Background: Bacterial fimbriae mediate binding to host tissue through specific interactions.
Results: ECP interacts with arabinosyl residues in pectin and other plant cell wall components.
Conclusion: ECP-arabinan interactions facilitate binding of E. coli to plant hosts.
Significance: The prevalence of arabinan targets in produce plants together with ECP expression may explain the association of pathogenic bacteria in edible plants.
Keywords: Adhesin, Bacterial Adhesion, Escherichia coli (E. coli), Glycobiology, Host-Pathogen Interaction, Ligand-binding Protein, Organelle, Plant Cell Wall, Arabinose, Glycan Array
Abstract
Outbreaks of verotoxigenic Escherichia coli are often associated with fresh produce. However, the molecular basis to adherence is unknown beyond ionic lipid-flagellum interactions in plant cell membranes. We demonstrate that arabinans present in different constituents of plant cell walls are targeted for adherence by E. coli common pilus (ECP; or meningitis-associated and temperature-regulated (Mat) fimbriae) for E. coli serotypes O157:H7 and O18:K1:H7. l-Arabinose is a common constituent of plant cell wall that is rarely found in other organisms, whereas ECP is widespread in E. coli and other environmental enteric species. ECP bound to oligosaccharides of at least arabinotriose or longer in a glycan array, plant cell wall pectic polysaccharides, and plant glycoproteins. Recognition overlapped with the antibody LM13, which binds arabinanase-sensitive pectic epitopes, and showed a preferential affinity for (1→5)-α-linked l-arabinosyl residues and longer chains of arabinan as demonstrated with the use of arabinan-degrading enzymes. Functional adherence in planta was mediated by the adhesin EcpD in combination with the structural subunit, EcpA, and expression was demonstrated with an ecpR–GFP fusion and ECP antibodies. Spinach was found to be enriched for ECP/LM13 targets compared with lettuce. Specific recognition of arabinosyl residues may help explain the persistence of E. coli in the wider environment and association of verotoxigenic E. coli with some fresh produce plants by exploitation of a glycan found only in plant, not animal, cells.
Introduction
There is mounting evidence that human enteric pathogens can utilize plants as hosts (1–4). Several bacterial pathogens have been involved in different foodborne outbreaks, including pathogenic Escherichia coli and Salmonella enterica (5–10). E. coli O157:H7, one of the most important causative agents of fresh produce-associated outbreaks, is often linked with contaminated lettuce and spinach (11, 12).
In mammalian hosts, bacterial adherence is often mediated by lectins present at the fimbrial tip that bind to complementary carbohydrates on the surface of the host tissues. Type 1 fimbriae and P fimbriae are the best characterized in the chaperone-usher family, encoding tip adhesins FimH and PapD, respectively. They recognize α-d-mannosylated proteins and α-d-galactopyranosyl-(1→4)-β-d-galactopyranoside receptor epitope in the globoseries of glycolipids, respectively (13, 14). Conversely, no specific adhesin targets in plant tissue have been elucidated either for phytopathogenic bacteria that encode chaperone-usher fimbriae or for human pathogenic bacteria that can colonize plants as secondary hosts.
The E. coli common pilus (ECP),3 originally termed meningitis-associated and temperature-regulated (Mat) fimbria, was first identified in newborn meningitis and septicemia E. coli isolate IHE3034 (O18:K1:H7) when it was grown at 20 °C (15). The ecp operon is ubiquitous across E. coli and even conserved for some other enteric species (15–19). ECP belongs to the chaperone-usher family encoded by the ecpRABCDE operon where EcpA encodes the pilin domain and EcpD encodes the polymerized tip adhesin. Unusually for classical tip adhesins, EcpD can be polymerized independently, which requires an N-terminal extension in EcpD, or with the major pilin domain (18). Several roles have been described for ECP, including binding to cultured human epithelial cells (16, 17, 20), colonization of infant mice (21), and biofilm development through interorganelle binding of EcpA (22). The regulator EcpR represses the flagellar master operon flhDC, supporting the role for EcpA in biofilms (23). Expression of ECP is strain-dependent in E. coli: some isolates belonging to the B2 phylogenetic group are able to produce these fimbriae in conditions mimicking those in the intestine or at 20 °C, whereas others do not retain this capability (24).
Plant cell walls are complex configurations of structures composed mostly of carbohydrates present in polysaccharides and highly glycosylated proteins. Polysaccharides are represented by three different families: cellulose, hemicellulose, and pectin. Cellulose is present as long chains of β-1,4-glucose, and hemicelluloses are branched polysaccharides containing backbones of neutral sugars. Pectins are defined by the presence of uronic acids divided in four polysaccharide “domains”: homogalacturonan (HG), rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II), and xylogalacturonan (25, 26). The polysaccharides are linked together in varying abundances with HG as the most abundant followed by RG-I (27). Cell walls also contain abundant hydroxyproline-rich glycoproteins (HRGPs), a superfamily that encompasses extensins, proline-rich proteins, and arabinogalactan proteins (AGPs). HRGPs are mainly O-glycosylated with arabinose and galactose (28, 29).
In the present study, we tested the hypothesis that bacteria interact with plant tissue by targeting specific glycans. We identified targets for two different ECP variants in plants by using high throughput plant glycan arrays and enzyme-linked immunosorbent assay (ELISA). We were able to capitalize on the extensive body of work for ECP/Mat from newborn meningitis and septicemia E. coli strain IHE3034 (O18:K1:H7) but expanded this to investigate the role of ECP from the human pathogen E. coli O157:H7 (strain Sakai). The interactions were characterized with plant polysaccharides and from functional in planta adhesion assays.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Media
E. coli O157:H7 strain Sakai (Shiga-toxin negative) (30) and E. coli K-12 strain JT1 (31) were grown in either Luria-Bertani (LB) broth or rich defined MOPS supplemented with 0.2% glucose, thiamine, and essential and non-essential amino acids (32). Antibiotics were included where necessary to maintain transformed plasmids or for selection with adherence assays at the following final concentrations: 50 μg ml−1 kanamycin, 12.5 μg ml−1 chloramphenicol, 50 μg ml−1 ampicillin, 10 μg ml−1 tetracycline. Induction of ecp genes in recombinant E. coli strains was carried out with 5 μm isopropyl β-d-thiogalactopyranoside. All media, antibiotics, and inducers were purchased from Sigma-Aldrich. E. coli strain JT1 was selected to overexpress ECP fimbriae. This strain lacks flagella and type 1 fimbriae, and it encodes a copy of the ecp cluster even though it belongs to serogroup K-12, which does not contain strong, active promoters for the expression of native ecp (24).
Cloning and Mutagenesis
ecpA (ECs0323) and ecpA–D (ECs0323–0320) deletions were constructed using allelic exchange as described previously (33). Primers used for crossover PCR are listed in Table 1. A PstI site in the ecpA upstream sequence required that the PCR products were blunt end-cloned via T4 polynucleotide kinase into the pTOF24 vector. A Flip recombinase target-flanked tetracycline cassette was subcloned into the NotI site introduced into pTOF24, creating allelic exchange vectors pAH002 for ecpA and pAH003 for ecpA–D. The promoter region of ecpR (440 bp of the 5′-UTR) was PCR-amplified from E. coli O157:H7 Sakai genomic DNA with primers 0324.5.XbaI and 0324.3.XbaI (Table 1) and cloned into pKC026 using XbaI, creating the transcriptional fusion pAH001. Two overexpression constructs for E. coli, ecpA–D and ecpA–E, were generated by cloning the regions into pSE380 (Table 2), generating pYR006 and pYR007, using HindIII-HindIII and XbaI-HindIII, respectively. Enzymes were purchased from New England Biolabs (Ipswich, MA) or Roche Applied Science. Plasmid clones were confirmed by appropriate restriction digests and Sanger sequencing. Mutants were confirmed by phenotype and diagnostic PCR.
TABLE 1.
Primer sequences used in this study
| Product | Primer name | Primer sequencea |
|---|---|---|
| ecpA–D | ecpAHindIII | CCCAAGCTTGTCCTCAATTCAACTCGG |
| ecpDHindIII | CCCAAGCTTGCCGACCGAAATCGCCTG | |
| ecpA–E | EcpAXbaI | GGACATCACGTCCTCTAGACAACTCGGG |
| EcpEHindIII | TCTGAGGTGGAAAGCTTCCCTCGA | |
| ecpR promoter | 0324.5.XbaI | GGTCTAGACTTTAATTATGACTC |
| 0324.3.XbaI | GGTCTAGATACTTTCCAAACCTG | |
| 5′-flanking region of ecpA for deletions | 0323.SalI.5F | GGAGAGTCGACGAAAAGATTTCGTGTTTATC |
| 0323.NotI.5R | CCGTTCCAAGCGGCCGCAAGAGCGTGTATTTCTTCCCGAGTTGAATTG | |
| 3′-flanking region of ecpA | 0323.NotI.3F | CGCTCTTGCGGCCGCTTGGAACGGTCTCTCTGATGTACCAGCAGGG |
| deletions | 0323.PstI.3R | GGTGCCTGCAGGGTACTGAAAGTGGTAGTTTG |
| 3′-flanking region of ecpD for deletions | 0320.Not1.3F | CGCTCTTGCGGCCGCTTGGAACGG TAAGGCCCTGCTGACAGCGGTCTG |
| 0320.PstI.3R | TTAATCTGCAGCTTAAACCACGTGTTGCCGGTG | |
| ΔecpA.NotI linker | 0323.SalI.5F | For splice overlap extension PCR of products ecpA.5.Not and ecpA.3.Not PCR products |
| 0323.PstI.3R | ||
| ΔecpA–D.NotI linker | 0323.SalI.5F | For splice overlap extension PCR of products ecpA.5.Not and ecpD.3.Not PCR products |
| 0320.PstI.3R |
a Restriction enzyme sequences are underlined.
TABLE 2.
Plasmids and strains
FRT, Flip recombinase target; Tet, tetracycline.
| Plasmid | Relevant features | Source/Ref. |
|---|---|---|
| pSE380 | Expression vector, trc promoter | Invitrogen |
| pYR006 | pSE380; AmpR; XbaI-HindIII insertion of EcpA–E (Sakai) | This study |
| pYR007 | pSE380; AmpR; HindIII -HindIII insertion of EcpA–D (Sakai) | This study |
| pMat3 | pSE380; AmpR; EcpA–E (IHE3034) | 15 |
| pTOF24 | Temperature-sensitive allelic exchange vector | 33 |
| pTOF1-TetR | FRT Tet cassette | Sean McAteer, unpublished |
| pKC26 | pAJR145 with P rpsM replaced by PgyrA. CamR | 77 |
| pAH001 | pKC26: PgyrA replaced with PecpR. CamR | This study |
| pAH002 | pTOF24: PstI-SalI blunt end insertion of ecpA.NotI linker + NotI insertion of Tet from pTOF1-TetR CamR TetR Ts SucS | This study |
| pAH003 | pTOF24: PstI-SalI blunt end insertion of ecpA–D.NotI linker PCR + NotI insertion of Tet from pTOF-TetR. CamR TetR Ts SucS | This study |
ECP Preparation and Antibodies
ECP from pYR006 (EcpA–E from E. coli O157:H7 Sakai) and pMat3 (EcpA–E from E. coli O18:K1:H7 IHE3034) transformed in E. coli JT1 were isolated as described with modifications (34). In short, bacteria were cultured in LB medium supplemented with isopropyl β-d-thiogalactopyranoside and ampicillin at 37 °C for 16 h in static conditions, harvested by centrifugation, resuspended in cold Tris-buffered saline (TBS), detached from the bacterial cell by using a blender (three times for 30 s), and centrifuged twice at 4,000 × g for 30 min. Ammonium sulfate was added slowly into the supernatant containing the fimbriae with vigorous stirring to achieve two-thirds saturation. After an overnight incubation at 4 °C, the fimbriae were harvested by centrifugation at 15,000 × g for 20 min at 4 °C and then suspended in cold TBS + 0.5% deoxycholate. The preparation was subjected to a sucrose gradient ultracentrifugation (60–10%) in an Optima L-80 XP centrifuge for 16 h at 20,000 rpm at 4 °C in an SW41 rotor and then fractioned in 1-ml lots. The fractions were visualized for density and absorbance at 280 nm. The fractions containing ECP were pooled, dialyzed against water, and freeze-dried. To confirm purity of the isolated ECP, the samples were subjected to SDS-PAGE after denaturation at low pH (35). The proteins were separated by 12.5% SDS-PAGE and electrotransferred to Hybond-P membrane (GE Healthcare). Protein concentrations were determined using the BCA protein assay (Thermo Fisher Scientific Inc., Waltham, MA). The predicted size of E. coli EcpA is 21 kDa. A mock fimbrial preparation was prepared in an identical manner using E. coli JT1 as a control. The anti-ECP fimbrial serum derived from E. coli strain JTI overexpressing pMat3 was obtained by immunizing rats according to standard procedures by Genosphere Biotechnologies (France). Antibodies used in this study were rabbit anti-E. coli (Abcam, Cambridge, UK; Ab137967), anti-rat IgG Alexa Fluor 568-conjugated antibodies, and anti-rabbit and anti-rat horseradish peroxidase (Sigma-Aldrich). For anti-plant polysaccharide/glycoprotein (Plantprobes, Leeds, UK) specificities and details, see Table 3. The presence of fimbriae was confirmed by electron microscopy. Fimbrial preparations were spotted (10 μl) to carbon-coated copper grids and incubated for 1 min, and the grid was transferred to a 100-μl drop of sterile distilled water and incubated for 2 s. Without drying, the grid was transferred to a 100-μl drop of 2% phosphotungstic acid, pH 7.0 and incubated for 2 min. Excess liquid was then removed, the grid allowed to dry, and the images were obtained with a JEOL JEM-1400 electron microscope (Japan).
