Abstract
Objective
To investigate the 5-year incidence and progression rate of primary open-angle glaucoma (POAG) in a health-center-based Korean population.
Methods
The study population comprised 5,021 subjects who participated in standardized health screening (including non-contact tonometry and fundus photography) at the Gangnam Healthcare Center during the period from January 2005 to December 2006 and again from January 2010 to December 2011. Among these subjects, 948 (18.9%) with findings suggestive of glaucoma were subjected to a comprehensive glaucoma evaluation, which included applanation tonometry and standard automated perimetry. Based on the results, the subjects were diagnosed as POAG suspect or definite POAG.
Results
The 5-year incidences of POAG suspect and definite POAG were 0.84% (42 subjects) and 0.72% (36 subjects), respectively. The rate of progression from POAG suspect to definite POAG was 4.75% per year. In subjects with a baseline intraocular pressure (IOP) >21 mmHg, the incidence of POAG suspect or definite POAG was significantly higher than in those with a baseline IOP≤21 mmHg (32% vs. 1.05%; P<0.001). A multivariate analysis showed that the progression from POAG suspect to definite POAG was significantly associated with older age (odds ratio [OR], 1.07; 95% confidence interval [CI], 1.03–1.10), higher baseline IOP (OR, 1.10; 95% CI, 1.01–1.24), higher body mass index (BMI) (OR, 1.15; 95% CI, 1.03–1.31), higher education level (OR, 1.57; 95% CI, 1.05–2.17), and higher hematocrit level (OR, 1.22; 95% CI, 1.08–1.43).
Conclusions
In the health-center-based Korean population, the 5-year incidence of POAG was 0.72%, and the rate of progression from POAG suspect to definite POAG was 4.75% per year. This study identified old age, high baseline IOP, high BMI, high level of education, and high hematocrit level as significant risk factors for incident POAG.
Introduction
Glaucoma is the leading cause of irreversible blindness, affecting 66.8 million people worldwide [1], [2]. As the population ages, this figure is expected to increase. Therefore, the epidemiology of glaucoma is of vital importance for establishing appropriate clinical and public health intervention strategies. The epidemiological features of primary open-angle glaucoma (POAG) have been identified in several population-based studies including the Visual Impairment Project in Melbourne, the Rotterdam Study in the Netherlands, the Los Angeles Eye Study in California, and the Barbados Incidence Study of Eye Disease [3]–[6]. Most studies, however, have focused on POAG prevalence, with relatively few investigating the incidence of POAG in longitudinal cohorts. Furthermore, few studies have focused on POAG incidence in Asian populations.
This study was designed to investigate POAG incidence and rate of progression in a health-screened Korean population. The information gained will be helpful for understanding whether or not there are ethnic differences in POAG occurrence, and could help identify risk factors associated with its progression. This large-scale, longitudinal cohort study should facilitate the design of strategies for the prevention of disease in cases with modifiable risk factors.
The Gangnam Eye Study is an ongoing cohort study designed in 2004. The study population comprises individuals who had participated in a glaucoma-screening program at the Gangnam Healthcare Center of Seoul National University Hospital. Its primary objective was to evaluate POAG prevalence and incidence during the longitudinal follow-up. The study’s secondary objectives included determining baseline demographics and systemic risk factors, quantifying the diagnostic performance of examination findings, identifying the relevant risk factors of POAG, and elucidating the clinical role of glaucoma-screening strategies.
