Abstract
Hydrogen sulfide (H2S) is a gaseous mediator synthesized in mammalian tissues by three main enzymes—cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate-sulfurtransferase—and its levels increase under inflammatory conditions or sepsis. Since H2S and H2S-releasing molecules afford inhibitory properties in leukocyte trafficking, we tested whether endogenous annexin A1 (AnxA1), a glucocorticoid-regulated inhibitor of inflammation acting through formylated-peptide receptor 2 (ALX), could display intermediary functions in the anti-inflammatory profile of H2S. We first investigated whether endogenous AnxA1 could modulate H2S biosynthesis. To this end, a marked increase in CBS and/or CSE gene products was quantified by quantitative real-time polymerase chain reaction in aortas, kidneys, and spleens collected from AnxA1−/− mice, as compared with wild-type animals. When lipopolysaccharide-stimulated bone marrow-derived macrophages were studied, H2S-donor sodium hydrosulfide (NaHS) counteracted the increased expression of inducible nitric oxide synthase and cyclooxygenase 2 mRNA evoked by the endotoxin, yet it was inactive in macrophages harvested from AnxA1−/− mice. Next we studied the effect of in vivo administration of NaHS in a model of interleukin-1β (IL-1β)–induced mesenteric inflammation. AnxA1+/+ mice treated with NaHS (100 μmol/kg) displayed inhibition of IL-1β-induced leukocyte adhesion/emigration in the inflamed microcirculation, not observed in AnxA1−/− animals. These results were translated by testing human neutrophils, where NaHS (10–100 μM) prompted an intense mobilization (>50%) of AnxA1 from cytosol to cell surface, an event associated with inhibition of cell/endothelium interaction under flow. Taken together, these data strongly indicate the existence of a positive interlink between AnxA1 and H2S pathway, with nonredundant functions in the control of experimental inflammation.
Introduction
Inflammatory process represents a complex network of events in the host response to infections and insults. This network is tightly regulated by engagement of a series of lipid mediators, cytokines, and adhesion molecules promoting leukocyte extravasation, immune cell trafficking, and removal of pathogens (Nathan, 2002; Nathan and Ding, 2010). Such a chain of events is somehow paralleled by release/biosynthesis of different mediators that stop cell trafficking, promote apoptosis and effer-ocytosis of extravasated cells, and lead to tissue repair (Serhan, 2011), known as effectors of resolution. The latter events are temporally and spatially regulated within the inflamed tissue site, in line with the appreciation that “the beginning programs the end” (Serhan and Savill, 2005). Annexin A1 (AnxA1), a 37-kDa calcium-binding protein, is involved in the resolution process together with other mediators regulating the proresolving response, such as lipoxin A4 (LXA4) or resolvins (Serhan, 2007). In particular, AnxA1 and LXA4 are prototypes of proresolving mediators in view of their nature (protein versus lipid), mode of production/release (cytoplasmic pool mobilization versus enzymatic biosynthesis), and engagement by widely used therapeutics (glucocorticoids for AnxA1 versus aspirin for LXA4). Interestingly, these two mediators share the activation of the same G protein–coupled receptor—namely, formyl-peptide receptor 2 (ALX)—through which they exert anti-inflammatory actions (Perretti et al., 2002; Brancaleone et al., 2011). AnxA1 is very abundant in human polymorphonuclear cells (PMNs), representing between 2 and 4% of total intracellular proteins (Rosales and Ernst, 1997). In resting PMNs, a proportion (~60%) of intracellular AnxA1 is localized in gelatinase and azurophilic granules (Perretti et al., 2000; Lominadze et al., 2005), with the remaining stored as a “free” cytosolic pool. PMN activation triggers a rapid mobilization of AnxA1 and its exposure to the cell surface, which in turn curtails the extent of neutrophil trafficking leading to a reduction of inflammation (Gastardelo et al., 2009). In particular, AnxA1 also exerts its effect by provoking the detachment of leukocytes from the vessel wall when administered during inflammation (Gavins et al., 2003; Brancaleone et al., 2011).
Among the wide range of signaling mediators that regulate organ and cell function, including inflammatory processes, hydrogen sulfide (H2S) represents a novel gasotransmitter. It is mainly synthesized by pyridoxal phosphate–dependent enzymes cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) from physiologic precursor l-cysteine (Stipanuk, 2004). Conversely, 3-mercaptopyruvate sulfurtransferase is a zinc-dependent enzyme mainly localized in mitochondria that also accounts for hydrogen sulfide biosynthesis (Shibuya et al., 2009).