TABLE 3.
Monoclonal antibodies
XXXG is a form of xyloglucan, where X refers to α-d-Xylp and G refers to β-d-Glcp.
| Antibody | Specificities | Ref. |
|---|---|---|
| LM1 | Extensin (plant glycoprotein) | 78 |
| LM2 | AGPs | 79 |
| LM5 | (1→4)-β-Galactan | 80 |
| LM6 | (1→5)-α-Arabinan and arabinogalactan (plant glycoprotein) | 39, 43 |
| LM13 | (1→5)-α-Arabinan | 38, 44, 45 |
| LM15 | Non-fucosylated xyloglucan (XXXG) | 56 |
| LM16 | Uncharacterized RG-I epitope | 38, 44 |
| LM21 | Heteromannan | 50 |
| LM25 | XXXG/galactosylated xyloglucan | 38 |
| JIM7 | Partially methyl-esterified homogalacturonan | 55 |
| JIM8 | AGPs | 45 |
| JIM19 | Extensin (plant glycoprotein) | 45 |
Plants and Growth Conditions
Spinach (Spinacia oleracea var. Amazon), lettuce (Lactuca sativa var. All Year Round) (Suttons Seeds, UK), and Nicotiana benthamiana plants expressing mGFP5-ER (36) were grown in individual pots in a glass house at 22 °C (16 h of light, 8 h of dark) with 130–150 μmol m−2 s−1 light intensity and 40% humidity. Regular compost was used for all experiments except for plant root polysaccharide extraction where vermiculite completed with Osmocote Start (6 weeks) containing 12%, 11%, and 17% nitrogen, phosphorus, and potassium, respectively, was used to avoid compost contamination (peat).
Spinach Protein Enrichment
Extracts enriched in glycoproteins from spinach leaves or roots were obtained as described by Karlova et al. (37). Briefly, plant material was ground in liquid nitrogen and thawed in extraction buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.5% Triton X-100, and 1× plant protease inhibitor mixture (Sigma-Aldrich). The samples were incubated on ice for 30 min and then centrifuged at 400 × g at 4 °C for 3 min. Protein concentrations were determined using the BCA protein assay. The proteins were kept at −80 °C for no longer than 1 week.
Cell Wall Polymer Extraction
Lettuce and spinach leaves or roots were ground by mortar and pestle to a fine powder in liquid nitrogen. Six volumes of ethanol 70% were added to 1 volume of powder and incubated at room temperature for 10 min with rotation and then centrifuged at 3,000 × g 10 min. This step was repeated five times. The pellet was rinsed twice with acetone and dried. This alcohol-insoluble residue preparation was then followed by sequential extraction using two solvents: 50 mm CDTA, pH 7.5 and 4 m NaOH. The two extraction solvents used are known to solubilize pectins and noncellulosic polysaccharides, respectively.
Enzymatic Treatment
Highly purified enzymes (Megazyme, Bray, Ireland) included endo-β-mannanase (Bacillus sp.) used in 0.1 m glycine, pH 8.8 for 2 h at 40 °C; arabinofuranosidase (Aspergillus niger) used in 200 mm acetate buffer, pH 4 for 2 h at 40 °C; arabinanase (recombinant from Cellvibrio japonicus) used in 100 mm potassium phosphate buffer, pH 7 for 2 h at 40 °C; and pectate lyase (C. japonicus) used in 50 mm CAPS buffer, pH 10 for 2 h at 40 °C. All enzymes were used at 10 units/ml.
Glycan Array Printing and Screening
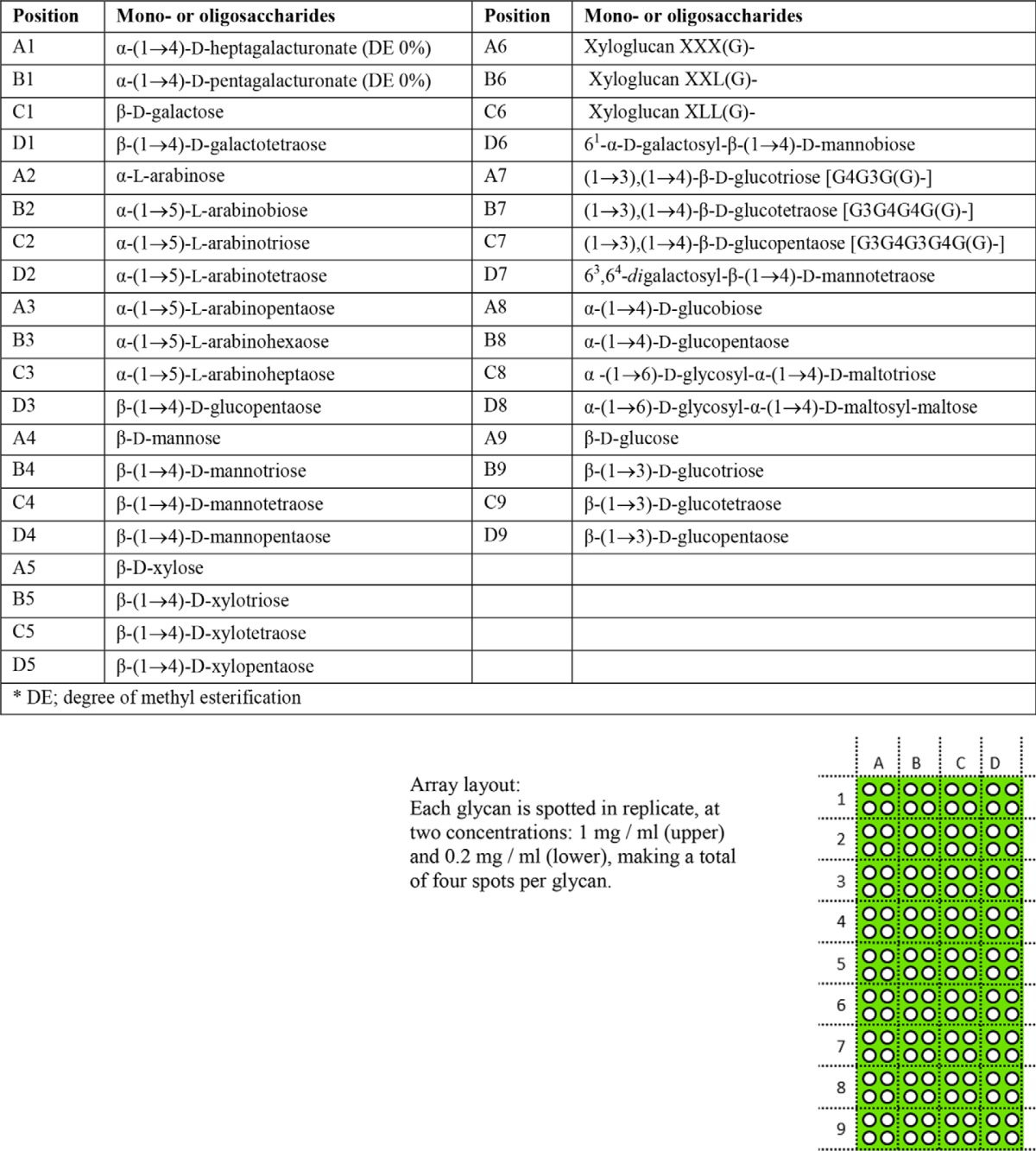
Glycan array printing was performed as described (38), and arrays were screened as described previously (4). For detailed information on all oligosaccharide samples, see Table 4. The results shown are based on six individual experiments. A competition assay with BSA-conjugated arabinoheptaose (Ara7) was performed by co-incubation with purified ECP (pYR006) at 20 μg/ml and was based on two independent experiments.
TABLE 4.
List of plant derived BSA-conjugated oligosaccharides and layout of the glycan array
X refers to xylose, L refers to galactose, and G refers to glucose.
ELISA and mAb Screening for Extract Characterization and Bacterial Interaction
ELISAs were performed in 96-well microtiter plates (Nunc, Maxisorb) coated with spinach proteins or arabinan at 50 μg/ml and at a 1:10 dilution of plant polysaccharide extract (in 0.1 m NaHCO3 buffer, pH 9.6) in a volume of 100 μl incubated at 4 °C overnight, similar to the concentrations used by others (39). The plates were washed three times with TBS and blocked with protein-free blocking buffer (Thermo Fisher Scientific Inc.) for 1 h at room temperature. Plates were again washed three times with TBS and then incubated for 2 h with 100 μl of bacteria at a concentration of ∼2 × 107 cfu/ml (A600 adjusted to 0.02 in TBS) or primary antibodies (Table 3) (at 1:20 for all plant polysaccharides/glycoproteins). After 2 h, the wells were washed three times with TBS and incubated with a rabbit anti-E. coli antibody (Abcam) diluted at 1:500 in TBS or incubated directly with a horseradish peroxidase anti-rat IgG conjugate (diluted at 1:1000). For detection of bacteria, after 1 h, the wells were washed three times with TBS and incubated with 100 μl of horseradish peroxidase anti-rabbit IgG conjugate (diluted at 1:1000). After 1 h, all secondary antibodies were washed three times with TBS. The color reaction was developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) solution: 22 mg of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma-Aldrich) diluted in 100 ml of citrate buffer (50 mm sodium citrate, 0.05% H2O2, pH 4.0). The absorbance at A405 was measured on a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA). Extracts enriched in glycoproteins were pipetted onto nitrocellulose membrane as immunodot blots at 100 μg each, allowed to dry, and probed with the monoclonal antibodies (Table 3) essentially as described for the ELISA with the following modifications: nonspecific interactions were blocked with 3% skimmed milk, primary antibodies were used at 1:100 dilution, the secondary anti-rat HRP conjugate was used at 1:5000 dilution, and the blots were washed with PBS containing 0.08% Tween 20. Gum arabic was used at 20 μg as a positive control. ECL substrate was used to detect binding (Millipore, Billerica, MA) and was exposed to ECL Hyperfilm for 10 min.
