Summary
Optical stimulation and silencing of neural activity is a powerful technique for elucidating the structure and function of neural circuitry. In most in vivo optogenetic experiments, light is delivered into the brain through a single optical fiber. However, this approach limits illumination to a fixed volume of the brain. Here a focused ion beam is used to pattern multiple light windows on a tapered optical fiber. We show that such fibers allow selective and dynamical illumination of different brain regions along the taper. Site selection is achieved by a simple coupling strategy at the fiber input, and the use of a single tapered waveguide minimizes the implant invasiveness. We demonstrate the effectiveness of this approach for multipoint optical stimulation in the mammalian brain in vivo by coupling the fiber to a microelectrode array and performing simultaneous extracellular recording and stimulation at multiple sites in the mouse striatum and cerebral cortex.
Introduction
The use of microbial opsins for optical stimulation and silencing of neuronal activity (optogenetics) facilitates understanding neural circuits and linking the activity of circuit elements to behavior (Alivisatos et al., 2013; Andrasfalvy et al., 2010; Boyden et al., 2005; Han and Boyden, 2007; Liu et al., 2012; Papagiakoumou et al., 2010; Prakash et al., 2012; Rickgauer and Tank, 2009; Zhang et al., 2007). Optogenetics has in turn created a demand for optical devices that target delivery of light to sub-regions of the living brain. Current spatially selective light-delivery devices for optogenetics are based on solid-state photonic waveguide array or integrated semiconductor light-emitting diodes (LEDs), each exciting a specific spot in the brain by exploiting the spatial distribution of multiple light emitters. This control has been achieved by means of several technological approaches, including amplitude or phase modulation (Anselmi et al., 2011; Grossman et al., 2010), glass-sharpened optrodes (Abaya et al., 2012a; Abaya et al., 2012b), arrayed optical fibers (Royer et al., 2010; Stark et al., 2012), multi-waveguide fabrication on a single substrate (Zorzos et al., 2010), endoscopic fiber bundles (Hayashi et al., 2012), LED-coupled tapered fiber arrays (Stark et al., 2012), and wireless micrometer-sized LEDs on flexible shafts (Kim et al., 2013). Recently, implantable three-dimensional sets of silicon oxynitride waveguides have been developed, raising the possibility of generating 3D distributed light patterns in the brain (Zorzos et al., 2012). Individual waveguides can be addressed by a matrix of micromirrors (Zorzos et al., 2012) or separately coupled to different light sources (Stark et al., 2012), allowing optical stimulation at each point with tunable wavelength and intensity.
While these methods allow spatially selective illumination, they require a complex fabrication process and/or coupling strategy at the distal end of the waveguides. Moreover, despite the wide range of proposed devices, only a few have been tested in vivo (Hayashi et al., 2012; Kim et al., 2013; Royer et al., 2010; Stark et al., 2012; Tamura et al., 2012). These devices are also quite invasive due to the large number of implanted waveguides, oversized optical components, blunt inserting edges and potentially high temperatures generated by implanted electronics.
Here we describe the implementation of a novel optogenetic tool based on a single waveguide that, by a simple optical strategy, can selectively and dynamically illuminate multiple brain regions. The device is minimally invasive because it comprises only one thin fiber with a sharp, tapered tip. To demonstrate the effectiveness of this device in vivo, we coupled it to a linear array of microelectrodes for simultaneous multi-site extracellular recording and optical stimulation in the brain of awake mice. In a proof-of-principle experiment to validate the methodology, we find that activation of GABAergic interneurons at different depths in primary motor cortex differentially modulate subsets of cortical neurons, suggesting cell-to-cell specificity of GABAergic inhibition in the living mammalian brain.
Results
A single core optical fiber with cladding (total diameter d0=125μm; see Experimental Procedures for further details) was tapered and, with exception of a 200 nm diameter circular area at the tip, was coated with gold as a reflective material (Figure 1A & B). The tapered shape allows selection and manipulation of propagating and evanescent modes, whereas the coating prevents leakage of light (Novotny and Hecht, 2006). Light emission is permitted at selected sites along the taper by locally removing the coating to create “windows”. Illumination with a well-defined modal set at the fiber input then addresses emission to specific windows along the fiber.
Figure 1. Multi-point emitting optical fibers.
(A) Schematic representation of a seven-window multi-point emitting optical fiber device. (B-D) SEM micrograph of the realized devices. The inset in panel B shows the circular aperture at the taper tip. The inset in panel C shows the smallest optical window realized in the case of a three-window multi-point emitting optical fiber. (E-F) A square (panel E) and circular (panel F) optical window realized on the taper edge.
The gradual taper angle (∼3°-6°) and the small external diameter of the tip (∼600nm) allow smooth insertion into the brain, thereby reducing tissue damage. To obtain multiple optical windows, the reflective coating along the taper and part of the underlying material are pierced at selected points, allowing light of specific modes to escape into the surrounding environment. In the following we discuss three different devices, displayed in Figures 1B-1D, in which two, three or seven optical windows were created by focused ion beam (FIB) milling (detailed geometrical parameters of each optical aperture are given in Experimental Procedures section). FIB is a particularly versatile technology for this purpose because it allows localized micromachining all along the fiber taper with a resolution better than 50nm. Thus optical apertures can be milled with different sizes and shapes (Figures 1E-1F), and relatively simple optical elements, such as diffraction gratings or metallic mirrors, can be fabricated using ion-or electron-beam induced deposition within the same system (Cheng and Steckl, 2002). We used square patterned windows for this study, but the approach can be easily extended to other types of optical elements along the taper.
