Abstract
Alkaline salt stress adversely affects rice growth, productivity and grain quality. However, the mechanism underlying this process remains elusive. We characterized here an alkaline tolerant mutant, alt1 in rice. Map-based cloning revealed that alt1 harbors a mutation in a chromatin remodeling ATPase gene. ALT1-RNAi transgenic plants under different genetic background mimicked the alt1 phenotype, exhibiting tolerance to alkaline stress in a transcript dosage-dependent manner. The predicted ALT1 protein belonged to the Ris1 subgroup of the Snf2 family and was localized in the nucleus, and transcription of ALT1 was transiently suppressed after alkaline treatment. Although the absorption of several metal ions maintained well in the mutant under alkaline stress, expression level of the genes involved in metal ions homeostasis was not altered in the alt1 mutant. Classification of differentially expressed abiotic stress related genes, as revealed by microarray analysis, found that the majority (50/78) were involved in ROS production, ROS scavenging, and DNA repair. This finding was further confirmed by that alt1 exhibited lower levels of H2O2 under alkaline stress and tolerance to methyl viologen treatment. Taken together, these results suggest that ALT1 negatively functions in alkaline tolerance mainly through the defense against oxidative damage, and provide a potential two-step strategy for improving the tolerance of rice plants to alkaline stress.
Introduction
The widely distributed salt and alkaline salts are important abiotic stress factors that greatly affect plant growth and development and severely threat crop productivity throughout the world [1]. Previous research has demonstrated that alkaline salt stress and neutral salt stress are two distinct kinds of stresses for plants, and should be called alkali stress and salt stress, respectively [2]. Alkaline salts (NaHCO3 and Na2CO3), which elevate soil pH, are much more destructive than neutral salts (NaCl and Na2SO4). So far, extensive studies have tried to uncover the signaling mechanisms and regulatory networks underlying plant tolerance to salt stress [3]–[6]. However, few regulators have been identified to function directly in the tolerance of plants to alkaline salt stress.
Alkaline salt stress involves multiple factors including osmotic stress, ion injury, and elevated soil pH (pH>8.5), which reduces iron (Fe) solubility [7]. Consequently, plants grown in calcareous soils often exhibit Fe deficiency symptoms of chlorosis [8], [9]. Therefore, various groups have sought to genetically engineer crop plants with improved Fe uptake under alkaline salt conditions, by introducing genes encoding iron transporters, iron reductases, and enzymes involved in phytosiderophore biosynthesis into plants [8]–[14]. For example, transgenic rice expressing the barley nicotianamine aminotransferase gene, HvNAAT1, showed enhanced Fe availability and higher grain yields in alkaline soils [8]. Similarly, ectopic expression of the yeast Fe3+-chelate-reductase gene refre1/372 greatly improved grain yield on calcareous soil-grown rice plants [9].
In addition to the strategies that overcome the Fe limitations of alkaline soils, a series of studies suggested that many other mechanisms appear to be involved in the tolerance to alkaline salt stress in plants. For example, analysis of the global gene expression profiles of Puccinellia tenuiflora, a widely distributed monocotyledonous alkaline-tolerant halophyte in the Songnen Plains of China, under alkaline salt stress (Na2CO3 or NaHCO3) revealed that the differentially expressed genes fell into a dozen functional categories, implying the complex mechanisms underlie alkaline salt tolerance in plants [15]. In Arabidopsis, protein kinase PKS5 was reported to negatively regulate tolerance to high external pH by interacting with chaperone J3 to inhibit plasma membrane H+-ATPase activity [16], [17]. In addition, microRNAs, which are ubiquitous regulators of gene expression in eukaryotic organisms, were also found to be involved in alkaline salt stress response [18], [19]. On the other hand, efforts have also been put to screen for natural plant germplasms with varied alkaline tolerance, and several associated quantitative trait loci (QTLs) have been roughly mapped in rice and soybean [20]–[23]. However, none of these genes have been cloned to date.
ATP-dependent chromatin remodeling complexes affect chromatin dynamics using the energy released by ATP hydrolysis to alter histone-DNA contacts, thereby making genomic regions more accessible to the transcriptional machinery or transcription factors [24]. Therefore, chromatin remodeling plays a central role in establishing specific gene expression patterns and maintaining transcriptional states in eukaryotes. Emerging evidence shows that the chromatin remodeling ATPases of Snf2 family not only play various roles in the regulation of plant development [25], but also function in different abiotic stress responses [26]. In Arabidopsis, knockout atchr12 plants shows tolerance to drought, salt and heat stresses [27], and AtBRM was reported to regulate drought tolerance [28]. The rice genome contains totally 40 Snf2 family proteins [29]. However, only one member, OsCHR4, was reported to function in early chloroplast development in adaxial mesophyll cells [30]. So far, no ATP-dependent chromatin remodeling enzymes have been reported to function in alkaline salt stress response.
Here we report the functional characterization of an alkaline tolerant mutant, alt1, in rice. We showed that alt1 contains a mutation in a Snf2 family chromatin remodeling ATPase gene that functions negatively in alkaline tolerance in rice. We found that the improved tolerance of the mutant to alkaline stress was largely due to the enhanced defense against oxidative damage. Our results suggest a potential two-step strategy for improving the tolerance of rice plants to alkaline salt stress.
Results
Phenotypic analysis of the alt1 mutant
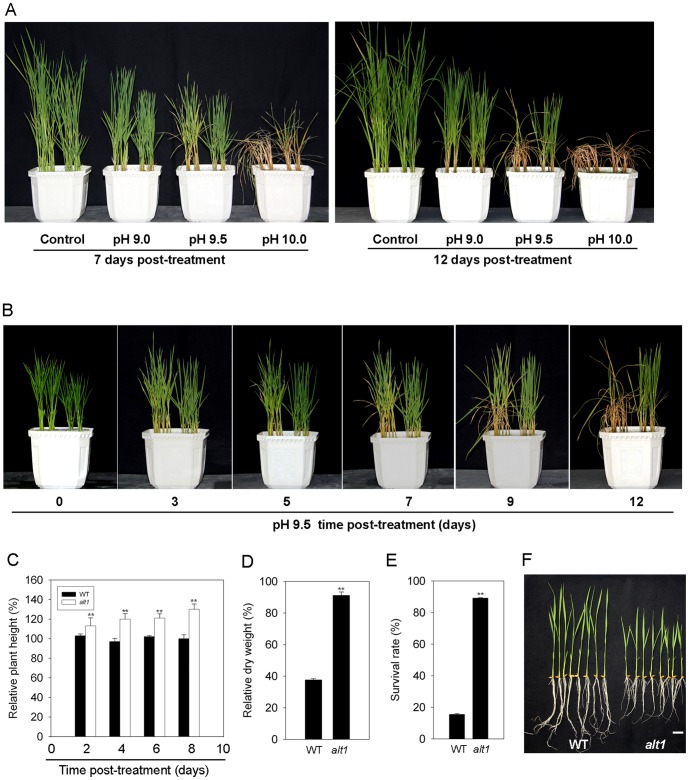
To gain insight into the molecular basis of tolerance to alkaline salt stress in rice, we screened a NaN3-mutagenized mutant population in the background of KY131, a widely cultivated variety in north China. From a total of 100,000 M2 individuals treated with NaHCO3-NaOH solution (pH 9.5), we identified a mutant, alt1 (for alkaline tolerance 1) with enhanced tolerance to alkaline stress. To obtain detailed information on the alkaline tolerance phenotype of the mutant, two-leaf stage alt1 and wild type (WT) plants were subjected to treatment with alkaline solutions ranging in pH from 9.0 to 10.0. The alt1 mutant showed less chlorosis than WT under all pH values (Figure 1A). At pH 9.0, plants of alt1 and WT grew similarly for the first 12 days of treatment (Figure 1A), but the mutant displayed an obvious tolerant phenotype 15 days after the stress (Figure S1). For pH 10.0, most of the alt1 seedlings survived the first 7 days, but plants of both alt1 and WT wilted completely by 12 days after treatment (Figure 1A).
Figure 1. Phenotypic analysis of the alt1 mutant.
Left part in each pot of (A) and (B): WT; Right part: alt1. (A) Two-leaf stage alt1 and WT seedlings were subjected to alkaline treatment with values 9.0, 9.5 and 10.0, respectively, and photographed at 7 (left) and 12 (right) days after treatment. (B) Time-course observation for tolerance phenotype of alt1 and WT at pH 9.5. (C) Comparison of relative plant height of alt1 and WT grown under pH 9.5 for the indicated number of days. Values are means ± SE (n = 20). **: P≤0.01. (D) Comparison of relative dry weight of alt1 and WT after 10 days of treatment at pH 9.5. Values are means ± SE (n = 3, with 15 plants in each repeat). **: P≤0.01. (E) Comparison of survival rate of alt1 and WT after 12 days of treatment at pH 9.5. Values are means ± SE (n = 3, with 50 plants in each repeat). **: P≤0.01. (F) Phenotypes of two-leaf stage seedlings of alt1 and WT. Bar = 2 cm.