Monosaccharide Analysis
Monosaccharide analysis of cell walls was carried out as described (40). Acid hydrolysis (2 m trifluoroacetic acid for 1 h at 120 °C) was carried out, and the samples were loaded on a high performance anion exchange chromatography column and run on a Dionex ICS3000 system with a Dionex AS autosampler fitted with a 25-μl loop. The monosaccharides fucose, galactose, glucose, xylose, mannose, galacturonic acid, and glucuronic acid were separated on 3 × 150-mm PA-20 column with a 3 × 30-mm guard column. Eluents were ultrapure water (18.2 megaohms), 200 mm NaOH, and 1 m sodium acetate as described. The monosaccharides arabinose and rhamnose were separated on 4 × 250-mm PA-100 column with a 4 × 50-mm guard column eluted isocratically at 1 ml min−1 with 200 mm NaOH. The flow rate was 0.4 ml min−1 (40). The data are presented as a percentage of the total (mg/mg dry weight) to allow direct comparison.
Bacterial Adherence Assays
Adherence assays were performed as described (4). Briefly, plant tissue was aseptically separated, washed, and incubated with a bacterial suspension at a concentration of ∼2 × 107 cfu/ml (A600 adjusted to 0.02 in PBS) for 2 h at 18 °C. The tissue was then washed three times and macerated, and the bacteria were enumerated on selective medium.
Confocal Microscopy
Plant tissues were analyzed as described (4). Briefly, plant tissues were fixed, deparaffinized, treated with enzymes, and incubated successively with protein-free blocking buffer and antibodies. Primary antibodies were used at 1:50 and secondary (anti-rat conjugated to Alexa Fluor 568) at 1:5000 (Sigma-Aldrich). The sample was incubated for 10 min with Calcofluor white and then mounted with Dako fluorescent mounting medium (Dako, Carpentaria, CA). Fresh leaves were stained with polyclonal antiserum against ECP for 1 h (1:500) or propidium iodide. After three washes in TBS, the leaf samples were incubated for 45 min with an Alexa Fluor 568-conjugated anti-rat antibody (1:5000). Imaging was performed on a Leica TCS-SP2 AOBS microscope (Leica Microsystems, Germany). Photoshop CS software (Adobe Systems) was used for postacquisition image processing.
Sequence and Phylogenetic Analysis
The ecpD ORF derived from E. coli isolate Sakai was used to carry out a BLASTn analysis against a range of E. coli sequences (supplemental Table 1). The ORFs were identified, and amino acid sequences were derived using a bioinformatics program (CLC Bio, Aarhus, Denmark). These sequences were aligned using ClustalW (41). Sequences that did not encode a full-length ecpD ORF were excluded from subsequent alignment.
Statistical Analyses
The means ± S.E. were calculated for each experimental group, and the statistical significance was evaluated with Student's t test or one-way analysis of variance. The results were considered as significant for a p value ≤0.05.
RESULTS
ECP Binds to Arabinose Oligosaccharides
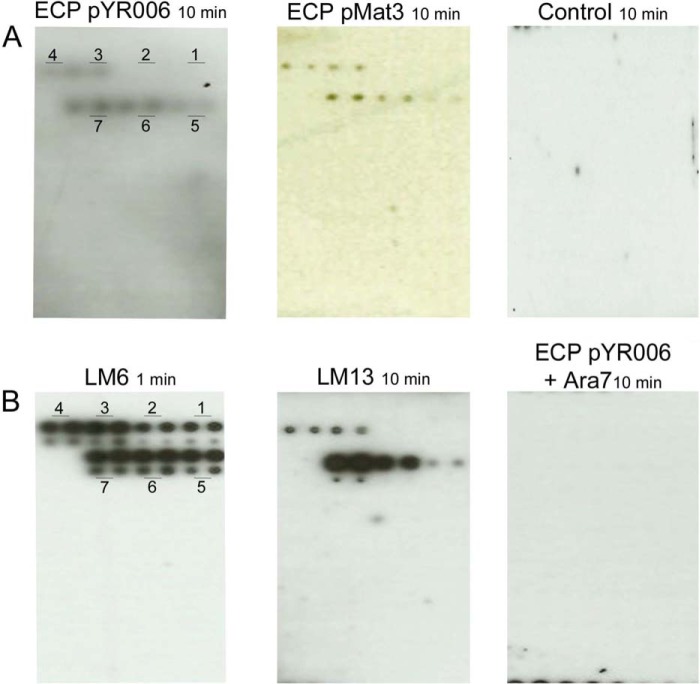
Recombinant ECP was purified from E. coli JT1 overexpressing ECP from high copy number plasmid pMat3 (ECP derived from newborn meningitis and septicemia E. coli strain IHE3034 (O18:K1:H7)) (15) to generate a polyclonal antibody. The antibody reacted with a protein of the predicted size of the main structural subunit, EcpA (Fig. 1, A–C). Induction of pMAT3 resulted in fimbrial elicitation with fine, hairlike structures (Fig. 1, D and E). The antibody reacted to a similar level to ECP derived from E. coli O157:H7 isolate Sakai, which encodes an identical copy of the mature peptide of the main structural subunit, EcpA, but not to the mock fimbrial preparation from E. coli JT1 (pSE380). Although the host strain encodes a native ecp cluster, it belongs to serogroup K-12, which expresses minimal or non-detectable levels of ECP (24). ECP derived from E. coli O157:H7 strain Sakai was cloned and purified. We used this strain because it was isolated from a massive foodborne outbreak in Japan in 1996 associated with white radish sprouts (42). Purified ECP fimbriae were used to screen a plant glycan array printed with oligosaccharides coupled to BSA including galacturonate, galactose, arabinose, mannose, xylose, xyloglucan, and glucose and probed with the ECP antibody (detailed in Table 4). ECPSakai (pYR006) and ECPIHE3034 (pMat3) interacted only with BSA conjugates with arabinose oligosaccharides from three (α-(1→5)-linked arabinotriose) to seven (α-(1→5)-linked arabinoheptaose) residues (Fig. 2A). Although detection required a relatively long exposure time (10 min), a specific signal was observed for the higher concentration of the conjugated oligosaccharides (1 mg ml−1) (Fig. 2A). No signal was detected with the negative control (no ECP). The signal intensity appeared to be proportional to the oligosaccharide complexities: the longer the oligosaccharides, the stronger the signal, and no signals were detected from complexes smaller than arabinotriose. To confirm the specificity, two arabinose-specific antibodies were used, LM6 and LM13. LM6 recognizes linear chains of (1→5)-α-arabinan from pectin and some AGPs (39, 43), whereas LM13 is specific to linear chains of (1→5)-α-arabinan from pectin but importantly only to oligosaccharides longer than arabinotriose (38, 44, 45). As expected, LM6 recognized arabinose residues on the glycan arrays of all sizes, whereas LM13 recognized residues from arabinotriose to arabinoheptaose present on the array (Fig. 2B). ECP was found to share the same specificity for longer arabinans as LM13. The ECP interaction was abrogated following co-incubation with free BSA-Ara7, confirming the interaction by competitive inhibition (Fig. 2B).
FIGURE 1.
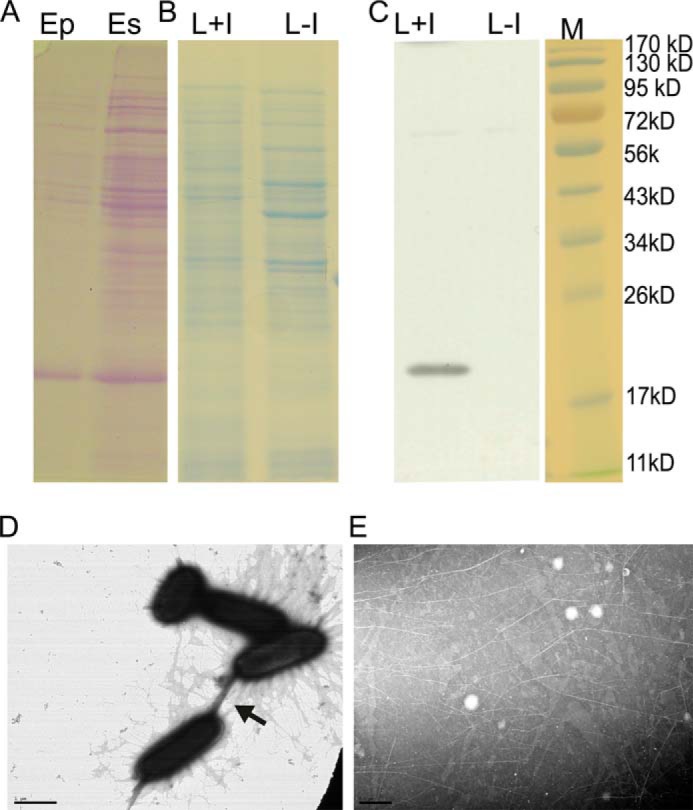
Purification of ECP. A, polyacrylamide gel showing ECP enrichment after shearing from the bacterial cells (Es) and purification (Ep). Shown are the polyacrylamide gel of bacterial lysates induced (L+I) for ECP expression or the negative control (L−I) (B) and the corresponding Western blot probed with ECP antiserum (C). M (kDa) indicates the migration of standard molecular mass markers. Electron micrographs of E. coli expressing ECPIHE3034 (pMat3) (D) and after fimbrial purification of ECPIHE3034 (pMat3) (E) are shown. The arrowhead indicates “bundling” of fimbriae. Scale bars, 1 μm.
FIGURE 2.
Plant glycan interactions with ECP. A, plant glycan arrays comprising 36 defined oligosaccharides probed with purified ECPSakai (pYR006) or ECPIHE3034 (pMat3) detected with a specific anti-ECP antibody on ECL film. The negative control, no ECP, is also shown. The glycans are arranged on a grid pattern, each spotted four times, twice at 1 mg ml−1 (upper) and twice at 0.2 mg ml−1 (lower); refer to Table 4 for detail and a pictorial map. B, glycan arrays probed with anti-arabinan antibodies LM6 and LM13 or co-incubated with purified ECPSakai (pYR006) and Ara7. Numbers 1–7 refer to the number of arabinosyl residues in each oligosaccharide.
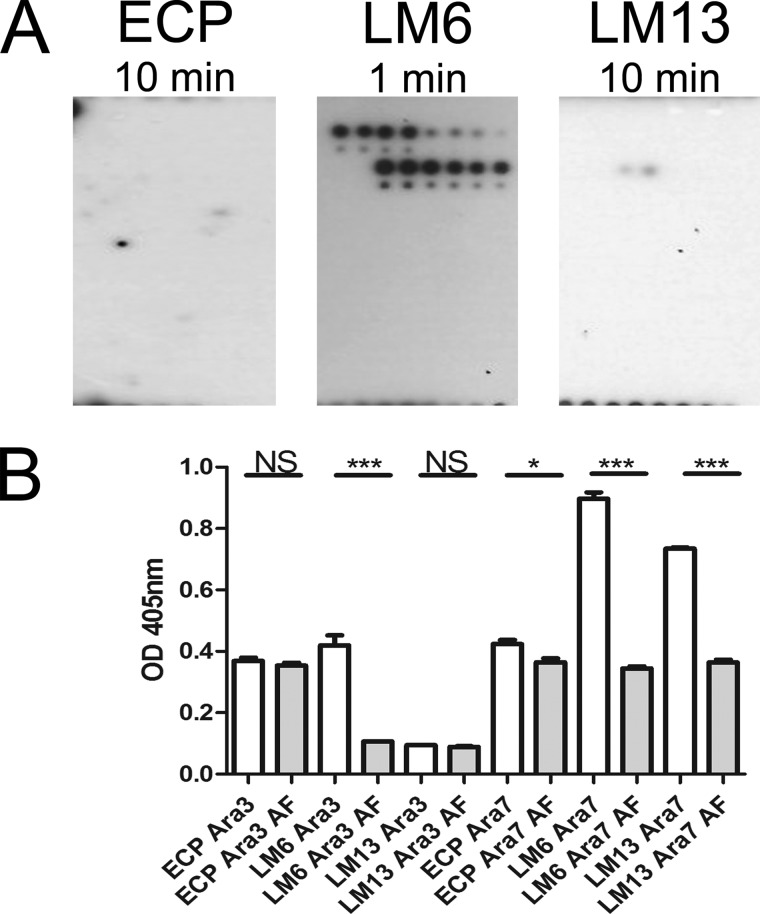
Arabinofuranosidase (AF) is a glycoside hydrolase that specifically targets α-l-arabinofuranosides containing (1→3) and/or (1→5) linkages found in RG-I as well as arabinoxylans and arabinogalactans. Pretreatment of the glycan array with AF prevented binding of purified ECPSakai (pYR006) to any residues and severely affected LM13 binding to Ara7 so that a signal was only just visible after a long exposure time (Fig. 3A). In contrast, LM6 detection occurred for all BSA-arabinose conjugates. No obvious interaction was detected between ECP and any other purified components on the array.
