Abstract
Background
Evidence suggests that high-dose statin pretreatment may reduce the risk of periprocedural myocardial infarction (PMI) and major adverse cardiac events (MACE) for certain patients; however, previous analyses have not considered patients with a history of statin maintenance treatment. In this meta-analysis of randomized controlled trials (RCTs), we reevaluated the efficacy of short-term high-dose statin pretreatment to prevent PMI and MACE in an expanded set of patients undergoing elective percutaneous coronary intervention.
Methods
We searched the PubMed/Medline database for RCTs that compared high-dose statin pretreatment with no statin or low-dose statin pretreatment as a prevention of PMI and MACE. We evaluated the incidence of PMI and MACE, including death, spontaneous myocardial infarction, and target vessel revascularization at the longest follow-up for each study for subgroups stratified by disease classification and prior low-dose statin treatment.
Results
Twenty-four RCTs with a total of 5,526 patients were identified. High-dose statin pretreatment was associated with 59% relative reduction in PMI (odds ratio [OR]: 0.41; 95% confidence interval [CI]: 0.34–0.49; P<0.00001) and 39% relative reduction in MACE (OR: 0.61; 95% CI: 0.45–0.83; P = 0.002). The benefit of high-dose statin pretreatment on MACE was significant for statin-naive patients (OR: 0.69; 95% CI: 0.50–0.95; P = 0.02) and prior low dose statin-treated patients (OR: 0.28; 95% CI: 0.12–0.65; P = 0.003); and for patients with acute coronary syndrome (OR: 0.52; 95% CI: 0.34–0.79; P = 0.003), but not for patients with stable angina (OR: 0.71; 95% CI 0.45–1.10; P = 0.12). Long-term effects on survival were less obvious.
Conclusions
High-dose statin pretreatment can result in a significant reduction in PMI and MACE for patients undergoing elective PCI. The positive effect of high-dose statin pretreatment on PMI and MACE is significant for statin-naïve patients and patients with prior treatment. The positive effect of high-dose statin pretreatment on MACE is significant for patients with acute coronary syndrome.
Introduction
Percutaneous coronary intervention (PCI) is an important method in the treatment of coronary artery disease. Periprocedural myocardial infarction (PMI), characterized by cardiac biomarker elevation, is a common complication of PCI [1]. Studies have suggested that side branch occlusion, distal embolism, coronary dissection, disruption of collateral flow, and inflammation can result in PMI [1], [2]. Although most patients who have PMI remain asymptotic and have no change in cardiac function, PMI has been associated with higher mortality [3]. A meta-analysis showed that even small increases in creatine kinase-MB (CK-MB) are associated with significantly higher risk of death during follow-up [4]. Several therapeutic strategies, including statins, antithrombotic agents [5], [6], and β-blockers [7], [8], have been suggested to reduce PMI. Statins, which inhibit hydroxymethylglutaryl-CoA reductase, have been demonstrated to have pleiotropic effects, including rapid anti-inflammatory and antithrombotic properties [9], [10], antioxidant effects [11], improvement of endothelial dysfunction [12], and stabilization of atherosclerotic plaques [13]. Therefore, statins are regarded as an important agent for the prevention of PMI.
A 2011 meta-analysis [14] comprising 3341 patients from 13 randomized, controlled trials (RCTs) indicated that high-dose statin pretreatment is associated with a significant reduction in PMI and major adverse cardiac events (MACE) in patients undergoing PCI within thirty days. However, more recent clinical trials showed that early use of high-dose statin before PCI did not reduce the occurrence of PMI or improve the long-term clinical outcomes [15]–[18]. Another more recent meta-analysis of 14 RCTs with 3146 patients [19] showed that high-dose statin loading prior to PCI reduced clinical events for patients with acute coronary syndrome (ACS) but not stable angina. However, the latter study was not exhaustive and did not consider subgroups receiving chronic statin therapy prior to the high dose pretreatment. Therefore, a more comprehensive analysis of the benefits of high-dose statin reloading before PCI, including patients under long-term statin therapy, is needed. Thus, we performed a meta-analysis of 24 RCTs comprising 5,526 patients to reevaluate the efficacy of a short-term high-dose statin pretreatment to prevent PMI and MACE in patients undergoing elective PCI.
Methods
Search strategy and inclusion criteria
In this meta-analysis, two authors independently conducted a thorough search of the PubMed/Medline database for the reports of all RCTs conducted until January 2014 that compare the clinical outcomes of high-dose statin pretreatment with no-statin or low-dose statin pretreatment in patients undergoing elective PCI. Search keywords were “statins”, “statin”, “atorvastatin”, “rosuvastatin”, “cervastatin”, “simvastatin”, “pravastatin”, “lovastatin”, “fluvastatin”, “hydroxymethylglutaryl-CoA”, “percutaneous coronary intervention”, “PCI”, “stent” and “randomized”. We also reviewed the references within related meta-analyses. The primary endpoints of the analysis were the incidences of PMI and MACE. The definition of PMI was taken from the original articles. MACE included death, spontaneous myocardial infarction (MI), and target vessel revascularization (TVR) at the longest follow-up for each study. Studies were included in the present meta-analysis if they met the following criteria: (1) the studies were written in English; (2) the studies were RCTs conducted on humans; (3) the studies did not include patients undergoing primary PCI and non-ST elevation acute coronary syndrome (NSTE-ACS) with high-risk features needing emergency coronary angiography; (4) the studies included a high-dose statin pretreatment therapy group and a no statin or low-dose statin pretreatment therapy group; (5) the definition of PMI in the studies was specified; (6) the incidence of PMI or MACE was specified or could be calculated.
Data extraction and quality assessment
The data extraction was independently performed by WL and PPA. Any disagreement was resolved by consensus. The risk of bias in each study was evaluated by using the Cochrane Collaboration's instructions [20]. Quality assessment was made on allocation concealment, similarity of baseline characteristics, eligibility criteria, blinding, completeness of follow-up and intention-to-treat analysis. Study quality was quantified using the Jadad score [21].
Statistical analysis
The statistical analyses were performed using Review Manager 5.2.0. To evaluate the clinical benefit of high-dose statin specifically for patients undergoing elective PCI, subgroup analyses were performed on trials that included only patients with stable angina or patients with ACS, only statin-naive patients, or only patients with prior low-dose statin treatment. The incidence of PMI and MACE was expressed in dichotomous variables, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. If heterogeneity existed a random-effect model was used; otherwise, a fixed-effect model was chosen. Potential publication bias was assessed with funnel plots. All tests were two-tailed, and a P value of less than 0.05 was regarded as statistically significant.
