Abstract
The agricultural pest Ceratitis capitata, also known as the Mediterranean fruit fly or Medfly, belongs to the Tephritidae family, which includes a large number of other damaging pest species. The Medfly has been the first non-drosophilid fly species which has been genetically transformed paving the way for designing genetic-based pest control strategies. Furthermore, it is an experimentally tractable model, in which transient and transgene-mediated RNAi have been successfully used. We applied Illumina sequencing to total RNA preparations of 8–10 hours old embryos of C. capitata, This developmental window corresponds to the blastoderm cellularization stage. In summary, we assembled 42,614 transcripts which cluster in 26,319 unique transcripts of which 11,045 correspond to protein coding genes; we identified several hundreds of long ncRNAs; we found an enrichment of transcripts encoding RNA binding proteins among the highly expressed transcripts, such as CcTRA-2, known to be necessary to establish and, most likely, to maintain female sex of C. capitata. Our study is the first de novo assembly performed for Ceratitis capitata based on Illumina NGS technology during embryogenesis and it adds novel data to the previously published C. capitata EST databases. We expect that it will be useful for a variety of applications such as gene cloning and phylogenetic analyses, as well as to advance genetic research and biotechnological applications in the Medfly and other related Tephritidae.
Introduction
Ceratitis capitata, the Mediterranean fruit fly (Medfly) is an agricultural pest insect of high economic importance. During the last centuries, this pest spread from Africa to other continents, and it is continuing to move and colonize new geographic areas such as more recently in China [1]–[4]. Because of its wide host range (more than 200 vegetable species), its prodigious reproductive capacity, and its surprising adaptability to new and even adverse ecological environments the Medfly is one of the world’s most destructive fruit pests, with the potential to cause up to billions of Euros losses within a few months at the national level [5], [6]. In contrast to other fruit flies, such as Drosophila melanogaster, which lays eggs on decaying fruit, C. capitata causes damage to fruit crops by laying eggs inside intact fruit [7].
The genetic knowledge gained from studies using the model insect Drosophila, was an important premise to develop genetic based methods for sexing males in C. capitata in pest control programs such as the Sterile Insect Technique (SIT) [8] and the Release of Insects carrying Dominant conditional Lethal genes (RIDL) [9]–[11]. In the last two decades, a comparative study based on the identification of candidate genes led to the identification of key C. capitata sex determining genes. This knowledge has been exploited to obtain male-only progeny by suppressing the female determining gene Cctransformer (or Cctransformer-2) by RNAi or by conditional lethality setting the stage for the insect biotechnology to control pest and disease vector insects in the field [8], [12]–[20]. Indeed, the private sector also moved into this field to develop C. capitata transgenic strains by introducing a conditional female-specific dominant lethal construct, [14]. Biotechnology based control strategies have been developed in pest and in animal disease vectors and some have been tested in the field [21], [22] and many more are expected to be realized in the near future [23]–[26].
Various differential expression techniques have been used to identify C. capitata genes which are potentially involved in reproduction, mating behaviour [27], in sexual maturation [5], in mating, post mating [28] and in olfaction [29]. Similar studies have been started recently in other related Tephritidae, such as Anastrepha suspensa and Bactrocera dorsalis [30], [31]. Other investigators applied molecular subtractive strategies isolating C. capitata genes either specifically expressed during the cellularization stage or which are male-specific [32; Salvemini et al., manuscript submitted]. Furthermore, gene promoters which are active in oogenesis and in early embryos have been identified both in C. capitata and in the disease vector Aedes aegypti. These regulatory regions can be used to construct transgenes which express maternal or early embryonic products [33], [34].
The cost- and time-effectiveness of Next Generation Sequencing technologies [35], [36] and the development of new reverse genetics tools (RNAi, TALENs and CRISPR-Cas9) allow many insect species to become model organisms in evolutionary and developmental studies as well as substrates for biotechnological applications [37]–[43]. Integrated international initiatives recently started to focus on comparative genomics studies in insects, which eventually will be very useful to develop alternative control strategies for pest species or disease vectors as well as to improve the existing ones [26], [44]–[49].
Detailed knowledge of stage-specific and tissues-specific transcription in C. capitata is, however desirable to broaden the range of biotechnical techniques which can be used for its control. For example, Medfly male development is based on switching OFF the transformer gene during embryogenesis. This masculinising event can be artificially induced in karyotypically females by RNAi based silencing of tra (or of its co-regulator tra-2) [15], [16], [50]–[54]. Previous studies have revealed that sex determination starts around blastoderm cellularization in C. capitata [15], [55], at the same stage as determined in other insect species, such as the dipterans, D. melanogaster [56] and Musca domestica [53], the lepidopteran Bombyx mori [57], the coleopteran Tribolium castaneum [58], the hymenopterans Apis mellifera [59] and Nasonia vitripennis [60], [61].
In this study we used, for the first time, Illumina short read sequencing technology for transcriptome analysis of mixed male and female embryos aged between 8–10 hours after egg lay), the stage at which sex becomes irreversibly fixed. Nearly 21 million 100-bp paired-end (PE) reads were used to assemble 42,614 transcripts, which clustered into 26,319 unigenes (MEET database; Medfly Early Embryonic Transcripts). Comparison with the previous Medfly EST database revealed that 78% of the assembled unigenes (20,400) were newly identified C. capitata transcripts. The MEET database and the Medfly genome and transcriptome data, which soon should be published by the Medfly International Consortium (Handler, AM:, pers. Comm.), will help to apply in silico approaches such as chromosome quotient or in silico subtraction to identify Y-linked genes, male-specific or male-biased genes and to search for the primary signal of the sex determination of Medfly, the Male Determining Factor (M-factor) [57], [62]–[64].
Results and Discussion
Sequencing and de novo assembly
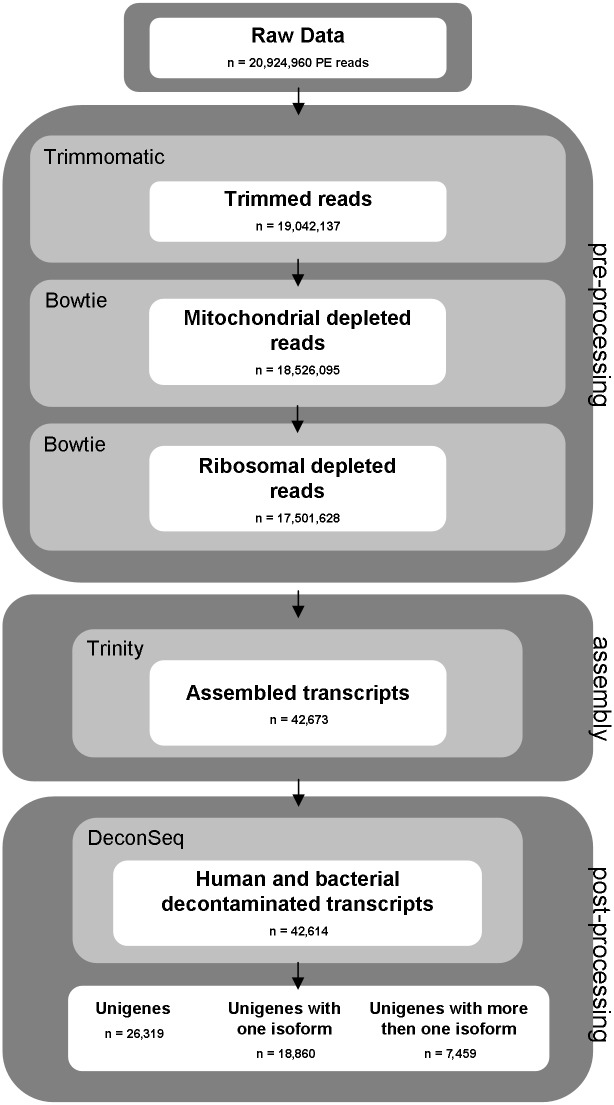
A cDNA library for paired-end (PE) Illumina sequencing was prepared from total RNA isolated from 8–10 hours after egg laying (AEL) embryos. Using an Illumina Genome Analyzer IIx platform, a total of 21 million (M) 100 bp-long PE reads with high sequence quality (Phred score > = 33) were obtained. Figure 1 shows an overview of the sequence analysis workflow of this study. De novo assembly of the Illumina reads was performed using Trinity, a de Bruijn graph based assembler which can reconstruct full-length transcripts and corresponding isoforms, without the need of a reference genome [65], [66]. As Trinity algorithm discards low coverage k-mers, no quality trimming of the reads is usually required [65]. However, we assessed if an adapter contamination removal and a quality control of raw reads were performed with three filtering software (NGS-QC-Toolkit, Trimmomatic and Galaxy) [67]–[69], this could improve the overall quality of the Trinity assembly. We compared the performance of these applications under four different conditions and found that Trimmomatic software was the most efficient (see Methods and Methods S1 for further details).
Figure 1. Filtering and assembling pipeline flowchart.
Data files are represented as white boxes. Software utilized for each step are represented as light grey boxes while dark grey boxes represent the phases of the filtering and assembling pipeline.
Reads derived from C. capitata mitochondrial genome and five ribosomal transcripts (2.71% and 5.38% of the total reads, respectively) were identified by BOWTIE [70] and discarded. The Trinity assembler reconstructed 42,673 transcripts, using a total of 48,815,521 nucleotides, which showed sequence length ranging between 200 bp and 10,000 bp, (average length = 1145 bp) and high N50 value (2,158). The number of assembled transcripts derived from bacterial, viral and human genomes (contamination) was negligible (0.14%) with only 56 transcripts identified by DeconSeq web-tool and discarded [71]. An additional BLAST analysis against the UniVec database found only three transcripts containing 34 bp long Illumina adapters. The final resulting 42,614 transcripts have been listed in the MEET dataset (Medfly Early Embryonic Transcriptome). Sequencing and assembly statistics are summarized in Table 1.
