated with LppA, GST-SidCP4C also bound to the spots initially occupied by PtdIns(3,4)P2, PtdIns(4,5)P2, or PtdIns(3,4,5)P3, indicating that PtdIns(4)P is the major PI product of the phosphatase. In parallel, the LppA-treated membrane was probed with GST-LpnE to detect PtdIns(3)P, yet the fusion protein bound only to the initial PtdIns(3)P spot, indicating that no PtdIns(3)P was produced by the phosphatase LppA (Fig. 2E). Thus, in vitro LppA also rapidly metabolizes polyphosphorylated PI lipids containing a 4-phosphate residue to yield PtdIns(4)P.
High Resolution Structure of L. pneumophila LppA
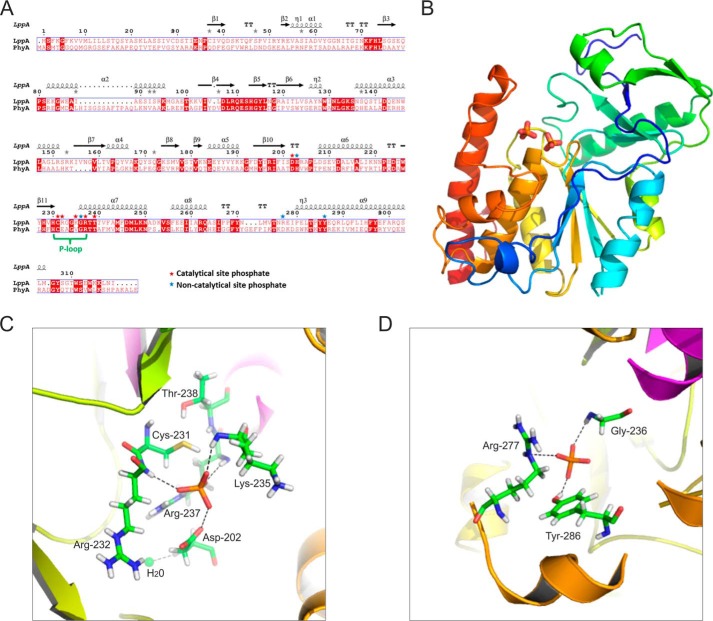
Toward understanding LppA function at the molecular level, we determined the crystal structure of the phytase apo-form by molecular replacement at 1.4 Å resolution. The data collection and refinement statistics are listed in Table 3, and a structure-based alignment of LppA with PhyA is shown in Fig. 3A. The current model of LppA consists of amino acid residues 26–314 (chain B in the unit cell tetramer) and reveals an overall structure of a mainly α-helical globular protein stabilized by four antiparallel β-sheets (Fig. 3B). The catalytic site forms a PTP-like fold characteristic of dual specificity Ser/Thr and Tyr protein phosphatases.
FIGURE 3.
Overall structure of L. pneumophila LppA binding two phosphates. A, structure-based alignment of L. pneumophila LppA with S. ruminantium PhyA. B, overall structure of LppA showing two bound phosphates (phosphorus (orange) and oxygen (red)). C, close-up view of the catalytic P-loop site of LppA coordinating a phosphate with the amino acids Asp202, Cys231, Arg232, Lys235, Arg237, and Thr238. D, close-up view of the second phosphate coordinated at a non-catalytic site by Gly236, Arg277, and Tyr286.
One remarkable feature of the LppA crystal structure is the binding of two phosphate moieties in a positively charged pocket formed by the basic amino acids His203, Arg232, Lys235, Arg237, and Arg277. This pocket probably accommodates the binding sites for two of the six phosphate residues of phytate. One phosphate localizes to the active site P-loop and is coordinated by the amino acids Cys231, Arg232, Lys235, and Arg237 (Fig. 3C). The position of Cys231 suggests a catalytic mechanism, where the thiolate anion of Cys231 is the nucleophile that attacks a phytate phosphate to form a cysteinyl-phosphate trigonal-bipyramidal pentavalent intermediate. The negative charge on the Sγ atom of Cys231 might be stabilized by the hydroxyl group of the conserved adjacent Thr238 (Fig. 2A). Also located in the catalytic pocket, Asp202 probably acts as a general acid and donates a proton to form myo-inositol pentakisphosphate. In the second step of the phosphatase reaction, Asp202 might act as a general base accepting a proton from a nearby water molecule (at a distance of 3.4 Å), which upon nucleophilic attack liberates phosphate and regenerates the active cysteinyl-phosphatase.
The second phosphate, which cannot be directly hydrolyzed, is coordinated by Gly236, Arg277, and Tyr286 and is further stabilized by His203 and the neighboring phosphate (Fig. 3D). The arrangement of the two adjacent phosphate moieties bound to LppA is in agreement with a model proposed for the phytase PhyA from S. ruminantium, suggesting that after hydrolytic removal of the first phosphate, myo-inositol pentakisphosphate rotates, and a second phosphate is placed in the vicinity of the catalytically active cysteine to be subsequently hydrolyzed in a stepwise manner (39).