Methods
This investigation was based on the Gangnam Eye Study, an ongoing cohort study conducted at the Gangnam Healthcare Center at Seoul National University Hospital. It was approved by the Institutional Review Board of Seoul National University Hospital and conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Study Population
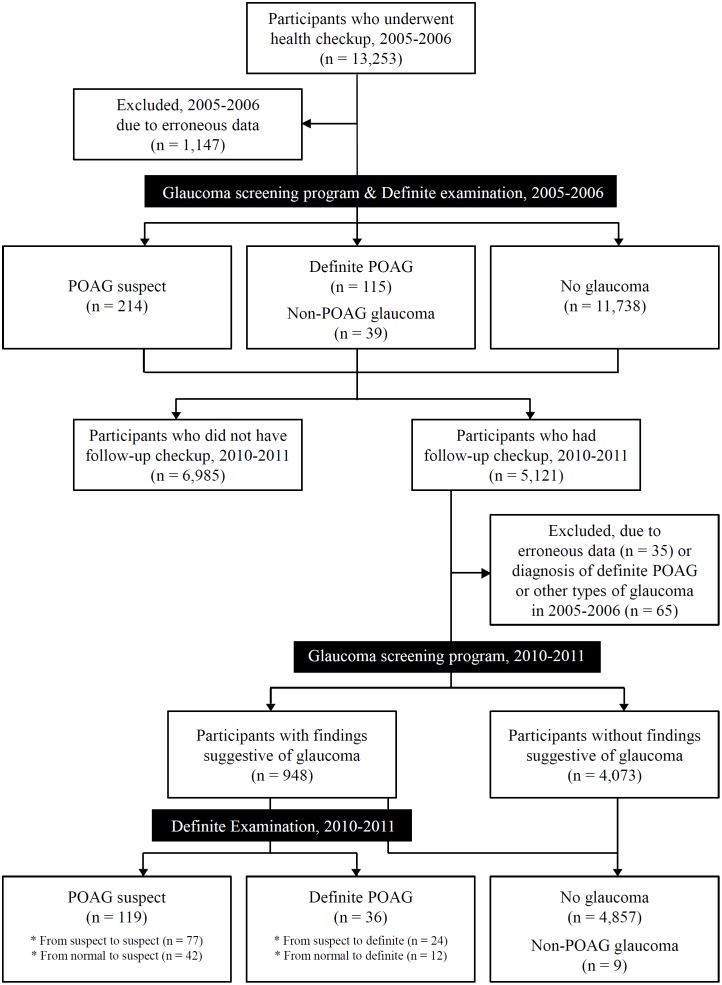
This study enrolled individuals who had been attending a healthcare center for general checkups. The inclusion criteria were as follows: participation in a glaucoma-screening program at the Gangnam Healthcare Center of Seoul National University Hospital during the period from January 2005 to December 2006 and then again during the period from January 2010 to December 2011, and age ≥40 years at the time of the first exam (Figure 1). Individuals were excluded if they had a history of intraocular surgery other than uncomplicated cataract surgery or of diseases that could affect the visual field (e.g., diabetic retinopathy, retinal vein occlusion, ischemic optic neuropathy, pituitary lesions, and demyelinating diseases).
Figure 1. Flow diagram of participant progress in Gangnam Eye Study.
Screening Examination
All of the subjects underwent a default screening test including measurements of body height, body weight, blood pressure (BP), and blood and urine sampling. Also, each subject answered a standard questionnaire, administered by trained research technicians, regarding the presence of systemic diseases (e.g., diabetes mellitus, arterial hypertension, coronary heart disease, asthma, hyperlipidemia). Each participant’s socioeconomic status was evaluated according to their level of education and monthly family income. The level of education was categorized as “primary school education,” “middle school education,” “high school education,” “college education,” or “graduate school education”. The level of monthly family income was categorized as “<$2000 (USD),” “$2000–5000 (USD),” “$5000–8000 (USD),” or “>$8000 (USD)”. Further, the level of smoking was categorized as “have never smoked,” “previously smoked but no longer smoking,” or “currently smoking,” and the level of alcohol consumption was categorized as “do not drink any,” “less than once a month,” “2–3 times a month,” “1–2 times a week,” “3–4 times a week,” or “almost every day”.
Glaucoma-Screening Program
The glaucoma screening included measurements of intraocular pressure (IOP) and fundus photography. IOP was measured using a non-contact tonometer (model CT10; Topcon Inc., Tokyo, Japan). Fundus photos were taken using a 45° digital non-mydriatic fundus camera (model EOS D60; Canon Inc., Utsunomiya, Japan). Two experienced ophthalmologists (Y.K.K. and H.J.C.), masked to the subjects’ identity, independently evaluated the fundus photographs to check for suspicious findings such as glaucomatous optic neuropathy (GON) or retinal nerve fiber layer (RNFL) defects. The criteria for suspicious findings were as follows:
Suspected GON: vertical cup-to-disc ratio (VCDR) ≥0.6, VCDR difference ≥0.2 between the eyes, minimal neural rim width <0.1 times the disc diameter, or disc hemorrhage.
Suspected RNFL defect: width greater than that of a major retinal vessel when measured at the disc edge, diverging in an arcuate or wedge shape.
Baseline IOP≥22 mmHg [7].
Definitive Examination
All of the subjects suspected to have glaucoma underwent a comprehensive glaucoma evaluation, including Goldmann applanation tonometry (model AT900; Haag-Streit, Köniz, Switzerland), Goldmann 3-mirror gonioscopy, stereoscopic disc photography, red-free RNFL photography (CF-60Uvi; Canon Inc., Utsunomiya, Japan), and standard automated perimetry (HFA II 720i; Carl Zeiss Meditec Inc., Dublin, CA). GONs and glaucomatous visual field losses (GVFLs) were evaluated by the same physicians. Any disagreements were settled via discussion; when necessary, an additional grader (K.H.P.) was consulted.