Table 1. Sequencing and assembly statistics.
| Number of input paired end reads | 20924960 |
| Low quality reads | 1882823 |
| Final HQ reads | 19042137 |
| % of Final HQ reads | 91 |
| Mitochondrial and ribosomal depleted reads | 17501628 |
| Number of assembled transcripts | 42673 |
| Number of cleaned assembled transcripts | 42614 |
| Number of assembled nt | 48815521 |
| N50 | 2158 |
| Average transcript lenght | 1145 |
| Number of transcripts>1 Kb | 16023 |
| Number of transcripts>2 Kb | 8136 |
| Number of transcripts>3 Kb | 3693 |
| Number of transcripts>5 Kb | 631 |
| Number of transcripts>10 Kb | 14 |
| Max transcript lenght | 10406 |
We selected the longest transcript for each Trinity component and obtained 26,319 clusters of which 18,860 (71.7%) have only 1 isoform while the remaining 7,459 (28.3%) have 2 or more isoforms. Accordingly to other authors, we named these clusters as “unigenes”. The unigenes showed an average length of 764 bp and the N50 value of 1,517; 5,436 (∼25%) reconstructed unigenes were larger than 1,000 bp. In comparison with other similar transcriptome studies [30], [31], [38], [48], the high N50 value and the number of assembled unigenes indicated that both the mRNA-seq and the assembly procedures were very effective. All sequencing reads were deposited into the Short Read Archive (SRA) of the NCBI (Accession Number: SRR1380982). The assembled MEET dataset is freely available at meetbase.evosexdevo.eu, following online registration.
Coverage assessment
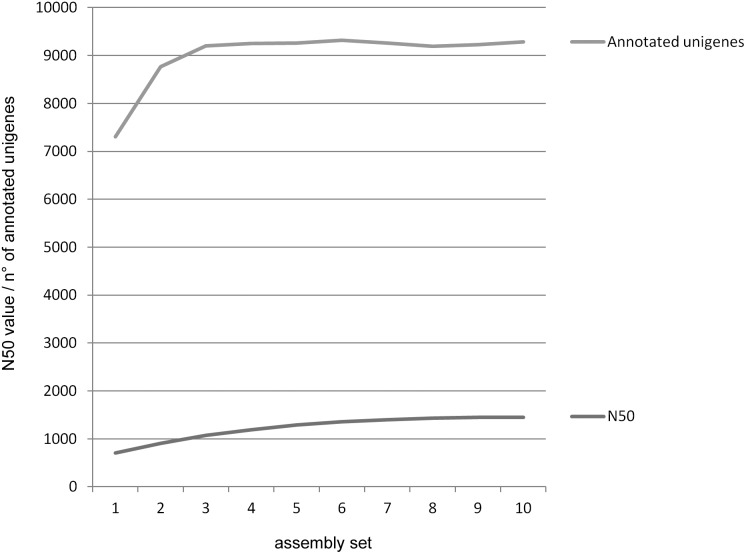
A transcriptome analysis is effective if the number of assembled transcripts and their lengths approximates the real values. To evaluate how thoroughly our RNA sequencing captured the real diversity of early embryonic Medfly transcripts, we assembled ten independent and progressively larger (2M for each step) sub-samples of randomly selected raw reads [37]. We counted the total number of unigenes annotated for each assembly using FastAnnotator [72] an automated web-based service, which integrates well-established annotation tools as Blast2GO, PRIAM and RPS BLAST to assign Gene Ontology (GO) terms, Enzyme Commission numbers (EC numbers) and functional domains to query sequences, respectively. The number of unigenes slowly plateaus after ∼8 M reads out of 21M (Figure 2 - green line). The N50 unigene length increases roughly linearly until ∼12M when started to plateau, although very slowly (Figure 2 - red line). These results suggest that the 21M reads is likely sufficient to represent most of the real complexity of embryonic transcriptome present between 8–10 hours AEL. However, we expect that additional mRNA sequencing and de novo assembly will slightly lengthen the unigene sequences, mainly in the UTR regions, because novel reads will cover the entire length of the purified and sequenced mRNA, especially for those genes having a lower expression.
Figure 2. Coverage assessment of the MEET data set.
The number of assembly obtained with a progressively large sub-samples of randomly selected raw reads (2M for each step) is plotted against the number of annotated unigenes by FastAnnotator software (using NCBI nr database) and the N50 value. The number of annotated unigenes reached a plateau after about 8M reads (green line) while the N50 unigene value continued to increase approximately linearly over the same range (red line).
To address how closely the assembled unigenes are to the real full-length transcripts, as a first approach, we performed gene-by-gene analyses on a small sample. For size comparison, we randomly selected 27 C. capitata cDNA sequences from GenBank, annotated as complete sequence, and compared them to the corresponding transcripts from our MEET dataset (see Table S1). Eight out of twenty-seven MEET transcripts showed the same length, six out of twenty-seven MEET transcripts were longer in size, extending by an average of 665 bp (range of 127–1,884 bp) (see Table S1). Thirteen out of twenty-seven transcripts were not found in the MEET dataset. These genes may not be expressed at the early embryonic stage, such as ceratotoxin A [73], farnesoic acid O-methyltransferase [74] and alpha-L-fucosidase [75]. These 13 mRNAs are indicated with an asterisk in Table S1.
As a second approach, we used the “ortholog hit ratio” (OHR), which provides an estimate of the length of the assembled transcripts relative to a reference genome by comparing only the coding regions [76]. We compared the number of bases in the hit region of C. capitata unigenes to the length of the best TBLASTX match with Drosophila transcripts, which correspond to orthologous coding regions. The idea of this approach is to test whether the coding regions of the unigene and the reference gene have the same length, An “OHR” value of 1 suggests that a transcript has been assembled to its correct size, while values lower than 1 suggest incomplete assembly (e.g. artificial deletions in the coding region as a result of low coverage).
To calculate the MEET “OHR” values we first identified the 1-to-1 orthologs comparing our data set and the D. melanogaster transcriptome (BDGP v5.22). We utilized a custom Perl script to perform a best reciprocal TBLASTX analysis resulting in 2,231 selected MEET unigenes. We observed that half of the selected unigenes cover at least 50% of corresponding Drosophila coding regions (average “OHR” and median “OHR” of 0.52 and 0.49, respectively – see Table S2). Similar values have been reported from transcriptome analyses of other insects [37], [76] indicating that the MEET assembly has a high length coverage.
Also, we compared transcript sequences with 5′ and 3′ untranslated regions. We performed BLASTN using the full length of the 2,231 MEET transcripts as queries and the full-length of their Drosophila orthologs as target subject sequences. We defined a “full-length hit ratio” metric (“FLHR”) as query length divided by subject length and we observed an average and median values both of 1.04, with about 60% of the 2,231 unigenes having a length equal or higher than the Drosophila orthologs (see Table S2). This observation is coherent with the previous OHR, indicating that the assembly was relatively efficient. In addition, for those genes coding for highly conserved proteins, the entire length of the transcript seems to be conserved during evolution even in distantly related species such as C. capitata and Drosophila (120 million of years, mya; [13], [77].
Comparisons with available medfly ESTs
Gomulski et al., (2008) [27] and Scolari et al., (2012) [28] have previously assembled 27,167 high-quality ESTs into a total of 15,229 C. capitata ESTs from 0–36 hours old embryos, dissected adult heads and testes and male accessory glands. To compare the MEET dataset to other publicly available medfly transcript data sets, all C. capitata ESTs deposited at dbEST/GenBank were downloaded (27,167 ESTs with an N50 of 740 bp, 2013.09.01). Since the assembled ESTs described in both papers were not deposited in public databases, we performed, as a first step in our comparative analysis, an assembly of the ESTs dataset using iAssembler software [78], obtaining comparable assembly results with respect to the results of the original papers (see Table S3).
We performed bidirectional BLASTN analyses of the Global EST dataset (13538 assembled ESTs from all tissues and stages) and embryonic EST sub-dataset (5754 assembled ESTs) with the novel MEET unigene dataset (26319 assembled unigenes 8–10 AEL) (Table 2), with a stringent cut-off value of 1e-100, a minimum coverage of the BLAST hit of 30% and a minimum of identity of the BLAST hit of 80%. It is important to notice that the Global EST dataset (and the embryonic EST sub-dataset) includes embryonic transcripts expressed between 0 and 36 hours, which is a much larger time range than the 8–10 hour window of the MEET dataset. Hence, we expected to find a higher complexity in this dataset than in the MEET dataset. Indeed, only 53% Global EST dataset and 83% of Embryonic EST sub-dataset had BLASTN hits to the MEET unigene dataset. Hence 47% of Global EST dataset (6,300 out of 13,538) and 17% of the Embryonic EST sub-dataset (1,004 out of 5,754) is absent in the MEET unigene dataset. The missing transcripts are likely to be expressed only at later stages of development. Interestingly, only 22% of the MEET unigenes had hits to the Global EST dataset. We concluded that 78% unigenes of the MEET unigene dataset (20,447) correspond to newly identified Medfly transcripts, an higher effectiveness of transcriptome analyses when performed by this novel RNA-seq de novo assembly approach. Interestingly, only 16% of the MEET unigenes had BLASTN hits to embryonic EST sub-dataset. Hence, 84% of the MEET unigenes (22,136) are new.