The most obvious difference in tolerance phenotype between alt1 and WT was observed for treatment at pH 9.5. The leaves of WT seedlings became chlorotic 3 days after the start of treatment, and wilted completely by 12 days (Figure 1B). In contrast, plants of alt1 exhibited much less chlorosis and grew well compared to WT under the same stress conditions (Figure 1B). Statistical analysis of the relative plant height (height of the treated plant/height of the plant before treatment), survival rate and relative dry weight (dry weight of the treated plant/dry weight of the plant before treatment) found that the relative plant height of alt1 increased by up to 30% under 8 days after treatment at pH 9.5, but the growth of WT plants was stunted (Figure 1C). In addition, the relative dry weight of the mutant was 2.4-fold greater than that of WT after 10 days of the treatment (Figure 1D). The survival rate of alt1 was 89% after 12 days of treatment, but only 15% for WT (Figure 1E). These data demonstrated that alt1 is an alkaline stress tolerant mutant.
To investigate whether the ALT1 mutation also affected other abiotic stress processes, we next examined the phenotype of alt1 and WT plants under salt or drought stress. No differences were found between the mutant and WT under NaCl or dehydration treatment (Figure S2). These observations suggested that tolerance to salt or drought stress was not enhanced in the alt1 mutant.
The most evident morphological alteration of the alt1 mutant is short roots during early development (Figure 1F). At the adult stage, no significant morphological alterations were found between alt1 and WT plants, with the exception of tiller number per plant (Table S1).
Map-based cloning and complementation test of alt1
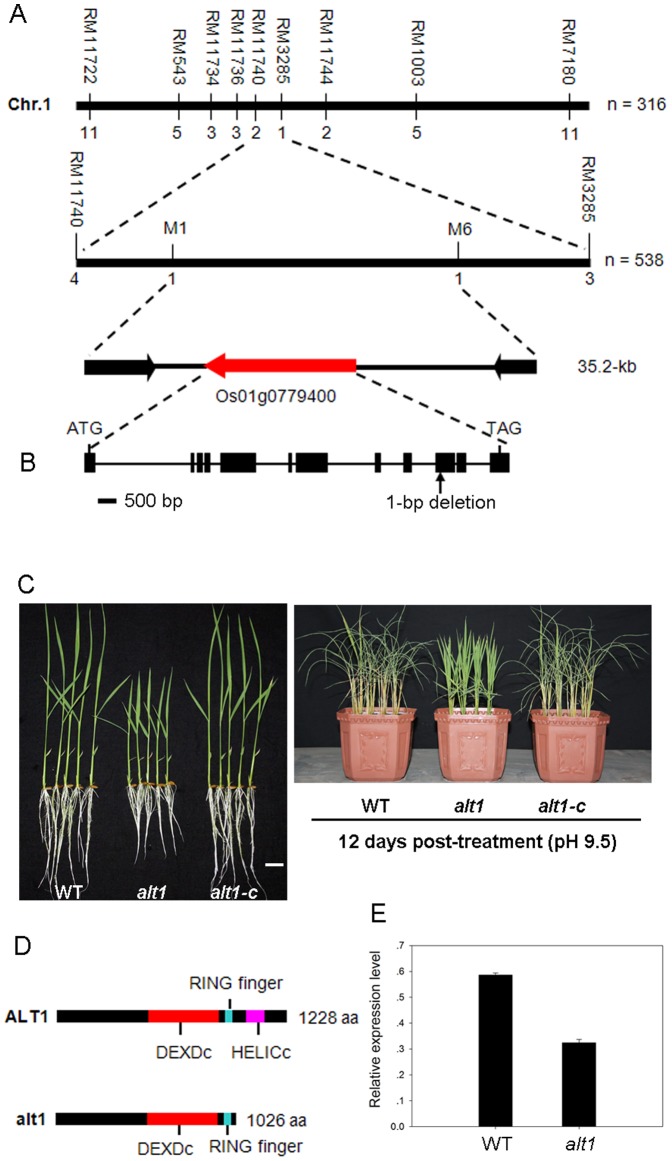
As described above, the alt1 mutant showed enhanced alkaline tolerance as well as phenotypes of short roots and low tiller number. Because individuals with the mutant short root phenotype could be easily distinguished among the segregating population, we used this trait for mapping analysis. Investigation of the short root phenotype in the F2 population derived from a cross between alt1 and WT revealed that alt1 is caused by a single recessive nuclear gene mutation (χ2 = 0.28<χ2 0.05 = 3.84; P>0.05). Therefore, we performed map-based cloning to isolate the underlying gene. Using 538 F2 mutant plants derived from the cross between alt1 and Kasalath, the candidate gene was narrowed down to a 35.2-kb region between STS markers M1 and M6 on the long arm of chromosome 1 (Figure 2A). Three predicted genes exist within this region. After sequencing these genes, only a single nucleotide deletion (G) was identified at the 3037th position of Os01g0779400 in alt1 (Figure 2B).
Figure 2. Map-based cloning of ALT1 and complementation analysis.
(A) Fine mapping of the ALT1 locus. Numbers below the horizontal line are the number of recombinants. The ALT1 locus was fine mapped to a 35.2-kb region between markers M1 and M6. Indicating three putative ORFs contained in this region. (B) Gene structure of ALT1. Black boxes indicate exons and lines between boxes indicate introns. There is 1-bp deletion in the 10th exon in the alt1 background. (C) Phenotypic analysis of two-leaf stage seedlings of WT, alt1 and alt1-c under normal (left) and alkaline (right, pH 9.5) stress conditions, respectively. Bar = 2 cm. (D) Protein structure of ALT1 and alt1. (E) Transcript levels of ALT1 in the roots of two-leaf-stage alt1 and WT seedlings. Actin was used as an internal control. Data shown are mean values of three biological repeats with SD.
To verify that Os01g0779400 is indeed ALT1, we performed a genetic complementation test in which alt1 plants were transformed with wild-type Os01g0779400, which consists of the full-length coding sequence of the gene driven by its native promoter (1.1-kb genomic DNA fragment upstream of the ATG start codon). All the 23 independent T2 transgenic lines showed root length comparable to that of control, and displayed alkaline stress (pH 9.5) response similar to that of the WT (Figure 2C). This result indicates that the mutation in Os01g0779400 is responsible for the phenotype of short root and alkaline tolerance exhibited in the alt1 mutant.
ALT1 is predicted to encode a core subunit of the Snf2 family chromatin remodeling ATPase, containing the featured Snf2 ATPase domains of DEXDc and HELICc (Figure 2D) that together have at least 12 characteristic sequence motifs with various roles in nucleic acid and/or nucleotide binding or hydrolysis [31]. The rice genome contains two paralogs of ALT1 (i.e., Os04g0629300 and Os08g0180300), with identities of 53% and 56% at the protein level, respectively. All these proteins belong to the Ris1 subgroup of the Snf2 family [29]. The nucleotide deletion in the mutant not only moderately affected ALT1 transcription (Figure 2E), but also created a premature stop codon in the predicted coding region (Figure S3), resulting in a truncated alt1 protein lacking the HELICc domain (Figure 2D).
dsRNAi knockdown transgenic plants mimic the alt1 phenotype
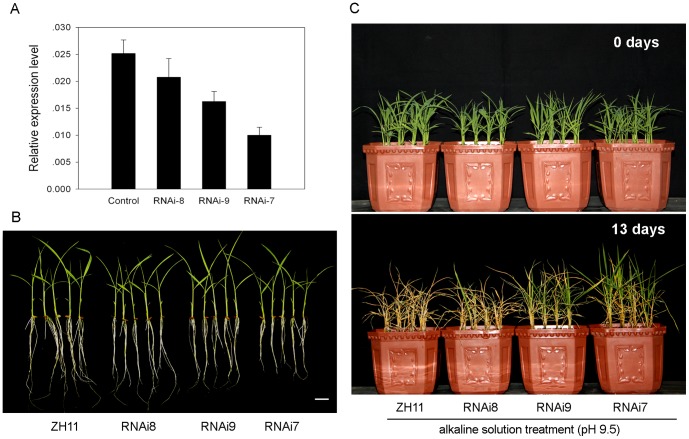
To further confirm the function of ALT1 in alkaline tolerance, we generated a double stranded RNA interference (dsRNAi) construct harboring a unique fragment from 3516 bp to 3882 bp downstream of the start code under ZH11 background. Among the 27 independent transgenic lines generated, three lines, designated as RNAi-8, RNAi-9, and RNAi-7, were selected for their variously down-regulated ALT1 transcription (20%, 40%, and 60%, in turn) (Figure 3A). The root length of the RNAi transgenic lines decreased in a transcript dosage-dependent manner (Figure 3B), with about 98%, 85%, and 70% of that of the WT roots. Alkaline stress (pH 9.5) treatment showed that the three RNAi transgenic plants displayed different degrees of alkaline tolerance compared with WT (Figure 3C). RNAi-7, which had the greatest reduction in transcript, showed the strongest tolerance to alkaline stress. In contrast, RNAi-8, which had the smallest reduction in transcript, displayed similar phenotype as the WT in terms of tolerance to alkaline stress (Figure 3C). After 13 days of alkaline treatment, the WT seedlings were completely wilted (Figure 3C), while the seedlings of the RNAi-7 and RNAi-9 transgenic lines remained a survival rate of 88% and 56%, respectively, under the same stress conditions. These data clearly demonstrated that ALT1 plays a role in regulating tolerance to alkaline stress.