Results
Characteristics of studies selected for meta-analysis
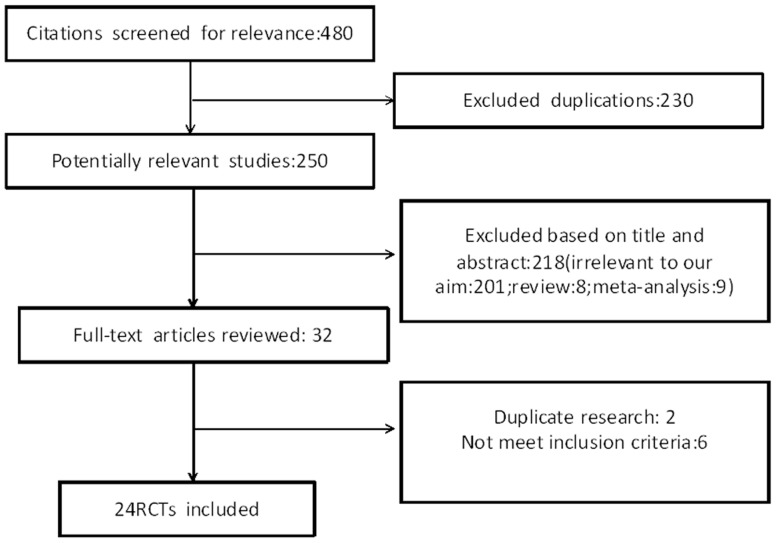
We identified 480 potentially relevant articles from the PubMed/Medline database (Figure 1). After reading the titles and abstracts of all potential articles, the full text of 32 articles was reviewed. By further evaluation, two articles were excluded as duplicate research; and six additional articles were excluded for not meeting our inclusion criteria. Finally, a total of 24 RCTs were examined.
Figure 1. Study selection diagram.
480 potentially relevant publications were identified from Pubmed/Medline search for key words and research references within related studies. Of these, 230 were duplications and 218 were excluded after further examination of the title and abstract. Of the 32 remaining articles, 2 were from duplicate RCTs and 6 did not meet our inclusion criteria, which left 24 RCTs that were used for our analysis.
Tables 1, 2 and 3 summarize the main characteristics of the 24 RCTs included in this study. All included studies were published between 2004 and 2013. 5,526 patients were included in the analysis: 2,867 were randomized to a high-dose statin group and 2,659 randomized to receive low-dose or no statin therapy (Control group). Approximately 73% of enrolled patients were male, 30% had diabetes, roughly two-thirds of the patients had chronic stable angina, and one-third of the patients presented with ACS. There were five trials [18], [22]–[25] with only prior low dose statin-treated patients, eighteen [16], [17], [26]–[41] with only statin-naïve patients, and one [42] with both prior low-dose statin-treated and statin-naïve patients. Twelve studies [16], [18], [22], [24], [26], [29], [30], [33], [34], [36], [39], [42] included patients with stable angina, seven studies [17], [28], [35], [37], [38], [40], [41] included patients with ACS, and five studies [23], [25], [27], [31], [32] included patients with mixed presentations. Fourteen trials [16]–[18], [22]–[28], [30], [31], [34], [37] used atorvastatin, eight [24], [33], [35], [38]–[42] used rosuvastatin, three [27], [29], [36] used pravastatin, one [27] used fluvastatin, and two [27], [32] used simvastatin. The duration of statin pretreatment ranged from two hours to four weeks. All patients in these trials received statin therapy after PCI, irrespective of the initial assignment. Eighteen studies [17], [18], [22], [23], [25]–[29], [31]–[34], [36], [37], [40]–[42] included short-term follow-up (in-hospital or up to 30 days), and six studies [16], [24], [30], [35], [38], [39] had follow-up duration between six months and forty-five months. The definition of PMI varied among the included studies as follows: Four studies [26], [28], [35], [37] demanded a CK-MB concentration greater than two times above the upper normal limit (ULN), thirteen studies [17], [18], [23], [24], [29]–[31], [33], [34], [38], [39], [41], [42] a CK-MB concentration greater than three times above ULN, and one study [27] a CK-MB concentration greater than five times above ULN. Other studies employed the Troponin I (TnI) concentration to define PMI. Three studies [16], [32], [40] required a TnI concentration greater than three times above ULN and two studies [25], [26] a TnI concentration greater than five times above ULN. Table 4 shows the quality characteristics of the included RCTs. Seventy-nine percent of the trials are high-quality trials, according to the Jadad score. Three trials [23], [25], [28] used allocation concealment and blinding.
Table 1. Characteristics of included studies.
| Trial | patient (n) | Type of population | Clinical presentation | Type of statin | Statin regime before PCI | Statin regime after PCI | Follow up | PMI definition |
| armyda | 153 | Statin naive | Stable angina | Atorvastatin | 7-day 40 mg/d before PCI VS placebo | Atorvastatin 40 mg/d | 30days | CK-MB>2UNL |
| Briguori et al. | 451 | Statin naive | 92% Stable angina/asymptomatic; 8% unstable angina | Atorvastatin29%;pravastatin,29%, simvastatin,39%, fluvstatin, 3% | 3-Day pretreatment(average 17 days) VS no statin pretreatment | Same statin as before PCI in the statin group and atorvastatin 20 mg/d in the control group | In-hospital | CK-MB>5UNL |
| armyda-acs | 171 | Statin naive | NSTE-ACS | Atorvastatin | 80 mg 12 h before PCI+40 mg 2 h before PCI VS placebo before PCI | Atorvastatin 40 mg/day | 30days | CK-MB>2UNL |
| Bozbas et al. | 93 | Statin naive | Stable angina | Pravastatin | 1-week pretreatment with 10 mg/d VS 40 mg/d before PCI VS no statin pretreatment | Pravastatin 10 to 40 mg/d | In-hospital | CK-MB>3UNL |
| Kinoshita et al. | 42 | Statin naive | Stable angina | Atorvastatin | 5–20 mg/d ≥2 wk before PCI to reach LDL<70 mg/dl VS 100 mg/dl | Atorvastatin 5 to 20 mg/d | 6months | CK-MB>3UNL |
| NAPLES II | 668 | Statin naive | 98% Stable angina/asymptomatic;2% unstable angina | Atorvastatin | 80 mg within 24 h before PCI VS no statin pretreatment | atorvastatin 20 mg/day | In-hospital | CK-MB>3UNL |
| armyda-recapture | 383 | Statin-treated | 53% stable angina and 47% NSTE-ACS | Atorvastatin | 80 mg 12 h before PCI+40-mg 2 h before PCI VS placebo | Atorvastatin 40 mg/day | 30days | CK-MB>3UNL |
| Jia et al. | 228 | Statin naive | 70.6% NSTE-ACS;29.4%STE-ACS | Simvastatin | 7-day pretreatment with 80 mg/d before PCI VS 20 mg/d before PCI | Simvastatin 20 mg/d | In-hospital | TnI>3UNL |
| Cay et al. | 299 | Statin naive | Stable angina | Rosuvastatin | Rosuvastatin 40 mg 24 h before PCI VS no statin pretreatment | Rosuvastatin 10–40 mg/d | In-hospital | CPK-MB>3UNL |
| Toso et al. | 161 | Statin naive | Stable angina | Atorvastatin | 80 mg within 48 h before PCI VS placebo within 48 h before PCI | NA | In-hospital | CPK-MB>3UNL |
| Yun et al. | 445 | Statin naive | NSTE-ACS | Rosuvastatin | 40 mg within 7–25 h before PCI VS no statin treatment before PCI | Rosuvastatin 10 mg/day | 12months | CK-MB>2UNL |
| Veselka et al. | 200 | Statin naive | Stable angina | Atorvastatin | 2-day pretreatment with atorvastatin 80 mg/day before PCI VS without atorvastatin pretreatment | atorvastatin 20 to 80 mg/day | 45months | TnI>3UNL |
PCI = percutaneous coronary intervention; CK-MB = creatine kinase-MB; UNL = upper normal limit of normal; NSTE-ACS = non-ST-segment elevation acute coronary syndrome;
Table 2. Characteristics of included studies.