Table 2. Comparison of the present C. capitata MEET dataset with the available C. capitata ESTs.
| e-value (1E-100) mincoverage (30%) minidentity (80%) | Global ESTs datasetversus MEETunigene dataset | MEET unigenedataset versusGlobal ESTs dataset | Embryonic ESTssub-dataset versusMEET unigene dataset | MEET unigenedataset versusEmbryonic ESTssub-dataset |
| BLASTN hits | 7155 | 5872 | 4750 | 4183 |
| total | 13538 | 26319 | 5754 | 26319 |
| % | 53 | 22 | 83 | 16 |
| average length of hits | 861 | 1639 | 802 | 1796 |
| N50 of hits | 828 | 2411 | 784 | 2480 |
Functional annotation
We annotated the MEET unigene dataset using FastAnnotator (Chen et al., 2012) obtaining 11,045 unigenes (42% of total 26,319 assembled unigenes) with a significant similarity (E-value<1e−5) to proteins present in the NCBI non-redundant (nr) database. Of these 9,098 unigenes (82%) had at least one functional annotation. In particular, 7,948 (87%) mapped to GO terms - level 2, by Blast2Go, 705 (8%) were identified to have at least one enzyme hit in the ENZYME database (http://enzyme.expasy.org/) and 5,965 (65%) had at least one domain, with domain coverage >50%, in PFAM database (http://pfam.sanger.ac.uk/). The 15,274 unigenes without annotation have an N50 value of only 398 bp, a very low value respect to the N50 of the 11,045 unigenes corresponding to putative protein coding genes (N50 = 2231 bp). These non-annotated transcripts could represent 1) non-coding genes having short transcripts and/or 2) non fully assembled 5′ and 3′ UTR regions of very low expressed genes. In the first case, it would be of interest to further investigate the nature and the possible function of this group of short transcripts, enlarging the transcriptome complexity. In the second case, the unigenes could correspond to genes already contained in the 11,045 annotated unigenes, without contributing to transcriptome complexity.
Among the GO 7,948 unigenes, a large diversity of functional categories was observed similar to those found in other dipteran insect transcriptomes. These unigenes were categorized into 53 main function groups of Gene Ontology database, belonging to molecular function, biological process and cellular functional component categories (Figure 3). In the molecular function classification, the most represented classes are “binding” and “catalytic activity” classifications, which contain respectively 45.8% and 33.4% of all unigenes. In the biological process classification the largest classes are “cellular process” (25.4%), “metabolic process” (19.5%) and “biological regulation” (13.8%). In the cellular component categories, “cell” and “organelle” result the most represented (45.3% and 31.8%, respectively).
Figure 3. Gene ontology distribution of the MEET data set.
GO term distribution, level 2, of our data set derived from the blast2go analysis of the FastAnnotator annotation pipeline. Percentages are indicated after each GO term.
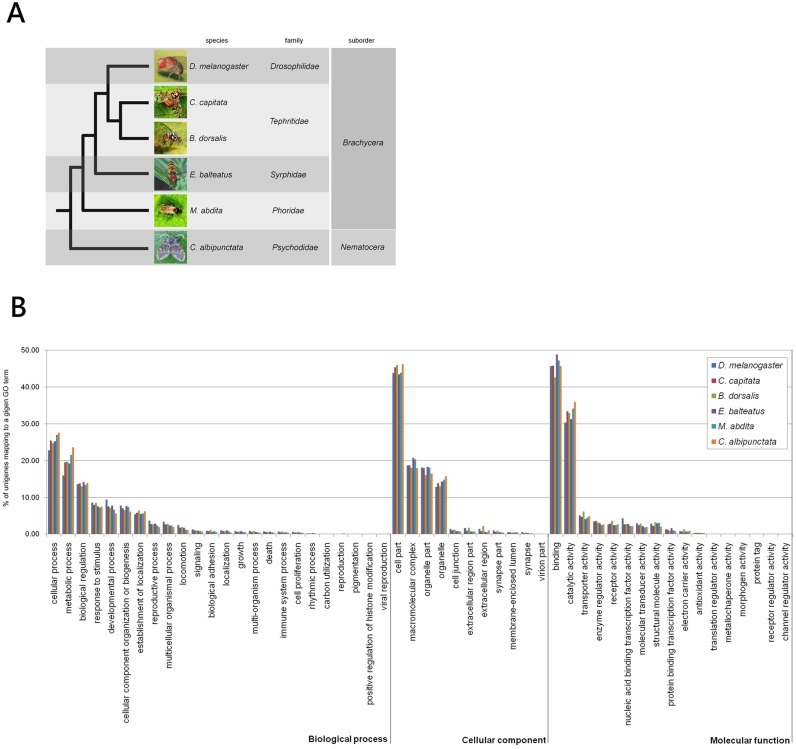
We further performed a comparative analysis of the MEET GO terms distribution with the GO terms obtained from available dipteran high-throughput embryonic transcriptome data of D. melanogaster (2–16hrs old embryos) [79], of the Tephritidae species B. dorsalis (0–24hrs old embryos) [80] and of three emerging dipteran experimental model systems: the moth midge Clogmia albipunctata (family: Psychodidae) (8–12hrs old embryos), the scuttle fly Megaselia abdita (family: Phoridae) (0–4hrs old embryos) [38], and the hoverfly Episyrphus balteatus (family: Syrphidae) (3–6hrs old embryos) [81]. These species span about 200 million of years of evolution in the Diptera order (Figure 4A). To obtain comparable datasets, raw data files for each species were downloaded and assembled by Trinity or by iAssembler software followed by a functional annotation step with FastAnnotator software.
Figure 4. Comparative analysis of GO term distribution in dipteran embryonic transcriptome.
(A) A schematic phylogenetic tree of the species included in the comparison of GO term distribution. Families and sub-orders are indicated. The analyzed species span approximately 200 million of years of evolution of the order Diptera [38], [102]. (B) Column heights represent the percentage of the annotated unigene in each assembly assigned to a give GO term, level 2. The relative percentage of unigenes included in each GO categories are comparable among all the analysed species.
Although the number of assembled unigenes differs from species to species the overall distribution of sequences which are grouped in “molecular function”, biological process” and “cellular component” categories, level 2, are similar for all tested species (Figure 4B).
For example, in the “molecular function” classification the categories of “binding” and of “catalytic activity” are the largest one (respectively, 46% +/−2% and 33% +/−2%) in all species. In the “biological process” classification the first two represented categories are “cellular process” (25% +/−1.7%) and “metabolic process” (20% +/−2.5%). In the “cellular component” classification the first two represented categories in all species are “cell part” (45% +/−1.2%) and “macromolecular complex” (19% +/−1.2%).
These findings indicated that the Medfly 8–10h MEET dataset contains, similar to other dipteran transcriptomes, a wide diversity of reconstructed transcripts from genes involved in a variety of conserved biological processes without any notable biases towards particular categories of genes. This result is expected, since early embryogenesis is highly conserved among dipterans. Differences between species are mostly due to temporal or spatial changes in gene expression [38], [82].
Expression level analysis
To evaluate the relative expression level of the transcripts assembled in the MEET dataset we applied the RSEM software [83] using the FPKM metric (Fragments Per Kilobase of transcript per Million mapped reads) which normalizes paired-end read counts of transcripts by both their length and the total number of mapped reads in the sample [84]. The FPKM values were calculated using the Illumina reads generated from the single library we have produced.
We obtained a wide range of expression levels from less than 1 FPKM to about 200,000 FPKM (see Table S4). We clustered transcripts and unigenes according to their FPKM expression levels as reported in table 3. We considered those transcripts (37%) and unigenes (16%) as not expressed having a FPKM value<1. We observed that about 55% of transcripts have a low expression level (FPKM 1–10); about 8% have a moderate expression level (FPKM 10–100); and only 1.5% exhibit a high to extremely high expression level with values of >100 FPKM. As validation of this in silico expression analysis, qPCR was performed on total RNA extracted for the 8–10 hours AEL embryos to evaluate expression levels of 10 C. capitata genes and compared them to their respective FPKM values. We chose four housekeeping genes superoxide dismutase (sod), ribosomal protein P1 (rpP1), ribosomal protein S21 (rps21) and glycerol-3-phosphate dehydrogenase (gpdh), and six developmental genes (tra-2, hopscotch (hop), outstretched (os), sisterless-A (sisA), deadpan (dpn) and virilizer (vir) genes), known to be expressed during embryogenesis from previous studies in Drosophila and C. capitata [27], [55]. We normalized the respective expression levels obtained by the two methods and we found a high correlation (R = 0.93, Pearson; p-value of 3.29e-4) (see Methods; Table S5).