Recent findings support involvement of hydrogen sulfide in maintenance of cardiovascular homoeostasis, as it exhibits vasodilator activity both in vitro and in vivo (Zhong et al., 2003; Ali et al., 2006; Qu et al., 2006) and modulates apoptosis in vascular smooth muscle cells (Zhao et al., 2001; Yang et al., 2004). Although hydrogen sulfide functions in the cardiovascular system have been widely studied, and diverse mechanisms have been addressed, there is still a lack of univocal information on its role in inflammation. Indeed, administration of hydrogen sulfide donors mitigates leukocyte-endothelium interaction in the inflamed microcirculation (Zanardo et al., 2006; Zhang et al., 2007b) and promotes tissue healing (Wallace et al., 2007, 2009), although there are reports of hydrogen sulfide increasing in septic shock conditions and promoting organ injury in endotoxemia (Hui et al., 2003; Collin et al., 2005). Consistently, lipopolysaccharide (LPS)-induced inflammation displays an increase in hydrogen sulfide levels and CSE expression, which is reverted by glucocorticoids (Zhu et al., 2010). Although recent literature seems to address hydrogen sulfide as a proresolutive mediator (Wallace et al., 2012), scientific evidence does not unambiguously clarify whether hydrogen sulfide biosynthesis is elevated to drive the inflammatory response or to limit tissue inflammation (Bhatia et al., 2005; Zanardo et al., 2006; Sivarajah et al., 2009; di Villa Bianca et al., 2010; Whiteman and Winyard, 2011). Here, we addressed this aspect by testing whether hydrogen sulfide modulates vascular inflammatory processes and determining whether the AnxA1 pathway might display intermediary functions in its signaling.
Materials and Methods
Animals
Male AnxA1+/+ or AnxA1−/− littermate mice (C57BL/6 background, 3–4 weeks old) were maintained on a standard chow pellet diet and had free access to water, with a 12-hour light-dark cycle. Animals were used according to the guidelines laid down by the Ethical Committee for the Use of Animals, Barts and The London School of Medicine on the basis of Animal Research: Reporting In Vivo Experiments protocol. Animal work was performed according to Home Office Regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986).
Bone Marrow–Derived Macrophage Preparation
Bone marrow–derived macrophages (BMDMs) were obtained from femurs and tibias of 4–6-week-old AnxA1+/+ or AnxA1−/− mice, using three or more mice per genotype. The marrow was flushed from the bone; washed; resuspended (2–3 × 106 cells/ml) in Dulbecco’s modified Eagle’s medium supplemented with l-glutamine, penicillin/streptomycin (Lonza Biologics, Slough, UK), 20% fetal calf serum, and 30% L929 conditioned medium; and incubated at 37°C over 5 days. Macrophages were treated with Escherichia coli LPS (0111:B4, 100 ng/ml, 6 hours; Sigma-Aldrich, St. Louis, MO) alone or in the presence of sodium hydrosulfide (NaHS; 100 μM, 1 hour before LPS). Cells were then collected and used for RNA extraction and real-time polymerase chain reaction (PCR) analysis.
Hydrogen Sulfide Quantification Assay
H2S determination in plasma samples was performed as previously described (d’Emmanuele di Villa Bianca et al., 2013). In brief, samples (200 μl) were added to Eppendorf tubes (Hamburg, Germany) containing trichloroacetic acid (10%, 300 μl) to allow protein precipitation. Supernatant was collected after centrifugation, and zinc acetate (1%, 150 μl) was then added. Subsequently, N,N-diphenylenediamine (20 mM, 100 μl) in 7.2 M HCl and iron chloride (III) (30 mM, 133 μl) in 1.2 M HCl were added to the reaction mixture, and absorbance was measured after 20 minutes at a wavelength of 668 nm. All samples were assayed in duplicate, and H2S concentration was calculated against a calibration curve of NaHS (3.12–250 μM).
