Abstract
Antisera specific for purified cell walls of Fusarium solani f. sp. pisi and phaseoli and of shrimp shell chitosan were utilized as immunochemical probes to determine the location of fungal components in the pea-Fusarium interaction.
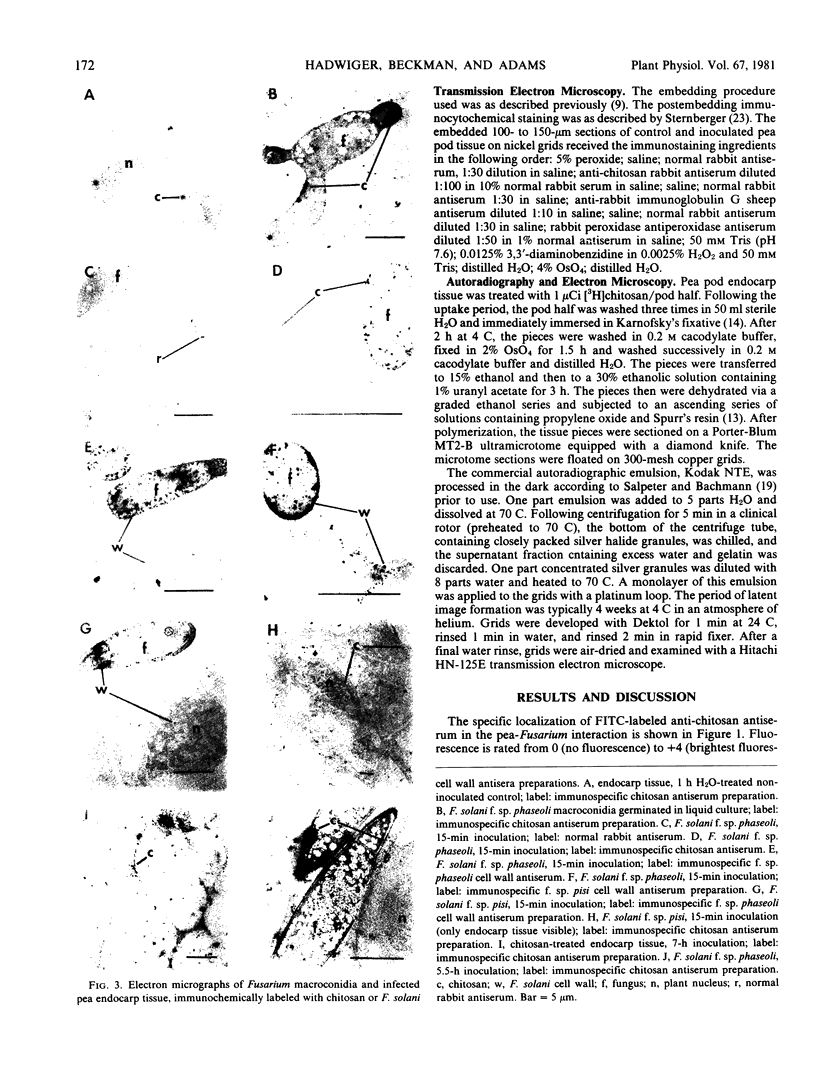
Within 15 minutes after inoculation, fungal cell wall components appear to enter the plant cell and to accumulate inside the plant cell wall as fungal growth on the plant tissue is inhibited. The accumulation patterns of chitosan and all components containing hexosamine polymers resembled those of the fungal wall components.
Chitosan is present on, and is released from, the outer surface of the fungal spore. Within 15 minutes after applying [3H]chitosan to the surface of the plant tissue, the label is readily detectable within the plant cytoplasm and conspicuously detectable within the plant nucleus. It is proposed that the potential for transport of chitosan between the spores of Fusarium solani and pea cells, in addition to its potential to inhibit fungal growth and elicit disease resistance responses, suggests chitosan has a major regulatory role in this host-parasite interaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Browder S. K., Beevers L. Incorporation of [C]Glucosamine and [C]Mannose into Glycolipids and Glycoproteins in Cotyledons of Pisum sativum L. Plant Physiol. 1980 May;65(5):924–930. doi: 10.1104/pp.65.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Wessels J. G., van den Ende H. The hyphal wall of Mucor mucedo. 2. Hexosamine-containing polymers. Eur J Biochem. 1977 Nov 1;80(2):621–626. doi: 10.1111/j.1432-1033.1977.tb11919.x. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A. Induction of phenylalanine ammonia lyase and pisatin by photosensitive psoralen compounds. Plant Physiol. 1972 May;49(5):779–782. doi: 10.1104/pp.49.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Jafri A., von Broembsen S., Eddy R. Mode of Pisatin Induction: Increased Template Activity and Dye-binding Capacity of Chromatin Isolated from Polypeptide-treated Pea Pods. Plant Physiol. 1974 Jan;53(1):52–63. doi: 10.1104/pp.53.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Schwochau M. E. Specificity of deoxyribonucleic Acid intercalating compounds in the control of phenylalanine ammonia lyase and pisatin levels. Plant Physiol. 1971 Mar;47(3):346–351. doi: 10.1104/pp.47.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALPETER M. M., BACHMANN L. AUTORADIOGRAPHY WITH THE ELECTRON MICROSCOPE. A PROCEDURE FOR IMPROVING RESOLUTION, SENSITIVITY, AND CONTRAST. J Cell Biol. 1964 Aug;22:469–477. doi: 10.1083/jcb.22.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander C., Hadwiger L. A. L-Phenylalanine ammonia-lyase and pisatin induction by 5-bromodeoxyuridine in Pisum sativum. Biochim Biophys Acta. 1979 Jul 26;563(2):278–292. doi: 10.1016/0005-2787(79)90047-9. [DOI] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Teasdale J., Daniels D., Davis W. C., Eddy R., Hadwiger L. A. Physiological and Cytological Similarities between Disease Resistance and Cellular Incompatibility Responses. Plant Physiol. 1974 Nov;54(5):690–695. doi: 10.1104/pp.54.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Kinoshita T., Hoshino M. Analytical chemical studies on amino sugars. II. Determination of hexosamines using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Chem Pharm Bull (Tokyo) 1969 Jul;17(7):1505–1510. doi: 10.1248/cpb.17.1505. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]