Table 3. FPKM distribution of the MEET data set.
| FPKM accumulation level | N° of transcripts | % of transcripts | N° of genes | % of genes |
| 0<FPKM<1, very low | 15248 | 35,78 | 4221 | 16,04 |
| 1<FPKM<10, low | 23162 | 54,35 | 18225 | 69,25 |
| 10<FPKM<10∧2 moderate | 3557 | 8,35 | 3217 | 12,22 |
| 10∧2<FPKM<10∧3, high | 529 | 1,24 | 542 | 2,06 |
| 10∧3<FPKM<10∧4, very high | 110 | 0,26 | 108 | 0,41 |
| FPKM>10∧4, extremely high | 8 | 0,02 | 6 | 0,02 |
| 42614 | 100,00 | 26319 | 100,00 |
To evaluate if the 647 transcripts with FPKM >100 have some enrichment in specific GO terms we performed a specific analysis. We compared their GO term frequencies for the molecular function category (LVL4) with the respect of those from the whole annotated MEET dataset (42,614 transcripts), using a custom R script (http://www.r-project.org/) which exploits the Fisher exact text. We considered in our analysis only the GO terms representing at least 1% of the total GO terms in our sample. We observed highly statistically supported enrichments (p-value 0.00001) for RNA binding (GO:0003723), hydrolase activity (GO:0016817) and unfolded protein binding (GO:0051082) (see Table S6). In contrast, the DNA binding (GO:0003677) term, often associated with transcription factors, was not enriched at this stage. The observation that RNA binding protein coding transcripts are highly expressed and overrepresented as GO term during this specific stage of embryogenesis is of particular interest considering that sex determination of insects is mostly based on sex-specific alternative splicing [85].
Long Non Coding RNAs
In contrast to the increasing number of reports on the presence of long non-coding RNA (ncRNA) in human, mouse and other vertebrate systems, there is little knowledge about their distribution and function in invertebrates. A study in Drosophila revealed that a large proportion of intergenic regions encode ncRNAs and that particularly early stages of development express most abundant species of ncRNAs [86]. A recent study showed that in Drosophila many of these long ncRNAs are male-biased and preferentially associate with autosomes [87]. In the lepidopteran species Bombyx mori, a W-linked long non-coding RNA has been even implicated in acting as the primary signal in sex determination [57].
We performed a predictive analysis of coding potential of 18,337 non-annotated MEET transcripts (corresponding to 15,274 non-annotated unigenes) to search for putative long non-coding transcripts. We used three different applications: the Coding Potential Calculator (CPC, [88]), which uses machine-learning methods; the Portrait tool [89], which uses support vector machine and is optimized for non-model organisms; and the alignment-independent Coding Potential Assessment Tool, which uses logistic regression [90]. To ensure a high level of accuracy, we set a very stringent threshold for all three software outputs (see Methods and Table S7). In total 861 putative long non-coding assembled transcripts were identified, corresponding to 846 unigenes. A very similar number was recently reported in Drosophila (528; [87]). Interestingly, the GC content of these transcripts is 27.99%, significantly lower with respect to that of whole MEET data set (37.35%). Low GC content is one of the emerging feature of the long non-coding transcripts at least in human [91] and hence, this finding supports reliability of our prediction.
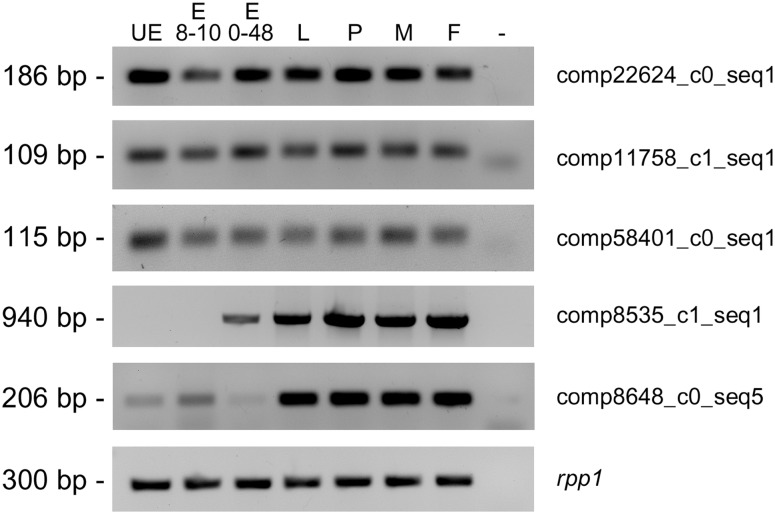
To verify whether the predicted long ncRNAs were true transcripts and to exclude them if they were assembly artifacts we selected seven putative long ncRNAs between the more expressed ones (FPKM>25) and the longest one (length>800 bp) and we performed an RT-PCR analysis on RNA from embryos at the stage of 8–10 hours after egg laying. We further decided to monitor the expression profile of the seven putative ncRNAs during development of Medfly using total RNA from unfertilized eggs, embryos at the stage of 0–48 hours after egg laying, third instar larvae, pupae, and adult males and females. We obtained amplification products of the expected size for five out of seven ncRNAs, which were cloned and sequenced (Figure 5). Three of these were highly expressed (comp22624_c0_seq1, comp11758_c1_seq1 and comp58401_c0_seq1) and present in all developmental stages tested. Expression of the other two putative ncRNAs appears to be regulated during development. For instance, the expression of transcript comp8535_c1_seq1 starts at the late embryonic stage and continues until adulthood The transcript comp8648_c0_seq5 is initially present at low levels in UE and embryos, but undergoes a marked increase in expression levels from larval stage until adulthood (Figure 5). The five validated long ncRNAs were utilized as queries in BLASTN searches against Drosophila genome and NONCODE v4 database, a database of all kinds of non-coding RNAs (noncode.org). We could not retrieve any significant matches.
Figure 5. Temporal expression analysis of putative non-coding RNAs.
UE = unfertilized eggs; E8–10h = embryos at the stage of 8–10 hours after egg laying (AEL); E0–48h = embryos at the stage of 0–48 hours AEL; L = third instar larvae; P = pupae; M = adult males; F = adult females. All the samples constituted individuals of both sexes.
Orthologs of Drosophila genes involved in sex determination
In Drosophila, female development is instructed by a double dose of the X chromosome. This signal activates a cascade of genes (Sxl > tra) which culminates in the female expression of the doublesex (dsx) gene at the bottom of the cascade. The presence of one X chromosome does not activate the cascade. As a consequence dsx is expressed in the male mode and male development follows. The sex determination pathway in C. capitata is only partially conserved with respect to that of Drosophila [15], [16]). While tra and dsx are structurally and functionally conserved it is not clear how tra is regulated in the Medfly. In particular, it is unclear how the male determining Y-linked M factor prevents activation of tra. Also, it is not known whether other Drosophila orthologs are involved as secondary players in the pathway [15], [16], [55].
To address these questions we searched for sex determining orthologs in the MEET dataset. We started with a list of 211 Drosophila genes from Flybase which include the key word “sex determination”. We filtered this initial set using “sex determination” as a GO term (GO:0007530) in Biological Process GO Class recovering a shortened list of 19 genes. We added to this list (Table 4), 6 more genes listed by Gomulski et al. (2008) as sex determining (see [27], table 2). We used the corresponding Drosophila proteins as queries to perform a TBLASTN analysis (with a cut-off value of 1e-10) against the MEET dataset. In case of genes encoding multiple isoforms, the protein having the longest ORF was selected. For the tra gene we used the C. capitata TRA protein as a query due to the very low level of similarity to the TRA protein of Drosophila [54]. Out of the initial set of 25 genes we identified Ceratitis orthologs for 20, including tra, tra-2, Sex-lethal (Sxl) and intersex (ix) genes (Table 4). Of the five genes, which were missing in our dataset, three genes are known not to be expressed at early embryonic stages in Drosophila: dissatisfaction (dsf); hermaphrodite (her); and CG3726.The dsx gene is expressed in C. capitata from 10h after oviposition [55], [92]–[94]. Although fru gene is expressed throughout Drosophila embryogenesis producing various isoforms [95], we failed to identify FRU encoding transcripts in the MEET dataset, most likely due to its narrow and specific temporal window.
Table 4. Sex determination orthologs identified in the MEET data set.