Quantitative Real-Time PCR
Quantization of the expression level of selected genes [CBS, CSE, cyclooxygenase 2 (COX2), inducible nitric oxide synthase (iNOS)] was performed by quantitative real-time PCR. Total RNA was isolated from liver, aorta, kidney, and spleen collected from AnxA1+/+ or AnxA1−/− mice using TRIzol reagent (Invitrogen, Carlsbad, CA). For real-time PCR, a 10-ng template was used in combination with each primer solution (Qiagen, Hilden, Germany) and Fast SYBR Green Master Mix solution (Applied Biosystems, Paisley, UK). All reactions were performed in a 7900HT Fast Real-Time PCR System instrument (Applied Biosystems). Relative expression (versus housekeeping gene glyceraldehyde-3-phosphate dehydrogenase) was reported in graphs as relative quantity (n = 3).
Intravital Microscopy in Mouse Mesenteric Microcirculation
Intravital microscopy was performed as previously reported (Gavins et al., 2003; Leoni et al., 2008). Mice were treated with interleukin-1β (IL-1β; 10 ng/mouse i.p., 2 hours; eBioscience, Hatfield, UK) alone or in combination with NaHS (100 μmol/kg s.c., 1 hour before IL-1β injection; Sigma-Aldrich), l-cysteine (1000 μmol/kg s.c., 1 hour before IL-1β injection; Sigma-Aldrich), or d,l-propargylglycine (PAG; 10 mg/kg i.p., 30 minutes before IL-1β injection; Sigma-Aldrich). In another set of experiments, NaHS was given at the same time as IL-1β or 1 hour after IL-1β injection. In all cases, AnxA1−/− or wild-type mice were anesthetized and placed in the supine position on a heating pad (37°C). A cautery incision was made along the abdominal region, and the vascular bed was exposed and positioned under the microscope while superfused with thermostated (37°C) bicarbonate-buffered solution gassed with 5% CO2/95% N2 at a rate of 2 ml/min; recording started after a 5-minute equilibration period, followed by offline analyses, as previously reported (Gavins et al., 2003). Such analyses were done in one to three randomly selected postcapillary venules (diameter, 20–40 μm; visible length >100 μm) for each mouse. Thus, rolling leukocyte flux was obtained by the number of leukocytes passing a reference point in the venule per minute (cells/min); leukocyte adhesion reflected cells stationary for 30 seconds or longer; leukocyte emigration was calculated by the number of cells in a 100 × 50-μm2 area on both sides of the 100-μm vessel segment. For all vessels, red blood cell centerline velocity was measured with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, Dallas, TX), and venular wall shear rate was determined based on the Newtonian definition: wall shear rate = 8000 [(red blood cell velocity/1.6)/venular diameter].
Human PMN Isolation and Membrane/Cytosolic Fractions Preparation
Peripheral blood was collected from healthy male volunteers by intravenous withdrawal in 3.2% sodium citrate solution (1:10). PMNs were isolated from blood by density centrifugation on a Histopaque 1119/1077 gradient (Sigma-Aldrich, Poole, UK) according to the manufacturer’s instructions, and were suspended in phosphate-buffered saline containing 0.5% bovine serum albumin. All healthy volunteers gave oral and written consent, and cell separation was covered by ethical approval 05/Q0603/34 (East London and The City Research Ethics Committee 1). Cells (3 × 106) from four different male volunteers were treated with NaHS (10–100 μM) or rolipram (1–100 μM; Tocris, Bristol, UK) for 30 minutes. After incubation, PMNs were centrifuged to separate cell pellet from supernatant, and cytosolic and membrane fractions were obtained for western blot analysis. In a separate experiment, PMNs were also screened for cAMP levels upon NaHS and/or rolipram administration (10 or 100 μM). In another set of experiments, PMNs were therefore treated with N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) (0.1 μM, 30 minutes; Sigma-Aldrich) in the presence of vehicle, NaHS (1–100 μM, 10 minutes before fMLP), or l-cysteine (1–100 μM, 10 minutes before fMLP), and cells were then analyzed to check CD11b surface expression by flow cytometry. After treatment, cells (4 × 106) were resuspended in lysis buffer and processed. Lysates were subsequently centrifuged for 2 minutes at 300g, and supernatants were centrifuged again for 45 minutes at 800g. The resultant supernatant (cytosolic fraction) was collected, and the remaining pellet resuspended in lysis buffer supplemented with 1% Triton X-100 for 15 minutes (membrane fraction) (Vong et al., 2007).