Comparison of L. pneumophila LppA and S. ruminantium PhyA
The phytase LppA is structurally similar to the S. ruminantium phytase PhyA in its apo-form (Protein Data Bank code 1U24). Cα superimposition of PhyA onto LppA results in a root mean square deviation of 1.6 Å over 254 residues and 28% sequence identity (Fig. 4A). Significant differences in structure are seen at the N and C termini that extend by 11 and 9 residues in PhyA, respectively. Furthermore, LppA is lacking a loop present in PhyA (amino acids 88–99). These results are in good agreement with the overall similarity of LppA and PhyA, which share 30% sequence identity.
FIGURE 4.
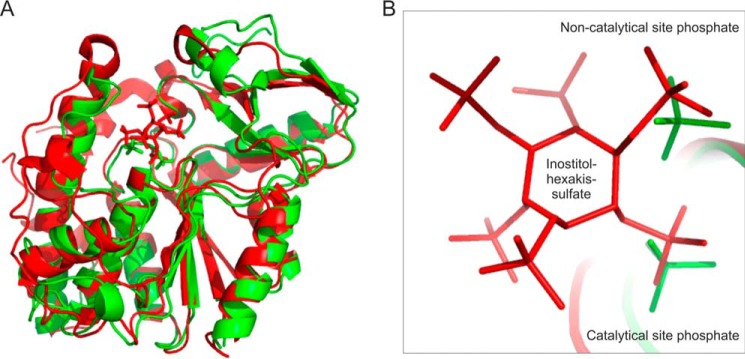
Comparison of overall structure and active sites of L. pneumophila LppA and S. ruminantium PhyA. A, overlay of the overall structures; B, close-up view of the catalytic P-loop sites of L. pneumophila LppA (green) and S. ruminantium PhyA (red) binding two phosphates (green) or the inhibitor myo-inositol hexasulfate (IHS; red), respectively.
The P-loop sites of LppA (HCRGGKGRT) and PhyA (HCEAGVGRT) are 66% identical, and the phosphate groups bound by LppA superimposes with S4 and S5 of the inhibitor myo-inositol hexasulfate (IHS) that was co-crystallized with PhyA from S. ruminantium (PDB code 1U26) (Fig. 4B). It is noteworthy that Arg232 and Arg277, coordinating in LppA the phosphate residues in the catalytic or the non-catalytic site, respectively, are replaced by acidic amino acids in PhyA (Glu242 and Asp289). Moreover, LppA Lys235 is not conserved in PhyA, and accordingly, its replacement by Ala did not affect the catalytic activity of LppA (Fig. 1D). In contrast, mutation of PhyA His213 or Tyr298, which form the substrate-binding pocket and are conserved in LppA (His187 and Tyr270), caused a significant decrease in phytase activity by 52 and 92%, respectively (39). In summary, these findings suggest a similar mechanism of phytate binding and catalysis of LppA and PhyA.
Phytate Reversibly Inhibits L. pneumophila Growth and Is Counteracted by LppA or Micronutrient Supplementation
To investigate the effect of phytate on the growth of L. pneumophila in AYE broth, the compound was added to a bacterial culture in early exponential growth phase (Fig. 5A). Under these conditions, 10 mm phytate caused growth stasis of L. pneumophila. The bacteria were subsequently pelleted, suspended in fresh AYE medium, and allowed to grow again. After phytate was removed, L. pneumophila resumed growth at the initial rate. Therefore, phytate is not toxic to L. pneumophila but reversibly inhibits growth of the bacteria and thus exerts a bacteriostatic rather than a bactericidal effect.
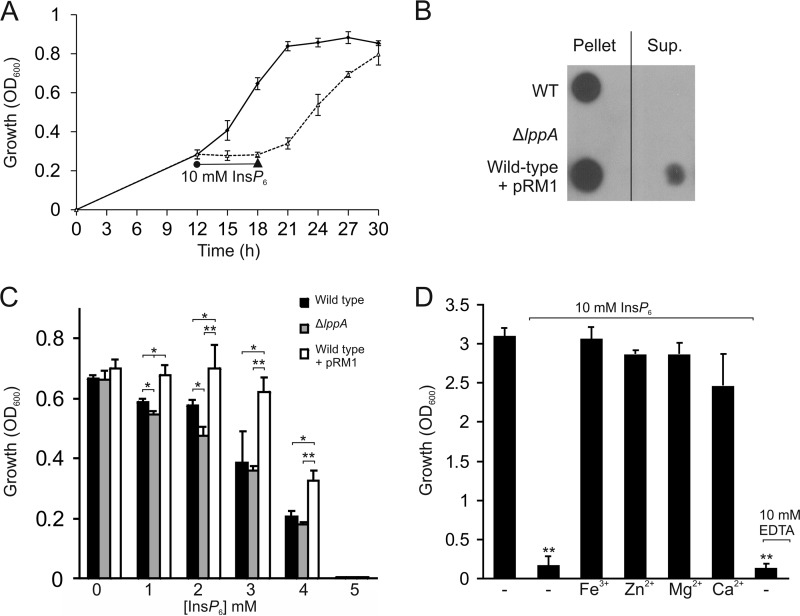
FIGURE 5.