| Trial | Patient (n) | Type of population | Clinical presentation | Type of statin | Statin regime before PCI | Statin regime after PCI | Follow up | PMI definition |
| Fujii et al. | 80 | Statin naive | Stable angina | Pravastatin | 4-week pretreatment with 20 mg/d before PCI VS no pretreatment | NA | In-hospital | TnI>5UNL |
| Yu et al. | 81 | Statin naive | NSTE-ACS | Atorvastatin | 80 mg 12 h before PCI+40 mg before PCI VS placebo group | Atorvastatin 20 mg/d | 30days | CK-MB>2UNL |
| Gao et al. | 117 | Statin naive | NSTE-ACS | Rosuvastatin | 20 mg 12 h before PCI +10 mg 2 h before PCI VS no treatment | Rosuvastatin 10 mg/d | 6months | CK-MB>3UNL |
| Jang et al. | 335 | Statin naive | NSTE-ACS | Atorvastatin | 80 mg 12 h and 40 mg 2 h before PCI VS no statin pretreatment | Atorvastatin 40 mg/day | 30days | CK-MB>3UNL |
| Zemánek et al. | 202 | Statin-treated | Stable angina | Atorvastatin | 7-day pre-treatment with 80 mg/d VS no statin pretreatment | NA | In-hospital | CK-MB>3UNL |
| ROMA Trial | 160 | Statin naive | Stable angina | Rosuvastatin | Rosuvastatin 40 mg within 24 h before PCI VS no statin | Rosuvastatin 20 mg/d | 12months | CK-MB>3UNL |
| ROMA II trial | 450 | Statin-treated | Stable angina | Atorvastatin; Rosuvastatin | Rosuvastatin 40 mg or Atorvastatin 80 mg 24 h before PCI VS no statin pretreatment | Rosuvastatin 20 mg/d Atorvastatin 40 mg/d | 12months | CK-MB>3UNL |
| Nafasi et al. | 190 | Statin-treated | Stable angina and recent MI | Atorvastatin | 80 mg within 24 h before PCI VS placebo within 24 h before PCI | NA | In-hospital | TnI>5UNL |
| Takano et al. | 210 | statin-treated and statin-naive | Stable angina | Rosuvastatin | 5–7day pretreatment with 20 mg/d before PCI VS 5–7day pretreatment with 2.50 mg before PCI | Rosuvastatin 10 mg/d AND rosuvastatin 2.5 mg/d | In-hospital | CK-MB>3UNL |
| Wang et al. | 125 | Statin naive | NSTE-ACS | Rosuvastatin | 20 mg 2–4 hours before PCI VS placebo before PCI | Rosuvastatin 10 mg/d | 30days | CK-MB>3UNL |
| Luo et al. | 67 | Statin naive | NSTE-ACS | Rosuvastatin | 20 mg 12 h before PCI+20 mg 2 h before PCI VS no Statin pretreatment | Rosuvastatin 10 mg/d | 30days | TnI>3UNL |
| Li et al. | 215 | statin-treated | Stable angina | Atorvastatin | 80 mg 12 h before PCI VS 20 mg 12 h before PCI | Atorvastatin 20 mg/d | 30days | NA |
PCI = percutaneous coronary intervention; CK-MB = creatine kinase-MB; UNL = upper normal limit of normal; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; ROMA trial = Rosuvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of periprocedural myocardial necrosis; ROMA II trial = Comparison of high reloading Rosuvastatin and Atorvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of periprocedural myocardial necrosis; NA = not available.
Table 3. Clinical and Procedural features in the overall population.
| Variables | High-dose statin | Control |
| Population size | 2867 | 2659 |
| Men | 2103(73) | 1921(72) |
| Family history of CAD | 536(19) | 405(15) |
| Hypertension | 1935(67) | 1736(65) |
| Diabetes Mellitus | 868(30) | 766(29) |
| Hyperlipidemia | 701(24) | 531(20) |
| Smoking | 772(27) | 689(26) |
| Previous myocardial infarction | 440(15) | 455(17) |
| Previous PCI | 219(8) | 194(7) |
| Previous CABG | 94(3) | 93(3) |
| Clinical presentation | ||
| Chronic stable angina | 1950(68) | 1739(66) |
| NSTEACS | 886(31) | 884(33) |
| STEACS | 31(1) | 36(1) |
| Medical therapy | ||
| β-blocker | 1318(46) | 1318(50) |
| ACEI/ARB | 1193(42) | 1196(45) |
| CCB | 331(12) | 326(12) |
| Vessel disease | ||
| Multiple vessel | 727(25) | 688(26) |
| B2/C lesion | 1289(45) | 1088(41) |
| Thrombus | 40(1.4) | 29(1.1) |
| Treated vessel | ||
| LM | 14(0.5) | 17(0.7) |
| LAD | 1250(47) | 1178(49) |
| LCX | 598(23) | 532(22) |
| RCA | 778(29) | 663(28) |
| Sapheonus vein graft | 6(0.2) | 11(0.5) |
| Multiple vessel PCI | 280(10) | 268(10) |
| Antithrombotic therapy | ||
| Aspirin | 2674(93) | 2416(91) |
| Clopidogrel | 2460(86) | 2188(82) |
| Glycoprotein IIb/IIIa inhibitors | 295(10) | 315(12) |
CAD = coronary artery disease; PCI = percutaneous coronary syndrome; CABG = coronary artery bypass graft; NSTEACS = non-ST segment elevation acute coronary syndrome; STEACS = ST segment elevation acute coronary syndrome; ACEI = angiotensin converting enzyme inhibitor; ARB = Angiotensin II receptor antagonist; CCB = calcium channel blocker; LM = left main; LAD = left anterior descending; LCX = left circumflex; RCA = right coronary artery.
Table 4. Quality of included RCTs.
| Author, Year | Jadad score | Allocation Concealment | Similarity of baseline characteristics | Eligibility Criteria | Blinding | Completeness of Follow-up | Intention-to-treat | ||
| Outcome Assessor | Care provider | Patient | |||||||
| ARMYDA,2004 | 5 | NA | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Briguori,2004 | 4 | Yes | Yes | Yes | No | No | No | Yes | Yes |
| ARMYDA-ACS 2007 | 6 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Kinoshita,2007 | 4 | NA | Yes | Yes | No | No | NA | Yes | Yes |
| Bozbas,2007 | 4 | NA | Yes | Yes | No | No | Yes | Yes | Yes |
| Naples II,2009 | 5 | Yes | Yes | Yes | No | No | No | Yes | Yes |
| ARMYDA-RECAPTURE,2009 | 6 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Jia,2009 | 4 | NA | Yes | Yes | Yes | No | No | Yes | Yes |
| Cay,2010 | 3 | NA | Yes | Yes | No | No | No | Yes | Yes |
| Toso,2011 | 3 | No | Yes | Yes | No | No | No | Yes | Yes |
| Yun,2011 | 4 | NA | Yes | Yes | No | No | No | Yes | Yes |
| Veselka,2011 | 3 | NA | Yes | Yes | No | No | No | Yes | Yes |
| Fujii,2011 | 4 | NA | Yes | Yes | No | No | No | Yes | Yes |
| Yu,2011 | 4 | NA | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Gao,2012 | 3 | NA | Yes | Yes | No | No | No | Yes | Yes |
| Jang,2013 | 4 | NA | Yes | Yes | No | No | Yes | Yes | Yes |
| Zemánek,2013 | 4 | NA | Yes | Yes | No | No | Yes | Yes | Yes |
| ROMA II,2013 | 5 | Yes | Yes | Yes | No | No | No | Yes | Yes |
| Nafasi,2013 | 6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ROMA,2013 | 4 | Yes | Yes | Yes | No | No | No | Yes | Yes |
| Takano,2013 | 3 | No | Yes | Yes | No | No | No | Yes | Yes |
| Wang,2013 | 4 | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Luo,2013 | 4 | NA | Yes | Yes | NA | NA | NA | Yes | Yes |
| Li,2013 | 4 | NA | Yes | Yes | NA | NA | NA | Yes | Yes |
NA = not available.