Figure 3. Suppression of ALT1 resulted in enhanced tolerance to alkaline stress.
(A) Transcript levels of ALT1 in the three selected RNAi transgenic lines and the vector control. Actin was used as an internal control. Data shown are mean values of three biological repeats with SD. (B) Morphology of two-leaf stage seedlings of the three RNAi transgenic lines and the control cultured in tap water under natural conditions. Bar = 2 cm. (C) Phenotypic analysis of alkaline tolerance. Two-leaf stage seedlings of the three RNAi transgenic lines and the vector control were treated with alkaline solution (pH 9.5), and photographed at 0 day and 13 day after the start of treatment, respectively.
Subcellular localization and transcriptional response of ALT1 to alkaline stress
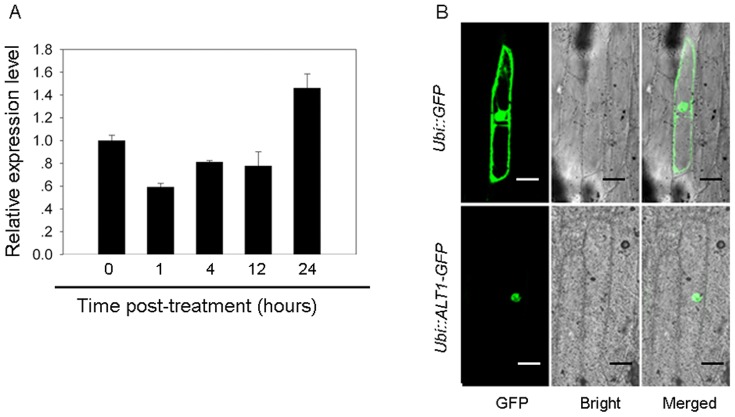
The data above showed that mutation of ALT1 enhances tolerance to alkaline stress. For functional characterization of ALT1, we investigated the transcriptional response of ALT1 to alkaline stress. The result showed that transcription of ALT1 was transiently and moderately suppressed after 1 hour of pH 10.0 treatment (Figure 4A), consistent with the negative function of ALT1 in alkaline tolerance.
Figure 4. Expression and subcellular localization of ALT1.
(A) Transcriptional response of ALT1 to alkaline stress. Two-leaf stage WT seedlings were treated with alkaline solution (pH 10.0), and ALT1 expression was monitored at the indicated time points by qRT-PCR analysis. Actin was used as internal control. Data shown are mean values of three biological repeats with SD. (B) Subcellular localization of ALT1. GFP and the ALT1-GFP fusion under the control of the maize Ubi promoter were transiently expressed in onion epidermal cells. Indicating the ALT1-GFP fusion protein was specifically expressed in the nucleus. Bars = 100 µm.
To determine the subcellular localization of ALT1, we fused in-frame of ALT1, driven by the maize ubiquitin promoter, to the upstream of the GFP-coding sequence, and transiently expressed the construct in onion epidermal cells. The green fluorescence signal resulting from ALT1::GFP was detected exclusively in the nucleus (Figure 4B), indicating that ALT1 is a nucleus localized protein.
alt1 shows stable capacity for ion absorption under alkaline stress
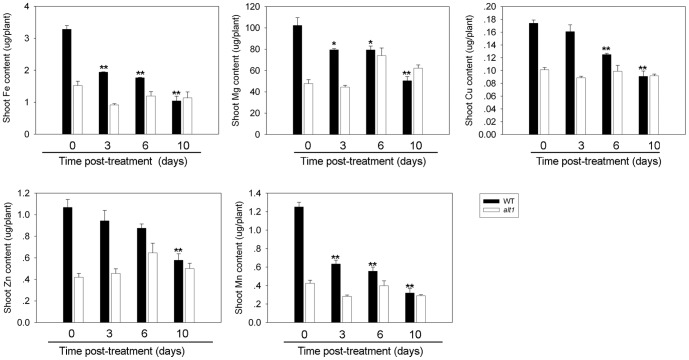
As alkaline stress severely affects the absorption of metal ions especially Fe [8], [9], [32], we quantified a series of metal ions (i.e., Fe, Mg, Cu, Zn, and Mn) in the shoots of alt1 and WT under alkaline stress (pH 9.5). The quantity of all metal ions decreased significantly in WT plants after alkaline treatment (Figure 5). After 10 days of treatment, the quantity of Fe, Mg, Cu, Zn, and Mn in WT plants decreased to 30%, 50%, 53%, 53% and 23% of the starting concentrations, respectively (Figure 5). The plants of alt1 contained lower levels of the five metal ions than the WT at the start of treatment, which should be due to the retarded growth of the mutant seedlings (Figure 1F). However, all these metal ions maintained at stable levels throughout the alkaline treatment in the mutant plants (Figure 5). These data suggest that the absorption of these metal ions, including Fe, was less affected in alt1 under alkaline conditions.
Figure 5. Metal ion quantification.
Two-leaf stage alt1 and WT seedlings grown hydroponically were subjected to alkaline solution (pH 9.5), and quantification of metal ions was carried out in the shoots of alt1 and WT plants on days 0, 3, 6 and 10, respectively. Values are means ± SE (n = 3). Asterisks denote significance compared with the control plants of day 0. *: P≤0.05, **: P≤0.01.
alt1 maintains intact root morphology under alkaline stress
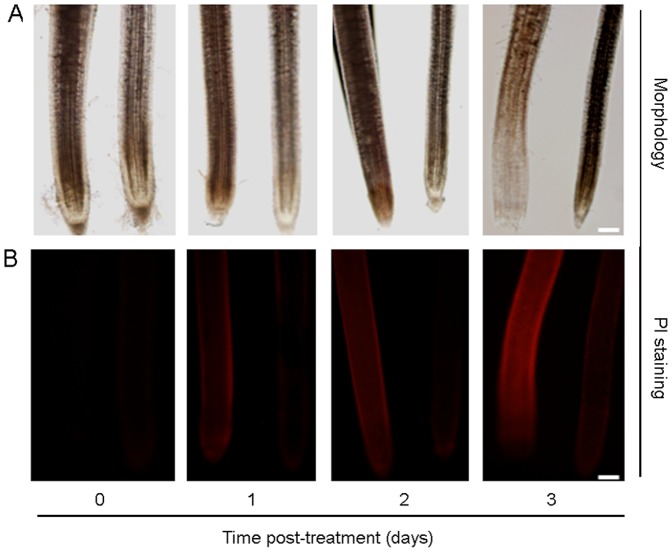
Previous studies showed that abiotic stresses such as drought and salt usually induce plant root death [33], [34]. Our data presented above show that alt1 had better growth (Figure 1C and 1D), and the mutant displayed a stable capacity for metal ion utilization under alkaline treatment (Figure 5). To determine whether root growth of alt1 was differentially impaired under alkaline stress, we first examined the root morphology of plants subjected to alkaline treatment. Because roots of WT collapsed rapidly upon treatment at pH 9.5 (data not shown), we treated the seedlings with pH 9.0 for detailed comparison. The results showed that roots of alt1 remained intact during a 3-day alkaline treatment (Figure 6A). In contrast, WT roots had obvious morphological defects after as short as 1 day of alkaline stress. Furthermore, root cap abscission was observed for WT plants after 2 days, and cells in the root tip collapsed after 3 days of treatment (Figure 6A). We next examined root vitality using PI staining, which labels dead or dying plant cells (Duan et al., 2010). Staining was detected on the root epidermis of WT after 1 day of treatment, and the stained area rapidly enlarged and intensified after the second day of treatment (Figure 6B). However, staining was consistently weak in the roots of the alt1 mutant (Figure 6B). These observations suggest that the mutant maintained intact root morphology under alkaline stress, which might explain why roots of alt1 are better able to absorb metal ions under such conditions.
Figure 6. alt1 showed intact root morphology under alkaline stress.
Two-leaf stage alt1 and WT seedlings were subjected to alkaline solution (pH 9.0) and evaluated over a time course. Left: WT; Right: alt1. (A) Root morphology of alt1 and WT under alkaline treatment. (B) Propidium iodide staining of root cells of alt1 and WT plants subjected to alkaline treatment. Bars = 2 mm.