Optical properties of multi-point emitting optical fibers in non-scattering medium
Each micro-machined window out-couples only a fraction of the light that is guided into the fiber. The remaining radiation propagates further into the taper and undergoes a modal manipulation and selection: the transversal component of the wave vector associated to the j-th mode (kjT) increases as the taper narrows (Figure 2A). As detailed in Experimental Procedures, this implies that the higher the kjT value of modes at the taper entrance, the shorter the propagation length of the j-th mode into the taper. Because the lower order modes propagate further down the taper than the higher order modes, this allows a strategy to out-couple light from specific optical windows along the taper. The simplest way to control kjT values at the taper input is to modify the input-coupling angle θ at the distal end of the fiber. The higher the input coupling angle θ, the higher the kT of the light guided into the fiber (Figure 2B; see Supplemental Information for theory related to these phenomena).
Figure 2. Modal evolution in multi-point emitting optical fibers.

(A) kjT(d) evolution as a function of the taper diameter d. Each color represents a different value of kjT(d0) at the taper entrance. The dashed black line represents the kj value into the taper. For kjT>kj, the j-th mode becomes evanescent and lies within the grey area. (B) kT of the most powerful mode injected in the core/cladding section of the optical fiber, as a function of the input coupling angle θ. Details on the calculations reported in are given in Supplemental Information.
In order to control the input light angle to the fiber, a λ0=473nm laser is reflected by a fixed mirror (M1) and a sliding mirror (M2), whose position defines the input coupling angle θ (Figure 3A). When the mirror M2 is in the Home position, the laser beam travels perpendicularly through the center of the lens L1, which focuses the optical radiation coaxially to the optical fiber axis (θ=0°). If the mirror is moved along the optical axis, the light is focused into the fiber coupler with a different θ.
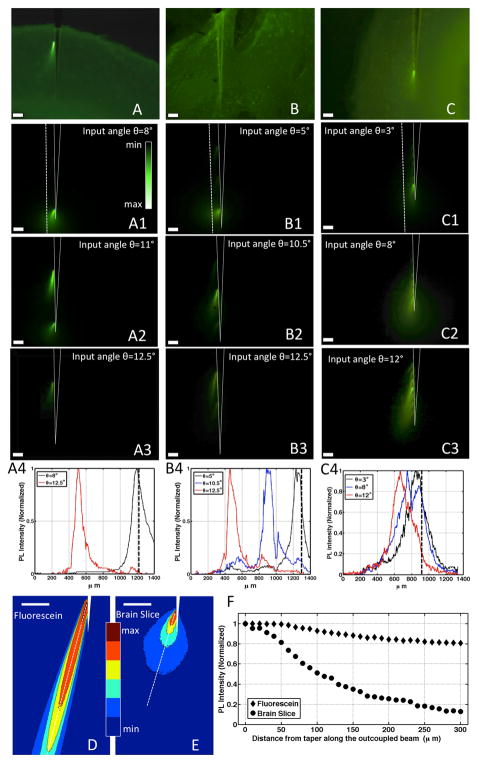
Figure 3. Optical properties of the multi-point emitting optical fiber in fluorescein solution.
(A) Optical setup used to modify the input-coupling angle θ. A CW λ0=473nm laser beam is reflected by a fixed mirror (M1) and a sliding mirror (M2) redirects it toward lens L1. When M2 is in the Home position, the laser beam travels perpendicularly to L1 and through its center, and is then focused onto the optical fiber. When M2 is moved by Pm2 along the optical axis of the setup, the laser beam is still perpendicular to L1 but it is focused into the optical fiber with an angle θ. To perform optical characterization of the device, the fiber taper was immersed in a fluorescein bath and the fluorescein emission collected by an optical microscope equipped with a FITC filter and a color CCD camera. (B-D) Light-microscope images of the 2-, 3- and 7- window devices immersed in a drop of Fluorescein:water solution with no laser coupled at its entrance. (B1-B3,C1-C3,D1-D3): Fluorescence images showing the taper emission for three different input-coupling angles. Scale bars are 100μm. Continuous white lines were added to highlight the taper profile. Dashed lines identify where the intensity profiles in panels B4, C4 and D4 were measured. (B4,C4,D4) Intensity profiles collected 100μm from the fiber taper, along the white dashed lines displayed in panels B1, C1 and D1. (B5,C5,D5) Output angle as a function of the input coupling angle θ for 2-, 3- and 7- window MPF, respectively. Output angle is defined in panel B2.