Definitions
The baseline IOP was defined as the IOP measured using a non-contact tonometer during the period from January 2005 to December 2006. For all subjects, glaucomatous optic neuropathy and GVFL were defined based on the following criteria [4]:
GON: VCDR≥0.7 or VCDR difference ≥0.2 between the eyes, or minimal neural rim width <0.1 times the disc diameter on fundus photos.
GVFL: ≥2 of the following 3 criteria: (1) the presence of a cluster of 3 points on a pattern deviation probability plot with P<0.05, one of which with P<0.01; (2) a pattern standard deviation with P<0.05; (3) glaucoma hemifield test results outside the normal limits.
Subjects were deemed to have definite POAG if they met both of the following criteria: (1) presence of typical GON with compatible GVFL; (2) normal anterior chamber angles. “POAG suspect” was defined as follows: (1) the presence of typical GON in the absence of compatible GVFL; (2) normal anterior chamber angles. “No glaucoma” was defined as follows: (1) the absence of GON or GVFL; (2) normal anterior chamber angles.
The incidence of POAG suspect was defined as its development during follow-up in at least 1 eye, with no glaucoma present at baseline in either eye. The incidence of definite POAG was defined as its development during follow-up in at least 1 eye, with POAG suspect or no glaucoma present in either eye at baseline. Progression from POAG suspect to definite POAG was defined as the development of the latter during follow-up in at least 1 eye, with POAG suspect in either eye at baseline.
Statistical Analyses
Statistical analyses were performed using a commercially available statistical software package (SPSS for Windows, version 19.0, IBM-SPSS, Chicago, IL). Chi-square tests were used to compare the proportions. Logistic regression models were utilized to analyze the associations between the incidence of POAG and any continuous or categorical independent variables such as age, gender, or personal history. The data were presented as mean values with standard deviations. All of the P-values were two-sided, and were considered significant when <0.05.
Results
A total of 13,253 subjects aged 40 years or older who had participated in a glaucoma-screening program at the Gangnam Healthcare Center, Seoul National University Hospital, during the period from 2005 to 2006 were enrolled. Of these, 238 subjects (1.7%) with a history of intraocular surgery other than uncomplicated cataract surgery, and 441 subjects (3.3%) with diseases that could affect the visual field, were excluded from further analysis, yielding a sample of 12,574 subjects. Another 468 subjects were excluded from the study owing to incomplete medical records (n = 204) or poor-quality fundus photography (n = 264). Ultimately, 12,106 subjects were included (Figure 1).
During the period from 2010 to 2011, 42.3% (5,121) of the 12,106 subjects were subjected to the same glaucoma-screening program described above. Excluding 35 subjects with incomplete medical records during 2010–2011, and excluding 65 subjects with definite POAG or non-POAG glaucoma (46 with definite POAG, 10 with primary angle-closure glaucoma, 4 with pseudoexofoliation glaucoma, 3 with pigmentary glaucoma, and 2 with uveitic glaucoma) during 2005–2006, 5,021 subjects ultimately were included. Among them, 948 subjects (18.9%) were referred for comprehensive glaucoma evaluations (Figure 1).
When comparing the baseline characteristics between the patients who presented for follow-up during 2010–2011 (n = 5,121) and those who did not (n = 6,985), there were no significant differences in age, sex, IOP, or personal history of diabetes mellitus, arterial hypertension, coronary heart disease, asthma, hyperlipidemia, aspirin use, or anti-coagulant use. Additionally, the baseline prevalences of glaucoma were similar (Table 1).
Table 1. Demographic Data of Study Participants.
| Characteristics at Baseline in 2005–2006 | Participants from2005–2006 and Includedin 2010–2011 | Participants from2005–2006 and NotParticipating in 2010–2011 | PValue | ||
| No. | 5,121 | 6,985 | |||
| Age (yrs) (mean±SD) | 51.35±13.12 | 53.39±14.01 | 0.062a | ||
| Women (%) | 39.19 | 42.55 | 0.087b | ||
| Intraocular pressure (mmHg) | 13.7±3.1 | 13.9±2.9 | 0.231b | ||
| History of arterial hypertension (%) | 14.82 | 12.66 | 0.091b | ||
| History of coronary heart disease (%) | 2.26 | 2.29 | 0.401b | ||
| History of asthma (%) | 2.78 | 2.57 | 0.388b | ||
| History of hyperlipidemia (%) | 5.50 | 6.14 | 0.102b | ||
| History of Aspirin use (%) | 8.34 | 7.78 | 0.165b | ||
| History of anti-coagulant use (%) | 2.34 | 2.39 | 0.222b | ||
| Prevalence in 2005–2006 | % | No. | % | No. | |
| POAG suspect | 1.98 | 101 | 1.62 | 113 | 0.218b |
| Definite POAG | 0.90 | 46 | 0.99 | 69 | 0.741b |
SD = standard deviation; POAG = primary open-angle glaucoma.