Phytate reversibly inhibits L. pneumophila growth and is counteracted by LppA or micronutrient supplementation. A, phytate is bacteriostatic for L. pneumophila. Wild type L. pneumophila was inoculated at an A600 of 0.1 in AYE medium, and growth was measured by A600 every 3 h from 12 to 30 h. At 12 h, 10 mm phytate (InsP6) was added for 6 h (dashed line), and the bacteria were pelleted, resuspended in fresh AYE medium, and allowed to grow again. The control without phytate was treated the same way. B, detection of LppA by Western blot in L. pneumophila wild type or ΔlppA harboring pCR33 (vector), wild type harboring pRM1 (M45-LppA), and culture supernatants. The data shown are representative of two independent experiments. C, growth inhibition by phytate is counteracted by LppA. L. pneumophila wild type or ΔlppA harboring pCR33 (vector) or wild type harboring pRM1 (M45-LppA) was grown in AYE supplemented with 0–5 mm phytate for 5 days at 24 °C. D, micronutrient supplementation of L. pneumophila cultures grown with 10 mm phytate. L. pneumophila was grown for 21 h in AYE containing 10 mm phytate or 10 mm of the micronutrients indicated (iron, zinc, magnesium, or calcium). 10 mm EDTA alone was used as a control for chelation. Data (A–C) represent means ± S.D. (error bars) of triplicates (*, p < 0.05; **, p < 0.005).
Next, we thought to assess the effect of LppA on growth inhibition by phytate. To this end, an L. pneumophila mutant strain lacking lppA (ΔlppA) was generated by double homologous recombination. Western blots using a polyclonal anti-LppA antibody revealed that the ΔlppA mutant strain did not produce LppA anymore, yet phytase production was restored upon expression of plasmid-encoded lppA (pWS31) (Fig. 5B) (data not shown). Moreover, upon overproduction of LppA by wild type L. pneumophila, some phytase was detected in the supernatants of bacterial cultures. Growth of L. pneumophila wild type, ΔlppA, or wild type overproducing LppA in AYE medium supplemented with 0–5 mm phytate was monitored by measuring the optical density of the culture. In the absence of phytate, the L. pneumophila strains grew indistinguishably. The addition of phytate inhibited bacterial growth in a dose-dependent manner, and a 5 mm concentration of the compound completely abolished bacterial replication (Fig. 5C). L. pneumophila lacking lppA was slightly more susceptible to phytate upon growth at 24 °C but not at 37 °C, regardless of whether complex AYE or chemically defined minimal medium was used (data not shown). On the other hand, the wild type strain overproducing LppA grew significantly better at phytate concentrations ranging from 1 to 4 mm. Thus, LppA phytase (released from the bacteria) counteracts the bacteriostatic effect of phytate (Fig. 5, B and C).
Phytate is a strong chelator and complexes iron, calcium, zinc, and magnesium among other micronutrients (37). In order to test whether the bacteriostatic effect of phytate on L. pneumophila is due to its chelating properties, we added the compound in the presence of equimolar concentrations of micronutrients (iron, zinc, magnesium, or calcium) to bacterial cultures growing in AYE medium and assessed growth. Whereas 10 mm phytate (or the positive control EDTA) completely blocked the growth of L. pneumophila, the concomitant addition of 10 mm micronutrients reversed the inhibition (Fig. 5D). In summary, supplementation of phytate with equimolar concentrations of metal ions reversed the bacteriostatic effects of phytate; therefore, growth inhibition is due to micronutrient deprivation by the chelator.
LppA Promotes Intracellular Replication of L. pneumophila in Phytate-loaded Amoebae
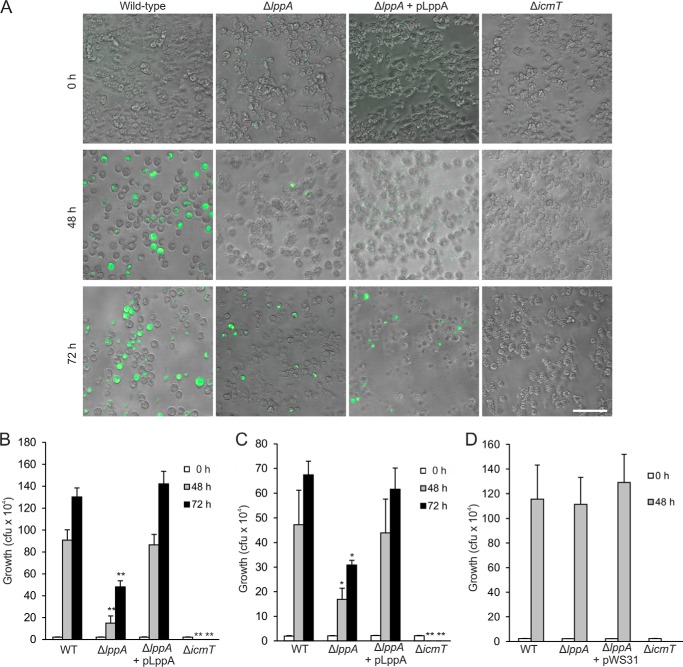
To determine whether phytate plays a role in intracellular replication of L. pneumophila, we preloaded A. castellanii or D. discoideum with the chelator. To this end, the amoebae were initially treated with 2.5 mm phytate, and the concentration was increased every 2 days up to 10 mm for A. castellanii or 5 mm for D. discoideum. Shortly before an experiment, the amoebae were washed and suspended in LoFlo medium. The phytate-loaded amoebae were then infected with GFP-producing L. pneumophila wild type, ΔicmT, ΔlppA, or complemented ΔlppA strains. Bright field and GFP fluorescence microscopy images were taken for infected A. castellanii.