When θ is changed, the out-coupled light intensity redistributes among the optical windows. At θ∼0°, most of the light is emitted from the fiber tip (Figure S4). However, as θ is increased above ∼2°, tip emission becomes negligible and most of the light is emitted by the side apertures. Emission evolves as a function of θ, demonstrated in Figure 3 for a 2-, 3- and 7-window multi-point emitting fibers (hereafter referred to as 2-, 3- and 7-MPF) immersed in a Fluorescein:water solution. At low θ, light is predominantly emitted by the holes closest to the tip, and in both 2- and 3-MPF non-negligible emission takes place only at window H1 (Figures 3B1 & C1). Progressively increasing θ directs light emission to window H2 for the 2-MPF (Figure 3B2 & B3) and to H2 and then H3 for the 3-MPF (Figure 3C2 & C3). This allows the independent control of two or three emitting points along 700μm, with spatially sharp emission profiles (Figures 3B4 & C4). In the case of the 7-MPF, the light emission is less selective among the closely-spaced windows, but increasing θ still shifts the maximum of the emitted light towards the windows further from the tip, and emission from all windows defines the point of maximum intensity (Figure 3D4).
These behaviors can be explained by the modal manipulation performed by the taper on kjT, whose values strongly influence the radiation efficiency of each side window (Figure S2). This is confirmed by the directionality of out-coupled light: increasing θ is accompanied by a moderate tilting of the output angle of the light beams (Figures 3B5, 3C5 and 3D5). The closer the window to the taper tip, the wider the tilting of the out-coupled beam, by virtue of the wider range of kjT values between θ∼0° and θ∼12.5°.
Due to modes evanescence, the maximum out-coupling efficiency is not the same for all the side windows. Thus, the input power needed in order to emit a constant power (Pout) at each window varies. We determined the input power (Pfc) and angle (θ) required at the fiber connector to output ∼0.1 mW at the selected window while maintaining extinction ratios (the ratios of powers emitted by non-selected and selected windows) below 1:10 for 2-MPF and 1:4 for 3-MPF. In the case of a 2-MPF, this occurred for H1 with θ=8° and PFC∼2.3mW and for H2 with θ=12.5° and PFC∼15mW. For the 3-MPF, this required PFC∼25mW at θ=5° (H1), PFC∼22mW at θ=10.5° (H2) and Pfc∼15mW at θ=12.5° (H3). In contrast, in the 7-MPF the emission of all apertures contributes to the total emitting power: the input power to obtain Pout∼0.1mW at 100μm from the taper was estimated to be Pfc∼1.5mW at θ=5°, PFC∼1mW at θ=9° and Pfc∼7mW at θ=11.5°.
Optical properties of multi-point emitting optical fibers in brain tissue
In the brain, scattering and absorption of light by tissue determine the distribution of photons emitted by an optical fiber (Yizhar et al., 2011). To test how light emitted from the small windows on the tapered fibers behaves in scattering medium, we measured the fluorescent profiles generated by 2-, 3- and 7-MPFs inserted into fluorescein-stained coronal mouse brain-slices (Figures 4A-C). For the 2- and 3-MPFs two main differences were observed compared to fluorescein in solution: (i) spreading of the emitted beam, as shown by the iso-intensity curves in Figures 4D-E, and (ii) faster decay of light intensity due to tissue absorption (Figure 4F). Nevertheless, the active window can still be selected by changing the input-coupling angle θ for both 2- and 3-MPF (Figures 4A4 & 4B4) with only slight variations in the angle dependence compared to fluorescein in solution (Figure S8). For 7-MPF, the spread of the beam emitted by a single window results in a strongly reduced influence of θ on the selection of the illuminated brain region (Figure 4C4) with respect to fluorescein in solution.
Figure 4. Optical properties of the multi-point emitting optical fiber in fluorescein-stained coronal brain slices.
(A-C) Light-microscope images of the 2-, 3- and 7- window devices inserted in fluorescein-stained mouse brain slices. (A1-A3,B1-B3,C1-C3) Fluorescence images showing the taper emission in fluorescein-stained mouse brain slices for three different input-coupling angles. Scale bars are 100μm. Continuous white lines were added to highlight the taper profile. Dashed lines identify where the intensity profiles in panels A4, B4 and C4 were measured. (A4,B4,C4) Intensity profiles collected 100μm from the fiber taper; black dashed line identify the position of taper tip. (D,E) Iso-intensity photoluminescence curves for a single optical window emitting in Fluorescein:water solution (panel D) and in fluorescein-stained brain slices (panel E). Scale bars are 100μm. Dashed lines identify where the intensity profiles in panel F were measured. (F) Photoluminescence intensity decay comparison in Fluorescein:water solution and brain tissue for windows H1 measured along the white dashed lines in panels D and E, respectively.
Use of multiple wavelengths and effect of extension fibers
MPFs also allow multiple θ and multiple wavelengths to be used at the same time, to enable simultaneous stimulation and inhibition of neural activity at two different wavelengths (Figure 5). We examined multi-source illumination using the optical setup schematized in Figure 5A, where a Fluorescein:TexasRed:water solution was prepared to highlight light radiation at different wavelengths. A yellow (λy=593nm) and a blue (λB=473nm) laser were coupled into a two-window optical fiber at two different angles (θy and θb, respectively). For θb=8° and θy=12.5° the yellow light escaped predominantly from H2 by virtue of its higher input coupling angle, while the blue light was efficiently emitted at the aperture H1, as shown in Figure 5B (the blue beam excites only Fluorescein, which emits at green wavelengths, while the yellow laser excites only TexasRed, emitting in the red). This suggests that the multi-point emission could be used to simultaneously elicit and inhibit neural activity in different portions of the neural tissue, through the same single waveguide. The two light sources can, moreover, emerge from the same window, as shown by the brown luminescence (combination of red and green) from H2 shown in Figure 5C (in supplemental information this is shown also for H1, as displayed in Figure S6B).