Independent-samples t-test.
Chi-square test.
Five-Year Incidence of Primary Open-Angle Glaucoma
At 5.1±0.5 years after the baseline screening, 78 subjects (95 eyes) in the study group were newly diagnosed as POAG suspect (42 subjects; 52 eyes) or definite POAG (36 subjects; 43 eyes). Additionally, 9 subjects (13 eyes) were newly diagnosed as non-POAG glaucoma: 6 (10 eyes) with primary angle closure glaucoma, 2 (2 eyes) with pseudoexofoliation glaucoma, and 1 (1 eye) with uveitic glaucoma. The 5-year incidence of POAG suspect was 0.84%, and that of definite POAG, 0.72% (Table 2). The 5-year incidence of primary angle-closure glaucoma was 0.12%, and that of pseudoexofoliative glaucoma, 0.04%.
Table 2. Five-Year Incidence of Primary Open-Angle Glaucoma (POAG) Stratified by Age and Gender.
| Normal to POAG Suspect | Normal to Definite POAG | POAG Suspect to Definite POAG | |||||
| Age group at Baseline (yrs) | Gender (No.) | No. | 5-yearIncidence (%) | No. | 5-yearIncidence (%) | No. | 5-yearIncidence (%) |
| 40–49 | Male (1,380) | 10 | 0.72 | 3 | 0.22 | 5 | 0.36 |
| Female (938) | 5 | 0.53 | 1 | 0.11 | 2 | 0.21 | |
| Combined (2,318) | 15 | 0.65 | 4 | 0.17 | 7 | 0.30 | |
| 50–59 | Male (1,182) | 13 | 1.10 | 3 | 0.25 | 6 | 0.51 |
| Female (796) | 5 | 0.63 | 2 | 0.25 | 2 | 0.25 | |
| Combined (1,978) | 18 | 0.91 | 5 | 0.25 | 8 | 0.40 | |
| 60–69 | Male (425) | 4 | 0.94 | 2 | 0.47 | 5 | 1.15 |
| Female (207) | 2 | 0.97 | 0 | 0.00 | 3 | 1.45 | |
| Combined (632) | 6 | 0.95 | 2 | 0.32 | 8 | 1.27 | |
| >70 | Male (64) | 1 | 1.56 | 1 | 1.56 | 0 | 0.00 |
| Female (29) | 2 | 6.90 | 0 | 0.00 | 1 | 3.45 | |
| Combined (93) | 3 | 3.23 | 1 | 1.08 | 1 | 1.08 | |
| Total | Male (3,051) | 28 | 0.92 | 9 | 0.29 | 16 | 0.52 |
| Female (1,970) | 14 | 0.71 | 3 | 0.15 | 8 | 0.41 | |
| Combined (5,021) | 42 | 0.84 | 12 | 0.24 | 24 | 0.48 | |
Rate of Progression from Primary Open-Angle Glaucoma Suspect to Definite Primary Open-Angle Glaucoma
In the study group, the prevalence of POAG suspects in 2005–2006 was 1.98% (n = 101). Of these, 23.77% (n = 24) had progressed to definite POAG by 2010–2011 (Table 2). The rate of progression from POAG suspect to definite POAG was 4.75% per year.
Association between Intraocular Pressure and Development of Primary Open-Angle Glaucoma
At baseline, the mean IOP in the subject eyes was 13.7±3.1 mmHg (13.6±3.0 mmHg in the right eye; 13.7±3.2 mmHg in the left eye). In the POAG suspect or definite POAG eyes (n = 147), the mean baseline IOP was 16.9±4.3 mmHg, which was greater than that in the eyes without progression (12.8±3.3 mmHg; n = 10,039; P = 0.012). In subject eyes with the baseline IOP>21 mmHg (n = 38), the incidence of POAG suspect or definite POAG was significantly higher than in those with the baseline IOP≤21 mmHg (n = 10,096; 32% vs. 1.05%; P<0.001) (Figure 2).
Figure 2. Distribution of baseline intraocular pressure (IOP) and 5-year incidence of primary open-angle glaucoma (POAG) suspect or definite POAG as related to IOP.