| Sex determination; Biological process; GO:0007530 | |||||||||||
| # | Dmsymbol | Dm name | Dmannotation id | meetBASEhortholog ID | Alignmentlenght (aa) | e-Value | Identity(%) | Similarity(%) | FPKM | FullORF | ORFlenght(aa) |
| 1 | da | daughterless | CG5102 | comp8485_c1_seq2 | 157 | 5,00E-66 | 51 | 60 | 53,09 | yes | 712 |
| 2 | dgrn | degringolade | CG10981 | comp9048_c0_seq8 | 56 | 1,00E-15 | 57 | 69 | 14,74 | yes | 369 |
| 3 | dpn | deadpan | CG8704 | comp11618_c0_seq5 | 224 | 1,00E-78 | 66 | 77 | 11,73 | no | 226 |
| 4 | dsf | dissatisfaction | CG9019 | - | - | - | - | - | - | - | - |
| 5 | dsx | doublesex | CG11094 | - | - | - | - | - | - | - | - |
| 6 | emc | extramacrochaetae | CG1007 | comp9421_c1_seq4 | 168 | 3,00E-41 | 55 | 64 | 703,73 | yes | 242 |
| 7 | fl(2)d | female lethal d | CG6315 | comp12209_c0_seq13 | 200 | 3,00E-75 | 93 | 97 | 16,14 | yes | 652 |
| 8 | fru | fruitless | CG14307 | - | - | - | - | - | - | - | - |
| 9 | her | hermaphrodite | CG4694 | - | - | - | - | - | - | - | - |
| 10 | hop | hopscotch | CG1594 | comp9764_c0_seq2 | 1165 | 0.0 | 39 | 57 | 30,53 | yes | 1149 |
| 11 | ix | intersex | CG13201 | comp7792_c0_seq1 | 143 | 8,00E-57 | 72 | 86 | 82,39 | yes | 139 |
| 12 | os (sisC) | outstretched | CG5993 | comp11260_c0_seq3 | 308 | 6,00E-47 | 43 | 57 | 13,02 | yes | 411 |
| 13 | sc (sisB) | scute | CG3827 | comp3154_c0_seq1 | 73 | 2,00E-16 | 69 | 83 | 2,94 | yes | 282 |
| 14 | sisA | sisterless A | CG1641 | comp10723_c0_seq1 | 142 | 1,00E-11 | 28 | 53 | 175,56 | yes | 184 |
| 15 | Stat92E | Signal-transducerand activatoroftranscriptionprotein at 92E | CG4257 | comp10855_c0_seq2 | 770 | 0.0 | 46 | 62 | 118,69 | yes | 725 |
| 16 | Sxl | Sex lethal | CG43770 | comp11506_c0_seq8 | 278 | 9,00E-104 | 73 | 78 | 102,41 | yes | 338 |
| 17 | tra | transformer* | CG16724 | comp11901_c1_seq5 | * | * | * | * | 10,56 | yes | 429 |
| 18 | tra2 | transformer-2 | CG10128 | comp10827_c1_seq5 | 104 | 4,00E-34 | 67 | 87 | 272,94 | yes | 251 |
| 19 | vir | virilizer | CG3496 | comp11922_c0_seq8 | 973 | 0.0 | 62 | 76 | 12,38 | yes | 1432 |
| 20 | gro | groucho | CG8384 | comp9374_c0_seq1 | 733 | 0.0 | 89 | 91 | 223,55 | yes | 724 |
| 21 | Mes-4 | Mes-4 | CG4976 | comp8295_c0_seq1 | 477 | 0.0 | 65 | 79 | 4,22 | yes | 507 |
| 22 | mod(mdg4) | modifier ofmdg4 | CG32491 | comp5246_c0_seq2 | 120 | 5,00E-64 | 89 | 96 | 356,86 | yes | 463 |
| 23 | lolal | lola-like | CG5738 | comp11328_c0_seq22 | 127 | 1,00E-72 | 100 | 100 | 166,07 | yes | 127 |
| 24 | lola | Longitudinalslacking | CG12052 | comp10714_c0_seq2 | 213 | 6,00E-74 | 71 | 76 | 545,70 | yes | 990 |
| 25 | CG3726 | - | CG3726 | - | - | - | - | - | - | - | - |
We found that practically all MEET reconstructed mRNAs (19 out of 20 orthologs) contained full length ORFs. All of the identified sex determination ortholog genes were expressed with an FPKM>1 with the top expressed genes corresponding to extra macrochaetae (emc - FPKM = 703.73), longitudinals lacking (lola - FPKM = 545.70), modifier of mdg4 (mod(mdg4)) - FPKM = 356.86), tra-2 (FPKM = 272.94), groucho (gro - FPKM = 223.55) and sis-A (FPKM = 175.56).
These top expressed sex determination genes encode DNA-binding proteins involved in transcriptional activity regulation except for the tra-2 gene, which encodes an RNA-binding protein involved in alternative splicing regulation. As in C. capitata also in Drosophila the orthologs of emc, lola, modg(mdg4), groucho and sis-A are expressed at relatively high levels during this specific embryonic period of time. Only the tra-2 gene has much higher expression when in C. capitata respect to Dmtra-2 at this cellularization stage. This is quite intriguing because we know that Cctra-2, differently to Drosophila, performs novel additional function in Ceratitis being required for the Cctra female-specific splicing and autoregulation [16].
In Drosophila the sis-A gene encodes a bZIP transcription factor (basic leucine zipper). sis-A is one of the 2 potent indicators of X chromosome dose (both X-linked, of course), together with sis-B, that are required for the female-specific early transcription of the Sex-lethal gene and the establishment of its female-specific splicing regulation, as well as, the female-specific repression of dosage compensation, required on the contrary in XY individuals. This gene is expressed in Drosophila only during embryogenesis and its functions are linked to 1) the female-specific activation of Sxl, as well as 2) to endoderm migration and midgut formation in both sexes [96]. This second function is vital for both sexes while the first function is vital only for female XX embryos. In C. capitata, as in many other non Drosophilidae species, the ortholog of Sxl is not sex-specifically regulated and the encoded protein product is present in embryos of both sexes (Saccone et al., 1998). Ceratitis SIS-A shows 26% aa identity and 49% similarity to the Drosophila SIS-A and, as in Drosophila, it is expressed during embryogenesis but not at adult stages. Most likely the C. capitata ortholog is still required in both sexes, for the vital function of midgut formation, but not for female-specific CcSxl regulation, which most likely evolved only before or during Drosophilidae radiation [97].
Drosophila lola gene has multiple key functions during development of the nervous system of embryos/larvae until adulthood in both sexes and it seems to be involved indirectly to control the number of male-specific sex combs required for proper mating [98]. Furthermore, Lola protein shares with FRU the same BTB domain conferring DNA-binding abilities to both proteins. In C. capitata lola is also expressed at high levels during embryogenesis as well as in adults of both sexes, with very complex pattern of alternatively spliced isoforms (data not shown).
Extra macrochaetae participates in sensory organ patterning by antagonizing the neurogenic activity of the achaete-scute complex (AS-C), which includes sis-B (sc) involved also in early female sex determination. EMC lacks a basic DNA-binding domain but is able to form heterodimers with other HLH proteins, such as SIS-B. Both sis-B and emc seem to be involved in the X:A counting mechanism composing the Drosophila sex determination primary signal, respectively as X-linked numerator and denominator. This model has been recently challenged by a new one based only on X-linked elements (XSE; [99]. It is difficult to imagine that the emc ortholog in C. capitata and/or sis-B could play a role in sex determination, being in Drosophila upstream of Sxl, a gene not required for sex determination in non Drosophilidae species.
Drosophila gro transcripts are expressed at high levels in 2–6 hr embryos, and in adult females. In C. capitata gro is also expressed at high levels in embryos and preliminary data suggest that is expressed also in adult flies but is female-biased (6 folds; unpub. data).
Conclusions
We produced a de novo transcriptome assembly of early C. capitata embryos (MEET; Medfly Early Embryonic Transcriptome) and identified 26,319 unigenes that seem to represent accurately the complexity present in 8–10 hours AEL embryos. Furthermore, the reconstructed transcripts seem to be not far off from their real full length in vivo, as suggested by three different analyses. We compared the sequence complexity of MEET and of reassembled C. capitata ESTs (from embryos, adults and tissues), concluding that we have identified ∼20,400 novel C. capitata unigenes (78%) 11,045 of which correspond to putative protein coding genes. The MEET contains, similarly to other dipteran transcriptome datasets, a broad diversity of transcripts from genes involved in a variety of different biological processes without any notable biases towards specific categories of genes. We identified 861 putative long ncRNAs and we validated their prediction on seven selected transcripts. We have also selected twenty C. capitata embryonic unigenes from the MEET, which are orthologs of Drosophila genes involved in sex determination (either directly or indirectly). We found that 19 out of 20 orthologs contained full length ORF. This de novo assembled transcriptome dataset is valuable tools for a wide range of different applications: developmental expression analyses, reverse genetic studies by RNAi, TALENs and CRISPR/cas-9, and improving transgenic strategies to produce male-only progeny. The MEET dataset will also be useful for the embryonic gene annotation in the on going Medfly Genome Project (Handler, A., USDA, pers. comm.).
Methods
Medfly rearing
C. capitata Benakeion adult flies were reared in standard laboratory conditions at 25°C, 70% relative humidity and 12∶12 h light-dark regimen. Flies were fed with yeast/sucrose powder (1∶2). A very large cross between about 800 males and 1600 virgin females was produced to obtain a high embryonic oviposition rate per hour. Embryos were collected in transparent plastic boxes filled with distilled water for two hour-long intervals. The collected embryos were allowed to age at 25°C for eight hours to get the synchronized embryonic sample of 8–10 hours after oviposition. Embryos were collected and transferred into a GIT extraction buffer (Guanidine isothiocyanate 4M, EDTA 5mM, NaAc 0.1M, Sarkosyl 0.5%) and stored at −20°C.
RNA extraction, Illumina paired-end cDNA library construction and sequencing
We produced a cDNA library for the 8–10h embryonic sample using the NEB-Next Ultra RNA Library Prep Kit (New England Biolabs, USA) according to the manufacturer’s instructions. Briefly, high quality total RNA was extracted in GIT buffer and purified by CsCl gradient ultracentrifugation method. The integrity and purity of total RNA were assessed using Agilent Bioanalyzer, ThermoScientific NanoDrop and gel electrophoresis analyses. Poly-A+ containing mRNA was extracted from 2 µg of total RNA using oligo(dT) magnetic beads and fragmented for 2 minutes into 200–500 bp pieces using an ultrasonicator (Covaris, USA). The cleaved RNA fragments were copied into first strand cDNA using reverse transcriptase and random primers. After second strand cDNA synthesis, fragments were end repaired, a-tailed and Illumina adapters were ligated. The products were purified using AMPure XP beads (Beckman Coulter) and enriched by running PCR for 10 cycles to create the final cDNA sequencing library. The cDNA library was used for 100 bp paired-end (PE) sequencing on an Illumina Genome Analyzer IIx (Otogenetics Corporation, USA) producing 20,924,960 PE reads.