FIGURE 3.
Effect of arabinofuranidase treatment on ECP interactions. A, plant glycan array treated with AF and probed with purified ECPSakai (pYR006), LM6, and LM13. B, interaction of E. coli JT1 expressing ECPIHE3034 (pMat3), LM6, or LM13 with α-(1→5)-l-arabinotriose (Ara3) and α-(1→5)-l-Ara7 (Ara7) ±AF treatment. Significance levels are as follows: ***, p ≤ 0.001; *, p ≤ 0.05; NS, non-significant. Error bars represent S.E.
Bacterial adhesion to BSA-conjugated arabinotriose and Ara7 mediated by ECP was confirmed by ELISA using E. coli JT1 overexpressing ECPIHE3034 (pMat3). Enzymatic treatment with AF to remove arabinans resulted in a significant decrease in the interaction between ECP and Ara7 (p < 0.05) but not for arabinotriose. The result was consistent with a decrease in the interaction of LM13 antibody with Ara7 and no interaction with arabinotriose (Fig. 3B). This assay was carried out with whole bacteria expressing ECP, which presumably accounts for the background level of binding to the substrate. As expected, LM6 antibody interacted with both arabinans, although enzymatic treatment was obviously not complete.
ECP Interaction with Spinach Arabinosyl Residues in Pectin
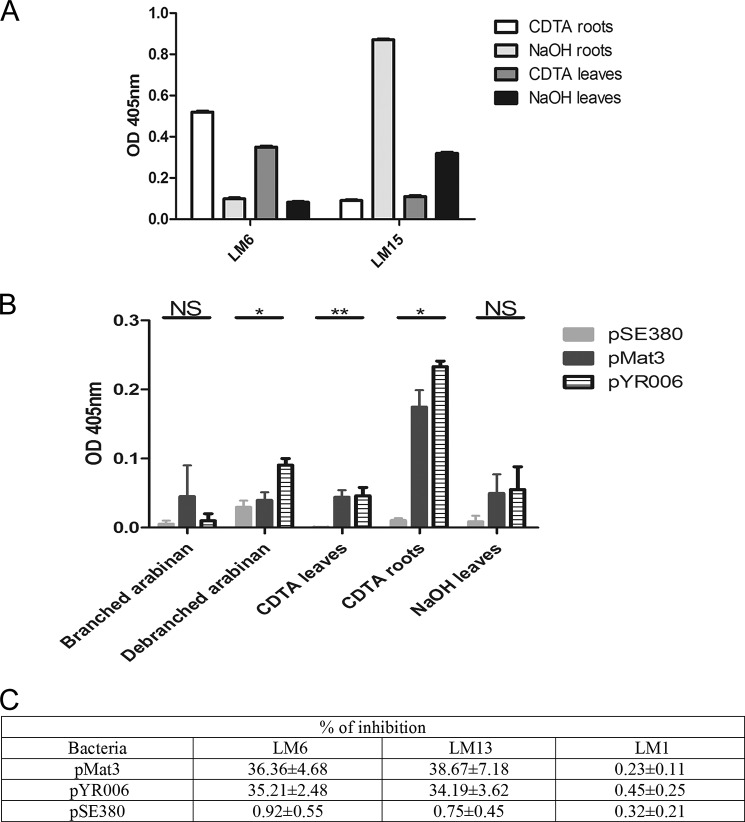
Because spinach (S. oleracea) accounts for a large proportion of E. coli O157:H7 fresh produce outbreaks (46–49), we assessed ECP binding to spinach plant cell wall constituents. Pectin and hemicellulose components were extracted from total spinach polysaccharides following CDTA and NaOH treatment, respectively, and probed with LM6 for pectin (arabinose) and LM15 for hemicellulose (xyloglucans) (Fig. 4A). As expected, arabinose was only detected in the pectin fractions of spinach leaves and roots, and xyloglucans were present only in hemicellulose fractions. To demonstrate tropism of ECP for pectin, adhesion of E. coli JTI expressing either variant of ECP (pMat3 or pYR006 with pSE380 as the negative control) was assessed on pectin fractions using hemicellulose as a negative control (Fig. 4B). Commercial purified branched and debranched long arabinan polymers (>110 residues/molecule), which were recognized by LM6 and LM13 (data not shown), were included to better characterize the interaction. Both ECP variants (Sakai and IHE3034) bound to spinach pectin and not to hemicellulose or branched arabinan, demonstrating a tropism for pectin. Adherence of ECP to isolated pectin from roots was >2-fold higher than on leaves presumably because this tissue type is richer in arabinan as shown with LM6 (Fig. 4B). Interestingly, ECPSakai expressed from E. coli JT1 (pYR006) was significantly more adherent to debranched arabinan ECPIHE3034 (pMat3) (p < 0.05) as well as pSE380. The ability of bacteria expressing ECP to inhibit antibody adhesion (LM1, LM6, and LM13) to the CDTA extract of spinach leaves was tested. The presence of bacteria expressing ECPSakai or ECPIHE3034 reduced LM6 and LM13 binding by ∼35% (Fig. 4C).
FIGURE 4.
ECP interactions with plant polysaccharides. A, ELISA of spinach polysaccharide extracts (CDTA and NaOH) from roots and leaves with LM6 (pectin) and LM15 (hemicellulose) antibodies. B, ELISA of spinach polysaccharide extracts (CDTA and NaOH) or long arabinan chains (branched and unbranched) with E. coli JT1 expressing ECPSakai (pYR006), ECPIHE3034 (pMat3), or the control plasmid (pSE380). Significance levels are as follows: **, p ≤ 0.005; *, p ≤ 0.05; NS, non-significant. C, inhibition of antibody binding to spinach leaf pectin with E. coli JT1 expressing ECP. “% of inhibition” represents the percentage of antibodies bound compared with the control value (i.e. no bacteria). Bacteria were added at an A600 of 0.8. Values are the average ± S.D. of three experiments. Error bars represent S.D.
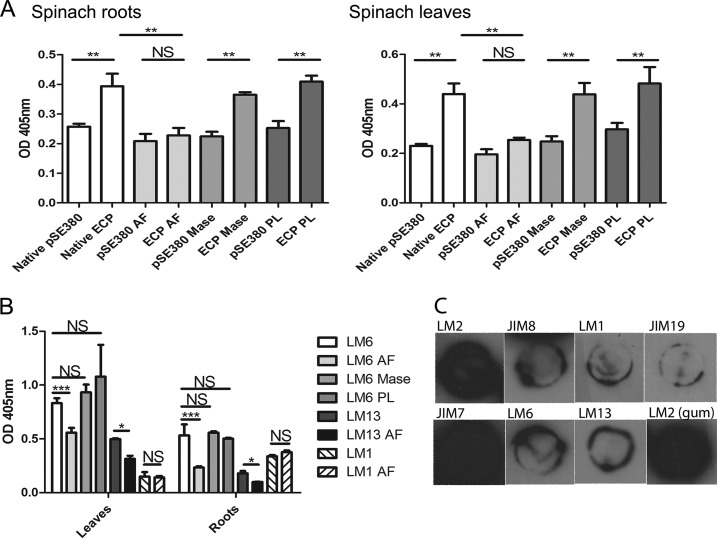
ECP Interaction with Spinach Arabinosyl Residues in Glycoproteins
An ELISA was performed with spinach leaf or root extracts enriched in glycoproteins and E. coli JT1 overexpressing ECPIHE3034 (from pMat3) or the empty plasmid (pSE380) as a control (Fig. 5A). Expression of ECPIHE3034 resulted in a ∼2-fold increase in adherence to the extracts from both leaves and roots compared with the control. Enzymatic treatment with AF abolished the increase in binding of ECP-expressing bacteria to levels similar to those seen with the pSE380 control, whereas treatment for mannans (mannanase) or pectins (pectin lyase (PL)) did not affect the interaction. The presence of arabinans in the spinach extracts was detected with antibodies LM6 and LM13 and was reduced by AF enzymatic treatment (Fig. 5B). There was no recognition of extensin using the LM1 antibody. Characterization of the extracts with the cell wall antibodies confirmed the presence of AGPs (with LM2 and JIM8) and showed the presence of the major pectin polysaccharide domain, homogalacturonan, partially methyl-esterified, using JIM7 (Fig. 5C). This is not unexpected as it is not possible to generate completely pure glycoprotein preparations. LM13 was detected albeit in relatively low amounts. A positive control for AGPs, gum arabic, was strongly bound by LM2 (not recognized by LM13 or LM6). The binding data together with the presence of antibody epitopes and response to enzymatic treatment indicate an interaction of ECP with AGPs.
FIGURE 5.
ECP interactions with spinach glycoproteins. Shown are the results of ELISA of spinach enriched with glycoproteins from roots or leaves with E. coli JT1 expressing ECPIHE3034 (pMat3) or the negative control (pSE380) (A) or with antibody LM6 (all arabinose), LM13 (arabinan), or LM1 (HRGPs) (B). Detection was carried out with or without (native) enzymatic treatments: AF, mannanase (Mase), and PL. Spinach root extracts were characterized from dot blots with the monoclonal antibodies (C). The labels refer to each antibody (Table 3), and “LM2 (gum)” refers to the interaction with gum arabic as a positive control. Significance levels are as follows: ***, p ≤ 0.001; **, p ≤ 0.005; *, p ≤ 0.05; NS, non-significant. Error bars represent S.E.
Functional Binding of ECP in Planta
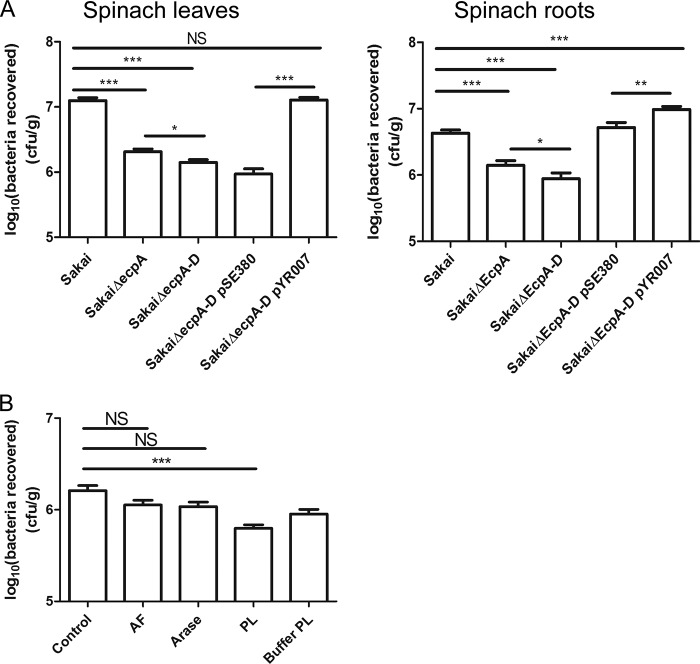
ECP knock-out mutants were constructed to determine whether ECP plays a functional role for E. coli O157:H7 in planta on roots and leaves. The main structural subunit, EcpA, is involved in biofilm formation and cell-cell binding; indeed, imaging of purified ECP fimbriae indicated organelle interactions and bundling (Fig. 1, D and E). Therefore, to distinguish the biofilm effect from a specific interaction, adherence to plants for an ecpA mutant was compared with that for a whole cluster ecpA–D mutant where structural and adhesin genes were removed. Deletion of ecpA in E. coli O157:H7 Sakai resulted in an ∼10-fold reduction in the number of bacteria recovered from spinach leaves that was further reduced with the removal of the whole cluster ecpA–D (p < 0.05) (Fig. 6A). A significant reduction in adherence for the ecpA and ecpA–D mutants was also observed in roots, although the magnitude of the decrease was not as great. Complementation of the ecpA–D mutant (with pYR007) restored the WT phenotype in leaves (p < 0.001) and roots (p < 0.005). Oddly, the mutant complemented with the control plasmid pSE380 became more adherent on spinach roots compared with Sakai WT, which we postulated was due to the effect of isopropyl β-d-thiogalactopyranoside (the inducing agent) elsewhere on the genome. Addition of isopropyl β-d-thiogalactopyranoside to E. coli O157:H7 Sakai WT and both ecp mutants resulted in an increase in bacterial adherence (data not shown), confirming this idea.