Images immediately following infection show an even distribution of amoebae and L. pneumophila (Fig. 6A). After 48 h, amoebae infected with wild type L. pneumophila or the complemented ΔlppA mutant strain predominantly had rounded up, which is a characteristic of advanced L. pneumophila infection. The bright field channel shows that the majority of the cells are filled with bacteria, many of which are producing GFP. The synthesis of GFP was rather low and heterogeneous for the complementation strain, producing a short lived GFP. In contrast, amoebae infected with L. pneumophila lacking lppA remained largely attached, the morphology of most amoebae was similar to cells infected with ΔicmT, and only a few amoebae were observably filled with bacteria. By 72 h postinfection, A. castellanii infected with wild type L. pneumophila were full of actively moving bacteria ready for exit (supplemental Movie S1), and many amoebae infected with the complemented ΔlppA mutant strain had burst, releasing the intracellular bacteria (supplemental Movie S2). Replication of the ΔlppA mutant strain increased over the 48 h time point but was still considerably lower than replication by wild type or complementation strains (supplemental Movie S3), and many cells resembled the ΔicmT-infected amoebae (supplemental Movie S4).
FIGURE 6.
LppA promotes intracellular replication of L. pneumophila under phytate load. A, A. castellanii amoebae cultured in the presence of 10 mm phytate were infected (MOI 1, 37 °C) with L. pneumophila wild type, ΔlppA, or ΔicmT harboring pNT28 (GFP) or ΔlppA harboring pWS31 (GFP and LppA). Shown are bright field and GFP fluorescence images for infected A. castellanii taken at 0, 48, or 72 h postinfection. Scale bar, 50 μm. B, cfu counts for intracellular replication of L. pneumophila strains corresponding to images in (A). C, cfu counts for intracellular replication of above-listed L. pneumophila strains at 23 °C in D. discoideum preloaded with 5 mm phytate. Data represent means ± S.D. (error bars) of triplicates and are representative of three independent experiments (*, p < 0.05; **, p < 0.005). D, A. castellanii cultured in the absence of phytate was infected (MOI 1, 37 °C) with L. pneumophila wild type, ΔlppA or ΔicmT harboring pNT28 (GFP), or ΔlppA harboring pWS31 (GFP and LppA), and intracellular growth was determined by cfu at 0 and 48 h postinfection.
Intracellular growth of the L. pneumophila strains in phytate-loaded A. castellanii or D. discoideum was also quantified by determining cfu (Fig. 6, B and C). Colony counts at the onset of infection were even across all strains, and ΔicmT mutant bacteria used as a negative control disappeared over time. Using cfu as readout for intracellular replication, significantly fewer bacteria lacking lppA were counted after 48 or 72 h of infection, and the growth defect was fully complemented by providing the gene on a plasmid.
We also tested the possible role of lppA in intracellular growth of L. pneumophila in A. castellanii or D. discoideum cultured in the absence of phytate. To this end, the amoebae were grown in standard medium and infected with L. pneumophila wild type, ΔicmT, ΔlppA, or complemented ΔlppA strains, and intracellular growth was determined by cfu after 48 h infection. However, under these conditions, the deletion or overexpression of lppA did not affect intracellular bacterial growth (Fig. 6D) (data not shown). Moreover, upon co-infection of A. castellanii with L. pneumophila wild type and ΔlppA at a 1:1 ratio, the mutant strain was not outcompeted for up to 18 days (data not shown). Finally, lppA did not affect the growth of L. pneumophila in RAW 264.7 macrophages, for which phytate was toxic (data not shown). In summary, these findings indicated that the L. pneumophila translocated phytase LppA provides an intracellular growth advantage in phytate-loaded amoebae, such as A. castellanii or D. discoideum.
LppA Does Not Play a Major Role in the Modulation of the LCV PI Pattern
LppA is translocated into host cells and in vitro efficiently hydrolyzes PI lipids to yield PtdIns(4)P (Fig. 2). Thus, we hypothesized that LppA might also modulate the LCV PI pattern in L. pneumophila-infected cells. To compare the dynamics of PtdIns(4)P accumulation on LCVs harboring wild type or ΔlppA mutant bacteria, we performed live cell imaging using specific PI probes (21). Upon infection of D. discoideum amoebae producing GFP-SidCP4C with L. pneumophila, LCVs harboring wild type or ΔlppA bacteria accumulated PtdIns(4)P to the same extent within 1 or 2 h postinfection (Fig. 7, A and B). The LCVs were similar in size and GFP-SidCP4C signal intensity, and PtdIns(4)P persisted on LCVs containing replicating bacteria. Similar results were obtained upon staining the endogenously produced PtdIns(4)P-binding effector SidC on LCVs harboring wild type or ΔlppA L. pneumophila (data not shown). Finally, LCVs harboring L. pneumophila wild type or ΔlppA mutant bacteria were decorated to the same extent with the ER marker calnexin (data not shown). Taken together, the translocated L. pneumophila phytase LppA does not appear to modulate the LCV PI pattern in infected cells.