De novo transcriptome assembly
The PE reads were de novo assembled using Trinity (release 2012-10-05) [65] on the ALAN Server at the Department of Biology, University of Naples Federico II (24 cores, 192 GB of memory). Trinity was run on the PE reads (raw or filtered) with the fixed default k-mer size of 25, minimum contig length of 200, maximum length expected between fragment pairs of 500, 22 CPUs, and a butterfly HeapSpace of 20 GB. We wished to verify if a filtering step could significantly improve the quality of our assembly, in terms of N50 value and average length of assembled transcripts, and hence we tested four filtering conditions with different stringency: n°1 (filtering condition named “trim”) trimming of 5 bp at 5′ and 3′ ends (where the lowest quality bases are usually located) of each read with Galaxy on-line tool [69], n°2 (named “trimmo”) quality control by sliding window analysis and adapter contamination removal by Trimmomatic software [68], n°3 (named “qc”) quality control on whole read length and adapter contamination removal with NGS-QC-Toolkit software [67] and n°4 (named “trim+qc”) trimming as for point 1 plus quality control and adapter contamination removal with NGS-QC-Toolkit software (see Methods S1). We produced four different Trinity assemblies and compared them with the assembly obtained by Trinity using raw reads. We took into account the following parameters: number of base pairs of reads utilized in the assembly (URbp), N50 value (N50), average transcript length (AVL), number of assembled transcripts (NoTr) and total number of base pairs of the assembled transcripts (ASbp). The rationale of our test is that if one of the filtering condition improves the quality of the assembly, we expect to obtain a reduction in URbp, ASbp and NoTR values but a significant improvement of N50 and AVL parameters. As expected, the URbp value decreases (from 10% to 48%) in the four filtered assemblies respect to the URbp of the assembly with raw data as well as the NoTr value (from 8% to 20%) (see Figure S1). The ASbp value instead decreases for all the filtering condition except for the “trimmo” condition where we observed a reduction of only 1%. Conversely, the N50 and the AVL values increase for the “trimmo” assembly (5.3% and 6.2% respectively). Based on this result we decided to use for our de novo transcriptome assembly the reads filtered with the “trimmo” condition.
Prior to the assembly step we depleted the filtered reads matching with the Medfly mitochondrial DNA sequence (GenBank acc. num.: AJ242872.1) and with the ribosomal RNA gene sequences (Cc18S, 5.8S, 16S and 28S; GenBank acc. num.: AF096450.2; AF189691; AY830884.1; KC177754.1) using the Bowtie aligner with zero mismatch allowed (-v 0 parameter) [70]. Assembled transcripts were blasted against NCBI UniVec database (http://www.ncbi.nlm.nih.gov/VecScreen/UniVec.html) to identify segments with adapter contamination. Human and bacterial sequence contamination was investigated using the web-based version of DeconSeq [71], with a query coverage and a sequence identity threshold of 90%.
Functional annotation
The assembled transcripts were annotated applying the FastAnnotator annotation pipeline with default parameters [72] to investigate and summarize their functional categories. The annotation in FastAnnotator includes four main parts: finding best hits in the NCBI nr database (December 2012 update) using LAST alignment algorithm, assigning GO terms, identifying enzymes, and identifying domains. FastAnnotator runs these four steps in parallel to speed up the annotation procedure.
To perform the comparative analysis of the MEET GO terms distribution with other dipteran high-throughput embryonic transcriptomic data, we downloaded SRA file for the following species: D. melanogaster (2–16h old embryos) (SRR042295 and SRR058885); B. dorsalis (0–24h old embryos) (SRR316210); C. albipunctata (8–12h old embryos) (ERR160071); M. abdita (0–4h old embryos) (ERR196167); E. balteatus (3–6h old embryos) (SRR190625). The raw data were assembled without any filtering step and with default parameters using Trinity. Functional annotations were performed for each species, applying the FastAnnotator pipeline with default parameters.
Evaluation of coding potential
The prediction of coding potential of transcripts not annotated in our MEET data set was performed using three independent different prediction methods: the Coding Potential Calculator [88], the Portrait software [89] and the Coding Potential Assessment Tool [90]. We applied available web-tools in CPC and Portrait, while the CPAT software was installed locally (v1.2.1) using D. melanogaster training data set as a reference. In CPC a positive coding potential score indicates a protein coding potential of the respective target transcript, whereas negative values predict non-coding potential of transcripts. In Portrait the coding and non-coding potential of transcripts are expressed in percentage while in CPAT the coding probability score ranges between 0 and 1 with coding probability cut-off usually set at ≥0.5. In order to extract potential non-coding transcripts with a high reliability from our dataset, we selected very stringent thresholds for the three prediction methods as following: CPC coding potential score <−1.2, Portrait non-coding probability >95% and CPAT coding probability <0.01. Only those transcripts in accordance, at the same time, with the three very conservative cut-offs were selected as putative early embryonic non-coding transcripts.
Expression analysis and Real Time-PCR validation
We applied the RSEM software and the BOWTIE aligner, as implemented in the Trinity software package, to assign reads to genes and isoforms and to compute transcript expression levels using FPKM metric. Transcripts with FPKM value above 100 were further investigated for Gene Ontology (GO) enrichment using the R language.
We validated expression levels of ten selected genes by Real-Time qPCR. We prepared cDNA using 50 ng of the polyA+ RNA utilized for the sequencing library preparation in a 60 µl reaction using the EuroScript M-MLV Reverse Transcriptase (Euroclone, ITALY) following the manufacturer’s instructions. The primers used in qPCR reactions were designed using the Primer Express software (Applied Biosystems, USA) and are listed in Methods S2. qPCR was performed in triplicate with 1 µl of a 1∶5 cDNA dilution for each target gene using Brilliant III Ultra-Fast SYBR Green qPCR Master Mix (Agilent Technologies, USA) and a 7500 Real-Time PCR System (Applied Biosystems, USA). Cycling parameters were: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 sec at 95°C and 60 sec at 60°C, 15 sec at 95°C, 1 min at 60°C, 30 sec at 95°C, 15 sec at 60°C. We applied the Rn method (Rn = Ro of the target gene/Ro of the normalizator gene) for relative gene expression analysis and the Real-Time PCR Miner tool [100] using the Medfly superoxide dismutase gene (GenBank acc. num.: L35494.1) as normalizator gene. We then compared, by performing a Pearson Correlation test in the R language, the Rn values of the nine selected genes with their normalized FPKM values (obtained by dividing the FPKM of the target gene by the FPKM value of the SOD gene).
RT-PCR validation of non-coding RNAs
Total RNA was extracted using TRIzol (Life Technologies, USA) from adult individuals and from unfertilized eggs, embryos, larvae and pupae of C. capitata. For the 8–10h old embryonic sample the same total RNA utilized for the sequencing library preparation was used. Oligo-dT-primed cDNA was prepared from 1 µg of DNAse I-treated total RNA using the EuroScript M-MLV Reverse Transcriptase (Euroclone, ITALY) following the manufacturer’s instructions. 1/20 v/v of the synthesised cDNA was amplified by PCR at non-saturating condition as described in Salvemini et al., (2006) [101]. RT-PCR products were analysed by agarose gel electrophoresis. Primers utilized in RT-PCR developmental expression analysis are listed in Methods S2.
Supporting Information
Filtering condition test for the Trinity de novo assembly of MEET data set. Comparison of the five assemblies obtained with raw data and the four different filtering conditions (trim, trimmo, qc and trim+qc). For each assembly: the blue line indicates the number of base pairs of Reads Utilized (URbp); the red line indicates the N50 values; the green line indicates the average transcript length (AVG); the purple line indicates the total Number of assembled TRanscripts (NoTR); the azure line indicates total number of base pairs of the ASsembled transcripts (ASbp). Values for the five parameters were arbitrarily corrected with correction factors indicated in the figure to obtain a clearer comparative graph.
(TIF)
Comparison with C. capitata full-length cDNAs.
(XLS)
OHR and FLHR analyses.
(XLS)
ESTs assembly statistics.
(XLS)
FPKM values of all assembled transcripts.
(XLS)
Comparison of RNA-seq and qPCR expression levels.
(XLS)
GO enrichment analysis.
(XLS)
Non-coding RNA prediction.
(XLS)
List of software utilized for the four tested filtering conditions.
(DOC)
List of primers utilized in this study.
(DOC)
Acknowledgments
We are deeply grateful to Stefano Perrini for the support in the preliminary Trinity assembly tests and for the access to his personal server. We thank Giuseppe Cicotti for the generous help with the meetBASE website development. We thank Scott Emrich (University of Notre Dame, U.S.) for the suggestion about the concept of OHR. We are deeply grateful to Dr. Rodrigo Nunes da Fonseca and the other anonymous reviewer, which helped with suggestions and corrections. GS and MS thanks Daniel Bopp (Zurich Univ.) for his generous inputs, suggestions and editing of the manuscript.
Dedication
This work is dedicated to the memory of our colleague Javaregowda Nagaraju, who unexpectedly passed away during the last phases of the research project and the preparation of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All sequencing raw data files are available from the BioProject-NCBI database (accession number PRJNA252550).
Funding Statement
Ministero dell’Università e della Ricerca Grant to GS (PRIN2012). University of Naples Federico II/ Fondazione Compagnia di Sanpaolo Grant to MS (STAR2014). Taskforce Project Grant from Department of Biotechnology, Government of India to KPA and JN.