Oxidative stress related genes are differentially expressed in the alt1 mutant
To explore the pathway for ALT1 to function in alkaline tolerance, a cDNA microarray analysis was performed to identify genes that are differentially expressed in alt1 and WT. Using the Rice Gene Expression 4×44 K Microarray (Agilent Technology), we compared the gene expression profiles in roots of alt1 and WT seedlings at the two-leaf stage under normal growth conditions. Totally, 641 transcripts were detected with differential expression levels (3-fold cutoff, P<0.01) (Table S2). Of these transcripts, 385 were up-regulated and 256 were down-regulated in alt1 compared with WT (Table S2). Because the mutant displayed stable levels of the five metal ions including Fe under alkaline treatment (Figure 5), and the improvement of Fe utilization has been regarded as the key factor for rice plants tolerant to alkaline stress [8], [9], we first supposed that ALT1 functions in alkaline tolerance by regulating the expression of metal ion utilization related genes. However, a survey of the microarray data found that, the known regulators functioning directly in metal ion utilization, such as OsIDEF1 (Os08g0101000), OsIDEF2 (Os05g0426200), OsIRO2 (Os01g0952800), OsNAS1 (Os03g0307300), OsNAS2 (Os03g0307200), OsNAS3 (Os07g0689600), OsNAAT1 (Os02g0306401), OsDMAS1 (Os03g0237100), OsTOM1 (Os12g0132650), OsYSL2 (Os02g0649900), OsYSL15 (Os02g0650300), OsIRT1 (Os03g0667500), and OsIRT2 (Os03g0667300) [32], could not be identified among the differentially expressed genes (Table S2).
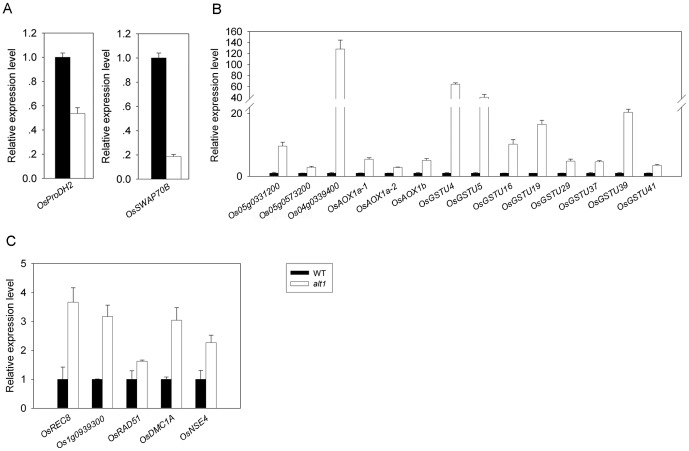
Functional classification of the differentially expressed genes revealed that about 78 of them are involved in abiotic stresses (Table S2). More importantly, 50 of the 78 genes appear to be involved in the defense against oxidative stress (Table 1). For example, OsSWAP70B (Os07g0138100) functions as a suppressor of ROS production [35], and OsProDH2 (Os10g0550900) appears to encode a proline oxidase that promotes ROS accumulation [36]. Transcription of both genes was significantly down-regulated in alt1 (Figure 7A). In addition, dozens of genes appear to protect plant cells from oxidative damage by scavenging ROS, such as a batch of Glutathione S-transferase genes, genes involved in the mitochondrial electron transport chain, genes encoding alcohol dehydrogenase-like proteins, an isoflavone reductase-like protein, and a cysteine proteinase-like protein [37]–[42]. Most of these genes were significantly up-regulated in the mutant (Figure 7B and Table 1). These data suggest that the quantity of ROS in alt1 might be maintained at lower levels by suppressing ROS production and enhancing ROS scavenging.
Table 1. Oxidative stress-related genes differentially expressed in alt1 as revealed by microarray analysis.
| Probe namea | Descriptionb | P-valuec | Fold changed |
| ROS producing | |||
| Os07g0138100 | Pleckstrin homology-type domain containing protein (OsSWAP70B) | 0.000420 | −5.29 |
| Os10g0550900 | Proline oxidase domain containing protein (OsProDH2) | 0.000020 | −6.01 |
| ROS scavenging | |||
| Os10g0527400 | Similar to Tau class GST protein 3 (OsGSTU19) | 0.000101 | 58.60 |
| Os01g0692100 | Glutathione S-transferase (OsGSTU39) | 0.000003 | 46.22 |
| Os10g0525600 | Similar to Tau class GST protein 3 | 0.000019 | 42.78 |
| Os10g0528300 | Tau class GST protein 4 (OsGSTU4) | 0.000161 | 36.84 |
| Os01g0949900 | Similar to Glutathione S-transferase (OsGSTU37) | 0.000007 | 35.86 |
| Os09g0367700 | Similar to GST6 protein (OsGSTU5) | 0.000059 | 25.58 |
| Os10g0368100 | Similar to Glutathione S-transferase GSTU35 | 0.000444 | 23.42 |
| Os01g0692000 | Similar to Glutathione S-transferase GST 26 | 0.000001 | 22.47 |
| Os05g0412800 | Similar to Glutathione S-transferase GST 41 (OsGSTU16) | 0.000014 | 20.62 |
| Os10g0528400 | Similar to Glutathione S-transferase (OsGSTU29) | 0.000071 | 11.62 |
| Os01g0950000 | Similar to Glutathione S-transferase GST 28 (OsGSTU41) | 0.000352 | 11.56 |
| Os03g0595600 | Similar to Glutathione S-transferase | 0.000001 | 9.45 |
| Os01g0949800 | Similar to Glutathione S-transferase GST 28 | 0.000001 | 6.75 |
| Os10g0530900 | Similar to Glutathione S-transferase GST 30 | 0.004421 | 6.11 |
| Os10g0528200 | Similar to Glutathione S-transferase TSI-1 | 0.000126 | 4.63 |
| Os01g0371200 | Similar to Glutathione-S-transferase 19E50 | 0.001183 | 3.87 |
| Os10g0525800 | Similar to Glutathione S-transferase GSTU31 | 0.008134 | 3.25 |
| Os10g0529500 | Similar to Glutathione-S-transferase 2 | 0.000013 | 3.23 |
| Os10g0529400 | Tau class GST protein 4 | 0.002258 | 3.23 |
| Os01g0369700 | Similar to Glutathione S-transferase GST 8 (OsGSTF5) | 0.001745 | 3.00 |
| Os05g0148900 | Similar to Glutathione-S-transferase 19E50 | 0.001472 | −3.29 |
| Os03g0643700 | Similar to GST6 protein | 0.002487 | −8.45 |
| Os02g0318100 | Alternative oxidase 1a (OsAOX1a-1) | 0.000669 | 23.84 |
| Os04g0600200 | Alternative oxidase 1a (OsAOX1a-2) | 0.000511 | 7.53 |
| Os05g0331200 | External rotenone-insensitive NADPH dehydrogenase | 0.000550 | 17.33 |
| Os08g0141400 | Similar to External rotenone-insensitive NADPH dehydrogenase | 0.000892 | 5.39 |
| Os01g0633500 | Similar to NADPH-dependent reductase A1 | 0.002659 | −4.34 |
| Os02g0794600 | Similar to Copper chaperone COX17-1 | 0.000085 | 7.34 |
| Os09g0370200 | Copper chaperone SCO1/SenC domain containing protein | 0.000104 | 4.78 |
| Os08g0496000 | Cytochrome oxidase assembly family protein | 0.002097 | 3.12 |
| Os02g0791400 | Cytochrome c oxidase, subunit VIb domain containing protein | 0.008472 | −3.26 |
| Os05g0573200 | NADP-isocitrate dehydrogenase | 0.001074 | 5.54 |
| Os04g0339400 | Aldo/keto reductase family protein | 0.000002 | 236.55 |
| Os12g0226900 | Similar to Allyl alcohol dehydrogenase | 0.000191 | 3.49 |
| Os11g0210300 | Alcohol dehydrogenase 1 | 0.000040 | 3.22 |
| Os02g0585700 | Quinonprotein alcohol dehydrogenase-like domain containing protein | 0.001116 | −5.40 |
| Os02g0586000 | Quinonprotein alcohol dehydrogenase-like domain containing protein | 0.000832 | −21.53 |
| Os01g0106400 | Similar to Isoflavone reductase homolog IRL (OsIRL) | 0.000257 | 14.03 |
| Os09g0381400 | Similar to Ervatamin C | 0.000013 | 4.33 |
| DNA repair | |||
| Os12g0497300 | Similar to DNA repair protein RAD51 homolog (OsRAD51) | 0.000015 | 8.34 |
| Os12g0143800 | Similar to Disrupted meiotic cDNA 1 protein (OsDMC1A) | 0.000016 | 8.18 |
| Os11g0146800 | Similar to Disrupted meiotic cDNA 1 protein (OsDMC1B) | 0.001043 | 4.79 |
| Os05g0580500 | Rad21/Rec8 like protein, N-terminal domain containing protein (OsREC8) | 0.000034 | 7.21 |
| Os06g0618000 | Nse4 domain containing protein (OsNSE4) | 0.000007 | 7.02 |
| Os01g0939300 | BRCT domain containing protein | 0.000063 | 5.44 |
| Os07g0209500 | DNA-directed DNA polymerase, family B domain containing protein (OsREV3) | 0.001388 | 5.03 |
| Os01g0801100 | Apurinic endonuclease-redox protein (OsARP) | 0.000007 | 4.50 |
| Os05g0498300 | DNA mismatch repair protein MutS, core domain containing protein (OsMSH5) | 0.000005 | 3.71 |
Name of probe set on Affymetrix Rice GeneChip.