Western Blot Analysis
Samples boiled in 6× Laemmli buffer were subjected to standard SDS-PAGE (12%) and electrophoretically blotted onto polyvinylidene difluoride membranes (Millipore, Watford, UK). Membranes were incubated with mouse monoclonal antibodies antihuman AnxA1 (clone 1B; dilution 1:1000) (Pepinsky et al., 1990) in Tris-buffer saline solution containing 0.1% Tween-20 and 5% (w/v) nonfat dry milk overnight at 4°C. Membranes were washed for 30 minutes with Tris-buffer saline solution containing 0.1% Tween-20, with the solution being changed at 10-minute intervals; membranes were then incubated with secondary antibody (horseradish peroxidase–conjugated goat anti-mouse 1:5000; Dako, Cambridge, UK), for 2 hours at room temperature. Proteins were then detected using the enhanced chemiluminescence detection kit and visualized on Hyperfilm (Amersham Biosciences, Amersham, UK).
Cyclic AMP Determination
Samples collected after NaHS and/or rolipram treatment were processed according to the manufacturer’s instructions to measure intracellular cAMP levels (Cayman, Ann Arbor, MI).
Flow Cytometry
Human PMN flow cytometry was performed to quantify the extent of CD11b expression on neutrophil as a marker of cell activation. Following staining with anti-CD11b (clone CBRM1/5; eBioscience), cell pellet was washed and fixed before sample analysis with a FACScalibur flow cytometer (BD Bioscience, Oxford, UK), acquiring >10,000 events. Results are reported as mean fluorescence intensity units of CD11b-positive events.
Data Analysis
All values are given as the mean ± S.E.M., with n indicating the number of animals for each experiment or the number of times the experiments were repeated. Statistical differences were determined by Student’s t test or repeated-measurement analysis of variance followed by the Dunnett’s post-test. P < 0.05 was considered to be significant. Analysis of the data and plotting of the figures were aided by GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA).
Results
Differential Levels of CBS/CSE mRNA and Hydrogen Sulfide in AnxA1−/− Mice
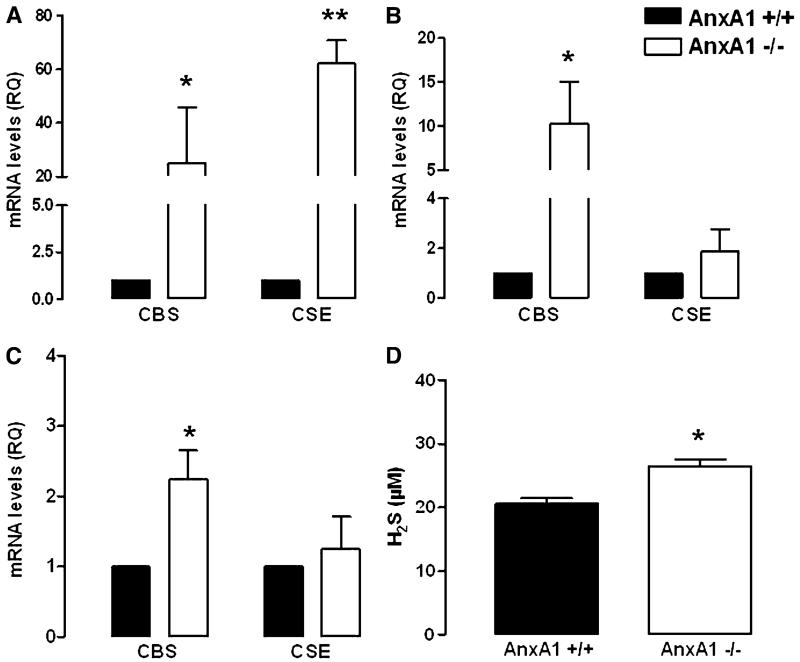
We first analyzed different tissues harvested from AnxA1−/− and AnxA1+/+ mice and compared their CBS and CSE mRNA content. In particular, we found an increase in mRNA expression for both CBS and CSE genes in the aorta (Fig. 1A), whereas only CBS was upregulated in the spleen and kidney (Fig. 1, B and C). No changes in mRNA levels were measured in liver samples (Supplemental Fig. 1).
Fig. 1.
Expression of mRNA levels in different tissues harvested from AnxA1+/+ and AnxA1−/− mice. mRNA levels expressed as relative quantity (RQ) in aorta (A), spleen (B), and kidney (C), respectively. Plasma H2S levels in AnxA1+/+ and AnxA1−/− mice (D). Statistical analysis was made using Student’s t test (*P < 0.05 versus AnxA1+/+; **P < 0.01 versus AnxA1+/+; n = 6).