Figure 5. Two-color emission and proof-of-principle for use of MPFs with freely behaving animals.
(A) Optical setup used to couple two different light beams into the optical fiber, with input coupling angles θB and θY for blue and yellow lasers, respectively (B) Fluorescence image of a 2-MPF immersed in a Fluorescein:TexasRed:water solution for θB∼8° and θY∼12.5°. The blue light excites only fluorescein, which emits at green wavelengths. The yellow light excites only TexasRed, emitting in the red. (C) Fluorescence image of a 2-MPF immersed in a Fluorescein:TexasRed:water solution for θB∼12.5° and θY∼12.5°. The superposition between green fluorescein luminescence and red TexasRed luminescence resulted in brown color at the CCD camera. (D) Schematic representation of the setup used to test MPF with warped fiber and extension cords. (E-H) Fluorescence images showing the taper emission for the configuration represented in panel D before (panels E and F) and after (panels G and H) warping the fiber, at two different θ. The fiber was rolled twice with a curvature radius of 4.5cm. Scale bars are 100μm. Continuous white lines were added to highlight the taper profile. Dashed line in panel E identify where the intensity profiles in panel I were measured. (I) Intensity profiles before (black) and after (red) warping the fiber measured along the white dashed line in panel E.
While all in vivo experiments reported below were carried out on head-restrained mice, the device can be easily adapted to freely behaving animals, where a short fiber stub is implanted into the moving mouse and an extension fiber is used to bring light to the stub. In order to demonstrate that the coupling at the fiber junction as well as fiber bending induced by mouse movement would not lead to significant modifications of MPFs light emission, we tested a warped 2-MPF using an extension fiber (Figure 5D). Emission properties were characterized before and after warping the fiber, to simulate animal movement. As shown in Figures 5E-H, windows H1 and H2 could still be independently addressed by changing θ, with only slight variations of emission profiles (Figure 5I). To assess warping effects on outcoupled power (Pout), Pout was set at 0.1mW before warping the fiber, and output power was then evaluated after warping. The rolling-unrolling experiment was performed six times. During fiber warping, at H1 we measured an average power output of 0.113mW (SD: 0.008mW), while at H2 we obtained 0.097mW (SD: 0.008mW). For comparison, the same evaluation on a standard core-cladding fiber without tapered region yielded Pout=0.100±0.002mW. Warping also had very little effect on outcoupling angles: in the same experiment we found that the output angles were 18.70°±0.21° for H1 and 14.61°±0.04° for H2 for the warped fiber, compared to 18.53° for H1 and 14.62° for H2 for the non-warped fiber. The minor effects of fiber warping on the outcoupling of light pave the way for application of MPFs to free-moving animals.
In vivo use of multi-point emitting optical fibers
To test the ability to address optical excitation to different parts of the brain along the length of the fiber, we coupled 2- and 7-MPFs to a linear array of microelectrodes on a silicon shank (Neuronexus Technologies, Inc.). The microstructured optical fiber was fixed in parallel to the shank of the multi-electrode (Figure 6A and Figure S9). To minimize light-induced artifacts in the recorded electrical signal (due to the Becquerel photoelectric effect) (Cardin et al., 2010; Han et al., 2009), the optical windows were oriented to illuminate the region immediately above the recording pads. To ensure that apertures were properly angled, the optrode was first tested in a phosphate buffered saline (PBS) solution. Photoelectrical artifacts were not detectable at any laser power tested or any input coupling angle, confirming that the fiber apertures did not directly illuminate the recording pads (Figure S10). For comparison, we used the same PBS bath to test a commercial optrode, with light delivery based on a single step-index optical fiber placed just above the last recording site (model A1×16-3mm-50-413-OA16-50 from Neuronexus Technologies, Inc.). Light-induced artifacts were observed during both OFF-ON and ON-OFF light transitions at laser powers PFC ≥33.9mW at the optical fiber input (Figure S10).
Figure 6. In vivo use of MPFs.

(A) Light-microscope image of the structured optical fiber fixed beside a linear array of electrodes designed for extracellular recording. Optical windows on the tapered fiber were oriented to shine light in the region just above the recording pads. (B) Overlay of sample spikes recorded at Ch2 with θ=3°. Blue curves are 1641 spikes recorded during light ON periods, while black lines are 146 spontaneous spikes recorded during light OFF periods. (C) In vivo recordings in striatum using a 7-MPF. (D) Schematic representation of the in vivo experiment carried out in motor cortex. (E) Representative spiking rate histograms recorded from single units when light was switched from OFF to H1 and from OFF to H2. (F) Summary of the units recorded during in vivo experiments in motor cortex and their sensitivity to light outcolupled form H1 and/or H2.