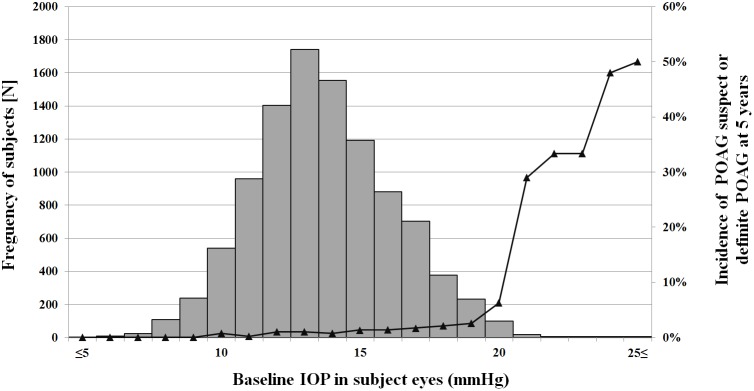
The bar and broken-line graphs respectively show the distribution of IOP and the 5-year incidence of POAG suspect or definite POAG.
Risk Factors Associated with the Progression of Primary Open-Angle Glaucoma
A univariate analysis showed that the progression from POAG suspect to definite POAG was significantly associated with older age (P = 0.021), higher baseline IOP (P = 0.023), higher body mass index (BMI) (P = 0.006), higher education level (P = 0.008), anti-coagulant use (P = 0.047), higher hematocrit level (P = 0.012), and higher eosinophil level (P = 0.048) (Table 3). A stepwise multivariate binary logistic regression analysis included all of the parameters for which the P-value of the association with the progression of POAG was ≤0.05 in the univariate analysis. The results revealed that the progression from POAG suspect to definite POAG remained significantly associated with older age (OR, 1.07; 95% CI, 1.03–1.10), higher baseline IOP (OR, 1.10; 95% CI, 1.01–1.24), higher BMI (OR, 1.15; 95% CI, 1.03–1.31), higher education level (OR, 1.57; 95% CI, 1.05–2.17), and higher hematocrit level (OR, 1.22; 95% CI, 1.08–1.43), whereas the associations with anti-coagulant use (P = 0.092) and higher eosinophil level (P = 0.103) were no longer significant (Table 4).
Table 3. Associations Between Glaucoma Progression and Systemic Parameters in Univariate Regression Analysis.
| Baseline systemic parameters | Progression from POAG suspect to POAG glaucoma | |||
| Odds Ratio | 95% Cl | P Value | ||
| Age (years) | 1.09 | 1.05 | 1.11 | 0.021 |
| Gender (men/women) | 0.79 | 0.67 | 0.88 | 0.789 |
| Intraocular pressure (mmHg) | 1.02 | 1.00 | 1.05 | 0.023 |
| Height (mm) | 0.98 | 0.68 | 1.40 | 0.975 |
| Body Weight (kg) | 1.16 | 0.92 | 1.47 | 0.242 |
| Body Mass Index (kg/m2) | 1.03 | 1.01 | 1.06 | 0.006 |
| Social history | ||||
| Level of Education | 1.15 | 1.07 | 1.23 | 0.008 |
| Income (US dollars) | 0.94 | 0.77 | 1.09 | 0.722 |
| Smoking (ever-current/never) | 0.91 | 0.46 | 2.03 | 0.182 |
| Consumption of alcohol (ever-current/never) | 1.55 | 0.82 | 2.03 | 0.521 |
| Personal history | ||||
| Diabetes mellitus (present/not present) | 0.83 | 0.67 | 1.02 | 0.661 |
| Arterial hypertension (present/not present) | 0.42 | 0.29 | 0.52 | 0.454 |
| Coronary heart disease (present/not present) | 1.31 | 1.16 | 1.46 | 0.109 |
| Asthma (present/not present) | 1.02 | 0.75 | 1.41 | 0.989 |
| Hyperlipidemia (present/not present) | 1.31 | 0.87 | 1.88 | 0.128 |
| Aspirin use (yes/no) | 2.03 | 1.21 | 2.65 | 0.772 |
| Anti-coagulant use (yes/no) | 1.70 | 1.47 | 1.97 | 0.047 |
| Systolic BP (mmHg) | 1.45 | 0.73 | 2.55 | 0.485 |
| Diastolic BP (mmHg) | 2.00 | 1.61 | 2.35 | 0.089 |
| Blood concentration of | ||||
| WBC (per µl) | 1.62 | 0.53 | 3.14 | 0.143 |
| Hematocrit (%) | 1.17 | 1.02 | 1.45 | 0.012 |
| Eosinophil (%) | 1.02 | 0.83 | 1.29 | 0.048 |
| Triglycerides (g/µL) | 1.05 | 0.70 | 1.49 | 0.181 |
| HDL (g/dL) | 1.56 | 1.09 | 2.08 | 0.840 |
| HBA1C (g/dL) | 0.93 | 0.71 | 1.32 | 0.491 |
| Urinary concentration of | ||||
| BUN (mg/dL) | 0.86 | 0.42 | 1.54 | 0.438 |
| Creatinine (mg/dL) | 0.91 | 0.74 | 1.92 | 0.854 |
| Hepatic functions | ||||
| ALT (mg/dL) | 1.55 | 0.83 | 2.88 | 0.387 |
| AST (mg/dL) | 1.01 | 0.78 | 1.31 | 0.467 |
| γ-GT (mg/dL) | 1.24 | 1.03 | 1.57 | 0.185 |
| Cancer markers | ||||
| AFP (mg/dL) | 0.91 | 0.65 | 1.31 | 0.668 |
| CEA (mg/dL) | 1.34 | 0.91 | 1.78 | 0.211 |
| Thyroid functions | ||||
| TSH (mg/dL) | 0.99 | 0.76 | 1.20 | 0.864 |
| Free T4 (mg/dL) | 1.51 | 0.81 | 2.73 | 0.392 |
POAG = primary open-angle glaucoma; CI = confidence interval; BP = blood pressure;
WBC = white blood cell; Hct = hematocrit; HDL = high-density lipoprotein;
BUN = blood urea nitrogen; ALT = alanine aminotransferase; AST = aspartate aminotransferase;
γ-GT = gamma glutamyl transpeptidase; AFP = alpha fetoprotein; CEA = carcinoembryonic antigen; TSH = thyroid-stimulating hormone.