Gene annotation in The Rice Annotation Project Database.
P-value of statistical Student's t-test.
Fold change of alt1 compared with WT. Values are calculated by R-software.
Figure 7. Expression analysis of oxidative stress-related genes.
qRT-PCR was conducted on the roots of hydroponically grown two-leaf stage alt1 and WT seedlings. Actin was used as an internal control. Data shown are mean values of three biological repeats with SD. (A) Genes related to ROS producing.- (B) Genes related to ROS scavenging. (C) Genes related to DNA repair.
Besides the genes involved in ROS homeostasis, a number of differentially expressed genes related to DNA repair were also identified in alt1, such as OsREC8 (Os05g0580500) [43], OsRAD51 (Os12g0497300) [44], OsARP (Os01g0801100) [45], OsMSH5 (Os05g0498300) [46], OsREV3 (Os07g0209500) [47], and OsDMC1A (Os12g0143800) [48]. Transcript levels of all these genes were significantly increased in the alt1 mutant (Figure 7C and Table 1), suggesting that DNA repair machinery might be highly activated in the mutant.
We also noticed that the expression of a dozen transcription factor (TF) genes related to abiotic stresses, such as NAC, WRKY, MYB, bZIP, and C2H2-type zinc finger [49], were significantly changed in the alt1 mutant (Table S2 and Figure S4). This result suggests that the TFs may also contribute to ALT1-mediated alkaline tolerance in rice.
alt1 exhibits tolerance to oxidative stress
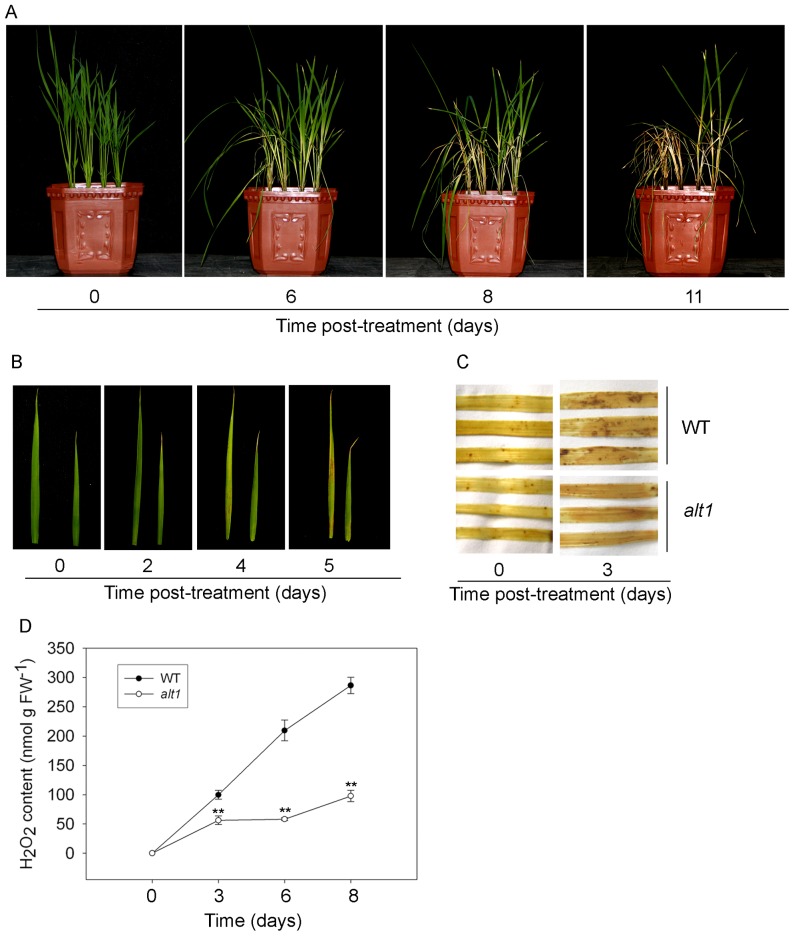
Abiotic stresses usually cause ROS accumulation, and excessive ROS results in oxidative stress, which ultimately causes cell death [50]. The microarray data above demonstrated that expression of a majority of genes involved in oxidative stress is favorably altered in the alt1 background, suggesting that the mutant might be tolerant to oxidative stress. To test this notion, we subjected alt1 and WT seedlings to 20 µM methyl viologen (MV), a well-known oxidative stress inducer for the production of ROS in chloroplasts under light [51]. Four days after MV treatment, necrotic spots were visible on the leaves of WT seedlings, and WT plants began wilting after 6 days and mostly died after 11 days of MV treatment (Figure 8A). In contrast, the leaves of alt1 were still green after 4 days of MV treatment (Figure 8B), and about half of the alt1 plants still survived 11 days under this conditions (Figure 8A). 3, 3′-diaminobenzidine (DAB) staining showed that brown precipitate, which is indicative of H2O2 accumulation, was distributed in the leaves of both alt1 and WT plants under MV treatment, but more precipitate was present in WT leaves than in those of alt1 (Figure 8C). We further examined whether the alkaline stress causes higher levels of oxidative stress in WT than alt1 by quantifying the level of H2O2. We found that the H2O2 content in WT leaves increased significantly from 0 to 300 nmol g FW−1 during the alkaline of pH 9.5 treatment (Figure 8D). In the leaves of the mutant, however, the level of H2O2 only reached 100 nmol g FW−1, one-third of that in WT after 8 days of treatment (Figure 8D). These data suggest that the oxidative stress induced by alkaline treatment is greatly alleviated in the alt1 mutant.
Figure 8. Phenotypic analysis of alt1 and WT plants under oxidative stress.
Two-leaf stage alt1 and WT seedlings grown hydroponically were subjected to 20 µM MV treatment. The left is WT and right is alt1 for (A) and (B), respectively. (A) Phenotypes of alt1 and WT at the indicated time points during MV treatment. (B) Leaf morphology of alt1 and WT during MV treatment. (C) DAB staining of alt1 and WT leaves from plants under normal (left) and stressed (right, pH 9.5) conditions, respectively. (D) Quantitative measurement of H2O2 in alt1 and WT leaves during pH 9.5 treatment. Values are means ± SD (n = 3).
Discussion
ALT1 functions in alkaline tolerance mainly via alleviating oxidative damage
The common feature for plants grown in calcareous soils is chlorosis, a typical symptom of Fe deficiency. Therefore, studies on the improvement of tolerance to the stress focused mostly on the enhancement of Fe uptake in the transgenic plants [8]–[14]. Although alt1 showed stable capacity of metal ion absorption including Fe under alkaline stress, none of the genes involved in metal ion utilization was differentially expressed between the mutant and WT (Table S2). In fact, whole genome transcriptional profiling of soybean under Fe deficiency [52] and Puccinellia tenuiflora under alkaline salt stress [15] revealed that the differentially expressed genes comprise various functional groups, including those related to ROS homeostasis and DNA repair. Besides, study of glutathione reductases, a large family of enzymes functioning in scavenging of ROS [50], in graminaceous plants found that expression of the related coding genes could be induced upon Fe deficiency [53], suggesting the involvement of ROS homeostasis in Fe-deficiency response. However, no direct evidence so far has been shown to connect ROS homeostasis with plant alkaline tolerance. Using microarray analysis, we found that transcription of 78 abiotic stress-related genes was significantly altered in the mutant background, with 50 out of them involving in oxidative stress response (Figure 7 and Table 1). Consistently, the mutant showed low levels of H2O2 accumulation and tolerance to oxidative stress (Figure 8). According to the report by Møller (2001) [54], strategies adopted by the plants to defend against oxidative stress include three lines of steps, i.e., avoid of ROS production, detoxification of ROS (ROS scavenging), and repair of ROS-mediated damage. The detected 50 oxidative stress-related genes in our study covered all the above three lines of defense steps (Table 1), suggesting the importance of ALT1 in defending against the oxidative damage induced by alkaline stress.