FIGURE 7.
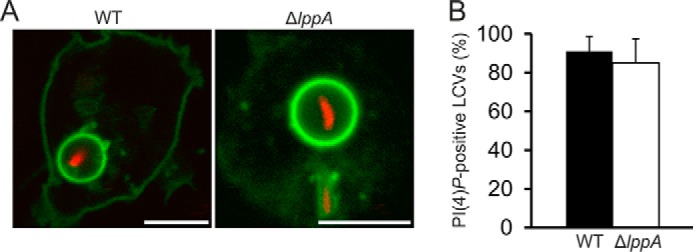
LppA does not influence the LCV PtdIns(4)P pattern in infected phagocytes. A, live cell imaging of D. discoideum producing GFP-SidCP4C infected with L. pneumophila wild-type or ΔlppA harboring pSW001 (DsRed) at MOI 10. Images were taken 2 h postinfection at 23 °C. Scale bars, 5 μm. B, quantification of PtdIns(4)P-positive LCVs. At least 100 LCVs were counted for each sample 2 h postinfection. Scale bar, 5 μm. Error bars, S.D.
DISCUSSION
In this study, we identified the chelator phytate as an intracellular bacteriostatic compound, and we provide evidence that the T4SS-translocated L. pneumophila phytase LppA counteracts bacterial growth restriction by phytate by hydrolyzing and thus inactivating the chelator. L. pneumophila requires iron for growth (53), and accordingly, the standard AYE growth medium contains 0.6 mm iron. The bacteria possess a number of iron uptake systems, including the siderophore legiobactin and the ferrous iron transmembrane transporter FeoB (54). The requirement for iron and possibly other micronutrients provides a rationale for the susceptibility of L. pneumophila to the chelator phytate (Fig. 5). The sensitivity of L. pneumophila to phytate also suggests that under the conditions tested, the bacteria do not use phytate as a source of phosphorus or as a siderophore for micronutrients, which has been described for X. oryzae (34) or P. aeruginosa (35, 36), respectively.
Upon extracellular growth in AYE broth, an L. pneumophila strain lacking lppA was only slightly more susceptible to phytate. The mild phenotype was observed only at a low growth temperature of 24 °C and not at 37 °C, regardless of whether complex AYE or chemically defined minimal medium was used (data not shown). In contrast, the wild type strain overproducing LppA grew significantly better in the presence of 1–4 mm phytate (Fig. 5C). The overproduction of LppA resulted in a portion of the phytase being released by the bacteria (Fig. 5B). Therefore, these findings are in agreement with the notion that LppA in the growth medium counteracts the bacteriostatic effect of phytate. The polyanionic compound phytate is expected to be largely membrane-impermeable and might not be taken up actively by L. pneumophila. Hence, under extracellular conditions where LppA is not translocated into a host cell, the absence of the phytase does not result in a pronounced growth defect.
Amoebae, in particular the social soil amoeba D. discoideum, produce phytate in the millimolar range (31–33). Phytate concentrations above 1–2 mm inhibit the extracellular growth of L. pneumophila (Fig. 5C), very likely due to the chelation of micronutrients (Fig. 5D). It is challenging to quantify the intracellular concentrations of micronutrients and the intracellular micronutrient requirements of L. pneumophila in host cells, yet the intracellular production of phytate in millimolar quantities seems sufficient to reduce or even deplete from pathogen-accessible intracellular compartments the micronutrients essential for L. pneumophila. We showed that D. discoideum as well as A. castellanii preloaded with phytate (but not amoebae grown in standard media) restrict intracellular growth of L. pneumophila in an lppA-dependent manner (Fig. 6). Perhaps the micronutrients available under the laboratory conditions used overcompensated the endogenous phytate produced by the amoeba.
Protozoa take up solutes via macropinocytic processes, and the macropinosomes formed probably communicate with LCVs. In support of this notion, L. pneumophila itself is taken up by phagocytes by macropinocytic rather than phagocytic processes (21, 55). Thus, the exogenously added phytate might reach the pathogen compartment through vesicle fusion, yet transmembrane transport processes might also play a role. Notably, the obligate intra-amoebal bacterium Candidatus Protochlamydia amoebophila, produces a putative cysteine phytase (32% identity with LppA) (29). This finding suggests that the phytate-containing compartment communicates with the bacterial symbiont and that degradation of intracellular phytate might also be beneficial for survival and replication of this bacterium.