Next, we evaluated circulating hydrogen sulfide levels in both AnxA1+/+ and AnxA1−/− mice. Our data revealed that H2S plasma levels were higher in AnxA1−/− compared with AnxA1+/+ mice (Fig. 1D), as a plausible outcome for higher CBS/CSE mRNA expression.
Hydrogen Sulfide Reverts LPS-Induced Inflammation in BMDMs
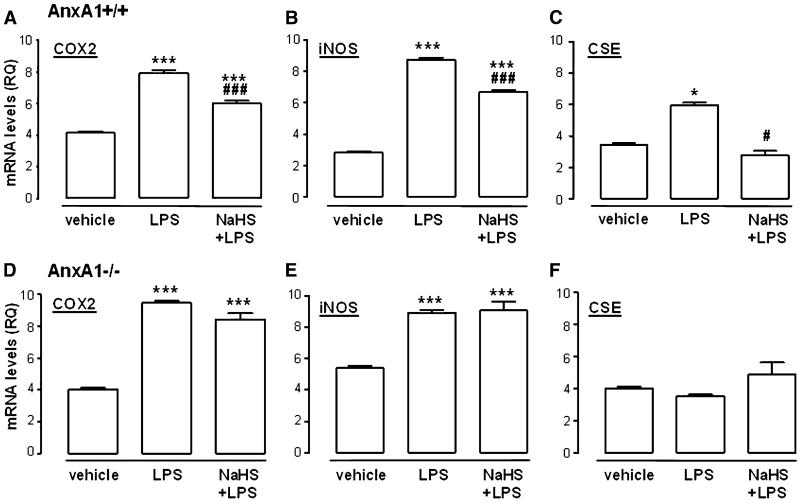
Based on differences in CBS/CSE expression and H2S levels observed in AnxA1−/− compared with AnxA1+/+ mice, we decided to use macrophages derived from bone marrow to investigate a possible anti-inflammatory effect mediated by hydrogen sulfide in this setting. LPS administration to cells derived from both AnxA1+/+ or AnxA1−/− mice showed a significant increase in COX2 (Fig. 2, A and D) and iNOS (Fig. 2, B and E) mRNA levels, used as established markers for inflammation. Furthermore, LPS treatment also increased CSE expression, in line with current literature (Fig. 2C) (Collin et al., 2005; Zhu et al., 2010). However, this effect was only observed in AnxA1+/+-derived macrophages. Exposure of the cells to exogenous hydrogen sulfide (100 μM NaHS) 1 hour prior to LPS administration significantly dampened the increase of COX2, iNOS, and CSE mRNA induced by LPS in AnxA1+/+ (Fig. 2, A–C), whereas administration of NaHS alone to unstimulated BMDMs was unappreciable in any of the gene products studied (Supplemental Fig. 2). Furthermore, opposite to AnxA1+/+ cells, administration of hydrogen sulfide in AnxA1−/−-derived macrophages did not affect COX2, iNOS, or CSE mRNA levels (Fig. 2, D–F). These data implicate AnxA1 in the effects driven by hydrogen sulfide.
Fig. 2.
Expression of mRNA levels in BMDMs from AnxA1+/+ or AnxA1−/− mice challenged with LPS (100 ng/ml, 6 hours) alone or in the presence of NaHS (100 μM, 1 hour before LPS). Effect of LPS/NaHS treatment in AnxA1+/+ BMDMs on levels of mRNA for COX2 (A), iNOS (B), and CSE (C). Effect of LPS/NaHS treatment in AnxA1−/− BMDMs on levels of mRNA for COX2 (D), iNOS (E) and CSE (F). Statistical analysis was made by using one-way analysis of variance with Dunnett’s post-hoc test (*P < 0.05, ***P < 0.001 versus vehicle; ###P < 0.001 versus LPS; n = 3). RQ, relative quantity.