Despite much progress, it still remains unclear how ROS affect the stress response of plants. In general, ROS is thought to affect stress responses by two different means [52], [55]. First, ROS react with cell structures, nucleic acids, lipids, and proteins [55], and may thus cause irreversible damage that can lead to tissue necrosis and ultimately plant death. Second, ROS influence the expression of a number of genes and signal transduction pathways, which activate and control various genetic stress response programs [52], [55]. Our results presented here revealed that ROS homoeostasis plays a critical regulatory role in the plant's tolerance to alkaline stress, which might involve both of the means described above. The most significant effect of the alt1 mutation on the plant's tolerance to alkaline stress is the reduction of ROS levels, which protects the roots of the mutant from damaging oxidative stress (Figure 6). On the other hand, the differentially expressed genes detected in our microarray assay (Table S2) suggest that the induced ROS may trigger alkaline stress responses in alt1 by modulating gene expression in an indirect manner. For example, except for the genes related to oxidative stress defense, many kinds of transcription factors involved in the abiotic stress response, such as WRKY, MYB, NAC, and bZIP, were found to be differentially regulated in the mutant (Table S2 and Figure S4). Furthermore, a series of protein kinases, including MAPK, was also identified in the microarray analysis (Table S2). Previous studies suggested that ROS generation induced by various stresses activates MAPK signaling cascades, and ROS-induced activation of MAPKs appears to be central for mediating cellular responses to multiple stresses [55]. The broadly affected genes by ALT1 suggest that the role of ROS in regulating tolerance to alkaline stress is complicated and involves many processes.
Although the enhanced defense against oxidative damage in alt1 gave plants tolerant to alkaline stress (Figure 1), the mutant showed similar response to drought and salt as WT (Figure S2). This observation suggests that the effects of oxidative stress might be different among abiotic stress conditions. So far, many studies have shown that ROS is involved in the response of plants to various abiotic stresses including drought and salt [56]. For example, mutation in ITN1 increased tolerance of plants to salt stress by enhancing ROS scavenging, but the mutant displayed a WT response when under drought conditions [57]. In addition, the reduction in scavenging of ROS resulted in dsm1 and dsm2 hypersensitive to drought stress, but the two mutants showed similar response as WT under cold or heat stress conditions [51], [58]. Furthermore, previous studies have shown that the oxidative stress induced by Fe deficiency is fundamentally different from that induced by other factors such as salt or heavy metal stress [53], [59]. Therefore, the differential responses of alt1 to alkaline and drought or salt observed in our study (Figure 1A and Figure S2) also support this notion that the oxidative stress induced by alkaline might be different from that induced by salt or drought. The cause for such difference, however, remained to be understood.
ALT1 provides a novel finding on the involvement of chromatin remodeling ATPase in plant alkaline tolerance
Chromatin remodeling plays a central role in establishing specific gene expression patterns and maintaining transcriptional states in eukaryotes [24], and loss of function of the component proteins usually results in alteration in multiple plant developmental processes [25]. Furthermore, emerging evidence shows that chromatin regulation also plays important functions in plant abiotic stress responses [26]. Interestingly, most of the core subunit ATPase of chromatin remodeling complex, such as AtCHR12 [27] and AtBRM [28] in Arabidopsis, function as negative regulators in abiotic stress tolerance. The rice genome contains 40 Snf2 family proteins [29]. However, only one member of OsCHR4 was reported to function in early chloroplast development [30]. Transcriptional profiling of the 40 genes found that some of the members respond to abiotic stresses such as draught, salt and cold [29]. However, how about the response of these genes to alkaline stress remained to be investigated. The ALT1 protein identified here belongs to the Ris1 subgroup of Snf2 family [29]. Similar to the various functions found for the Snf2 family ATPases [25], knockout alt1 plants displayed multiple morphological alterations, including reduced root length and plant height at early vegetative stage and low tiller number at adult stage (Figure 2C and Table S1). However, unlike the function of any other Snf2 family proteins reported so far [25], ALT1 functions negatively in stress tolerance to alkaline but not salt or drought (Figure 1 and Figure S2), consistent with the report that ALT1 was excluded from the Snf2 family genes that show transcriptional response to the stresses of salt and drought [29]. Therefore, our results provided a novel finding on the involvement of chromatin remodeling complex in alkaline stress response. The modulation of the expression of several batches of genes (Table S2) suggests that ALT1 might be a key regulator that occupies a critical site in the gene transcriptional regulatory network by modulating chromatin structure. However, we still have much to learn about the molecular mechanisms of the chromatin remodeling complexes and their biochemical capabilities [25], and further studies need to be performed to reveal the molecular function of ALT1 in alkaline stress tolerance.
ALT1 provides a potential two-step strategy for improving the tolerance of rice to severe alkaline stress
The underground part of roots is the first organ to perceive most of the abiotic stresses such as drought, salt or alkaline. Although the roots could also signal the stress to the aerial parts of the plants via various pathways [60], its basic function of water and nutrient absorption is indispensable for any other parts of the plant. However, almost all these stresses would inevitably induce excess accumulation of ROS within the plants when under severe stress conditions, and the over-accumulated ROS would react with cell structures, nucleic acids, lipids and proteins, which ultimately lead to tissue necrosis and plant death [50]. Therefore, although the low Fe availability is considered to be the main reason for the reduced crop yield and quality on calcareous soils [8], [9], a survey of the related reports revealed that almost all of the researches were conducted under the mild alkaline condition of pH 8.5 [8]–[14]. This could be due to the fact that under alkaline stress higher than pH 9.0, cell death would occur rapidly in the roots of rice plants, as that observed for WT roots in our study (Figure 6). Therefore, it would be of little effect to change the insoluble Fe into an absorbable form for plants without functional roots under such alkaline conditions. On the other hand, although mutation in ALT1 could keep the roots of the plants functionally in nutrient uptake (Figure 5), the mutant could not survive the alkaline stress of pH 9.5 until adult stage (data not shown). This could be explained by that only a very limited absorbable free form of Fe2+ could be utilized under such conditions [32], [61], [62], which is far from sufficient for developmental, physiological and biochemical processes of the growing alt1 plants. From these points of view, the strategy for genetic improvement of rice plants tolerant to severe alkaline stress should combine both steps described above, i.e., the first step of utilizing mutated form of ALT1 to ensure normal root function by alleviating oxidative damage, and the second step of exploiting genes to increase the production and secretion of mugineic acid family phytosiderophores to chelate the large amount of oxidized form of Fe3+ [32]. Verification of this hypothesis is now underway.
Materials and Methods
Plant materials and growth conditions
The rice mutant alt1 is in the KY131 background. Rice plants were cultivated in an experimental field at the Institute of Genetics and Developmental Biology (IGDB) in Beijing. An F2 mapping population was constructed by crossing alt1 (Japonica) with Kasalath (Indica). Rice plants used for the experiments were cultured in Kimura's culture solution B with the following composition: 0.18 mM (NH4)2SO4, 0.27 mM Mg(SO4)2, 0.091 mM KNO3, 0.091 mM KH2PO4, 0.046 mM K2SO4, 0.18 mM Ca(NO3)2, and 0.04 mM EDTA-Fe, and grown under natural conditions.
Stress treatment
For alkaline stress treatment, seedlings of two-leaf stage WT, alt1, and RNAi transgenic plants were transferred to Kimura's culture solution B, and the solution was adjusted to pH 9.0 by adding 25 mM NaHCO3 and 1.765 mM NaOH, to pH 9.5 by adding 25 mM NaHCO3 and 5 mM NaOH, and to pH 10.0 by adding 25 mM NaHCO3 and 10.7 mM NaOH. For other stresses, two-leaf stage alt1 and WT seedlings were subjected to Kimura's culture solution B with the addition of 80, 120, 150, and 180 mM NaCl for salt treatment, and 20 µM methyl viologen (MV) for oxidative stress treatment, respectively. These solutions were renewed every 3 days. For drought treatment, alt1 and WT seedlings were grown to the two-leaf stage in pots filled with nutrient soil, and water was withheld to allow drought stress to develop for 5, 6 and 7 days before irrigation, respectively.
For investigation of transcriptional response of ALT1 to alkaline stress, roots of two-leaf-stage WT seedlings under alkaline stress of pH 10.0 were sampled at 0, 1, 4, 12 and 24 hours after the start of treatment, respectively.
Map-based cloning
The alt1 locus was firstly mapped between markers RM11740 and RM3285 on chromosome 1 using 316 F2 mutant plants derived from cross of alt1 with Kasalath. For fine mapping, two other molecular markers were developed and the underlying gene was further narrowed down to a 35.2-kb region between markers M1 and M6 using 538 F2 mutant plants. Primers used in this study are listed in Table S3. Three predicted ORFs within this region were then sequenced and compared with WT and Nipponbare (http://rapdb.dna.affrc.go.jp/).
Plasmid construction and transgenic experiments
For complementation test, the full-length coding sequence of Os01g0779400 driven by its native promoter (1.1-kb region upstream of the ATG start codon) was ligated into the binary vector pZH2B, and the plasmid was then transformed into Agrobacterium tumefaciens AGL-1. Plant transformation was conducted as described previously [51].
The ALT1-RNAi construct was generated by inserting a hairpin sequence with two 367-bp cDNA inverted repeats targeting the sequence from 3516–3882 bp downstream of the start codon of ALT1. The 367-bp fragment was ligated into pZH2Bi and driven by the Ubiquitin promoter. ZH11 (Japonica) was used as the receptor plant for transformation by Agrobacterium. Primers used for vector construction are listed in Table S3.