Although LppA hydrolyzes phytate as well as polyphosphorylated PI lipids in vitro, only the phytase activity appears to be relevant in infected cells (Figs. 6 and 7). Other L. pneumophila Icm/Dot substrates, such as the PI phosphatases SidF and SidP, as well as host PI-metabolizing enzymes seem to modulate and define the LCV PI pattern. This is consistent with the notion that LppA apparently plays only a minor if any role in LCV formation. Cysteine phytases are characterized by the catalytic motif HCX2GX2R, of which the Cys and Arg residues are catalytically essential and His and Gly are important for conformation of the P-loop (39). Whereas the catalytic motif of LppA (HCRGGKGRT) is 66% identical to PhyA (HCEAGVGRT), it shares 89% identity with the mammalian PI 3-phosphatase PTEN (HC(R/K)AGKGRT) (Fig. 2A). Because both PTEN and LppA (Fig. 2B) effectively metabolize PtdIns(3,4,5)P3 and the main PI product of LppA in vitro is PtdIns(4)P (Fig. 2, D and E), it is tantalizing that LppA apparently does not function as a PI phosphatase in infected cells.
The results obtained in this study emphasize the critical role of micronutrients for intracellular pathogens. As an antibacterial strategy against vacuolar pathogens, micronutrient depletion by the chelator phytate might function in parallel with transporters that remove metal ions from the pathogen vacuole. Accordingly, the transmembrane proteins Nramp-1 and Nramp-2 have been shown to pump iron from vacuoles (LCVs) to the cytoplasm of D. discoideum (56, 57). Whereas the eukaryotic cell limits the availability of micronutrients by producing chelators and ion pumps, the intracellular pathogen L. pneumophila developed means to counteract the bacteriostatic strategy. Thus, our study reveals the potential to exploit intracellular micronutrient deprivation as an antibacterial strategy. Specifically, phytases or other microbial chelator antagonists might represent targets to control intracellular growth and virulence of bacterial pathogens.
Supplementary Material
Acknowledgments
We thank Aline Kessler and Sabrina Heiny for constructing pAK7 and pSH108, respectively.

This article contains supplemental Movies S1–S4.
- LCV
- Legionella-containing vacuole
- AYE
- ACES yeast extract
- T4SS
- type IV secretion system
- PI
- phosphoinositide
- PtdIns
- phosphatidyl-inositol
- PTEN
- phosphatase and tensin homologue deleted on chromosome 10
- PTP
- protein-tyrosine phosphatase
- Kan
- kanamycin
- β-ME
- β-mercapthoethanol
- MOI
- multiplicity of infection.
REFERENCES
- 1. Newton H. J., Ang D. K., van Driel I. R., Hartland E. L. (2010) Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23, 274–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hilbi H., Hoffmann C., Harrison C. F. (2011) Legionella spp. outdoors: colonization, communication and persistence. Environ. Microbiol. Rep. 3, 286–296 [DOI] [PubMed] [Google Scholar]
- 3. Whiley H., Bentham R. (2011) Legionella longbeachae and legionellosis. Emerg. Infect. Dis. 17, 579–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann C., Harrison C. F., Hilbi H. (2014) The natural alternative: protozoa as cellular models for Legionella infection. Cell Microbiol. 16, 15–26 [DOI] [PubMed] [Google Scholar]
- 5. Isberg R. R., O'Connor T. J., Heidtman M. (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hubber A., Roy C. R. (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26, 261–283 [DOI] [PubMed] [Google Scholar]
- 7. Hilbi H., Haas A. (2012) Secretive bacterial pathogens and the secretory pathway. Traffic 13, 1187–1197 [DOI] [PubMed] [Google Scholar]
- 8. Itzen A., Goody R. S. (2011) Covalent coercion by Legionella pneumophila. Cell Host. Microbe 10, 89–91 [DOI] [PubMed] [Google Scholar]
- 9. Sherwood R. K., Roy C. R. (2013) A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host. Microbe 14, 256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rothmeier E., Pfaffinger G., Hoffmann C., Harrison C. F., Grabmayr H., Repnik U., Hannemann M., Wölke S., Bausch A., Griffiths G., Müller-Taubenberger A., Itzen A., Hilbi H. (2013) Activation of Ran GTPase by a Legionella effector promotes microtubule polymerization, pathogen vacuole motility and infection. PLoS Pathog. 9, e1003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann C., Finsel I., Otto A., Pfaffinger G., Rothmeier E., Hecker M., Becher D., Hilbi H. (2014) Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol. 16, 1034–1052 [DOI] [PubMed] [Google Scholar]
- 12. Simon S., Wagner M. A., Rothmeier E., Müller-Taubenberger A., Hilbi H. (2014) Icm/Dot-dependent inhibition of phagocyte migration by Legionella is antagonized by a translocated Ran GTPase activator. Cell Microbiol. 16, 977–992 [DOI] [PubMed] [Google Scholar]
- 13. Hilbi H., Rothmeier E., Hoffmann C., Harrison C. F. (2014) Beyond Rab GTPases: Legionella activates the small GTPase Ran to promote microtubule polymerization, pathogen vacuole motility, and infection. Small GTPases, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ham H., Sreelatha A., Orth K. (2011) Manipulation of host membranes by bacterial effectors. Nat. Rev. Microbiol. 9, 635–646 [DOI] [PubMed] [Google Scholar]