We tested the performance of the combined tapered fiber-multielectrode assembly with in vivo recordings in the brains of awake, head-restrained transgenic mice. PFC was tuned in order to have ∼0.1mW at the selected stimulation site. To confirm selective activation of spatially separate cells, Channelrhodopsin 2 (Chr2) was expressed in striatal spiny projection neurons of the indirect pathway (iSPNs), and an optrode based on 7-MPF was used to elicit neural activity.
The tapered-fiber optrode was inserted into the mouse brain through a craniotomy, and neural activity was recorded at the multiple electrode contacts. Once the device was inserted into the striatum to a tip depth >1500μm, the optrode was immobilized and left in a steady position for about 10 minutes to let the tissue settle. In contrast to recordings in PBS, photoelectric effects were observed at the onset light pulses in some recordings (Figure S11 and S12), possibly due to scattering of light onto the recording pads by brain tissue. Although some neuronal spikes could have been obscured by brief photoelectric effects at the light transitions, single units could be identified and clustered by principle component analysis. Evoked spike waveforms were unchanged during the laser ON and OFF periods (Figure 6B), indicating that the same unit was observed in both conditions. Because ChR2 is restricted to iSPNs, which are GABAergic, all fast excitation must be via direct ChR2 excitation. Any synaptic excitation would have to be at least disynaptic, acting via disinhibition, and would appear delayed. Thus a fast, sustained increase in neuronal firing during illumination of the GABAergic iSPNs was considered evidence of a directly activated cell.
During periods of inactivity iSPNs fire infrequently, facilitating identification of directly activated ChR2-expressing neurons. Isolated single units were observed on four recording sites, located 50 μm, 100 μm, 200 μm and 450 μm proximal to the multielectrode tip, and were selected to evaluate the performance of the device; the sites are hereafter referred as Ch1, Ch2, Ch3 and Ch4, respectively (Figure 6C). Consistent with previous reports (Kravitz et al., 2013), baseline firing rates on all channels were low (0.8 to 6.6 Hz) but could be modulated robustly by illuminating with 473nm light. For small input coupling angles (θ∼3°), optically stimulated neuronal activity was detected at Ch1 and Ch2, with firing rates increasing more than 10× at each channel (P<0.05; t-test on the trial-by-trial firing rates). In contrast, negligible modulation of neuronal firing was detected at the other channels (2.6× and 0.58× changes in firing rate; P>0.1). With a coupling angle of ∼8°, light-induced neuronal modulation was weak or absent at Ch1 (0.7 fold increase; P>0.1) and Ch2 (3.4× increase; P<0.05), but was robust at Ch3 (11× increase; P<0.05) (Figure 6C).
In contrast to the unlayered structure of the striatum, cerebral cortex provides a naturally laminated structure in which the ability to steer light through different windows permits the activation of functionally distinct cell classes. We exploited this property to ask if the modulation of cortical neurons by local GABAergic interneurons depends on the depth of the interneuron. We used the VGAT-ChR2 mouse line, in which ChR2 is expressed in all inhibitory interneurons. 2-MPF-based optrodes were used to activate inhibitory neurons in deep or shallow layers of mouse primary motor cortex while measuring the effects on activity of cortical neurons (Figure 6D). The light stimulus was either delivered superficially (125μm above top recording site, <200μm below the pial surface) via “H2” or deep (between the 2nd and 3rd recording site, 800-1000μm below surface) via “H1” (Figure 6F).
Most activity was detected in deeper layers, consistent with a bias towards large pyramidal cell recordings in motor cortex. Illumination from each window was calibrated to deliver 0.1mW of light. More than 100 pulses of light (1-10s in duration) were delivered at the H1 and H2 windows. A unit was considered modulated by a given window if the firing rate in the first 200ms after illumination was significantly different from a 1.5s baseline period (p<0.05, T-Test). Across 5 recordings from 2 animals, 61 units were recorded, 32 of which were stably isolated across the entire recording session (representative responses are displayed in Figure 6E).
Of the 32 stably isolated units, 13 units were not inhibited at all, and one unit was excited by H1 but not H2, presumably reflecting direct excitation of a ChR2-expressing interneuron located near H1. The remaining 18 units were inhibited, presumably through excitation of GABAergic interneurons. Five units were inhibited by light delivered through either window, and 9 units were inhibited only by light delivered from H1 and not H2. Four units were inhibited by light from H2 and not H1, even though those units were located in deep cortical layers near H1, while H2 was located in superficial cortical layers (Figure 6E & F, and see Supplemental Figure S13 for the specific changes in spike rates). Moreover, units selectively inhibited by light delivered through H1 were found on the same recording channel as those inhibited selectively by H2. Although these experiments were intended to validate the in vivo application of MPFs in layered brain regions, the data suggest that local inhibitory projections in cortex are not universally one-to-all. Rather, some neurons receive inhibitory input that is more spatially defined, such as the deep-layer units in our study that only received inhibitory input from superficial layers. While more experiments will be needed to test this hypothesis definitively, the experiment illustrates the general utility of using the multipoint emitting fibers in parallel with electrophysiological recording to reveal novel aspects of local brain circuits: the experiment would not have been possible without spatially precise light stimulation of multiple locations in cortex.