FIGURE 6.
Functional adherence of ECP to plant tissue. A, bacteria recovered from spinach leaves and roots following a 2-h adherence assay for E. coli O157:H7 Sakai WT, mutants ΔecpA and ΔecpA–D, and the complemented ΔecpA–D mutant with plasmid pYR007 or the control pSE380. The average number of bacteria is expressed as log10 cfu/g of fresh tissue. B, bacteria recovered from spinach roots following a 2-h adherence assay with AF, arabinanase (Arase), PL, and PL buffer treatments for E. coli O157:H7 Sakai WT. Significance levels are as follows: ***, p ≤ 0.001; **, p ≤ 0.005; *, p ≤ 0.05; NS, non-significant. Error bars represent S.E.
Pretreatment of spinach roots with AF, arabinanase, PL, or mannanase reduced the ability of E. coli O157:H7 Sakai to adhere, although the greatest difference was observed with PL treatment (p < 0.005), indicating the possibility of multiple interactions, including arabinose-dependent ECP (Fig. 6B). The PL buffer (pH 10) was included because the high pH has been demonstrated previously to degrade pectin backbones that break down under basic conditions (50). Moreover, arabinan can contain various esters (e.g. acetyl and feroyl) that are also base-sensitive (51, 52).
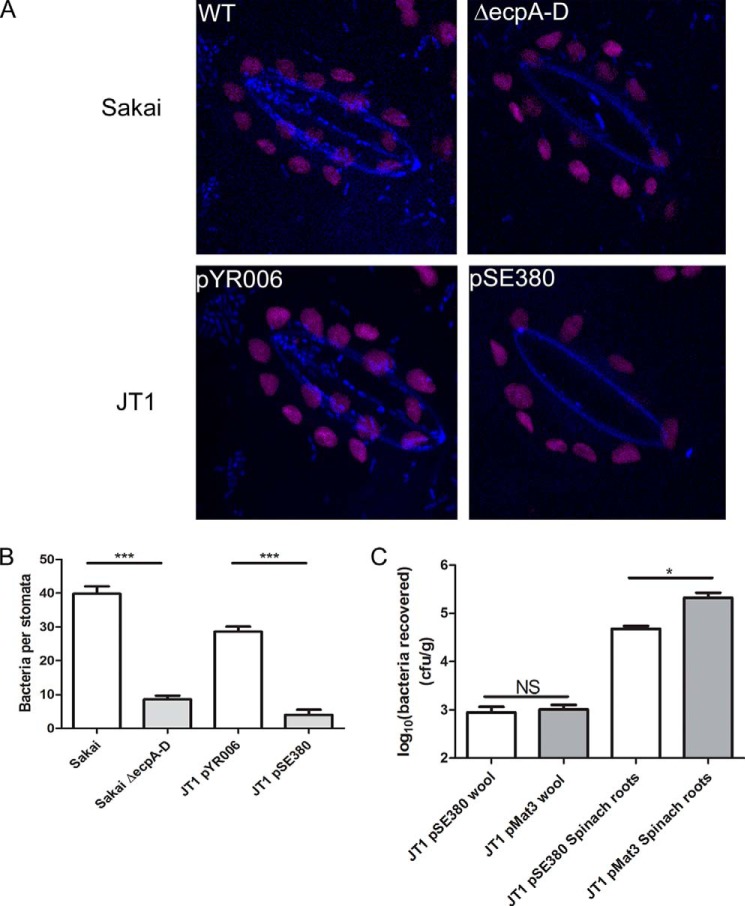
In plant leaves, stomatal guard cell walls are rich in pectin and contain arabinan epitopes (53, 54). Expression of functional ECP fimbriae significantly increased the number of bacteria associated with stomata for both E. coli O157:H7 isolate Sakai and E. coli JT1 overexpressing ECPSakai (from pYR006) (Fig. 7, A and B). To identify unspecific molecular binding mediated by ECP, natural wool was used as an inert, biological substrate to mimic plant root morphology in the absence of any plant glycans (Fig. 7C). There was no significant difference in the number of E. coli JT1 ± ECP (pMat3 or pSE380) recovered from wool (p = 0.721) in contrast to a significant increase exhibited by ECPIHE3034 (pMat3) on spinach roots (p < 0.05).
FIGURE 7.
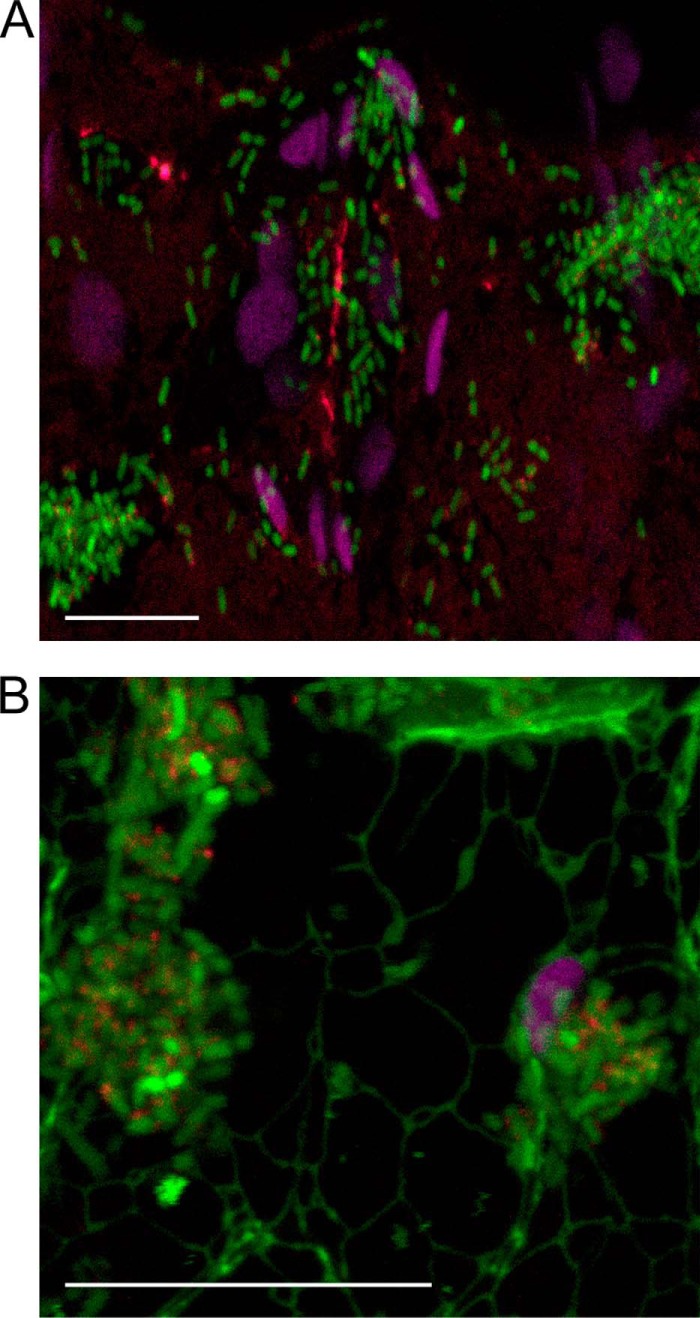
Interaction of E. coli with guard cells. A, confocal microscopy images of a stoma of a fresh spinach leaf inoculated with E. coli O157:H7 Sakai WT, E. coli O157:H7 Sakai ΔecpA–D, or E. coli JT1 expressing ECPSakai (pYR006) or the control plasmid (pSE380). Bacteria are false colored blue, and chloroplast autofluorescence is purple. B, quantification of the different bacteria strains from five different stomata, expressed as the average number of bacteria per stoma. C, bacteria recovered from wool or spinach roots following a 2-h adherence assay for E. coli JT1 expressing ECPIHE3034 (pMat3) or the control plasmid (pSE380). The average number of bacteria is expressed as log10 cfu/g of fresh tissue. Significance levels are as follows: ***, p ≤ 0.001; *, p ≤ 0.05; NS, non-significant. Error bars represent S.E.
Identification of LM13 Epitopes in Spinach
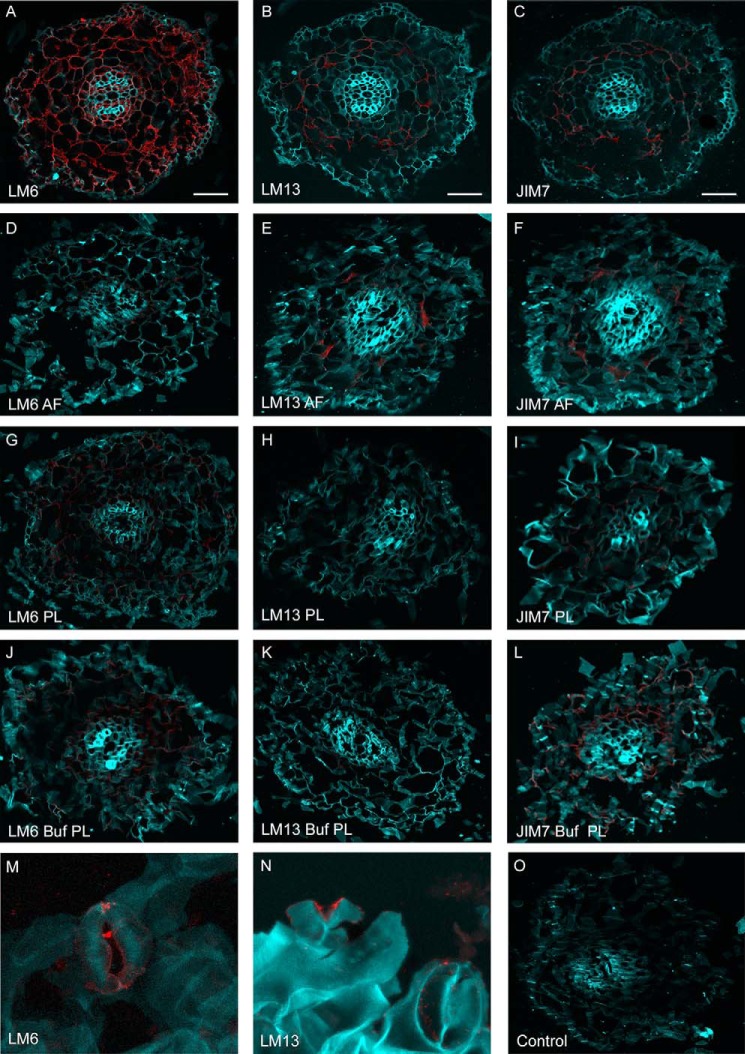
The distributions of LM13 (long arabinans) and LM6 (total arabinans) epitopes in spinach roots were identified using antibody probes together with enzymatic treatment (Fig. 8). Cortical cells appeared to be enriched in pectin as indicated by LM6 and LM13 detection and AF and PL treatment effects (Fig. 8, D, E, G, and H). Although AF treatment reduced signals from all of the antibody probes, PL treatment had a greater effect on LM13 detection. JIM7 (homogalacturonan) (55) and PL buffer treatment alone were included as controls. Epitopes for LM13 and LM6 were also identified in spinach guard cells (Fig. 8, M and N). It was not possible to assess enzymatic treatment on guard cells because of their destructive effects on stomata (53, 54).