Effect of statin pretreatment on clinical outcome
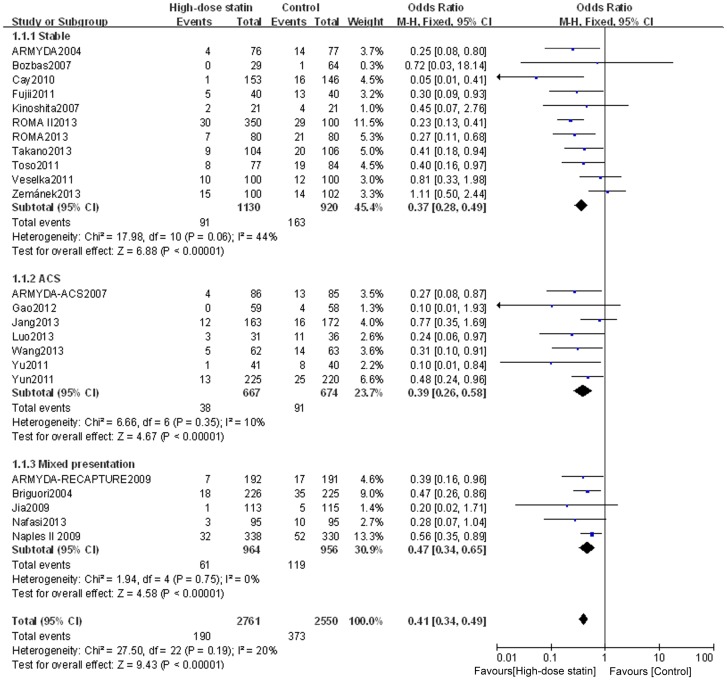
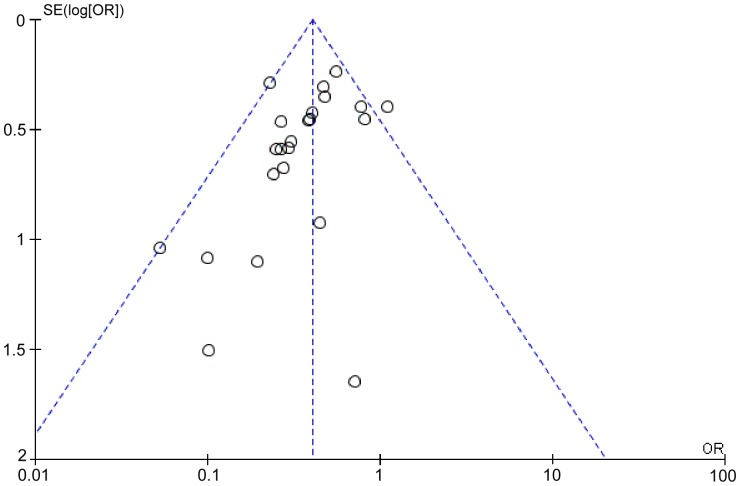
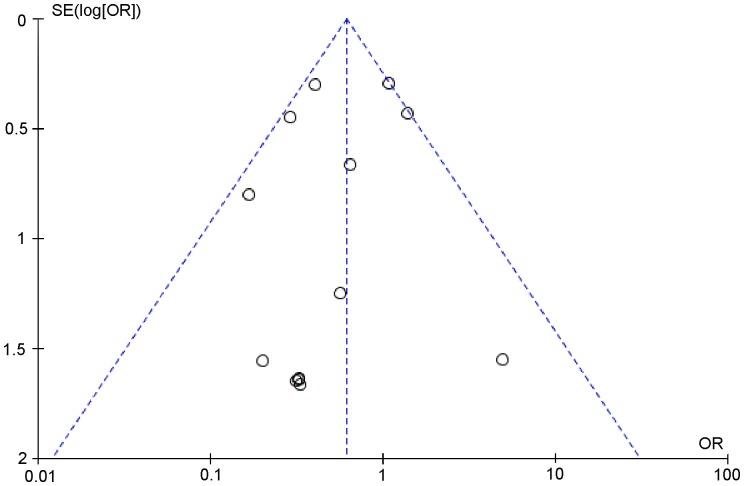
Outcome data for PMI were available from 23 RCTs, but not from the study by Li Q et al. [22]. The overall outcomes, based on the fixed effects model, showed that high-dose statin pretreatment was associated with a 59% relative reduction in PMI (OR = 0.41, 95% CI 0.34–0.49, P<0.00001; I2 = 20%) (Figure 2). The relative symmetry in the funnel plot shows that there was no evidence to suggest any publication bias (Figure 3).
Figure 2. Forest plots for PMI incidence for patients stratified by different clinical presentation.
Forest plots were generated using Review Manager 5.2.0 for patients with stable angina, ACS or mixed disease presentation from individual and pooled trials. The incidence of PMI was expressed as a dichotomous variable, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. Because the heterogeneity was low, a fixed-effect model was used.
Figure 3. Funnel plot for PMI incidence to rule out publication bias.
Funnel plot was generated using a fixed-effect model by Review Manager 5.2.0.
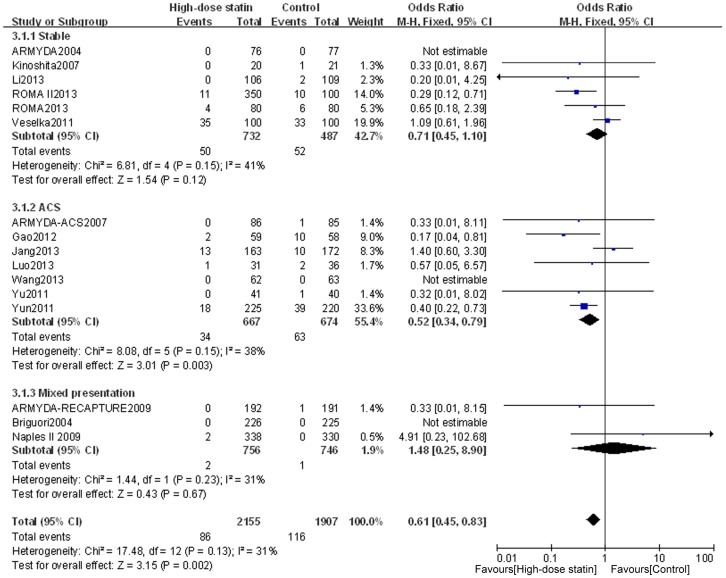
Outcome data for MACE were available from 16 RCTs, whereas the other 8 RCTs (by Bozbas et al. [29], Jia et al. [32], Cay et al. [33], Toso et al. [34], Fujii et al. [36], Zemanek et al. [18], Nafasi et al. [25], and Takano et al. [42]) only collected data about PMI. The overall outcomes, based on the fixed effects model, showed that high-dose statin pretreatment was associated with a 39% relative reduction in MACE (OR = 0.61, 95% CI 0.45–0.83, P = 0.002; I2 = 31%) (Figure 4). There was no evidence of significant heterogeneity among the 16 trials (P = 0.13; I2 = 31%), and the funnel plot analysis did not suggest the presence of any publication bias (Figure 5). These data confirm the reliability of this set of data and also verify the overall beneficial effects of high-dose statin loading on PMI and MACE using a greatly expanded data set relative to previous meta-analyses [14], [19].