Discussion
Here we demonstrate a novel, fiber-optic based approach by which light can be delivered into the brain via multiple ports in a user-selectable manner. The advantages of this approach are that multiple regions of the brain can be stimulated via a single fiber, the light-delivery device is easily adapted for use with “optrodes”, and that multiple wavelengths of light can be independently delivered via a single fiber.
In vivo validation
We demonstrated the utility and selectivity of the device in vivo by optogenetic activation of distinct populations of neurons via multiple optical windows on a single fiber. Within the striatum, we separately activated different populations of neurons along the length of the fiber by only changing the input angle of the ChR2-activating light to the fiber. We chose the striatum to test the spatial selectivity of the MPFs, because the baseline firing rate of striatal spiny projection neurons is low, allowing easy identification of activated ChR2-expressing neurons. Furthermore, since SPNs are inhibitory there is no possibility of intra-striatal polysynaptic excitation of non-ChR2 expressing neurons.
We also demonstrated the utility of the device for analyzing cortical microcircuits. Inhibition in cortex is critical for sensory and motor processing (Isaacson and Scanziani, 2011), yet the organization of inhibitory cortical microcircuits is unclear. There is both evidence that inhibitory neurons non-specifically interact with all nearby pyramidal cells (Packer and Yuste, 2011) and that inhibitory neurons can have asymmetric effects (Adesnik et al., 2012). Here we recorded in vivo from neurons in primary motor cortex of an awake mouse while activating GABAergic interneurons. We found that activation of superficial vs. deep interneurons can modulate different and sparse population of pyramidal neurons, consistent with high point-to-point specificity of inhibitory microcircuits. Although this preliminary result will require additional validation, it was made possible by the highly localized and spatially controlled light delivery system presented here.
Generalization of approach
The method proposed in this paper can be customized for specific experimental needs by adjusting the size, shape, or relative placement of the windows. The key to design the multipoint emitting optical fibers is to choose the correct size of the apertures, the appropriate fiber-taper diameter and the correct input-coupling angles. The combination of these two last parameters defines the wave-vector transversal component kjT all along the taper, allowing independent control of two or three windows along the taper length.
In summary, we developed a novel optogenetic device based on a tapered optical fiber that allows light to be directed to specific sites in the brain along the length of the fiber. Light escapes through a series of optical windows on the taper's edge, with the windows fabricated in a single technological step. In contrast with previously reported methods, dynamic stimulation of different brain sites was achieved by means of a single optical waveguide, thus reducing the invasiveness of the device in the brain. Site selection was achieved by a very simple optical strategy -- adjusting the angle of the incident light on the input facet of the fiber. In vivo experiments, performed by coupling the optical device to a linear electrode array for extracellular signal recording, demonstrated the effectiveness of the device for switching optical activation and indirect silencing of neurons between proximal or distal recording sites in real time. The result is a powerful, versatile and minimally invasive tool for the causal manipulation of neural circuits.
Experimental Procedures
Optical fiber processing
Tapered optical fibers were purchased from Nanonics Imaging Ltd. (core diameter a=50μm, cladding diameter d0=125μm, core refractive index n1=1.464, cladding refractive index n2=1.447, numerical aperture N.A.=0.22, taper angle between 3° and 6°, gold reflective coating thickness ∼300nm, aperture diameter at taper tip ∼200nm). Since the sharpened edge is obtained by a “heating and pulling” method, core and cladding are melted and mixed together, resulting in a homogeneous medium all along the taper. To open the optical windows, the optical fiber was inserted in a combined focused ion beam/scanning electron microscope system (FEI® Helios™ NanoLab™ 600i DualBeam™, equipped with the Tomahawk FIB column). For each window, a square area was scanned by the Ga+ ion beam perpendicularly to the fiber axis (acceleration potential 30 keV, probe current 9.3nA, dwell time 1μs, process time up to 20 minutes for a 25μm × 25μm window and to reach a milled depth of about 6 μm). Each window was milled with sub-micrometer precision at the position targeted by SEM inspection, using the 16-bit scan/pattern generator and the integrated CAD. The scanning direction was along the positive z-axis, since this produced the cleanest top sidewall of the windows, nearly free from redeposition artifacts. In the case of 2-MPFs, H1 was realized by milling an area of 15μm × 15μm to a depth of 4μm at a taper diameter of 25μm, while H2 was 25μm × 25μm × 6 μm (width × length × depth) at a taper diameter of 80μm. Distance between H1 and H2 was about 700μm. For the 3-MPF, H1 was 5μm × 5μm × 4μm (taper diameter 25μm), H2 15μm × 15μm × 4 μm (taper diameter 50μm), H3 25μm × 25μm × 6 μm (taper diameter 85μm). The distance between adjacent windows was ∼400μm. For the 7-MPF all windows were realized by milling an area of 25μm × 25μm to a depth of 6μm and a window-to-window spacing of 100μm.
Repeatability of the fabrication process
Given a certain taper angle, the process of FIB-induced milling is highly repeatable in terms of both size and optical quality of the windows, in particular because the windows milled on the cylindrical surface are two or three times smaller than the taper diameter. The distance between windows is related to the taper diameter at a given section, and thus the position of the windows has to be designed depending on the taper angle and launching parameters. Because the typical taper angle is between 3-6°, each multi-point emitting optical fiber must be characterized (e.g., in fluorescein solution) before use in vivo. However, we find that ∼10% of the commercially obtained tapered fibers show abrupt angle variations along the taper. These fibers are discovered by SEM inspection before FIB patterning, and are discarded. Further optimization of the tapering process, such as using a laser puller to improve the reproducibility of the taper, should reduce the number of discarded fibers.