- 15. Hilbi H., Weber S., Finsel I. (2011) Anchors for effectors: subversion of phosphoinositide lipids by Legionella. Front. Microbiol. 2, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haneburger I., Hilbi H. (2013) Phosphoinositide lipids and the Legionella pathogen vacuole. Curr. Top. Microbiol. Immunol. 376, 155–173 [DOI] [PubMed] [Google Scholar]
- 17. Weber S. S., Ragaz C., Reus K., Nyfeler Y., Hilbi H. (2006) Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ragaz C., Pietsch H., Urwyler S., Tiaden A., Weber S. S., Hilbi H. (2008) The Legionella pneumophila phosphatidylinositol-4-phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol. 10, 2416–2433 [DOI] [PubMed] [Google Scholar]
- 19. Brombacher E., Urwyler S., Ragaz C., Weber S. S., Kami K., Overduin M., Hilbi H. (2009) Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284, 4846–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoebel S., Blankenfeldt W., Goody R. S., Itzen A. (2010) High-affinity binding of phosphatidylinositol 4-phosphate by Legionella pneumophila DrrA. EMBO Rep. 11, 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weber S., Wagner M., Hilbi H. (2014) Live cell imaging of phosphoinositide dynamics and membrane architecture during Legionella infection. mBio 5, e00839–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dolinsky S., Haneburger I., Cichy A., Hannemann M., Itzen A., Hilbi H. (2014) The Legionella longbeachae Icm/Dot substrate SidC selectively binds PtdIns(4)P with nanomolar affinity and promotes pathogen vacuole-ER interactions. Infect Immun. 82, 4021–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weber S. S., Ragaz C., Hilbi H. (2009) The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell Microbiol. 11, 442–460 [DOI] [PubMed] [Google Scholar]
- 24. Jank T., Böhmer K. E., Tzivelekidis T., Schwan C., Belyi Y., Aktories K. (2012) Domain organization of Legionella effector SetA. Cell Microbiol. 14, 852–868 [DOI] [PubMed] [Google Scholar]
- 25. Finsel I., Ragaz C., Hoffmann C., Harrison C. F., Weber S., van Rahden V. A., Johannes L., Hilbi H. (2013) The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell Host Microbe 14, 38–50 [DOI] [PubMed] [Google Scholar]
- 26. Hsu F., Zhu W., Brennan L., Tao L., Luo Z. Q., Mao Y. (2012) Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase. Proc. Natl. Acad. Sci. U.S.A. 109, 13567–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toulabi L., Wu X., Cheng Y., Mao Y. (2013) Identification and structural characterization of a Legionella phosphoinositide phosphatase. J. Biol. Chem. 288, 24518–24527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner B. L., Papházy M. J., Haygarth P. M., McKelvie I. D. (2002) Inositol phosphates in the environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 449–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim B. L., Yeung P., Cheng C., Hill J. E. (2007) Distribution and diversity of phytate-mineralizing bacteria. ISME J. 1, 321–330 [DOI] [PubMed] [Google Scholar]
- 30. Rao D. E., Rao K. V., Reddy T. P., Reddy V. D. (2009) Molecular characterization, physicochemical properties, known and potential applications of phytases: an overview. Crit. Rev. Biotechnol. 29, 182–198 [DOI] [PubMed] [Google Scholar]
- 31. Martin J. B., Foray M. F., Klein G., Satre M. (1987) Identification of inositol hexaphosphate in 31P-NMR spectra of Dictyostelium discoideum amoebae. Relevance to intracellular pH determination. Biochim. Biophys. Acta 931, 16–25 [DOI] [PubMed] [Google Scholar]
- 32. Stephens L. R., Irvine R. F. (1990) Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature 346, 580–583 [DOI] [PubMed] [Google Scholar]
- 33. Pisani F., Livermore T., Rose G., Chubb J. R., Gaspari M., Saiardi A. (2014) Analysis of Dictyostelium discoideum inositol pyrophosphate metabolism by gel electrophoresis. PLoS One 9, e85533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatterjee S., Sankaranarayanan R., Sonti R. V. (2003) PhyA, a secreted protein of Xanthomonas oryzae pv. oryzae, is required for optimum virulence and growth on phytic acid as a sole phosphate source. Mol. Plant Microbe Interact. 16, 973–982 [DOI] [PubMed] [Google Scholar]
- 35. Smith A. W., Poyner D. R., Hughes H. K., Lambert P. A. (1994) Siderophore activity of myo-inositol hexakisphosphate in Pseudomonas aeruginosa. J. Bacteriol. 176, 3455–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirst P. H., Riley A. M., Mills S. J., Spiers I. D., Poyner D. R., Freeman S., Potter B. V., Smith A. W. (1999) Inositol polyphosphate-mediated iron transport in Pseudomonas aeruginosa. J. Appl. Microbiol. 86, 537–543 [DOI] [PubMed] [Google Scholar]
- 37. Urbano G., López-Jurado M., Aranda P., Vidal-Valverde C., Tenorio E., Porres J. (2000) The role of phytic acid in legumes: antinutrient or beneficial function? J. Physiol. Biochem. 56, 283–294 [DOI] [PubMed] [Google Scholar]