Figure 4. Forest plots for MACE incidence for patients stratified by different clinical presentation.
Forest plots were generated using Review Manager 5.2.0 for patients with stable angina, ACS or mixed disease presentation from individual and pooled trials. The incidence of MACE was expressed as a dichotomous variable, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. Because the heterogeneity was low, a fixed-effect model was used.
Figure 5. Funnel plot for MACE incidence to rule out publication bias.
Funnel plot was generated using a fixed-effect model by Review Manager 5.2.0.
Analysis of subgroups with different types of disease presentation
To determine whether the outcome of high-dose statin pretreatment prior to PCI differs for patients with stable angina, ACS, or a mixed disease presentation, we assessed subgroups of patients according to their disease classification. High-dose statin pretreatment was associated with 63% relative reduction in PMI for patients with stable angina (OR = 0.37, 95% CI 0.28–0.49, P<0.00001; I2 = 44%); 61% relative reduction in PMI for patients with ACS (OR = 0.39, 95% CI 0.26–0.59, P<0.00001; I2 = 10%); and 53% relative reduction in PMI for patients with mixed presentation (OR = 0.47, 95% CI 0.34–0.65, P<0.00001; I2 = 0%) (Figure 2).
We also assessed the stable angina, ACS, and mixed disease subgroups for their effect on MACE. For the subgroup with ACS, the incidence of MACE was significantly lower in the high-dose statin group than in the control group (OR = 0.52, 95% CI 0.34–0.79, P = 0.003; I2 = 38%); however, for patients with stable angina or mixed presentation, there was no significant difference in MACE between the high-dose statin group and the control group (OR = 0.71, 95% CI 0.45–1.10, P = 0.12; I2 = 41%; and OR = 1.48, 95% CI 0.25–8.90, P = 0.67; I2 = 31%, respectively) (Figure 4). These findings confirm and extend previous findings that suggest that high dose statin loading is only effective for ACS patients on MACE [19].
Analysis of subgroups according to prior low-dose statin use
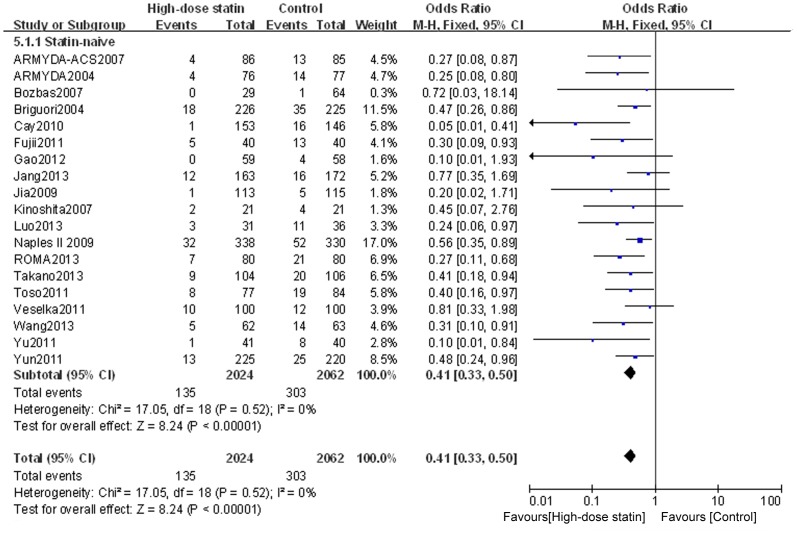
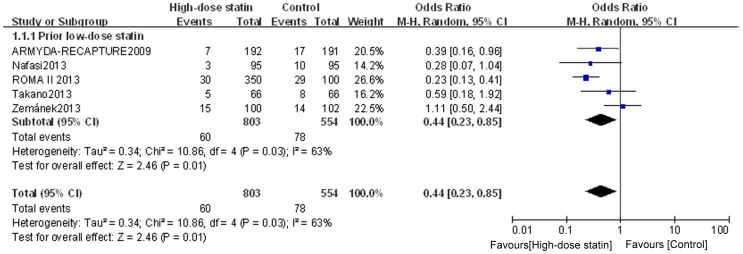
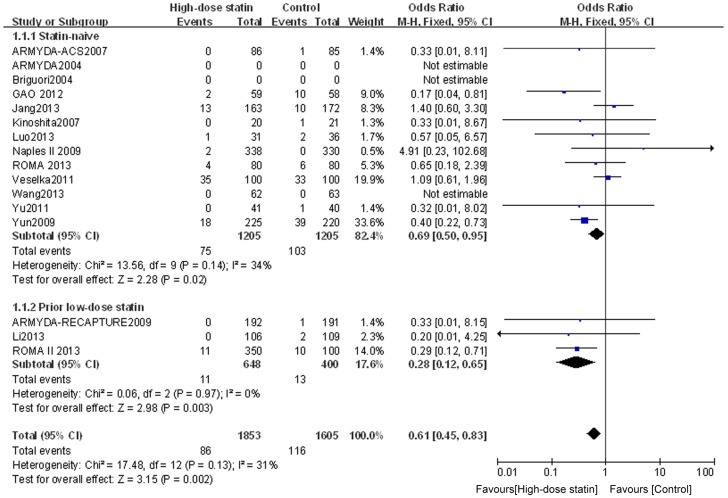
To determine whether chronic low-dose statin therapy prior to the high-dose PCI pretreatment affects clinical outcome, we grouped the patients according to whether or not they had a history of low-dose statin treatment prior to the high-dose pre-PCI treatment. For the subgroup of statin-naïve patients, high-dose statin pretreatment was associated with a lower incidence of PMI (OR = 0.41, 95% CI 0.33–0.50, P<0.00001; I2 = 0%) (Figure 6). For the subgroup with prior low-dose statin treatment, heterogeneity was found across trials (I2 = 63%), and a randomized model was therefore used. Similar to the results for the statin-naïve patients, the incidence of PMI for the prior low-dose statin patients was lower in the high-dose statin group than that in the control group (OR = 0.44, 95% CI 0.23–0.85, P = 0.01) (Figure 7). Furthermore, assessment of the effects on MACE suggested that high-dose statin loading is beneficial for both statin-naïve patients (OR = 0.69, 95% CI 0.50–0.95, P = 0.02; I2 = 34%) and prior low-dose statin patients (OR = 0.28, 95% CI 0.12–0.65, P = 0.003; I2 = 0%) (Figure 8). These data provide evidence using meta-analysis or multiple RCTs to support the use of high-dose statin loading prior to PCI for patients having prior low-statin chronic therapeutic use.
Figure 6. Funnel plot for PMI incidence for statin naïve patients.
Forest plot was generated using Review Manager 5.2.0 for statin naïve patients from individual and pooled trials. The incidence of PMI was expressed as a dichotomous variable, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. Because the heterogeneity was low, a fixed-effect model was used.
Figure 7. Funnel plot for PMI incidence for patients with prior low-dose statin use.