Emitted power estimation
To estimate the power emitted by the optical windows, indirect measurements based on the induced fluorescence intensity were performed. The fluorescence signal of the Fluorescein:water bath was calibrated using a core/cladding fiber optic without tapered region, emitting a set of known powers. FITC-filtered fluorescence images were acquired with an 8-bit CCD camera. For each known power emission, we measured the sum of the intensity values registered by the CCD over a line just outside the fiber termination, to construct a power versus intensity dependence. This procedure was repeated for different CCD acquisition parameters (gain and exposure time). The power emitted by the optical windows was then estimated through these curves. For the 2- and 3-MPFs, we recorded fluorescence images for PFC=350μW, and used the sum of the CCD counts over an imaginary line placed parallel to the taper and just outside the windows. For 7-MPFs, intensity profile was evaluated 100μm from the taper, the same distance at which we placed the linear multielectrode array in the optrodes. Extinction ratio for 2-MPW and 3-MPF device were computed using the estimated output powers.
Evolution of modes in the fiber taper
We express the propagation vector of the j-th mode at the taper entrance kj as the vectorial sum of its axial (kjA(d0)) and transversal (kjT(d0)) components, i.e., kj = kjT(d0) + kjA(d0), with their moduli related by
and with
constant all along the taper (n defines the refractive index within the taper). In general, the higher the order of the mode, the higher the transversal component of its propagation vector, i.e., kjT(d0)≤ k(j+1)T(d0). All the modes excited at the distal end of the fiber propagate up to the tapered section, where their behavior is strongly modified. The transversal component of the propagation vector of the j-th mode is a function of the taper diameter d:
where d0=125μm is the fiber diameter at the taper entrance, so that the narrower the taper, the higher the kjT(d) value. For d small enough to have kjT(d)>kj, becomes imaginary and the j-th mode thus becomes evanescent. This behavior is graphically described in Figure 2A: the dashed black line represents kj, whereas the other curves represent the evolution of kjT(d) for different kjT(d0). The higher the propagation-vector transversal component at the taper entrance kjT(d0), the higher the diameter at which kjT(d) overtakes kj, thus implying evanescence of the modes in sections closer to the taper entrance. It is worth noting that modal functions as well as kjT are influenced only by the shape and size of the waveguide in a particular section, while the propagation direction (forwards and backwards) has no influence on that parameter (Snyder and Love, 1983).
Tuning of the input-coupling angle
The optical setup used to modulate the input-coupling angle is shown in Figure 3A, with additional details given in Figure S3. By sliding the mirror M2 along the optical path by Pm2, the input-coupling angle θ is modified, as θ=atan(|PM2|/f1). Because the diameter of L1 is 50.8 mm, the maximum θ achievable is θmax∼14°, well above the limit given by the optical fiber numerical aperture (αmax=12.7°). The beam waist at L1, hereafter referred to as Wl1, defines instead the angular aperture of the focalized beam, as φ=atan[(WL1-WC)/f1)], where Wc is the waist of the focused beam at the fiber entrance. In our case we found φ∼0.4°.
To examine the radiation from the optical windows, the fiber taper was immersed into a fluorescein bath (in water). Fluorescein emission excited by the aperture on the taper's edge was detected by a Zeiss microscope equipped with a FITC filter, and imaged by a CCD camera. For experiments on brain slices, brain tissue was fixed with 4% paraformaldehyde and immersed in a Fluorescein bath in PBS for one day. The fluorescent tissue was placed on a microscope coverslip, the tapered optical fiber was inserted by a micromanipulator, and optical analysis was performed following the same procedures as in the fluorescein-bath experiments.
Animal Procedures
All animal procedures were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and conformed to National Institutes of Health Guidelines. Custom- made titanium head plates were surgically implanted on the skull of adult C57BL/6 mice expressing either cre recombinase in the indirect pathway spiny projection neurons (iSPNs), under the adenosine2A promoter (Adora2A-cre, GENSAT KG139), or expressing Channelrhodopsin in all inhibitory neurons (VGAT-ChR2, B6.Cg-Tg(Slc32a1-COP4*H134R/EYFP)8Gfng/J, Jackson Laboratory). In the same surgery, 1μl of adeno associated virus (AAV) expressing cre dependent ChR2 (AAV5.EF1.dflox.hChR2(H134R)-mCherry.WPRE.hGH vector from University of Pennsylvania Vector Core, AV-5-20297P) was injected into the dorsal striatum of the iSPN-Cre animal (coordinates 0.9mm anterior 1.7mm Lateral and 2.4mm deep). After recovery the animal was habituated to head restraint for up to 90 minutes per day over the course of 3 weeks. For an unrelated task the mouse was trained to press a lever to receive a water reward. 24 hours before the first recording, the injection craniotomy was reopened and enlarged (>1mm diameter) to allow acute electrical recordings from the motor cortex or striatum. Between uses the craniotomy was cleaned and covered.