FIGURE 8.
Epitope detection of spinach root and leaf sections. Shown are confocal microscopy images of fixed spinach root sections either untreated (A–C) or treated with AF (D–F), PL (G–I), or PL buffer (Buf) (J–L) and then probed with antibody LM6 (all arabinose; A, D, G, and J), LM13 (arabinan; B, E, H, and K), or JIM7 (homogalacturonan; C, F, I, and L). Spinach leaves were probed with LM6 (M) or LM13 (N). Root and leaf tissues were stained with Calcofluor white (light blue), and antibodies were detected with the secondary Alexa Fluor 568-conjugated antibody (red). Scale bars, 8 μm.
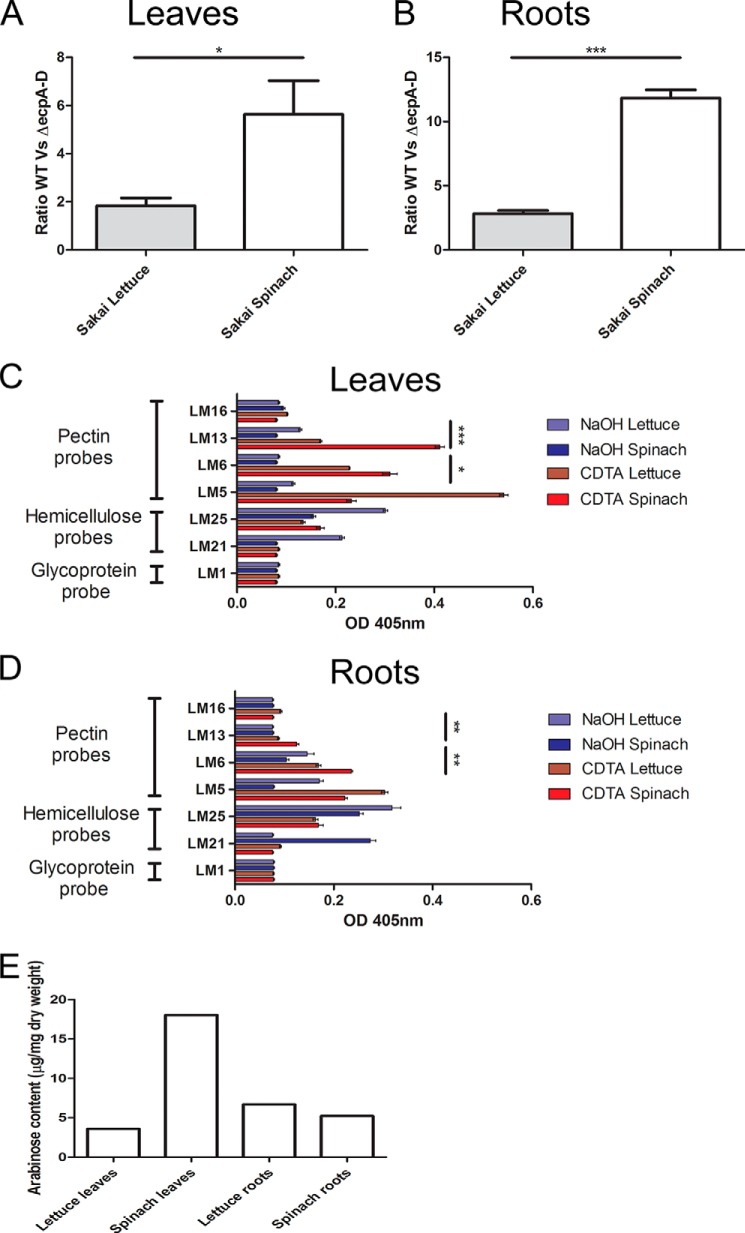
Comparative adherence assays for E. coli O157:H7 WT and ΔecpA–D showed a more pronounced role for ECP to spinach compared with lettuce (L. sativa) (Fig. 9) (the data are expressed as a ratio to compare plant species). To determine whether increased ECP-dependent adherence was a result of greater availability and/or abundance of the ECP target in spinach, cell wall polysaccharides from the roots and leaves of both plant species were analyzed using a range of antibodies to detect epitopes for pectin (LM5, LM6, and LM13), hemicellulose (LM21 and LM25), and extensin (LM1) (Table 3). As expected, the pectin probes reacted most strongly with CDTA-derived extracts, whereas the hemicellulose probes reacted with NaOH-derived extracts (Fig. 9, C and D). LM13 and LM6 gave signals in both plant species, but higher levels were detected in spinach. The trend was the same for both leaves and roots albeit with differences in the complement of the specific residues. Monosaccharide quantification of plant extracts, including arabinose (Table 5), determined from HPLC analysis correlated with the epitope presence (Fig. 9E).
FIGURE 9.
Plant species-dependent differences for adherence and polysaccharide composition. A and B, bacteria recovered from lettuce and spinach leaves or roots following a 2-h adherence assay for E. coli O157:H7 Sakai WT and ΔecpA–D. The average number of bacteria recovered is expressed as a ratio of WT versus ΔecpA–D mutant. C and D, ELISA of lettuce and spinach polysaccharide extracts (CDTA and NaOH) from roots and leaves with LM16 (uncharacterized RG-I epitope), LM13 (arabinan), LM6 (all arabinose), LM5 (galactan), LM25 (galactosylated xyloglucan), LM21 (mannan), or LM1 (HRGPs). E, HPLC analysis of arabinose monosaccharides present in a total polysaccharide extraction of lettuce and spinach root and leaves. Significance levels are as follows: ***, p ≤ 0.001; **, p ≤ 0.005; *, p ≤ 0.05. Error bars represent S.E.
TABLE 5.
Monosaccharide composition and content (%) of spinach and lettuce tissue
| Monosaccharide | Lettuce leaves | Spinach leaves | Lettuce roots | Spinach roots |
|---|---|---|---|---|
| Fucose | 0.31 | 1.17 | 0.13 | 0.98 |
| Galactose | 1.06 | 1.57 | 5.25 | 1.83 |
| Glucose | 54.54 | 25.94 | 29.90 | 5.77 |
| Xylose | 1.27 | 4.50 | 2.57 | 2.48 |
| Mannose | 5.50 | 2.96 | 2.63 | 7.39 |
| Galacturonic acid | 4.78 | 5.18 | 1.30 | 3.62 |
| Glucuronic acid | 3.62 | 1.83 | 0.61 | 12.39 |
| Arabinose | 24.82 | 47.23 | 47.08 | 63.83 |
| Rhamnose | 4.10 | 9.61 | 10.54 | 1.71 |
Functional Expression of ECP in Planta
E. coli O157:H7 has been shown to encode an active promoter site for the ecp operon (24). To observe ECP transcription and production in planta, we examined spinach leaves inoculated with E. coli O157:H7 Sakai transformed with a plasmid-encoded ecpR-GFP transcriptional fusion after anti-ECP detection. Expression of ecpR-GFP (green) was evident in most cells, and elicitation of ECP fimbriae (red) was also observed. Differences in the green fluorescence levels suggested that ecpR transcription may be heterogeneous in planta (Fig. 10). A different plant species was evaluated (a) to confirm that ECP expression is not restricted to spinach plants and (b) to observe any co-localization with particular plant tissues. N. benthamiana line mGFP5-ER expresses GFP fused to an endoplasmic reticulum protein (36). Inspection of a guard cell shows the green network of the endoplasmic reticulum together with bacteria expressing ecpR (green) and ECP fimbriae (red) adjacent to a chloroplast (purple) and on the leaf epidermis (Fig. 10B).
FIGURE 10.
ECP expression and elicitation in planta. Shown are confocal images of spinach (A) or N. benthamiana expressing mGFP5-ER (B) leaves inoculated with E. coli O157:H7 Sakai WT expressing ecpR-GFP (green) and ECP fimbriae (red) 4 h postinoculation. Chloroplast autofluorescence is false colored purple, and endoplasmic reticulum is green for N. benthamiana. Scale bars, 20 μm.
EcpD Sequence Variation
ECP fimbriae are almost ubiquitous in E. coli and are also encoded by related members of the Enterobacteriaceae, although the EcpA (main structural subunit) and EcpD (adhesin) coding sequences are not completely conserved. Alignment of 66 E. coli EcpD amino acid sequences highlighted variable residues (Table 6). The types of substitutions for the most frequent changes included conserved (e.g. L145M); non-polar aliphatic to polar, uncharged (e.g. R232L); and vice versa (e.g. A117T). The mature peptide of EcpD from E. coli from isolates Sakai and IHE3034 (used in this study) contains three amino acid substitutions.
TABLE 6.
EcpD sequence variation
A sequence comparison of 66 E. coli EcpD amino acid sequences is shown. The frequency of each substitution is shown as a percentage of the total number of occurrences in this group. AA, amino acid.
| AA positiona | Consensus | Substitution | Percentage (n = 66) |
|---|---|---|---|
| −15 | Thr | Ala | 43.9 |
| −5 | Val | Ala | 39.4 |
| −3 | Ala | Glu | 19.7 |
| 14 | Arg | Ser | 1.5 |
| 51 | Asn | Thr | 42.4 |
| 117 | Ala | Thr | 18.2 |
| 130 | Ala | Thr | 3 |
| 145 | Leu | Met | 15.2 |
| 157 | Thr | Ile | 1.5 |
| 170 | Ala | Asn | 10.6 |
| 180 | Ser | Thr | 1.5 |
| 222 | Ala | Thr | 1.5 |
| 229 | Ile | Met | 1.5 |
| 232 | Arg | Leu | 21.2 |
| 252 | Thr | Ile | 1.5 |
| 319 | Leu | Gln | 25.8 |
| 354 | Ile | Leu | 7.6 |
| 380 | Thr | Ala | 1.5 |
| 392 | Thr | Ile | 1.5 |
| 441 | Thr | Ala | 9.1 |
| 448 | Thr | Ile | 1.5 |
a Assumes a signal peptide from position −1 to −23.
DISCUSSION
Identification and characterization of the molecular targets underpinning the adhesion of bacteria (phytopathogenic or human pathogenic) to plants is limited compared with that for mammalian and animal hosts (57, 58). So far, only an interaction between E. coli flagella and ionic lipids has been described where flagella can intercalate into plant plasma membranes (4). The interaction between α-(1→5)-l-arabinose in plant cell walls and ECP derived from a well known human pathogen associated with outbreaks from fresh produce is the first description of its type. l-Arabinose is synthesized by the plant kingdom where it is very common, whereas only some bacterial species synthesize d-arabinose polysaccharides (59). l-Arabinose is present in homo- as well as heteropolysaccharides and is O-linked on HRGPs (60). Interaction with ECP required oligomerization of at least three residues of arabinose, and specificity correlated with the antibody LM13. LM13 primarily recognizes linear epitopes of (1→5)-α-l-arabinans present in RG-I polysaccharides of pectin as well as other arabinose residues, e.g. in AGPs where the structural context is correct (45). The overlap in recognition shows that ECP recognized pectic arabinans and potentially O-glycosylated hydroxyproline proteins (AGPs), which contain arabinose and galactose, ranging from a single residue to up to 75 arabinogalactan residues (28, 61).