Table 4. Associations Between Glaucoma Progression and Systemic Parameters in Multivariate Logistic Regression Analysis.
| Baseline systemic parameters | Progression from POAG suspect to definite POAG | |||
| Odds Ratio | 95% Cl | P-Value | ||
| Age (years) | 1.07 | 1.03 | 1.10 | 0.011 |
| Intraocular pressure (mmHg) | 1.10 | 1.01 | 1.24 | 0.037 |
| Body Mass Index (kg/m2) | 1.15 | 1.03 | 1.31 | 0.029 |
| Level of Education | 1.57 | 1.05 | 2.17 | 0.017 |
| Anti-coagulant (yes/no) | 1.13 | 0.83 | 1.43 | 0.092 |
| Hematocrit (%) | 1.22 | 1.08 | 1.43 | 0.008 |
| Eosinophil (%) | 1.39 | 1.12 | 1.85 | 0.103 |
POAG = primary open-angle glaucoma; CI = confidence interval; Hct = hematocrit.
Discussion
This study investigated the incidence of POAG in an East Asian population. Although there have been numerous epidemiologic studies on glaucoma in comparable populations, the focus in most cases was glaucoma prevalence, not incidence [8]–[11].
Compared with previous population-based studies, this study has several notable strengths: (1) artificial manipulation could be minimized, because the follow-up was performed at the participant’s discretion; (2) various tests could be conducted, including overall physical measurements and check-up examinations on various organs; (3) marked reductions in the cost and the overall time required for the analysis.
This study found that baseline age is a significant risk factor for progression from POAG suspect to definite POAG. Our results suggest that a 1-year difference in age at baseline is associated with a 7% increase in the risk of POAG. Our observations are consistent with previous studies [3], [4], [12], [13]. In the Barbados Incidence Study of Eye Disease and the Rotterdam Eye Study, the risk of developing glaucoma increased 4 and 6%, respectively, in subjects who were 1 year older at baseline [12], [14].
The overall mean baseline IOP of the eyes in this study was 13.7±3.1 mmHg, which is similar to that reported by the Namil study (13.5±2.9 mmHg) [9]. In the POAG suspect or definite POAG eyes, the mean baseline IOP was 16.9±4.3 mmHg, significantly higher than that of the healthy normal eyes (12.8±3.3 mmHg). These results are in agreement with those of the Tajimi study, where IOP was identified as a risk factor for POAG, despite the fact that most (92%) of the POAG patients had IOP≤21 mmHg [15]. Our findings suggest that IOP, even within the normal pressure range, plays a vital role in the pathogenesis of glaucoma. Furthermore, the 5-year incidence of POAG suspect or definite POAG in eyes with the baseline IOP>21 mmHg was significantly higher than in those with the baseline IOP≤21 mmHg (32 vs. 1.05%; P<0.001) (Figure 2). The 5-year incidences of normal-tension/low-tension POAG were 0.51 and 0.20%, respectively.
The present study demonstrated a positive association between BMI and progression of POAG, which finding corresponds well with those of the relevant earlier studies [16], [17]. This connection between obesity and POAG might be related to the presence of excess intraorbital fat tissue, increased episcleral venous pressure, and/or increased blood viscosity with increased resistance to outflow in the episcleral veins [18], [19]. In fact, several previous reports have confirmed the correlation between obesity and increased IOP [20]–[22].