Forest plot was generated using Review Manager 5.2.0 for patients with prior low-dose statin use from individual and pooled trials. The incidence of PMI was expressed as a dichotomous variable, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. A random-effect model was used for the subgroup with prior low-dose statin treatment because heterogeneity existed for this group.
Figure 8. Forest plots for MACE incidence for patients stratified by prior low-dose statin use.
Forest plots were generated using Review Manager 5.2.0 for statin naïve patients or patients with prior low-dose statin use from individual and pooled trials. The incidence of MACE was expressed as a dichotomous variable, and the results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Heterogeneity across trials was evaluated with I2 statistic, defined as I2>50%. Because the heterogeneity was low, a fixed-effect model was used.
Analysis of the effects of high-dose statins on follow-up clinical events for subgroups of patients
To determine the effects of high-dose statin loading on follow-up clinical events, we assessed the number of patients undergoing death, spontaneous MI, TVR or MACE events in the follow-up period after PCI. High-dose statin treatment did not significantly reduce overall mortality or spontaneous MI (P = 0.15 and P = 0.37, respectively), with similar findings for subgroups stratified according to disease type or prior statin use (Table 5). TVR was significantly reduced by high-dose statin pretreatment (overall P = 0.02), with high-dose statin pretreatment most effective for the ACS group and the prior low-dose statin group (P = 0.01 and P = 0.04, respectively). A statistical difference in the incidence of MACE was also observed overall (P = 0.002), for both statin-naïve (P = 0.02) and prior low-dose statin (P = 0.003) patients, and for patients with ACS (P = 0.003), but not for patients with stable angina (P = 0.12). These results provide additional support for the applicability of high-dose statins for patients with ACS, for both statin-naïve and prior low-dose statin patients.
Table 5. Clinical events in follow-up.
| Events | High-dose statin n (%) | Control n (%) | P |
| Stable | |||
| Death | 12(1.6) | 13(2.7) | 0.67 |
| Spontaneous MI | 7(1.0) | 7(1.3) | 0.50 |
| TVR | 31(4.2) | 32(6.6) | 0.23 |
| MACE | 50(6.8) | 52(10.7) | 0.12 |
| ACS | |||
| Death | 3(0.4) | 9(1.3) | 0.12 |
| Spontaneous MI | 15(2.2) | 20(3.0) | 0.40 |
| TVR | 16(2.4) | 34(5.0) | 0.01 |
| MACE | 34(5.0) | 63(9.3) | 0.003 |
| Mixed presentation | |||
| Death | 1(0.1) | 1(0.1) | 0.50 |
| Spontaneous MI | 1(0.1) | 0(0) | 0.51 |
| TVR | 0(0) | 0(0) | NA |
| MACE | 2(0.2) | 1(0.1) | 0.67 |
| Statin-naive | |||
| Death | 13(1.1) | 20(1.6) | 0.28 |
| Spontaneous MI | 21(1.7) | 24(2.0) | 0.80 |
| TVR | 41(3.4) | 59(4.9) | 0.09 |
| MACE | 75(6.2) | 103(8.5) | 0.02 |
| Prior low-dose statin | |||
| Death | 3(0.5) | 3(0.8) | 0.25 |
| Spontaneous MI | 2(0.3) | 3(0.8) | 0.07 |
| TVR | 6(0.9) | 7(1.7) | 0.04 |
| MACE | 11(1.7) | 13(3.3) | 0.003 |
| Overall | |||
| Death | 16(0.7) | 23(1.2) | 0.15 |
| Spontaneous MI | 23(1.1) | 27(1.4) | 0.37 |
| TVR | 47(2.2) | 66(3.5) | 0.02 |
| MACE | 86(4.0) | 116(6.1) | 0.002 |
Spontaneous MI = spontaneous myocardial infarction; ACS = acute coronary syndrome;
TVR = target vessel revascularization; MACE = major adverse cardiovascular events;
NA = not available.
Discussion
This meta-analysis indicated that high-dose statin pretreatment can result in a significant reduction of PMI and cardiovascular events in patients undergoing elective PCI. This basic finding is consistent with previous meta-analysis studies [14], [19]; however, our study includes more RCTs and more than 1200 additional patients and thus provides increased statistical power. When stratified according to clinical presentation, the positive effect was significant for patients with ACS, as previously suggested [19]. However, to our knowledge, our study is unique in that we provide the first meta-analysis showing that the positive effect of high-dose statin pretreatment on PMI and MACE (excluding PMI) is significant for both statin-naive patients and patients with prior low-dose statin therapy.
Previous meta-analyses show that the elevation of cardiac markers after PCI correlates with an increase of clinical events during follow-up [4], [43]. There is still no consensus on the definition of PMI; however, we have elected to analyze all relevant studies with quality data to provide the most comprehensive analysis possible. Despite variability in the definition of PMI, this meta-analysis demonstrated that high-dose statin pretreatment before PCI reduces the incidence of PMI. The positive effect of high-dose statin pretreatment on PMI was consistent in patients with stable and unstable coronary artery disease as well as in statin-naïve patients and prior statin-treated patients. In addition, high-dose statin pretreatment was associated with a 39% relative reduction in clinical events. The benefit in clinical events was predominantly driven by the reduction in TVR, especially for patients with ACS. The incidence of death and spontaneous MI was lower in the high-dose statin group. High-dose statin loading prior to PCI did not reduce the clinical events in patients with stable coronary artery disease. Our study confirms that reloading high-dose statin improves the clinical outcome in patients undergoing long-term therapy with statins.
Although the exact mechanisms underlying the early protective effects of high dose stains in cardiovascular events remain undetermined, the benefit of statins can be attributed to its pleiotropic effects, which include anti-inflammation, anti-platelet aggregation, improvement of endothelial function, and plaque stability. Periprocedural inflammatory status predicts early and late adverse outcomes after PCI [44]–[47]. Studies showed that a reduction of periprocedural myocardial injury after high-dose pretreatment is associated with attenuated inflammatory response [40], [48], [49]. This may explain why patients with ACS may benefit most from high-dose statins therapy before PCI. A clinical study [50] found that long-term treatment with statins did not result in a reduction of PMI in patients undergoing elective PCI. Animal experiments showed that the cardioprotection of statins disappears with time and that high-dose statin reloading before ischemia/reperfusion can restore the waning protection [51]. This may suggest that high-dose statin pretreatment is needed to reach the desired pleiotropic effects for patients under long-term low-dose statin treatment.
The main conclusion of this meta-analysis was similar with that from Patti et al. [14] and Alexandre et al. [19]. However, these other results should be interpreted with caution. Compared with the meta-analysis by Patti et al., we excluded the STATIN STEMI trial [52]. To our knowledge, it is the only study to evaluate the impact of high-dose statin pretreatment before primary PCI in patients with STEMI. The trial could not provide sufficient power to determine a significant difference in PMI between high-dose and low-dose statin. In their meta-analysis, Patti et al. demonstrated that high-dose statin loading before PCI significantly reduced PMI and MACE within thirty days. The clinical benefit was mainly driven by the reduction in PMI. However, when PMI was excluded from the end points, there was no significant difference in the rate of MACE within thirty days between high-dose statin and control groups (OR = 0.44, P = 0.05). Our study demonstrates that high-dose statin pretreatment before PCI significantly reduces clinical events, including spontaneous MI, death, and TVR. Furthermore, we showed that high-dose statin reloading before PCI significantly reduces the occurrence of PMI and MACE during follow-up in prior low-dose statin-treated patients. Unlike the meta-analysis by Alexandre et al. [19], our study included patients under long-term low-dose statin therapy, RCTs that compared high-dose with low-dose statin treatment, and RCTs that compared different types of statin. In clinical practice, patients under long-term statin therapy constitute a large proportion of patients who undergo elective PCI. Moreover, our study included recent large scale trials [17], [24]. Therefore, our study confirms and extends the conclusion of the meta-analysis by Alexandre et al.