- 38. Mullaney E. J., Ullah A. H. (2003) The term phytase comprises several different classes of enzymes. Biochem. Biophys. Res. Commun. 312, 179–184 [DOI] [PubMed] [Google Scholar]
- 39. Chu H. M., Guo R. T., Lin T. W., Chou C. C., Shr H. L., Lai H. L., Tang T. Y., Cheng K. J., Selinger B. L., Wang A. H. (2004) Structures of Selenomonas ruminantium phytase in complex with persulfated phytate: DSP phytase fold and mechanism for sequential substrate hydrolysis. Structure 12, 2015–2024 [DOI] [PubMed] [Google Scholar]
- 40. Norris F. A., Wilson M. P., Wallis T. S., Galyov E. E., Majerus P. W. (1998) SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. U.S.A. 95, 14057–14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wishart M. J., Dixon J. E. (2002) PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol. 12, 579–585 [DOI] [PubMed] [Google Scholar]
- 42. Grundner C., Ng H. L., Alber T. (2005) Mycobacterium tuberculosis protein tyrosine phosphatase PtpB structure reveals a diverged fold and a buried active site. Structure 13, 1625–1634 [DOI] [PubMed] [Google Scholar]
- 43. Beresford N., Patel S., Armstrong J., Szöor B., Fordham-Skelton A. P., Tabernero L. (2007) MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem. J. 406, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tiaden A., Spirig T., Weber S. S., Brüggemann H., Bosshard R., Buchrieser C., Hilbi H. (2007) The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol. 9, 2903–2920 [DOI] [PubMed] [Google Scholar]
- 45. Studier F. W. (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 46. Chen J., de Felipe K. S., Clarke M., Lu H., Anderson O. R., Segal G., Shuman H. A. (2004) Legionella effectors that promote nonlytic release from protozoa. Science 303, 1358–1361 [DOI] [PubMed] [Google Scholar]
- 47. Kessler A., Schell U., Sahr T., Tiaden A., Harrison C., Buchrieser C., Hilbi H. (2013) The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ. Microbiol. 15, 646–662 [DOI] [PubMed] [Google Scholar]
- 48. Puhl A. A., Gruninger R. J., Greiner R., Janzen T. W., Mosimann S. C., Selinger L. B. (2007) Kinetic and structural analysis of a bacterial protein tyrosine phosphatase-like myo-inositol polyphosphatase. Protein Sci. 16, 1368–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heidtman M., Chen E. J., Moy M. Y., Isberg R. R. (2009) Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell Microbiol. 11, 230–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burstein D., Zusman T., Degtyar E., Viner R., Segal G., Pupko T. (2009) Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 5, e1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu W., Banga S., Tan Y., Zheng C., Stephenson R., Gately J., Luo Z. Q. (2011) Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One 6, e17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lifshitz Z., Burstein D., Peeri M., Zusman T., Schwartz K., Shuman H. A., Pupko T., Segal G. (2013) Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc. Natl. Acad. Sci. U.S.A. 110, E707–E715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reeves M. W., Pine L., Hutner S. H., George J. R., Harrell W. K. (1981) Metal requirements of Legionella pneumophila. J. Clin. Microbiol. 13, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cianciotto N. P. (2007) Iron acquisition by Legionella pneumophila. Biometals 20, 323–331 [DOI] [PubMed] [Google Scholar]
- 55. Peracino B., Balest A., Bozzaro S. (2010) Phosphoinositides differentially regulate bacterial uptake and Nramp1-induced resistance to Legionella infection in Dictyostelium. J. Cell Sci. 123, 4039–4051 [DOI] [PubMed] [Google Scholar]
- 56. Bozzaro S., Buracco S., Peracino B. (2013) Iron metabolism and resistance to infection by invasive bacteria in the social amoeba Dictyostelium discoideum. Front. Cell Infect. Microbiol. 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peracino B., Buracco S., Bozzaro S. (2013) The Nramp (Slc11) proteins regulate development, resistance to pathogenic bacteria and iron homeostasis in Dictyostelium discoideum. J. Cell Sci. 126, 301–311 [DOI] [PubMed] [Google Scholar]
- 58. Segal G., Shuman H. A. (1998) Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 30, 197–208 [DOI] [PubMed] [Google Scholar]
- 59. Sadosky A. B., Wiater L. A., Shuman H. A. (1993) Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61, 5361–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wiater L. A., Sadosky A. B., Shuman H. A. (1994) Mutagenesis of Legionella pneumophila using Tn903dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11, 641–653 [DOI] [PubMed] [Google Scholar]
- 61. Mampel J., Spirig T., Weber S. S., Haagensen J. A. J., Molin S., Hilbi H. (2006) Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72, 2885–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 65. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) MolProbity. All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duarte J. M., Srebniak A., Schärer M. A, Capitani G. (2012) Protein interface classification by evolutionary analysis. BMC Bioinformatics 13, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.