Stomatal guard cells, which are rich in pectic arabinan (53, 54) and have been shown to be a target for bacteria (4, 62), were targeted by bacteria expressing ECP. ECP fimbriae bound preferentially to pectin fractions in polysaccharides extracted from spinach compared with long oligomers of arabinan purified from beet roots that were either branched or unbranched (63). Sugar beet arabinan consists of an α-(1→5)-linked backbone of l-arabinosyl residues that are either single or double substituted (64). Treatment of unbranched arabinan to remove (1→2)- and (1→3)-α-l-arabinofuranosyl branches revealed a specificity of ECPSakai, indicating a potential affinity for α-(1→5)-linked l-arabinosyl residues and longer chains of arabinan. This may explain why ECPSakai was slightly more adherent on pectin polysaccharides extracted from spinach roots than ECPIHE3034. The significance of structural context may explain differences in the extent of ECP binding to plant extracts compared with that on the purified oligosaccharides.
Sequences of EcpD, the adhesin, are extremely well conserved in all E. coli genomes and especially between the serotype O157:H7 strains. However, differences in the EcpD mature protein may be sufficient to alter receptor specificity as has been observed for type 1 fimbriae (65). ECP tropism for pectic arabinan was confirmed with pectate lyase treatment, which degrades homogalacturonan chains, the main component of pectin polysaccharides (66), and releases the arabinose-substituted pectin polysaccharides. The high potency of pectate lyase activity means that it may have some arabinase activity even at low concentrations. Furthermore, because of the many intermolecular connections between cell wall components, enzymatic treatments that do not target arabinan specifically may nonetheless impact its availability. ECP interaction with spinach extracts enriched in glycoproteins was only affected by arabinofuranosidase treatment. The data indicate that despite the presence of HG backbone in the extracts pectic arabinans were not abundant and that ECP also interacts with AGPs. The apparent lack of pectate lysase activity on the HG component can be accounted for by the requirement for Ca2+ for catalysis; Ca2+ ions are absent in the methylated form of HG (67). In summary, it appears that arabinan from pectin polysaccharides and possibly plant glycoproteins play a role in E. coli ECP-dependent adhesion in planta. In turn, the distribution of arabinan could affect tropism for the bacteria: toward guard cells in leaves and cortical cells within root tissue just as is seen for PapD-dependent tropism to galactans in renal tissue.
Functional binding of ECP to plant tissue appears to be conferred by the adhesin, EcpD, and is supported by the presence of the main structural subunit, EcpA. Because ECP did not significantly contribute to binding to wool (non-plant substrate with a morphology broadly similar to plant root), we concluded that a biofilm-associated function of EcpA is negligible during short term incubation on plants. EcpD can be secreted and polymerized/oligomerized in the absence of EcpA (18) as an “afimbrial” adhesin, although the processes may not be as efficient as in the presence of EcpA. Moreover, as with type 1 fimbriae, EcpA itself could play a role in the specificity (68). Our data show that the EcpA fimbrial structure is required for presentation of EcpD to target cell wall arabinans during interactions with plant hosts. The fact that bacterial binding to plant tissue was not completely inhibited by removal of the ECP cluster or enzymatic treatment points to a role for other functional adherence factors, including flagella, to mediate plant interactions.
Functional adherence of ECP in planta occurred at submammalian host temperatures and was coupled with expression of the ecpR regulator. This supports the reports of low temperature expression at 20 °C (15, 24). Variation in the level of ecpR expression may in turn reflect the amount of ECP organelle expressed from this polycistronic operon. This finding is in line with the reported alternative transcriptional start sites for ecpR (24).
Both ECP variants from E. coli isolates Sakai and IHE3034 demonstrated enhanced adherence to spinach root polysaccharides compared with leaves, which supports previous findings of increased colonization in the roots (69). Furthermore, higher levels of ECP-mediated adherence of E. coli O157:H7 occurred in spinach compared with lettuce, which may be a result of the reduced arabinan in lettuce. It is of note that verotoxigenic E. coli are isolated from spinach plants at higher rates compared with lettuce (70, 71) and that consumption of raw spinach has risen by 180% in the United States from 1992 to 2005 (49). This information together with ECP specificity for arabinose may go some way to explain why verotoxigenic E. coli outbreaks are more frequently associated with spinach.
Demonstration of adherence mediated by flagella together with specific recognition of a plant oligosaccharide by an adhesin (4) suggests a stepwise process of E. coli adherence to plant cells. First, a broad molecular recognition occurs between flagella and plasma membranes without any particular tropism. This is then followed by a specific, adhesin-mediated adherence, e.g. ECP to cell wall pectin polysaccharides. Exploitation of such an abundant plant-specific glycan may aid the spread of E. coli in the environment, and given the ubiquity of ECP, it could be a reasonably common mechanism of binding to plants (and for related enterobacteria). However, differences in ecp cluster expression and EcpD amino acid sequence are likely to affect both the occurrence of binding and the affinity.
The ability of mammalian pathogenic bacteria to target an intermediate host for survival and transmission is not without precedent: the spread of Vibrio cholerae, a well known human pathogen, through freshwater and marine environments (72, 73) is dependent on its interaction with chitin polysaccharide in zooplankton (74, 75). Furthermore, pathogenic strains of V. cholerae show increased fitness in bivalves compared with environmental isolates (76). Similarly, ECP-arabinan interactions may play an important role in allowing pathogenic E. coli to use plants as a secondary host and as a vehicle to spread to other primary hosts such as cattle or humans.
Supplementary Material
Acknowledgments
We thank Dr. Timo K. Korhonen for the kind gift of pMat3, Dr. Sean McAteer for the kind gift of pTOF1, Dr. Rob Hancock for help with the HPLC analysis, and Dr. Kath Wright and Dr. Sean Chapman for help with the microscopy analysis.
This work was supported by Leverhulme Trust Grant RPG-096 and the Biotechnology and Biological Sciences Research Council Grant BB/I014179/1.

This article contains supplemental Table 1.
- ECP
- E. coli common pilus
- Mat
- meningitis-associated and temperature-regulated
- HG
- homogalacturonan
- RG
- rhamnogalacturonan
- HRGP
- hydroxyproline-rich glycoprotein
- AGP
- arabinogalactan protein
- CDTA
- diaminocyclohexanetetraacetic acid
- Ara7
- arabinoheptaose
- AF
- arabinofuranosidase
- PL
- pectin lyase.
REFERENCES
- 1. Holden N., Pritchard L., Toth I. (2009) Colonization outwith the colon: plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol. Rev. 33, 689–703 [DOI] [PubMed] [Google Scholar]
- 2. Berger C. N., Sodha S. V., Shaw R. K., Griffin P. M., Pink D., Hand P., Frankel G. (2010) Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 12, 2385–2397 [DOI] [PubMed] [Google Scholar]
- 3. Brandl M. T. (2006) Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44, 367–392 [DOI] [PubMed] [Google Scholar]
- 4. Rossez Y., Holmes A., Wolfson E. B., Gally D. L., Mahajan A., Pedersen H. L., Willats W. G., Toth I. K., Holden N. J. (2014) Flagella interact with ionic plant lipids to mediate adherence of pathogenic Escherichia coli to fresh produce plants. Environ. Microbiol. 16, 2181–2195 [DOI] [PubMed] [Google Scholar]
- 5. Horby P. W., O'Brien S. J., Adak G. K., Graham C., Hawker J. I., Hunter P., Lane C., Lawson A. J., Mitchell R. T., Reacher M. H., Threlfall E. J., Ward L. R., and PHLS Outbreak Investigation Team (2003) A national outbreak of multi-resistant Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol. Infect. 130, 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doyle M.P., Erickson M. C. (2008) Summer meeting 2007—the problems with fresh produce: an overview. J. Appl. Microbiol. 105, 317–330 [DOI] [PubMed] [Google Scholar]
- 7. Hanning I. B., Nutt J. D., Ricke S. C. (2009) Salmonellosis outbreaks in the United States due to fresh produce: sources and potential intervention measures. Foodborne Pathog. Dis. 6, 635–648 [DOI] [PubMed] [Google Scholar]
- 8. Sivapalasingam S., Friedman C. R., Cohen L., Tauxe R. V. (2004) Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67, 2342–2353 [DOI] [PubMed] [Google Scholar]
- 9. Rangel J. M., Sparling P. H., Crowe C., Griffin P. M., Swerdlow D. L. (2005) Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11, 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frank C., Werber D., Cramer J. P., Askar M., Faber M., an der Heiden M., Bernard H., Fruth A., Prager R., Spode A. (2011) Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365, 1771–1780 [DOI] [PubMed] [Google Scholar]
- 11. Delaquis P., Bach S., Dinu L.-D. (2007) Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Prot. 70, 1966–1974 [DOI] [PubMed] [Google Scholar]
- 12. Valentin-Bon I., Jacobson A., Monday S. R., Feng P. C. (2008) Microbiological quality of bagged cut spinach and lettuce mixes. Appl. Environ. Microbiol. 74, 1240–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choudhury D., Thompson A., Stojanoff V., Langermann S., Pinkner J., Hultgren S. J., Knight S. D. (1999) X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 285, 1061–1066 [DOI] [PubMed] [Google Scholar]
- 14. Dodson K. W., Pinkner J. S., Rose T., Magnusson G., Hultgren S. J., Waksman G. (2001) Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105, 733–743 [DOI] [PubMed] [Google Scholar]
- 15. Pouttu R., Westerlund-Wikström B., Lång H., Alsti K., Virkola R., Saarela U., Siitonen A., Kalkkinen N., Korhonen T. K. (2001) matB, a common fimbrillin gene of Escherichia coli, expressed in a genetically conserved, virulent clonal group. J. Bacteriol. 183, 4727–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avelino F., Saldaña Z., Islam S., Monteiro-Neto V., Dall'Agnol M., Eslava C. A., Girón J. A. (2010) The majority of enteroaggregative Escherichia coli strains produce the E. coli common pilus when adhering to cultured epithelial cells. Int. J. Med. Microbiol. 300, 440–448 [DOI] [PubMed] [Google Scholar]
- 17. Rendón M. A., Saldaña Z., Erdem A. L., Monteiro-Neto V., Vázquez A., Kaper J. B., Puente J. L., Girón J. A. (2007) Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad Sci. U.S.A. 104, 10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garnett J. A., Martínez-Santos V. I., Saldaña Z., Pape T., Hawthorne W., Chan J., Simpson P. J., Cota E., Puente J. L., Girón J. A., Matthews S. (2012) Structural insights into the biogenesis and biofilm formation by the Escherichia coli common pilus. Proc. Natl. Acad Sci. U.S.A. 109, 3950–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alcántar-Curiel M. D., Blackburn D., Saldaña Z., Gayosso-Vázquez C., Iovine N. M., De la Cruz M. A., Girón J. A. (2013) Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 4, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saldaña Z., Erdem A. L., Schüller S., Okeke I. N., Lucas M., Sivananthan A., Phillips A. D., Kaper J. B., Puente J. L., Girón J. A. (2009) The Escherichia coli common pilus and the bundle-forming pilus act in concert during the formation of localized adherence by enteropathogenic E. coli. J. Bacteriol. 191, 3451–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lasaro M. A., Salinger N., Zhang J., Wang Y., Zhong Z., Goulian M., Zhu J. (2009) F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl. Environ. Microbiol. 75, 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehti T. A., Bauchart P., Heikkinen J., Hacker J., Korhonen T. K., Dobrindt U., Westerlund-Wikström B. (2010) Mat fimbriae promote biofilm formation by meningitis-associated Escherichia coli. Microbiology 156, 2408–2417 [DOI] [PubMed] [Google Scholar]