This study also identified high education level as a risk factor for glaucoma progression. However, in the overall analysis, there was no significant association with income, smoking, or consumption of alcohol. These results partially agree with those of previous investigations, such as the Los Angeles Latino Eye Study and the Rotterdam Study, which showed no significant association with education, employment status, income, or insurance status [23], [24]. Nonetheless, considering that a higher level of education is associated with increased myopic refractive error, [25] these factors might yet play a role in the development or progression of glaucoma. Certainly, further study will be necessary to elucidate the complex relationships between socioeconomic status and POAG.
Among the other results of the present study, POAG progression was associated with high hematocrit levels. A previous study attributed development of POAG to blood viscosity [26]. Another study reported that patients with normal-tension glaucoma exhibited abnormal hemorrheological parameters when compared with normal subjects. However, Sekeroglu et al [19]. reported no such differences among patients with POAG, exfoliation glaucoma, or exfoliation syndrome, when compared with normal subjects. Further study will be needed to clarify this issue.
Health screening helps with the early detection of diseases and allows for early access to the proper treatment, which in turn leads to reduced incidence and overall morbidity [27]. In 2011, about 10 million people underwent health screening in Korea. This number is equivalent to 73.7% of all individuals aged ≥40 years. In fact, since the Gangnam Healthcare Center was first established, approximately 400,000 people have presented for checkups, with a yearly average of 41,000 over the last five years. Baseline checkups typically are done 1–5 years later. Among those who presented for health screening during the period from 2005 to 2006, 85.3% presented for more than one follow-up examination. Although the actual follow-up rate in the present study was 42.3%, the cumulative rate of return at any of the 5 years after baseline (85.3%) was similar to the follow-up rates in previous population-based longitudinal studies (76.6–85%) [3]–[5], [28], [29].
In this study, a stepwise glaucoma-screening program was conducted. That is, a general screening (including non-contact tonometry and fundus photography) was initially performed. Based on the results obtained in that general screening, a selective comprehensive glaucoma evaluation was conducted for certain patients. The stepwise screening program followed in this study has several strengths. First, by selecting the subjects who were to undergo comprehensive glaucoma evaluations after the general screening, we were able to reduce the number of unnecessary evaluations. We were also able to detect a considerable number of glaucoma patients without any subjective symptoms. Therefore, stepwise glaucoma-screening strategies (i.e., selective comprehensive glaucoma evaluation following a general screening program) can be useful in clinical and public health interventions.
The Gangnam Eye Study eligibility criteria were formulated with the purpose to select subjects at moderate-to-high risk of developing glaucoma. Since numerous previous studies used the “IOP greater than 21 mmHg” criterion for elevated IOP, we used the cut-off of 22 mmHg in the initial screening. If we used only IOP criteria during the screening process, it would have been possible to underestimate the incidence of normal-tension/low-tension POAG. However, since the glaucoma-screening program included optic disc, RNFL and IOP criteria, we are confident that the incidence of POAG, including normal-tension/low-tension POAG, was not underestimated during the screening procedure.
This study has several limitations. First, the study design was not population based, and less than half (42.3%) of the individuals examined at baseline received a follow-up examination. Selection bias might have been operative, because subjects who take care of their own health tend to participate more often than those who do not. Second, the glaucoma-screening program did not include measurements of refractive error or visual field examinations. Particularly, the lack of initial visual field examinations might have resulted in an underestimation of glaucoma prevalence. Third, IOP was measured using a non-contact tonometer during the screening procedure. Only subjects with findings suggestive of glaucoma were offered a comprehensive glaucoma evaluation including Goldmann applanation tonometry. Moreover, the glaucoma-screening program did not include pachymetry measurement. Finally, the study findings cannot be extrapolated to draw conclusions with respect to the prevalence of POAG in any specific region or ethnicity.
In conclusion, in a health-screened Korean population, the 5-year incidence of POAG was 0.72%. Additionally, the rate of progression from POAG suspect to definite POAG was 4.75% per year. This study identified old age, high baseline IOP, high BMI, high level of education, and high hematocrit level as significant risk factors for progression to POAG. It seems clear that stepwise glaucoma-screening strategies can be useful in clinical and public health interventions.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data cannot be made publicly available because they contain identifying patient information. Data are available upon request from the Gangnam Healthcare Center. Data requests can be sent to Prof. Hyuk Jin Choi (docchoi@hanmail.net).
Funding Statement
The authors have no support or funding to report.