Electrical recording, real-time signal processing and data analysis
Acute extracellular single units recordings were made on 3 consecutive days through a craniotomy using a Neuronexus 16 channel probe model A1×16 – 5mm – 50 – 177 – A16 fitted with the optic fiber. Data was filtered (300hz to 10khz) and acquired by either an A-M systems model 3600 extracellular amplifier (A-M systems inc, Sequim, WA) equipped with a Cambridge Electoronic Design Power1401 interface (CED, Cambridge, England) or a Plexon Inc. Omniplex system. Putative spikes were identified offline via level crossing, and sorted by waveform based on principle component analysis using Offline sorter (Plexon Inc) and Spike2 software (Cambridge Electoronic Devices). Waveforms clusters that were not synchronous across all channels, not time locked to other triggers and not part of the ‘noise cluster’ were identified as units and analyzed further. All analyzed units fulfilled the following criteria: <2% inter spike interval violation within 2ms, and a continuous distribution of waveforms separate from the ‘noise cluster’ in PCA space. Further analysis of waveform shape and firing rates was performed using custom scripts in IGOR Pro (wavemetrics).
Supplementary Material
Highlights.
Dynamic stimulation of different brain regions with a single optical fiber;
Micro-machining of the tapered fiber using a focused ion beam;
Window-specific addressable light emission by selection of modal subsets at the fiber input;
In vivo demonstration of spatially selective optical stimulation in awake mice.
Acknowledgments
This work was partially supported by: PON project “ITEM” and NIH grants NRSA F31--MH093026--01A1 to I.A.O and R01 NS046579 to B.L.S. We thank the Department of Neurobiology of Harvard Medical School, for the use of the Neuro Imaging Core Facility, which provided fluorescent imaging service. Neuro Imaging Core Facility is supported in part by NINDS P30 #NS072030.
Footnotes
Supplemental Information: Supplemental Information includes Supplemental Experimental Procedures and thirteen supplemental figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abaya T, Blair S, Tathireddy P, Rieth L, Solzbacher F. A 3D glass optrode array for optical neural stimulation. Biomed Opt Express. 2012a;3:3087–3104. doi: 10.1364/BOE.3.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaya TVF, Diwekar M, Blair S, Tathireddy P, Rieth L, Clark GA, Solzbacher F. Characterization of a 3D optrode array for infrared neural stimulation. Biomed Opt Express. 2012b;3:2200–2219. doi: 10.1364/BOE.3.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos AP, Andrews AM, Boyden ES, Chun M, Church GM, Deisseroth K, Donoghue JP, Fraser SE, Lippincott-Schwartz J, Looger LL, et al. Nanotools for Neuroscience and Brain Activity Mapping. ACS Nano. 2013;7:1850–1866. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Zemelman BV, Tang J, Vaziri A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proceedings of the National Academy of Sciences. 2010;107:11981–11986. doi: 10.1073/pnas.1006620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Ventalon C, Bègue A, Ogden D, Emiliani V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proceedings of the National Academy of Sciences. 2011;108:19504–19509. doi: 10.1073/pnas.1109111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Steckl A. Focused ion beam fabricated microgratings for integrated optics applications. Selected Topics in Quantum Electronics, IEEE Journal of. 2002;8:1323–1330. [Google Scholar]
- Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Palmini RB, Gong Z, Drakakis EM, Neil MA. Multi-site optical excitation using ChR2 and micro-LED array. Journal of neural engineering. 2010;7:016004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-Color Optical Activation, Silencing, and Desynchronization of Neural Activity, with Single-Spike Temporal Resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian XF, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Tagawa Y, Yawata S, Nakanishi S, Funabiki K. Spatio-temporal control of neural activity in vivo using fluorescence microendoscopy. Eur J Neurosci. 2012;36:2722–2732. doi: 10.1111/j.1460-9568.2012.08191.x. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Ti, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim RH. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain research. 2013;1511:21–32. doi: 10.1016/j.brainres.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny L, Hecht B. Principles of nano-optics. Cambridge university press; 2006. [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? The Journal of Neuroscience. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiakoumou E, Anselmi F, Bègue A, de Sars V, Glückstad J, Isacoff EY, Emiliani V. Scanless two-photon excitation of channelrhodopsin-2. Nat Methods. 2010;7:848–854. doi: 10.1038/nmeth.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer JP, Tank DW. Two-photon excitation of channelrhodopsin-2 at saturation. Proceedings of the National Academy of Sciences. 2009;106:15025–15030. doi: 10.1073/pnas.0907084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsaki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279–2291. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AW, Love J. Optical waveguide theory. Vol. 190. Springer; 1983. [Google Scholar]
- Stark E, Koos T, Buzsaki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol. 2012;108:349–363. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Ohashi Y, Tsubota T, Takeuchi D, Hirabayashi T, Yaguchi M, Matsuyama M, Sekine T, Miyashita Y. A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures. Journal of Neuroscience Methods. 2012;211:49–57. doi: 10.1016/j.jneumeth.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett. 2010;35:4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt Lett. 2012;37:4841–4843. doi: 10.1364/OL.37.004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.