- 23. Lehti T. A., Bauchart P., Dobrindt U., Korhonen T. K., Westerlund-Wikström B. (2012) The fimbriae activator MatA switches off motility in Escherichia coli by repression of the flagellar master operon flhDC. Microbiology 158, 1444–1455 [DOI] [PubMed] [Google Scholar]
- 24. Lehti T. A., Bauchart P., Kukkonen M., Dobrindt U., Korhonen T. K., Westerlund-Wikström B. (2013) Phylogenetic group-associated differences in regulation of the common colonization factor Mat fimbria in Escherichia coli. Mol. Microbiol. 87, 1200–1222 [DOI] [PubMed] [Google Scholar]
- 25. Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J., Osborne E., Paredez A., Persson S., Raab T., Vorwerk S., Youngs H. (2004) Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211 [DOI] [PubMed] [Google Scholar]
- 26. Cosgrove D. J. (2005) Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861 [DOI] [PubMed] [Google Scholar]
- 27. Mohnen D. (2008) Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 [DOI] [PubMed] [Google Scholar]
- 28. Ellis M., Egelund J., Schultz C. J., Bacic A. (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol. 153, 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kieliszewski M. J., Lamport D. T. (1994) Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 5, 157–172 [DOI] [PubMed] [Google Scholar]
- 30. Hayashi T., Makino K., Ohnishi M., Kurokawa K., Ishii K., Yokoyama K., Han C. G., Ohtsubo E., Nakayama K., Murata T., Tanaka M., Tobe T., Iida T., Takami H., Honda T., Sasakawa C., Ogasawara N., Yasunaga T., Kuhara S., Shiba T., Hattori M., Shinagawa H. (2001) Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8, 11–22 [DOI] [PubMed] [Google Scholar]
- 31. Westerlund-Wikström B., Tanskanen J., Virkola R., Hacker J., Lindberg M., Skurnik M., Korhonen T. K. (1997) Functional expression of adhesive peptides as fusions to Escherichia coli flagellin. Protein Eng. 10, 1319–1326 [DOI] [PubMed] [Google Scholar]
- 32. Neidhardt F. C., Bloch P. L., Smith D. F. (1974) Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merlin C., McAteer S., Masters M. (2002) Tools for characterization of Escherichia coli genes of unknown function. J. Bacteriol. 184, 4573–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. (1980) New method for isolation of immunologically pure pili from Escherichia coli. Infect. Immun. 27, 569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McMichael J. C., Ou J. T. (1979) Structure of common pili from Escherichia coli. J. Bacteriol. 138, 969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz M. T., Voinnet O., Baulcombe D. C. (1998) Initiation and maintenance of virus-induced gene silencing. Plant Cell 10, 937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karlova R., Boeren S., Russinova E., Aker J., Vervoort J., de Vries S. (2006) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell 18, 626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pedersen H. L., Fangel J. U., McCleary B., Ruzanski C., Rydahl M. G., Ralet M.-C., Farkas V., von Schantz L., Marcus S. E., Andersen M. C., Field R., Ohlin M., Knox J. P., Clausen M. H., Willats W. G. (2012) Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 287, 39429–39438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willats W. G., Marcus S. E., Knox J. P. (1998) Generation of monoclonal antibody specific to (1–5)-α-L-arabinan. Carbohydr. Res. 308, 149–152 [DOI] [PubMed] [Google Scholar]
- 40. Ross H. A., Morris W. L., Ducreux L. J., Hancock R. D., Verrall S. R., Morris J. A., Tucker G. A., Stewart D., Hedley P. E., McDougall G. J. (2011) Pectin engineering to modify product quality in potato. Plant Biotechnol. J. 9, 848–856 [DOI] [PubMed] [Google Scholar]
- 41. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 42. Michino H., Araki K., Minami S., Takaya S., Sakai N., Miyazaki M., Ono A., Yanagawa H. (1999) Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150, 787–796 [DOI] [PubMed] [Google Scholar]
- 43. Lee K. J., Sakata Y., Mau S.-L., Pettolino F., Bacic A., Quatrano R. S., Knight C. D., Knox J. P. (2005) Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 17, 3051–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verhertbruggen Y., Marcus S. E., Haeger A., Verhoef R., Schols H. A., McCleary B. V., McKee L., Gilbert H. J., Knox J. P. (2009) Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 59, 413–425 [DOI] [PubMed] [Google Scholar]
- 45. Moller I., Marcus S. E., Haeger A., Verhertbruggen Y., Verhoef R., Schols H., Ulvskov P., Mikkelsen J. D., Knox J. P., Willats W. (2008) High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj. J. 25, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention (CDC) (2006) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach-United States, September 2006. MMWR Morb. Mortal. Wkly. Rep. 55, 1045–1046 [PubMed] [Google Scholar]
- 47. Grant J., Wendelboe A. M., Wendel A., Jepson B., Torres P., Smelser C., Rolfs R. T. (2008) Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New Mexico, 2006. Emerg Infect. Dis. 14, 1633–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wendel A. M., Johnson D. H., Sharapov U., Grant J., Archer J. R., Monson T., Koschmann C., Davis J. P. (2009) Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August-September 2006: the Wisconsin investigation. Clin. Infect. Dis. 48, 1079–1086 [DOI] [PubMed] [Google Scholar]
- 49. Calvin L. (2007) Outbreak linked to spinach forces reassessment of food safety practices. Amber Waves 5, 24–31 [Google Scholar]
- 50. Marcus S. E., Blake A. W., Benians T. A., Lee K. J., Poyser C., Donaldson L., Leroux O., Rogowski A., Petersen H. L., Boraston A., Gilbert H. J., Willats W. G., Knox J. P. (2010) Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 64, 191–203 [DOI] [PubMed] [Google Scholar]
- 51. Friedman M., Jürgens H. S. (2000) Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 48, 2101–2110 [DOI] [PubMed] [Google Scholar]
- 52. Levigne S. V., Ralet M.-C., Quéméner B. C., Pollet B. N., Lapierre C., Thibault J.-F. (2004) Isolation from sugar beet cell walls of arabinan oligosaccharides esterified by two ferulic acid monomers. Plant Physiol. 134, 1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jones L., Milne J. L., Ashford D., McCann M. C., McQueen-Mason S. J. (2005) A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta 221, 255–264 [DOI] [PubMed] [Google Scholar]
- 54. Jones L., Milne J. L., Ashford D., McQueen-Mason S. J. (2003) Cell wall arabinan is essential for guard cell function. Proc. Natl. Acad. Sci. U.S.A. 100, 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Clausen M. H., Willats W. G., Knox J. P. (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338, 1797–1800 [DOI] [PubMed] [Google Scholar]
- 56. Marcus S. E., Verhertbruggen Y., Hervé C., Ordaz-Ortiz J. J., Farkas V., Pedersen H. L., Willats W. G., Knox J. P. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mhedbi-Hajri N., Jacques M.-A., Koebnik R. (2011) in Bacterial Adhesion: Chemistry, Biology and Physics (Linke D., Goldman A., eds) pp. 71–89, Springer-Verlag, Berlin [Google Scholar]
- 58. Rodríguez-Navarro D. N., Dardanelli M. S., Ruíz-Saínz J. E. (2007) Attachment of bacteria to the roots of higher plants. FEMS Microbiol. Lett. 272, 127–136 [DOI] [PubMed] [Google Scholar]
- 59. Brennan P. J., Crick D. C. (2007) The cell-wall core of Mycobacterium tuberculosis in the context of drug discovery. Curr. Top. Med. Chem. 7, 475–488 [DOI] [PubMed] [Google Scholar]
- 60. Etzler M. E., Mohnen D. (2009) Chapter 22: Viridiplantae in: Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 61. Kieliszewski M. J., O'Neill M., Leykam J., Orlando R. (1995) Tandem mass spectrometry and structural elucidation of glycopeptides from a hydroxyproline-rich plant cell wall glycoprotein indicate that contiguous hydroxyproline residues are the major sites of hydroxyproline O-arabinosylation. J. Biol. Chem. 270, 2541–2549 [DOI] [PubMed] [Google Scholar]
- 62. Berger C. N., Shaw R. K., Ruiz-Perez F., Nataro J. P., Henderson I. R., Pallen M. J., Frankel G. (2009) Interaction of enteroaggregative Escherichia coli with salad leaves. Environ. Microbiol. Rep. 1, 234–239 [DOI] [PubMed] [Google Scholar]
- 63. Weinstein L., Albersheim P. (1979) Structure of plant cell walls IX. Purification and partial characterization of a wall-degrading endo-arabanase and an arabinosidase from Bacillus subtilis. Plant Physiol. 63, 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Westphal Y., Kühnel S., de Waard P., Hinz S. W., Schols H. A., Voragen A. G., Gruppen H. (2010) Branched arabino-oligosaccharides isolated from sugar beet arabinan. Carbohydr. Res. 345, 1180–1189 [DOI] [PubMed] [Google Scholar]
- 65. Kisiela D. I., Chattopadhyay S., Libby S. J., Karlinsey J. E., Fang F. C., Tchesnokova V., Kramer J. J., Beskhlebnaya V., Samadpour M., Grzymajlo K. (2012) Evolution of Salmonella enterica virulence via point mutations in the fimbrial adhesin. PLoS Pathog. 8, e1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ridley B. L., O'Neill M. A., Mohnen D. (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967 [DOI] [PubMed] [Google Scholar]
- 67. Knox J. P., Linstead P. J., King J., Cooper C., Roberts K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521 [DOI] [PubMed] [Google Scholar]
- 68. Duncan M. J., Mann E. L., Cohen M. S., Ofek I., Sharon N., Abraham S. N. (2005) The distinct binding specificities exhibited by enterobacterial type 1 fimbriae are determined by their fimbrial shafts. J. Biol. Chem. 280, 37707–37716 [DOI] [PubMed] [Google Scholar]
- 69. Wright K. M., Chapman S., McGeachy K., Humphris S., Campbell E., Toth I. K., Holden N. J. (2013) The endophytic lifestyle of Escherichia coli O157:H7: quantification and internal localization in roots. Phytopathology 103, 333–340 [DOI] [PubMed] [Google Scholar]
- 70. Feng P. C., Councell T., Keys C., Monday S. R. (2011) Virulence characterization of Shiga-toxigenic Escherichia coli isolates from wholesale produce. Appl. Environ. Microbiol. 77, 343–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feng P. C., Reddy S. (2013) Prevalences of Shiga toxin subtypes and selected other virulence factors among Shiga-toxigenic Escherichia coli strains isolated from fresh produce. Appl. Environ. Microbiol. 79, 6917–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. (1980) Cholera—a possible endemic focus in the United States. N. Engl. J. Med. 302, 305–309 [DOI] [PubMed] [Google Scholar]
- 73. Colwell R. R., Kaper J., Joseph S. W. (1977) Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198, 394–396 [PubMed] [Google Scholar]
- 74. Huq A., Sack R. B., Nizam A., Longini I. M., Nair G. B., Ali A., Morris J. G., Jr., Khan M. N., Siddique A. K., Yunus M., Albert M. J., Sack D. A., Colwell R. R. (2005) Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71, 4645–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lutz C., Erken M., Noorian P., Sun S., McDougald D. (2013) Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 4, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Collin B., Rehnstam-Holm A.-S., Lindmark B., Pal A., Wai S. N., Hernroth B. (2012) The origin of Vibrio cholerae influences uptake and persistence in the blue mussel Mytilus edulis. J. Shell Res. 31, 87–92 [Google Scholar]
- 77. Holden N., Totsika M., Dixon L., Catherwood K., Gally D. L. (2007) Regulation of P-fimbrial phase variation frequencies in Escherichia coli CFT073. Infect. Immun. 75, 3325–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smallwood M., Martin H., Knox J. P. (1995) An epitope of rice threonine-and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 196, 510–522 [DOI] [PubMed] [Google Scholar]
- 79. Yates E. A., Valdor J. F., Haslam S. M., Morris H. R., Dell A., Mackie W., Knox J. P. (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6, 131–139 [DOI] [PubMed] [Google Scholar]
- 80. Jones L., Seymour G. B., Knox J. P. (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiol. 113, 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.