References
- 1. Diamantidis AD, Carey JR, Nakas CT, Papadopulos NT (2011) Population-specific demography and invasion potential in medfly. Ecol Evol 1:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li B, Ma J, Hu X, Liu H, Zhang R (2009) Potential geographical distributions of the fruit flies Ceratitis capitata, Ceratitis cosyra, and Ceratitis rosa in China. J Econ Entomol 102:1781–1790. [DOI] [PubMed] [Google Scholar]
- 3. Malacrida AR, Gasperi G, Zacharopoulou A, Torti C, Francos ER, et al. (1992) Evidence for a genetic duplication involving alcohol dehydrogenase genes in Ceratitis capitata. Biochem Genet 30:35–48. [DOI] [PubMed] [Google Scholar]
- 4. Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, et al. (2007) Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica 131:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Gomulski LM, Dimopoulos G, Xi Z, Scolari F, Gabrieli P, et al. (2012) Transcriptome profiling of sexual maturation and mating in the Mediterranean fruit fly, Ceratitis capitata. PLoS ONE 7:e30857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klassen W, Lindquist AD, Buyckx EJ (1994) Overview of the Joint FAO/IAEA Division’s involvement in fruit fly sterile insect technique programs. In Fruit flies and the Sterile Insect Technique, edited by: Calkins, O. C, Klassen, W., Liedo P. Boca Raton, FL, USA: CRC Press. pp. 3–26.
- 7. Carey RJ (2011) Biodemography of the Mediterranean fruit fly: aging, longevity and adaptation in the wild. Exp Gerontol 46:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson SA (2002) Genetic sexing strains in medfly, Ceratitis capitata, sterile insect technique programmes. Genetica 116:5–13. [DOI] [PubMed] [Google Scholar]
- 9. Dafa'alla T, Fu G, Alphey L (2010) Use of a regulatory mechanism of sex determination in pest insect control. J Genet 89:301–305. [DOI] [PubMed] [Google Scholar]
- 10. Koukidou M, Alphey L (2014) Practical applications of insects' sexual development for pest control. Sex Dev 8:127–136. [DOI] [PubMed] [Google Scholar]
- 11. Nagaraju J, Saccone G (2010) How is sex determined in insects? Preface. J Genet 89:269–270. [DOI] [PubMed] [Google Scholar]
- 12. Loukeris TG, Livadaras I, Arcà B, Zabalou S, Savakis C (1995) Gene transfer into the medfly, Ceratitis capitata, with a Drosophila hydei transposable element. Science 270:2002–2005. [DOI] [PubMed] [Google Scholar]
- 13. Saccone G, Peluso I, Artiaco D, Giordano E, Bopp D, et al. (1998) The Ceratitis capitata homologue of the Drosophila sex-determining gene sex-lethal is structurally conserved, but not sex-specifically regulated. Development 125:1495–1500. [DOI] [PubMed] [Google Scholar]
- 14. Fu G, Condon KC, Epton MJ, Gong P, Jin L, et al. (2007) Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol 25:353–357. [DOI] [PubMed] [Google Scholar]
- 15. Pane A, Salvemini M, Delli Bovi P, Polito C, Saccone G (2002) The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129:3715–3725. [DOI] [PubMed] [Google Scholar]
- 16. Salvemini M, Robertson M, Aronson B, Atkinson P, Polito LC, et al. (2009) Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int J Dev Biol 53:109–120. [DOI] [PubMed] [Google Scholar]
- 17. Zwiebel LJ, Saccone G, Zacharopoulou A, Besansky NJ, Favia G, et al. (1995) The white gene of Ceratitis capitata: a phenotypic marker for germline transformation. Science 270:2005–2008. [DOI] [PubMed] [Google Scholar]
- 18. Handler MA, Harrell AR 2nd (2001) Transformation of the Caribbean fruit fly, Anastrepha suspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem Mol Biol 31:199–205. [DOI] [PubMed] [Google Scholar]
- 19. Franz G, Savakis C (1991) Minos, a new transposable element from Drosophila hydei, is a member of the Tc1-like family of transposons. Nucleic Acids Res 19:6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ha Harris AF, Nimmo D, McKemey AR, Kelly N, Scaife S, et al. (2011) Field performance of engineered male mosquitoes. Nat Biotechnol 29:1034–1037. [DOI] [PubMed] [Google Scholar]
- 21. Lacroix R, McKemey AR, Raduan N, Kwee Wee L, Hong Ming W, et al. (2012) Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE 7:e42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrison NI, Simmons GS, Fu G, O'Connell S, Walker AS, et al. (2012) Engineered repressible lethality for controlling the pink bollworm, a lepidopteran pest of cotton. PLoS ONE 7:e50922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papathanos PA, Bossin HC, Benedict MQ, Catteruccia F, Malcolm CA, et al. (2009) Sex separation strategies: past experience and new approaches. Malar J 8 Suppl 2S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wimmer AE (2013) Insect biotechnology: controllable replacement of disease vectors. Curr Biol 23:R453–456. [DOI] [PubMed] [Google Scholar]
- 25. Hokanson KE, Dawson WO, Handler AM, Schetelig MF, St Leger RJ (2013) Not all GMOs are crop plants: non-plant GMO applications in agriculture. Transgenic Res. 2014 Dec 23(6):1057–68. [DOI] [PubMed] [Google Scholar]
- 26. Alphey L (2014) Genetic control of mosquitoes. Annu Rev Entomol 59:205–224. [DOI] [PubMed] [Google Scholar]
- 27. Gomulski LM1, Dimopoulos G, Xi Z, Soares MB, Bonaldo MF, et al (2008) Gene discovery in an invasive tephritid model pest species, the Mediterranean fruit fly, Ceratitis capitata. Bmc Genomics 9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scolari F, Gomulski LM, Ribeiro JM, Siciliano P, Meraldi A, et al. (2012) Transcriptional profiles of mating-responsive genes from testes and male accessory glands of the Mediterranean fruit fly, Ceratitis capitata. PLoS ONE 7:e46812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siciliano P, Scolari F, Gomulski LM1, Falchetto M, Manni M, et al (2014) Sniffing out chemosensory genes from the Mediterranean fruit fly, Ceratitis capitata. PLoS ONE 9:e85523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nirmala X, Schetelig FM, Yu F, Handler AM (2013) An EST database of the Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae). Gene 517:212–217. [DOI] [PubMed] [Google Scholar]
- 31. Zheng W, Peng T, He W, Zhang H (2012) High-throughput sequencing to reveal genes involved in reproduction and development in Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 7:e36463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schetelig MF, Caceres C, Zacharopoulou A, Franz G, Wimmer EA (2009) Conditional embryonic lethality to improve the sterile insect technique in Ceratitis capitata (Diptera: Tephritidae). BMC Biol 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akbari OS, Papathanos PA, Sandler JE, Kennedy K, Hay BA (2014) Identification of germline transcriptional regulatory elements in Aedes aegypti. Sci Rep 4:3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogaugwu EC, Schetelig FM, Wimmer EA (2013) Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochem Mol Biol 43:1–8. [DOI] [PubMed] [Google Scholar]
- 35. Metzker LM (2010) Sequencing technologies - the next generation. Nat Rev Genet 11:31–46. [DOI] [PubMed] [Google Scholar]
- 36. Zhou J, Xu B (2010) Recent patents of nanopore DNA sequencing technology: progress and challenges. Recent Pat DNA Gene Seq 4:192–201. [DOI] [PubMed] [Google Scholar]
- 37. Ewen-Campen B, Shaner N, Panfilio KA, Suzuki Y, Roth S, et al. (2011) The maternal and early embryonic transcriptome of the milkweed bug Oncopeltus fasciatus. Bmc Genomics 12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiménez-Guri E, Huerta-Cepas J, Cozzuto L, Wotton KR, Kang H, et al. (2013) Comparative transcriptomics of early dipteran development. Bmc Genomics 14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vicoso B, Bachtrog D (2013) Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499:332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bachtrog D (2013) Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat Rev Genet 14:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heffer A, Pick L (2013) Conservation and variation in Hox genes: how insect models pioneered the evo-devo field. Annu Rev Entomol 58:161–179. [DOI] [PubMed] [Google Scholar]
- 42. Peel DA (2008) The evolution of developmental gene networks: lessons from comparative studies on holometabolous insects. Philos Trans R Soc Lond B Biol Sci 363:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans JD, Brown SJ, Hackett KJ, Robinson G, Richards S, et al. (2013) The i5K Initiative: advancing arthropod genomics for knowledge, human health, agriculture, and the environment. J Hered 104:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crisanti A (2013) INFRAVEC: research capacity for the implementation of genetic control of mosquitoes. Pathog Glob Health 107:458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Criscione F, Qi Y, Saunders R, Hall B, Tu Z (2013) A unique Y gene in the Asian malaria mosquito Anopheles stephensi encodes a small lysine-rich protein and is transcribed at the onset of embryonic development. Insect Mol Biol 22:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schetelig MF1, Scolari F, Handler AM, Kittelmann S, Gasperi G, et al (2009) Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc Natl Acad Sci U S A 106:18171–18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Catteruccia F, Crisanti A, Wimmer EA (2009) Transgenic technologies to induce sterility. Malar J 8 Suppl 2S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-Barnetche J, Gómez-Barreto RE, Ovilla-Muñoz M, Téllez-Sosa J, García López DE, et al. (2012) Transcriptome of the adult female malaria mosquito vector Anopheles albimanus. Bmc Genomics 13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Topalis P, Mitraka E, Dritsou V, Dialynas E, Louis C (2013) IDOMAL: the malaria ontology revisited. J Biomed Semantics 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burghardt G, Hediger M, Siegenthaler C, Moser M, Dübendorfer A, et al. (2005) The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Dev Genes Evol 215:165–176. [DOI] [PubMed] [Google Scholar]
- 51. Lagos D, Koukidou M, Savakis C, Komitopoulou K (2007) The transformer gene in Bactrocera oleae: the genetic switch that determines its sex fate. Insect Mol Biol 16:221–230. [DOI] [PubMed] [Google Scholar]
- 52. Schetelig MF, Milano A, Saccone G, Handler AM (2012) Male only progeny in Anastrepha suspensa by RNAi-induced sex reversion of chromosomal females. Insect Biochem Mol Biol 42:51–57. [DOI] [PubMed] [Google Scholar]