References
- 1. Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leske MC (1983) The epidemiology of open-angle glaucoma: a review. Am J Epidemiol 118:166–191. [DOI] [PubMed] [Google Scholar]
- 3. Mukesh BN, McCarty CA, Rait JL, Taylor HR (2002) Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology 109:1047–1051. [DOI] [PubMed] [Google Scholar]
- 4. de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Hofman A, et al. (2005) Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology 112:1487–1493. [DOI] [PubMed] [Google Scholar]
- 5.Varma R, Wang D, Wu C, Francis BA, Nguyen BB, et al. (2012) Four-year incidence of open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Am J Ophthalmol 154: 315–325 e311. [DOI] [PMC free article] [PubMed]
- 6. Wu SY, Nemesure B, Leske MC (2001) Observed versus indirect estimates of incidence of open-angle glaucoma. Am J Epidemiol 153:184–187. [DOI] [PubMed] [Google Scholar]
- 7. Leydhecker W, Akiyama K, Neumann HG (1958) [Intraocular pressure in normal human eyes]. Klin Monbl Augenheilkd Augenarztl Fortbild 133:662–670. [PubMed] [Google Scholar]
- 8. Hong C, Joo JH, Shin KH, Song KY (1987) Clinical study of Korean glaucomatous patients. Korean J Ophthalmol 1:41–46. [DOI] [PubMed] [Google Scholar]
- 9. Kim CS, Seong GJ, Lee NH, Song KC, Namil Study Group KGS (2011) Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 118:1024–1030. [DOI] [PubMed] [Google Scholar]
- 10. Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, et al. (2004) The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 111:1641–1648. [DOI] [PubMed] [Google Scholar]
- 11. Jonas JB, Xu L, Wang YX (2009) The Beijing Eye Study. Acta Ophthalmol 87:247–261. [DOI] [PubMed] [Google Scholar]
- 12. Leske MC, Connell AM, Wu SY, Nemesure B, Li X, et al. (2001) Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol 119:89–95. [PubMed] [Google Scholar]
- 13. Ekstrom C (2008) Incidence of open-angle glaucoma in central Sweden. Acta Ophthalmol 86:747–754. [DOI] [PubMed] [Google Scholar]
- 14. Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, et al. (1994) The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology 101:1851–1855. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki Y, Iwase A, Araie M, Yamamoto T, Abe H, et al. (2006) Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology 113:1613–1617. [DOI] [PubMed] [Google Scholar]
- 16. Klein BE, Klein R, Linton KL (1992) Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 33:2224–2228. [PubMed] [Google Scholar]
- 17. Mori K, Ando F, Nomura H, Sato Y, Shimokata H (2000) Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol 29:661–666. [DOI] [PubMed] [Google Scholar]
- 18. Lee EJ, Kim TW, Park KH, Seong M, Kim H, et al. (2009) Ability of Stratus OCT to detect progressive retinal nerve fiber layer atrophy in glaucoma. Invest Ophthalmol Vis Sci 50:662–668. [DOI] [PubMed] [Google Scholar]
- 19. Sekeroglu MA, Irkec M, Mocan MC, Ileri E, Dikmenoglu N, et al. (2011) The association of ocular blood flow with haemorheological parameters in primary open-angle and exfoliative glaucoma. Acta Ophthalmol 89:429–434. [DOI] [PubMed] [Google Scholar]
- 20. Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD (2011) The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology 118:1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higginbotham EJ (2006) How much influence do socioeconomic factors have on glaucoma prevalence and therapeutic outcomes? Arch Ophthalmol 124:1185–1186. [DOI] [PubMed] [Google Scholar]
- 22. Magalhaes da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, et al. (2012) Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene 502:142–146. [DOI] [PubMed] [Google Scholar]
- 23.Doshi V, Ying-Lai M, Azen SP, Varma R, Los Angeles Latino Eye Study G (2008) Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology 115: 639–647 e632. [DOI] [PubMed]
- 24. Ramdas WD, Wolfs RC, Hofman A, de Jong PT, Vingerling JR, et al. (2011) Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Arch Ophthalmol 129:767–772. [DOI] [PubMed] [Google Scholar]
- 25. Xu L, Wang YX, Jonas JB (2010) Level of education associated with ophthalmic diseases. The Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol 248:49–57. [DOI] [PubMed] [Google Scholar]
- 26. Wu ZJ, Li MY (1993) [Blood viscosity and related factors in patients with primary open-angle glaucoma]. Zhonghua Yan Ke Za Zhi 29:353–355. [PubMed] [Google Scholar]
- 27. Frame PS (1989) Clinical prevention in primary care–the time is now! J Fam Pract. 29:150–152. [PubMed] [Google Scholar]
- 28. Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, et al. (1991) A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol 134:1102–1110. [DOI] [PubMed] [Google Scholar]
- 29. Varma R, Ying-Lai M, Francis BA, Nguyen BB, Deneen J, et al. (2004) Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology 111:1439–1448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data cannot be made publicly available because they contain identifying patient information. Data are available upon request from the Gangnam Healthcare Center. Data requests can be sent to Prof. Hyuk Jin Choi (docchoi@hanmail.net).