Hydrogen Sulfide Exerts Anti-Inflammatory Properties through the AnxA1 Pathway
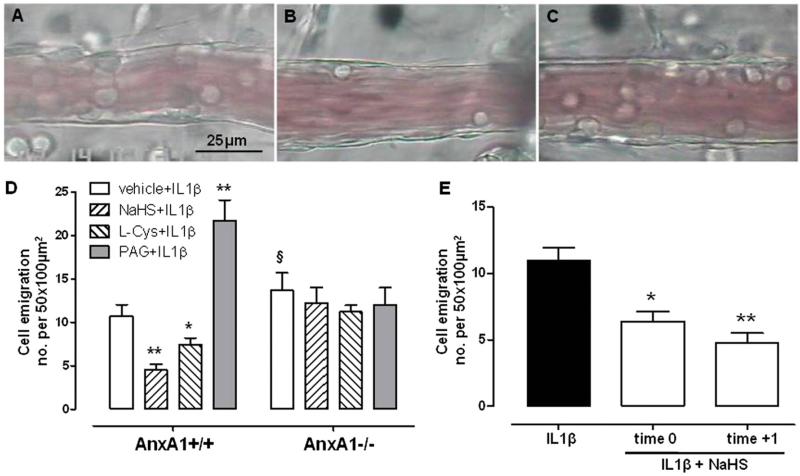
To better define the role of AnxA1 in the anti-inflammatory action of H2S, we performed intravital microcopy analysis in mouse mesenteric microcirculation, and the number of emigrated cells was evaluated. In untreated animals, the baseline cell number is within the 0–1 range. Inflammation was induced by injecting IL-1β (10 ng/mouse) into the mouse peritoneal cavity 1 hour after vehicle, NaHS (100 μmol/kg), or l-cysteine (1000 μmol/kg) treatment. Pretreatment of AnxA1+/+ mice with hydrogen sulfide significantly reduced cell emigration in postcapillary venules compared with the vehicle-treated group (Fig. 3, A and B). Similar effects were also observed for cell adhesion (Supplemental Fig. 3). Conversely, the same protocol, when performed in AnxA1−/− mice, did not display any anti-inflammatory effect following IL-1β injection (Fig. 3C). The same results were obtained using l-cysteine (Fig. 3D). Furthermore, pretreatment with CSE inhibitor PAG significantly exacerbated the inflammatory pattern observed in AnxA1+/+ animals, confirming a tonic role for H2S in the control of inflammation (Fig. 3D) (Asimakopoulou et al., 2013). To establish whether H2S could also revert inflammation, besides preventing it, we carried out a different approach where NaHS was injected into wild-type mice at the same time as (time 0) or 1 hour after (time +1) IL-1β. In both cases, the systemic administration of exogenous hydrogen sulfide significantly reduced cell emigration (Fig. 3E). It is noteworthy that NaHS, l-cysteine, and PAG only exerted their effects in the presence of inflammation, since their administration did not alter, per se, baseline levels of leukocytes (Supplemental Fig. 4).
Fig. 3.
Intravital microscopy analysis of postcapillary venules in AnxA1+/+ or AnxA1−/− mice showing emigrated leukocytes. (A) Representative picture of postcapillary venule in AnxA1+/+ animal treated with IL-1β (10 ng/mouse i.p., 2 hours). (B) Representative picture of postcapillary venule in AnxA1+/+ animal treated with IL-1β (10 ng/mouse i.p., 2 hours), where NaHS (100 μmol/kg s.c.) was given 1 hour before IL-1β injection. (C) Representative picture of postcapillary venule in AnxA1−/− animal treated with IL-1β (10 ng/mouse i.p., 2 hours), where NaHS (100 μmol/kg s.c.) was given 1 hour before IL-1β injection. Mice were pretreated with vehicle, NaHS (100 μmol/kg s.c.), or l-cysteine (L-cys; 1000 μmol/kg s.c.) 1 hour before stimulation with IL-1β (10 ng/mouse i.p., 2 hours), and the number of adherent leukocytes was analyzed (expressed as number of cells per 50 × 100 μm2). (D) Pretreatment with PAG (10 mg/kg i.p.) was performed 30 minutes before IL-1β injection. (E) Number of emigrated leukocytes in AnxA1+/+ animal treated with IL-1β (10 ng/mouse i.p., 2 hours), where NaHS (100 μmol/kg s.c.) was administered at the same time as IL-1β (time 0) or 1 hour after its injection (time +1). Statistical analysis was made using two-way analysis of variance (*P < 0.05, **P < 0.01 versus vehicle; §P < 0.05 versus AnxA1+/+; n = 6).