- 53. Hediger M, Henggeler C, Meier N, Perez R, Saccone G, et al. (2010) Molecular characterization of the key switch f provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics 184:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saccone G, Salvemini M, Polito LC (2011) The transformer gene of Ceratitis capitata: a paradigm for a conserved epigenetic master regulator of sex determination in insects. Genetica 139:99–111. [DOI] [PubMed] [Google Scholar]
- 55. Gabrieli P, Falaguerra A, Siciliano P, Gomulski LM, Scolari F, et al. (2010) Sex and the single embryo: early development in the Mediterranean fruit fly, Ceratitis capitata. BMC Dev Biol 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cline WT (1978) Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90:683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, et al. (2014) A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 509:633–636. [DOI] [PubMed] [Google Scholar]
- 58. Shukla NJ, Palli RS (2012) Doublesex target genes in the red flour beetle, Tribolium castaneum. Sci Rep 2:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gempe T, Hasselmann M, Schiøtt M, Hause G, Otte M, et al. (2009) Sex determination in honeybees: two separate mechanisms induce and maintain the female pathway. PLoS Biol 7:e1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Verhulst EC, Beukeboom LW, van de Zande L (2010) Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 328:620–623. [DOI] [PubMed] [Google Scholar]
- 61. Verhulst EC, Lynch JA, Bopp D, Beukeboom LW, van de Zande L (2013) A new component of the Nasonia sex determining cascade is maternally silenced and regulates transformer expression. PLoS ONE 8:e63618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hall AB1, Qi Y, Timoshevskiy V, Sharakhova MV, Sharakhov IV, et al (2013) Six novel Y chromosome genes in Anopheles mosquitoes discovered by independently sequencing males and females. Bmc Genomics 14:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arunkumar PK, Mita K, Nagaraju J (2009) The silkworm Z chromosome is enriched in testis-specific genes. Genetics 182:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carvalho BA, Clark GA (2013) Efficient identification of Y chromosome sequences in the human and Drosophila genomes. Genome Res 23:1894–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel KR, Jain M (2012) NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 7. [DOI] [PMC free article] [PubMed]
- 68.Bolger MA, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. [DOI] [PMC free article] [PubMed]
- 69. Blankenberg D, Gordon A, Von Kuster G, Coraor N, Taylor J, et al. (2010) Manipulation of FASTQ data with Galaxy. Bioinformatics 26:1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmieder R, Edwards R (2011) Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS ONE 6:e17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen TW, Gan RC, Wu TH, Huang PJ, Lee CY, et al. (2012) FastAnnotator–an efficient transcript annotation web tool. Bmc Genomics 13 Suppl 7S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marchini D, Manetti AG, Rosetto M, Bernini LF, Telford JL, et al. (1995) cDNA sequence and expression of the ceratotoxin gene encoding an antibacterial sex-specific peptide from the medfly Ceratitis capitata (diptera). J Biol Chem 270:6199–6204. [DOI] [PubMed] [Google Scholar]
- 74. Vannini L, Ciolfi S, Dallai R, Frati F, Hoffmann KH, et al. (2010) Putative-farnesoic acid O-methyltransferase (FAMeT) in medfly reproduction. Arch Insect Biochem Physiol 75:92–106. [DOI] [PubMed] [Google Scholar]
- 75. Intra J, Perotti EM, Pasini ME (2011) Cloning, sequence identification and expression profile analysis of alpha-L-fucosidase gene from the Mediterranean fruit fly Ceratitis capitata. J Insect Physiol 57:452–461. [DOI] [PubMed] [Google Scholar]
- 76. O'Neil ST, Dzurisin JD, Carmichael RD, Lobo NF, Emrich SJ, et al. (2010) Population-level transcriptome sequencing of nonmodel organisms Erynnis propertius and Papilio zelicaon. Bmc Genomics 11:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Beverley MS, Wilson CA (1984) Molecular evolution in Drosophila and the higher Diptera II. A time scale for fly evolution. J Mol Evol 21:1–13. [DOI] [PubMed] [Google Scholar]
- 78. Zheng Y, Zhao L, Gao J, Fei Z (2011) iAssembler: a package for de novo assembly of Roche-454/Sanger transcriptome sequences. BMC Bioinformatics 12:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Daines B, Wang H, Wang L, Li Y, Han Y, et al. (2011) The Drosophila melanogaster transcriptome by paired-end RNA sequencing. Genome Res 21:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shen GM, Dou W, Niu JZ, Jiang HB, Yang WJ, et al. (2011) Transcriptome analysis of the oriental fruit fly (Bactrocera dorsalis). PLoS ONE 6:e29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lemke S, Antonopoulos DA, Meyer F, Domanus MH, Schmidt-Ott U (2011) BMP signaling components in embryonic transcriptomes of the hover fly Episyrphus balteatus (Syrphidae). Bmc Genomics 12:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Davis GK, Patel NH (2002) Short, long, and beyond: molecular and embryological approaches to insect segmentation. Annu Rev Entomol 47:669–699. [DOI] [PubMed] [Google Scholar]
- 83. Li B, Dewey NC (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628. [DOI] [PubMed] [Google Scholar]
- 85. Bopp D, Saccone G, Beye M. (2014) Sex determination in insects: variations on a common theme. Sex Dev 8:20–28. [DOI] [PubMed] [Google Scholar]
- 86. Li Z, Liu M, Zhang L, Zhang W, Gao G, et al. (2009) Detection of intergenic non-coding RNAs expressed in the main developmental stages in Drosophila melanogaster. Nucleic Acids Res 37:4308–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gao G, Vibranovski MD, Zhang L, Li Z, Liu M, et al. (2014) A long-term demasculinization of X-linked intergenic noncoding RNAs in Drosophila melanogaster. Genome Res 24:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, et al. (2007) CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res 35:W345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arrial RT, Togawa RC, Brigido Mde M (2009) Screening non-coding RNAs in transcriptomes from neglected species using PORTRAIT: case study of the pathogenic fungus Paracoccidioides brasiliensis. BMC Bioinformatics 10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang L, Park HJ, Dasari S, Wang S, Kocher JP, et al. (2013) CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res 41:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Niazi F, Valadkhan S. (2012) Computational analysis of functional long noncoding RNAs reveals lack of peptide-coding capacity and parallels with 3′ UTRs. Rna 18:825–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tomancak P, Beaton A, Weiszmann R, Kwan E, Shu S, et al. (2002) Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol 3:RESEARCH0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weiszmann R, Hammonds AS, Celniker SE (2009) Determination of gene expression patterns using high-throughput RNA in situ hybridization to whole-mount Drosophila embryos. Nat Protoc 4:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Frise E, Hammonds AS, Celniker SE (2010) Systematic image-driven analysis of the spatial Drosophila embryonic expression landscape. Mol Syst Biol 6:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Salvemini M, Polito C, Saccone G (2010) fruitless alternative splicing and sex behaviour in insects: an ancient and unforgettable love story? J Genet 89:287–299. [DOI] [PubMed] [Google Scholar]
- 96. Walker JJ, Lee KK, Desai RN, Erickson JW (2000) The Drosophila melanogaster sex determination gene sisA is required in yolk nuclei for midgut formation. Genetics 155(1):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bopp D, Calhoun G, Horabin JI, Samuels M, Schedl P (1996) Sex-specific control of Sex-lethal is a conserved mechanism for sex determination in the genus Drosophila. Development 122(3):971–982. [DOI] [PubMed] [Google Scholar]
- 98. Spletter ML, Liu J, Liu J, Su H, Giniger E, et al. (2007) Lola regulates Drosophila olfactory projection neuron identity and targeting specificity. Neural Dev 16 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Erickson JW, Quintero JJ (2007) Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol 5(12):e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhao S, Fernald RD (2005) Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 12:1047–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Salvemini M, Mauro U, Velaeti S, Polito C, Saccone G (2006) A new Minos vector for eye-specific expression of white+ marker in Ceratitis capitata and in distantly related dipteran species. Insect Mol Biol 15:341–349. [DOI] [PubMed] [Google Scholar]
- 102. Wiegmann BM, Trautwein MD, Winkler IS, Barr NB, Kim JW, et al. (2011) Episodic radiation in the fly tree of life. PNAS 108:5690–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Filtering condition test for the Trinity de novo assembly of MEET data set. Comparison of the five assemblies obtained with raw data and the four different filtering conditions (trim, trimmo, qc and trim+qc). For each assembly: the blue line indicates the number of base pairs of Reads Utilized (URbp); the red line indicates the N50 values; the green line indicates the average transcript length (AVG); the purple line indicates the total Number of assembled TRanscripts (NoTR); the azure line indicates total number of base pairs of the ASsembled transcripts (ASbp). Values for the five parameters were arbitrarily corrected with correction factors indicated in the figure to obtain a clearer comparative graph.
(TIF)
Comparison with C. capitata full-length cDNAs.
(XLS)
OHR and FLHR analyses.
(XLS)
ESTs assembly statistics.
(XLS)
FPKM values of all assembled transcripts.
(XLS)
Comparison of RNA-seq and qPCR expression levels.
(XLS)
GO enrichment analysis.
(XLS)
Non-coding RNA prediction.
(XLS)
List of software utilized for the four tested filtering conditions.
(DOC)
List of primers utilized in this study.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All sequencing raw data files are available from the BioProject-NCBI database (accession number PRJNA252550).