ALT1 subcellular localization
To investigate the subcellular localization of ALT1, the Ubi::ALT1-sGFP plasmid and empty vector were introduced into onion epidermal cells using particle bombardment with a PDS-1000/He (BIO-RAD). After overnight incubation at 25°C in darkness, bombarded tissues were examined by confocal laser-scanning microscopy (Leica TCS SP5) using 488-nm excitation and 500- to 530-nm emissions pass filters.
3,3′-diaminobenzidine (DAB) and propidium iodide (PI) staining
DAB staining was carried out by the method of Ning et al. (2010) [51], and PI staining was performed as described previously [35] with slight modifications. Briefly, roots of seedlings were immersed in the solution containing 1 µg mL−1 PI in distilled water, incubated for 1 min at room temperature, and then washed with 1× phosphate buffered saline (PBS) several times. Staining was observed using fluorescence microscopy (OLYMPUS DP72).
Metal ion concentration measurement
Shoots of two-leaf stage alt1 and WT seedlings were collected at day 0, 3, 6, and 10 after alkaline stress treatment (pH 9.5), and dried in a 65°C incubator for 1 week. The samples (100–150 mg) were then wet-ashed with 13 mL of 11 M HNO3 and 2 mL of 30% H2O2 according to the following procedure: 110°C for 10 min, 130°C for 10 min, 150°C for 15 min, and 150°C for 60 min. The metal ion concentration was measured using an Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES, Perkin Elmer, USA) at wavelengths of 238.204 nm (Fe), 279.077 nm (Mg), 327.393 nm (Cu), 206.200 nm (Zn), and 257.610 nm (Mn), respectively, with three independent biological replicates.
Microarray analysis
Roots of two-leaf stage alt1 and WT seedlings grown under normal conditions were sampled for microarray analysis. The transcriptomic profiles were investigated using an Agilent-015241 Rice Gene Expression 4×44 K Microarray (Agilent Technology) containing 32,325 probes corresponding to cDNA, 6,934 probes corresponding to expressed sequence tags (ESTs), and 2,612 probes corresponding to gene predicted loci, respectively, with three independent biological replicates. All microarray procedures and data analyses were performed according to the manufacturer's instructions. The microarray data were deposited in Gene Expression Omnibus of NCBI under accession number: GSE61788 (http://www.ncbi.nlm.nih.gov/geo/info/linking.html). Analysis was performed using an ANOVA-false discovery rate (ANOVA-FDR) p-value of <0.01. Spots with changes in expression were extracted based on at least a threefold increase or decrease in expression. Functional classification of the differentially expressed genes was carried out using the tools for GO categories and revised manually.
Quantitative real-time RT-PCR (qRT-PCR) analysis
Total RNA was extracted using a TaKaRa RNAiso Plus Kit. For qRT-PCR, the RNA was pre-treated with DNase I (Fermentas), and first-strand cDNA was synthesized from 1 µg total RNA according to manufacturer's protocol (Promega). qRT-PCR was performed with the Lightcycler 480 SYBR Green I Master according to the manufacturer's protocol. Three repeats were carried out for each gene. For normalization, the Actin gene was used as the endogenous control. Primers used for qRT-PCR are listed in Table S3.
H2O2 measurement
The level of H2O2 was assessed as described [63]. Briefly, 200 mg of fresh leaf tissue from alt1 and WT seedlings subjected to alkaline stress treatment was extracted in 2.0 ml of TCA (0.1% w/v) on ice, and the homogenate was then centrifuged at 13,000 g for 15 min. After the addition of 0.3 ml of 10 mM sodium phosphate buffer (pH 7.5) and 0.6 ml of 1 M potassium iodide to 0.3 ml of the above supernatant, the absorbance of the samples was measured at 390 nm. H2O2 content, expressed as nmol g−1 fresh weight, was determined based on the standard curve generated from known concentrations of H2O2.
Supporting Information
Phenotypic analysis of the alt1 mutant. Two-leaf stage WT (left) and alt1 (right) seedlings were subjected to alkaline treatment with pH 9.0, and photographed at 15 days after treatment.
(TIF)
The alt1 mutant showed a normal response to salt and drought stresses. Left part: WT; Right part: alt1. Two-leaf stage alt1 and WT seedlings were subjected to NaCl and drought treatments, respectively.
(TIF)
Comparison of the predicted amino acid sequence of ALT1 and the truncated alt1 protein in the mutated region. The amino acids of the mutated region from 720 aa to 1228 aa of ALT1 are shown. The truncated alt1 stopped at 1085 aa.
(TIF)
Expression analysis of selected TF genes. qRT-PCR was conducted on the roots of hydroponically grown two-leaf stage alt1 and WT seedlings. Actin was used as an internal control. Data shown are mean values of three biological repeats with SD.
(TIF)
Comparison of agronomic traits between alt1 and WT.
(DOCX)
List of genes up/down-regulated 3 fold more in alt1 as revealed by microarray analysis.
(DOCX)
Primers used in this study.
(DOCX)
Acknowledgments
We wish to thank Prof. Hongqing Ling and Ms. Shiqin Jia (IGDB, CAS, China) for help with the metal ion quantification and GFP observation, respectively.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. The microarray data were deposited in Gene Expression Omnibus of NCBI under accession number: GSE61788.
Funding Statement
This work was supported by grants from Chinese Academy of Sciences (KSCX2-YW-N-079), Hundred Talents Program of Chinese Academy of Sciences (KSCX2-YW-076), National “973” Program (2013CBA01405) and State Key Laboratory of Plant Genomics. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO AGL (2000) Extent and causes of salt affected soils in participating countries. Available: http://www.fao.org/ag/agl/agll/spush/topic2.htm. Accessed: 2014 Nov 3.
- 2. Shi DC, Yin LJ (1993) Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora (Griseb.) Scribn. et Merr. plants. Acta Bot Sin 35:144–149. [Google Scholar]
- 3. Ren ZH, Gao JP, Li LG, Cai XL, Huang W, et al. (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37:1141–1146. [DOI] [PubMed] [Google Scholar]
- 4. Hu H, Dai M, Yao J, Xiao B, Li X, et al. (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103:12987–12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, et al. (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Z, Wang J, Zhang R, Huang R (2012) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis . Plant J 71:273–287. [DOI] [PubMed] [Google Scholar]
- 7. Yang C, Chong J, Li C, Kim C, Shi D, et al. (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276. [Google Scholar]
- 8. Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469. [DOI] [PubMed] [Google Scholar]
- 9. Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, et al. (2007) Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc Natl Acad Sci USA 104:7373–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi T, Ogo Y, Itai RN, Nakanishi H, Takahashi M, et al. (2007) The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Natl Acad Sci USA 104:19150–19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi T, Nakanishi H, Takahashi M, Mori S, Nishizawa NK (2008) Generation and field trials of transgenic rice tolerant to iron deficiency. Rice 1:144–153. [Google Scholar]
- 12. Ogo Y, Itai RN, Kobayashi T, Aung MS, Nakanishi H, et al. (2011) OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol Biol 75:593–605. [DOI] [PubMed] [Google Scholar]
- 13. Gómez-Galera S, Sudhakar D, Pelacho AM, Capell T, Christou P (2012) Constitutive expression of a barley Fe phytisiderophore transporter increases alkaline soil tolerance and results in iron partitioning between vegetative and storage tissues under stress. Plant Physiol Biochem 53:46–53. [DOI] [PubMed] [Google Scholar]
- 14. Lee S, Kim YS, Jeon US, Kim YK, Schjoerring JK, et al. (2012) Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cells 33:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Wei L, Wang Z, Wang T (2013) Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J Integr Plant Biol 55:262–276. [DOI] [PubMed] [Google Scholar]
- 16. Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, et al. (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant cell 19:1617–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Y, Qin Y, Xie C, Zhao F, Zhao J, et al. (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant cell 22:1313–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao P, Bai X, Yang L, Lv D, Li Y, et al. (2010) Over-expression of osa-MIR396c decreases salt and alkali stress tolerance. Planta 231:991–1001. [DOI] [PubMed] [Google Scholar]
- 19. Gao P, Bai X, Yang L, Lv D, Pan X, et al. (2011) osa-MIR393: a salinity- and alkaline stress-related microRNA gene. Mol Biol Rep 38:237–242. [DOI] [PubMed] [Google Scholar]
- 20. Lin S, Cianzio S, Shoemaker R (1997) Mapping genetic loci for iron deficiency chlorosis in soybean. Mol Breed 3:219–229. [Google Scholar]
- 21. Charlson DV, Bailey TB, Cianzio SR, Shoemaker RC (2005) Molecular marker Satt481 is associated with iron-deficiency chlorosis resistance in a soybean breeding population. Crop Sci 45:2394–2399. [Google Scholar]