The results of our meta-analysis are not consistent with the conclusions of Veselka et al. [15], [16], Jang et al. [17] and Zemanek et al. [18], which showed that high-dose statin pretreatment before PCI did not reduce the incidence of PMI and MACE in patients undergoing PCI. We think that this disagreement can be explained by different doses and durations of statins. As the optimal doses and duration of statins have not been determined, different therapeutic application may result in different results. Another potential explanation for the controversial outcome of the studies by Veselka et al. and Zemanek et al. is that the cardiac marker was examined at a later point in time (16 to 24 hours after PCI), potentially missing the opportunity to identify PMI. In addition, the discordant results may be attributed to a lower incidence of increased CK-MB in the high-dose statin loading group, compared to the control group.
There are several limitations in this meta-analysis. First, the studies used different definitions of PMI and different treatment strategies. To date, it is not possible to find an ideal marker for a universally accepted definition of PMI. Different doses, durations and types of statins may have different effects on PMI and MACE. A standardized statin protocol would help to eliminate the profounder. Secondly, although many studies provided data about PMI and MACE, one out of the twenty-four studies did not report the incidence of PMI, and eight out of twenty-four studies did not provide the incidence of MACE. Finally, we did not have access to patient-level data; therefore, we did not exclude the influence of different antithrombotic strategies.
Conclusions
High-dose statin pretreatment can result in a significant reduction in PMI and cardiovascular events in patients undergoing elective PCI. The positive effects of high-dose statin pretreatment on PMI and MACE are significant for statin-naive and prior low-dose statin-treated patients. The positive effect of high-dose statin pretreatment on MACE is significant for patients with ACS. The information provided in this analysis should be useful in the selection of patients who have the greatest potential for success in the use of high-dose statin loading prior to PCI. In particular, we suggest that high-dose pretreatment should be used regardless of prior low-dose statin use for patients with ACS, but that the high-dose statin loading can be eliminated for patients with stable angina to avoid unnecessary statin exposure and unwarranted expenditure on statins.
Supporting Information
PRISMA Checklist.
(DOC)
Acknowledgments
We thank Mr. Zhizhong Gong for his kind help with statistical methods. We thank LetPub (www.letpub.com) for linguistic assistance during the preparation of this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China NO. 81270285 and NO. 81100198, the National Construction Program of Key Clinical specialist facility Project and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support code: ZY201303 (http://www.bjah.gov.cn/gzdt/kyjy/xzzq/201403/P020140304488636040596.pdf). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Herrmann J (2005) Peri-procedural myocardial injury: 2005 update. Eur Heart J 26:2493–2519. [DOI] [PubMed] [Google Scholar]
- 2. Lansky AJ, Stone GW (2010) Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ Cardiovasc Interv 3:602–610. [DOI] [PubMed] [Google Scholar]
- 3. Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, et al. (2013) Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur Heart J 34:1662–1669. [DOI] [PubMed] [Google Scholar]
- 4. Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, et al. (2013) Prognostic value of creatine kinase-myocardial band isoenzyme elevation following percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 81:959–967. [DOI] [PubMed] [Google Scholar]
- 5. Di Sciascio G, Patti G, Pasceri V, Gatto L, Colonna G, et al. (2010) Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention: results of the ARMYDA-5 PRELOAD (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) randomized trial. J Am Coll Cardiol 56:550–557. [DOI] [PubMed] [Google Scholar]
- 6. Blankenship JC, Tasissa G, O'Shea JC, Iliadis EA, Bachour FA, et al. (2001) Effect of glycoprotein IIb/IIIa receptor inhibition on angiographic complications during percutaneous coronary intervention in the ESPRIT trial. J Am Coll Cardiol 38:653–658. [DOI] [PubMed] [Google Scholar]
- 7. Ellis SG, Brener SJ, Lincoff AM, Moliterno DJ, Whitlow PL, et al. (2001) beta-blockers before percutaneous coronary intervention do not attenuate postprocedural creatine kinase isoenzyme rise. Circulation 104:2685–2688. [DOI] [PubMed] [Google Scholar]
- 8. Park H, Otani H, Noda T, Sato D, Okazaki T, et al. (2013) Intracoronary followed by intravenous administration of the short-acting beta-blocker landiolol prevents myocardial injury in the face of elective percutaneous coronary intervention. Int J Cardiol 167:1547–1551. [DOI] [PubMed] [Google Scholar]
- 9. Macin SM, Perna ER, Farias EF, Franciosi V, Cialzeta JR, et al. (2005) Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J 149:451–457. [DOI] [PubMed] [Google Scholar]
- 10. Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, et al. (2005) Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation 111:412–419. [DOI] [PubMed] [Google Scholar]
- 11. Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M (2000) Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol 20:61–69. [DOI] [PubMed] [Google Scholar]
- 12. Califf RM, Abdelmeguid AE, Kuntz RE, Popma JJ, Davidson CJ, et al. (1998) Myonecrosis after revascularization procedures. J Am Coll Cardiol 31:241–251. [DOI] [PubMed] [Google Scholar]
- 13. Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, et al. (2001) Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103:926–933. [DOI] [PubMed] [Google Scholar]
- 14. Patti G, Cannon CP, Murphy SA, Mega S, Pasceri V, et al. (2011) Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation 123:1622–1632. [DOI] [PubMed] [Google Scholar]
- 15. Veselka J, Zemanek D, Hajek P, Maly M, Adlova R, et al. (2009) Effect of two-day atorvastatin pretreatment on the incidence of periprocedural myocardial infarction following elective percutaneous coronary intervention: a single-center, prospective, and randomized study. Am J Cardiol 104:630–633. [DOI] [PubMed] [Google Scholar]
- 16. Veselka J, Zemanek D, Hajek P, Maly M, Adlova R, et al. (2011) Effect of two-day atorvastatin pretreatment on long-term outcome of patients with stable angina pectoris undergoing elective percutaneous coronary intervention. Am J Cardiol 107:1295–1299. [DOI] [PubMed] [Google Scholar]
- 17. Jang Y, Zhu J, Ge J, Kim YJ, Ji C, et al. (2013) Preloading with atorvastatin before percutaneous coronary intervention in statin-naive Asian patients with non-ST elevation acute coronary syndromes: A randomized study. J Cardiol 63:335–343. [DOI] [PubMed] [Google Scholar]
- 18. Zemanek D, Branny M, Martinkovicova L, Hajek P, Maly M, et al. (2013) Effect of seven-day atorvastatin pretreatment on the incidence of periprocedural myocardial infarction following percutaneous coronary intervention in patients receiving long-term statin therapy. A randomized study. Int J Cardiol 168:2494–2497. [DOI] [PubMed] [Google Scholar]