- 22. Qi D, Guo G, Lee M, Zhang J, Cao G, et al. (2008) Identification of quantitative trait loci for the dead leaf rate and the seedling dead rate under alkaline stress in rice. J Genet Genomics 35:299–305. [DOI] [PubMed] [Google Scholar]
- 23. Tusen DD, Lal SK, Xu DH (2010) Identification of a major QTL allele from wild soybean (Glycine soja Sieb. & Zucc.) for increasing alkaline salt tolerance in soybean. Theor Appl Genet 121:229–236. [DOI] [PubMed] [Google Scholar]
- 24. Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273–304. [DOI] [PubMed] [Google Scholar]
- 25. Kwon CS, Wagner D (2007) Unwinding chromatin for development and growth: a few genes at a time. Trends Genet 23:403–412. [DOI] [PubMed] [Google Scholar]
- 26. Kim J-M, To TK, Nishioka T, Seki M (2010) Chromatin regulation functions in plant abiotic stress responses. Plant Cell Environ 33:604–611. [DOI] [PubMed] [Google Scholar]
- 27. Mlynárová L, Nap JP, Bisseling T (2007) The SWI/SNF chromatin-remodeling gene AtCHR12 mediates temporary growth arrest in Arabidopsis thaliana upon perceiving environmental stress. Plant J 51:874–885. [DOI] [PubMed] [Google Scholar]
- 28. Han SK, Sang Y, Rodrigues A, BIOL425 F2010, Wu M-F, et al. (2012) The SWI2/SNF2 chromatin remodeling ATPase BRAHMA represses abscisic acid responses in the absence of the stress stimulus in Arabidopsis . Plant Cell 24:4892–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu Y, Zhu N, Wang X, Yi Q, Zhu D, et al. (2013) Analysis of rice Snf2 family proteins and their potential roles in epigenetic regulation. Plant Physiol Biochem 70:33–42. [DOI] [PubMed] [Google Scholar]
- 30. Zhao C, Xu J, Chen Y, Mao C, Zhang S, et al. (2012) Molecular cloning and characterization of OsCHR4, a rice chromatin-remodeling factor required for early chloroplast development in adaxial mesophyll. Planta 236:1165–1176. [DOI] [PubMed] [Google Scholar]
- 31. Hopfner K-P, Gerhold C, Lakomek K, Wollmann P (2012) Swi2/Snf2 remodelers: hybrid views on hybrid molecular machines. Curr Opin Struct Biol 22:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi T, Nakanishi H, Nishizawa NK (2010) Recent insights into iron homeostasis and their application in graminaceous crops. Proc Jap Acad 86:900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan Y, Zhang W, Li B, Wang Y, Li K, et al. (2010) An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis . New Phytol 186:681–695. [DOI] [PubMed] [Google Scholar]
- 34. Ogawa D, Abe K, Miyao A, Kojima M, Sakakibara H, et al. (2011) RSS1 regulates the cell cycle and maintains meristematic activity under stress conditions in rice. Nat Commun 2:278 10.1038/ncomms1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamaguchi K, Imai K, Akamatsu A, Mihashi M, Hayashi N, et al. (2012) SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J 70:389–397. [DOI] [PubMed] [Google Scholar]
- 36. Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. [DOI] [PubMed] [Google Scholar]
- 37. Sunkar R, Bartels D, Kirch HH (2003) Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464. [DOI] [PubMed] [Google Scholar]
- 38. Athar H-R, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Env Exp Bot 63:224–231. [Google Scholar]
- 39. Kim SG, Kim ST, Wang Y, Kim SK, Lee CH, et al. (2009) Overexpression of rice isoflavone reductase-like gene (OsIRL) confers tolerance to reactive oxygen species. Physiol Plant 138:1–9. [DOI] [PubMed] [Google Scholar]
- 40. Jain M, Ghanashyam C, Bhattacharjee A (2010) Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genomics 11:73 10.1186/1471-2164-11-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bourens M, Fontanesi F, Soto IC, Liu J, Barrientos A (2012) Redox and reactive oxygen species regulation of mitochondrial cytochrome c oxidase biogenesis. Antioxid Redox Signal 19:1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li CR, Liang DD, Li J, Duan YB, Li H, et al. (2013) Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice ALTERNATIVE OXIDASE 1 genes. Plant Cell Environ 36:775–788. [DOI] [PubMed] [Google Scholar]
- 43. Shao T, Tang D, Wang K, Wang M, Che L, et al. (2011) OsREC8 is essential for chromatid cohesion and metaphase I monopolar orientation in rice meiosis. Plant Physiol 156:1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Markmann-Mulisch U, Wendeler E, Zobell O, Schween G, Steinbiss HH, et al. (2007) Differential requirements for RAD51 in Physcomitrella patens and Arabidopsis thaliana development and DNA damage repair. Plant Cell 19:3080–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Córdoba-Cañero D, Roldán-Arjona T, Ariza RR (2011) Arabidopsis ARP endonuclease functions in a branched base excision DNA repair pathway completed by LIG1. Plant J 68:693–702. [DOI] [PubMed] [Google Scholar]
- 46. Lu X, Liu X, An L, Zhang W, Sun J, et al. (2008) The Arabidopsis MutS homolog AtMSH5 is required for normal meiosis. Cell Res 18:589–599. [DOI] [PubMed] [Google Scholar]
- 47. Wang S, Wen R, Shi X, Lambrecht A, Wang H, et al. (2011) RAD5a and REV3 function in two alternative pathways of DNA-damage tolerance in Arabidopsis . DNA Repair 10:620–628. [DOI] [PubMed] [Google Scholar]
- 48. Deng ZY, Wang T (2007) OsDMC1 is required for homologous pairing in Oryza Sativa . Plant Mol Biol 65:31–42. [DOI] [PubMed] [Google Scholar]
- 49. Lindemose S, O'Shea C, Jensen MK, Skriver K (2013) Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 14:5842–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. [DOI] [PubMed] [Google Scholar]
- 51. Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Rourke JA, Charlson DV, Gonzalez DO, Vodkin LO, Graham MA, et al. (2007) Microarray analysis of iron deficiency chlorosis in near-isogenic soybean lines. BMC Genomics 8:476 10.1186/1471-2164-8-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bashir K, Nagasaka S, Itai RN, Kobayashi T, Takahashi M, et al. (2007) Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol Biol 65:277–284. [DOI] [PubMed] [Google Scholar]
- 54. Møller IM (2001) Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Mol Biol 52:561–591. [DOI] [PubMed] [Google Scholar]
- 55. Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. [DOI] [PubMed] [Google Scholar]
- 56. Huang GT, Ma SL, Bai LP, Zhang L, Ma H, et al. (2012) Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep 39:969–87. [DOI] [PubMed] [Google Scholar]
- 57. Sakamoto H, Matsuda O, Iba K (2008) ITN1, a novel gene encoding an ankyrin-repeat protein that affects the ABA-mediated production of reactive oxygen species and is involved in salt-stress tolerance in Arabidopsis thaliana . Plant J 56:411–422. [DOI] [PubMed] [Google Scholar]
- 58. Du H, Wang N, Cui F, Li X, Xiao J, et al. (2010) Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol 154:1304–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SY, Lim JH, Park MR, Kim YJ, Park TI, et al. (2005) Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. J Biochem Mol Biol 38:218–224. [DOI] [PubMed] [Google Scholar]
- 60. Schmidt R, Caldana C, Mueller-Roeber B, Schippers JH (2014) The contribution of SERF1 to root-to-shoot signaling during salinity stress in rice. Plant Signal Behav 9: pii:e27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bughio N, Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S (2002) Cloning an iron-regulated metal transporter from rice. J Exp Biol 53:1677–1682. [DOI] [PubMed] [Google Scholar]
- 62. Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, et al. (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ . Plant J 45:335–346. [DOI] [PubMed] [Google Scholar]
- 63. Garg B, Jaiswal JP, Misra S, Tripathi BN, Prasad M (2012) A comprehensive study on dehydration-induced antioxidative responses during germination of Indian bread wheat (Triticum aestivum L. em Thell) cultivars collected from different agroclimatic zones. Physiol Mol Biol Plants 18:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic analysis of the alt1 mutant. Two-leaf stage WT (left) and alt1 (right) seedlings were subjected to alkaline treatment with pH 9.0, and photographed at 15 days after treatment.
(TIF)
The alt1 mutant showed a normal response to salt and drought stresses. Left part: WT; Right part: alt1. Two-leaf stage alt1 and WT seedlings were subjected to NaCl and drought treatments, respectively.
(TIF)
Comparison of the predicted amino acid sequence of ALT1 and the truncated alt1 protein in the mutated region. The amino acids of the mutated region from 720 aa to 1228 aa of ALT1 are shown. The truncated alt1 stopped at 1085 aa.
(TIF)
Expression analysis of selected TF genes. qRT-PCR was conducted on the roots of hydroponically grown two-leaf stage alt1 and WT seedlings. Actin was used as an internal control. Data shown are mean values of three biological repeats with SD.
(TIF)
Comparison of agronomic traits between alt1 and WT.
(DOCX)
List of genes up/down-regulated 3 fold more in alt1 as revealed by microarray analysis.
(DOCX)
Primers used in this study.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. The microarray data were deposited in Gene Expression Omnibus of NCBI under accession number: GSE61788.