- 19. Benjo AM, El-Hayek GE, Messerli F, Dinicolantonio JJ, Hong MK, et al. (2013) High dose statin loading prior to percutaneous coronary intervention decreases cardiovascular events: A meta-analysis of randomized controlled trials. Catheter Cardiovasc Interv [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Deng SB, Xia S, Du JL, She Q (2013) Impact of intensive statin use on the level of inflammation and platelet activation in stable angina after percutaneous coronary intervention: a clinical study. Med Clin (Barc) 140:532–536. [DOI] [PubMed] [Google Scholar]
- 23. Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, et al. (2009) Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol 54:558–565. [DOI] [PubMed] [Google Scholar]
- 24. Sardella G, Lucisano L, Mancone M, Conti G, Calcagno S, et al. (2013) Comparison of high reloading ROsuvastatin and Atorvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of MyocArdial periprocedural necrosis. The ROMA II trial. Int J Cardiol 168:3715–3720. [DOI] [PubMed] [Google Scholar]
- 25. Nafasi L, Rahmani R, Shafiee A, Salari A, Abdollahi A, et al. (2014) Can a high reloading dose of atorvastatin prior to percutaneous coronary intervention reduce periprocedural myocardial infarction? Curr Med Res Opin 30:381–386. [DOI] [PubMed] [Google Scholar]
- 26. Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, et al. (2004) Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation 110:674–678. [DOI] [PubMed] [Google Scholar]
- 27. Briguori C, Colombo A, Airoldi F, Violante A, Focaccio A, et al. (2004) Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J 25:1822–1828. [DOI] [PubMed] [Google Scholar]
- 28. Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, et al. (2007) Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 49:1272–1278. [DOI] [PubMed] [Google Scholar]
- 29. Bozbas H, Yildirir A, Mermer S, Konas D, Atar I, et al. (2007) Does pravastatin therapy affect cardiac enzyme levels after percutaneous coronary intervention? Adv Ther 24:493–504. [DOI] [PubMed] [Google Scholar]
- 30. Kinoshita M, Matsumura S, Sueyoshi K, Ogawa S, Fukuda K (2007) Randomized trial of statin administration for myocardial injury: is intensive lipid-lowering more beneficial than moderate lipid-lowering before percutaneous coronary intervention? Circ J 71:1225–1228. [DOI] [PubMed] [Google Scholar]
- 31. Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, et al. (2009) Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. J Am Coll Cardiol 54:2157–2163. [DOI] [PubMed] [Google Scholar]
- 32. Jia XW, Fu XH, Zhang J, Gu XS, Fan WZ, et al. (2009) Intensive cholesterol lowering with statin improves the outcomes of percutaneous coronary intervention in patients with acute coronary syndrome. Chin Med J (Engl) 122:659–664. [PubMed] [Google Scholar]
- 33. Cay S, Cagirci G, Sen N, Balbay Y, Durmaz T, et al. (2010) Prevention of peri-procedural myocardial injury using a single high loading dose of rosuvastatin. Cardiovasc Drugs Ther 24:41–47. [DOI] [PubMed] [Google Scholar]
- 34. Toso A, Leoncini M, Maioli M, Gallopin M, Tedeschi D, et al. (2011) Short-term high-dose atorvastatin for periprocedural myocardial infarction prevention in patients with renal dysfunction. J Cardiovasc Med (Hagerstown) 12:318–321. [DOI] [PubMed] [Google Scholar]
- 35. Yun KH, Oh SK, Rhee SJ, Yoo NJ, Kim NH, et al. (2011) 12-month follow-up results of high dose rosuvastatin loading before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 146:68–72. [DOI] [PubMed] [Google Scholar]
- 36. Fujii K, Kawasaki D, Oka K, Akahori H, Iwasaku T, et al. (2011) The impact of pravastatin pre-treatment on periprocedural microcirculatory damage in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 4:513–520. [DOI] [PubMed] [Google Scholar]
- 37. Yu XL, Zhang HJ, Ren SD, Geng J, Wu TT, et al. (2011) Effects of loading dose of atorvastatin before percutaneous coronary intervention on periprocedural myocardial injury. Coron Artery Dis 22:87–91. [DOI] [PubMed] [Google Scholar]
- 38. Gao Y, Jia ZM, Sun YJ, Zhang ZH, Ren LN, et al. (2012) Effect of high-dose rosuvastatin loading before percutaneous coronary intervention in female patients with non-ST-segment elevation acute coronary syndrome. Chin Med J (Engl) 125:2250–2254. [PubMed] [Google Scholar]
- 39. Sardella G, Conti G, Donahue M, Mancone M, Canali E, et al. (2013) Rosuvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of myocardial periprocedural necrosis: the ROMA trial. Catheter Cardiovasc Interv 81:E36–43. [DOI] [PubMed] [Google Scholar]
- 40. Luo J, Li J, Shen X, Hu X, Fang Z, et al. (2013) The effects and mechanisms of high loading dose rosuvastatin therapy before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 167:2350–2353. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Dai H, Xing M, Yu Z, Lin X, et al. (2013) Effect of a single high loading dose of rosuvastatin on percutaneous coronary intervention for acute coronary syndromes. J Cardiovasc Pharmacol Ther 18:327–333. [DOI] [PubMed] [Google Scholar]
- 42. Takano H, Ohba T, Yamamoto E, Miyachi H, Inui K, et al. (2013) Usefulness of rosuvastatin to prevent periprocedural myocardial injury in patients undergoing elective coronary intervention. Am J Cardiol 111:1688–1693. [DOI] [PubMed] [Google Scholar]
- 43. Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, et al. (2011) Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 77:1020–1030. [DOI] [PubMed] [Google Scholar]
- 44. Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, et al. (1999) Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol 34:1512–1521. [DOI] [PubMed] [Google Scholar]
- 45. Walter DH, Fichtlscherer S, Sellwig M, Auch-Schwelk W, Schachinger V, et al. (2001) Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol 37:839–846. [DOI] [PubMed] [Google Scholar]
- 46. Delhaye C, Maluenda G, Wakabayashi K, Ben-Dor I, Lemesle G, et al. (2010) Long-term prognostic value of preprocedural C-reactive protein after drug-eluting stent implantation. Am J Cardiol 105:826–832. [DOI] [PubMed] [Google Scholar]
- 47. Delhaye C, Sudre A, Lemesle G, Marechaux S, Broucqsault D, et al. (2009) Preprocedural high-sensitivity C-reactive protein predicts death or myocardial infarction but not target vessel revascularization or stent thrombosis after percutaneous coronary intervention. Cardiovasc Revasc Med 10:144–150. [DOI] [PubMed] [Google Scholar]
- 48. Patti G, Chello M, Pasceri V, Colonna D, Nusca A, et al. (2006) Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J Am Coll Cardiol 48:1560–1566. [DOI] [PubMed] [Google Scholar]
- 49. Patti G, Chello M, Gatto L, Alfano G, Miglionico M, et al. (2010) Short-term atorvastatin preload reduces levels of adhesion molecules in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Results from the ARMYDA-ACS CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J Cardiovasc Med (Hagerstown) 11:795–800. [DOI] [PubMed] [Google Scholar]
- 50. Gordin J, Haider A, Swaminathan RV, Kim LK, Minutello RM, et al. (2012) Impact of long-term statin therapy on postprocedural myocardial infarction in patients undergoing nonemergency percutaneous coronary intervention. Am J Cardiol 110:1397–1404. [DOI] [PubMed] [Google Scholar]
- 51. Mensah K, Mocanu MM, Yellon DM (2005) Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten? J Am Coll Cardiol 45:1287–1291. [DOI] [PubMed] [Google Scholar]
- 52. Kim JS, Kim J, Choi D, Lee CJ, Lee SH, et al. (2010) Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv 3:332–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.