Hydrogen Sulfide Activates the AnxA1 Pathway
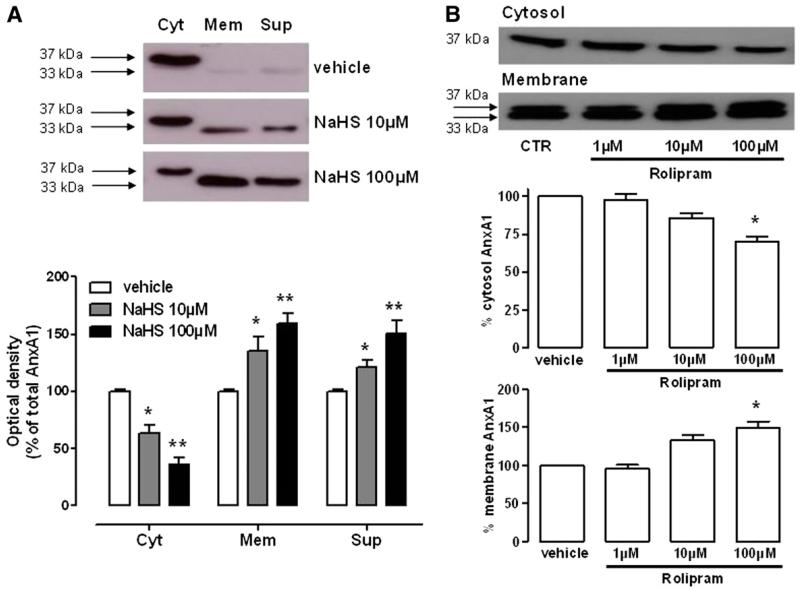
To provide translational impact to these findings, the last series of experiments was performed with human granulocytes. Resting PMNs retain the majority of AnxA1 in the cytosol as granular or free stores, which are mobilized by agonist-induced mechanisms or cell activation (Brancaleone et al., 2011). Treatment with NaHS (10–100 μM, 30 minutes) induced externalization of intracellular AnxA1 from cytosol toward the membrane surface and its further release in the cell culture medium (Fig. 4A). One of the mechanisms engaged by PMN to release AnxA1 relies on the phosphorylation of AnxA1, and this involves a mechanism based on agonist-receptor interaction (Solito et al., 2003; Yazid et al., 2010). Nonetheless, hydrogen sulfide is also known as a nonspecific phosphodiesterase (PDE) inhibitor (Bucci et al., 2010). Therefore, we supposed that inhibition of PDE4, the predominant PDE in granulocytes (Smolen and Geosits, 1984; Grady and Thomas, 1986; Wang et al., 1999), might have a role in AnxA1 mobilization triggered by hydrogen sulfide. To test this hypothesis, we stimulated PMN with PDE4 inhibitor rolipram (1–100 μM, 30 minutes) and monitored for both AnxA1 mobilization and cAMP levels. As shown in Fig. 4B, rolipram, similar to NaHS, significantly increased AnxA1 migration from cytosol to membrane in a dose-dependent fashion. In addition, cAMP levels were increased upon treatment with NaHS (100 μM), rolipram (100 μM), or their combination (both at 10 μM) (Table 1).
Fig. 4.
AnxA1 mobilization within human PMN and underlying mechanisms. (A) AnxA1 expression in cytosolic (Cyt), membrane (Mem), or supernatant (Sup) fractions from human PMN upon NaHS (10–100 μM, 30 minutes) challenge. Optical density is expressed as the percentage of total AnxA1. (B) AnxA1 expression in cytosolic and membrane fractions from human PMN upon treatment with PDE4 inhibitor rolipram (1–100 μM, 30 minutes). Statistical analysis was made using one-way analysis of variance (*P < 0.05, **P < 0.01 versus vehicle; n = 4).
TABLE 1.
Determination of cAMP levels in PMN treated with NaHS (100 μM), rolipram (100 μM), or NaHS + rolipram (both at 10 μM) for 30 minutes Data are presented as the mean ± S.E.M. for n = 3 replicated experiments. Statistical analysis was determined using Student’s t test.
| Treatment | cAMP |
|---|---|
| pmol/106 cells | |
| Vehicle | 2.01 ± 0.56 |
| NaHS | 6.74 ± 1.56* |
| Rolipram | 10.46 ± 2.38* |
| NaHS + rolipram | 15.66 ± 2.02**,# |
Statistically significant (P < 0.05) versus vehicle
statistically significant (P < 0.01) versus vehicle
statistically significant (P < 0.05) versus NaHS.
Hydrogen Sulfide Reduces the Expression of CD11b
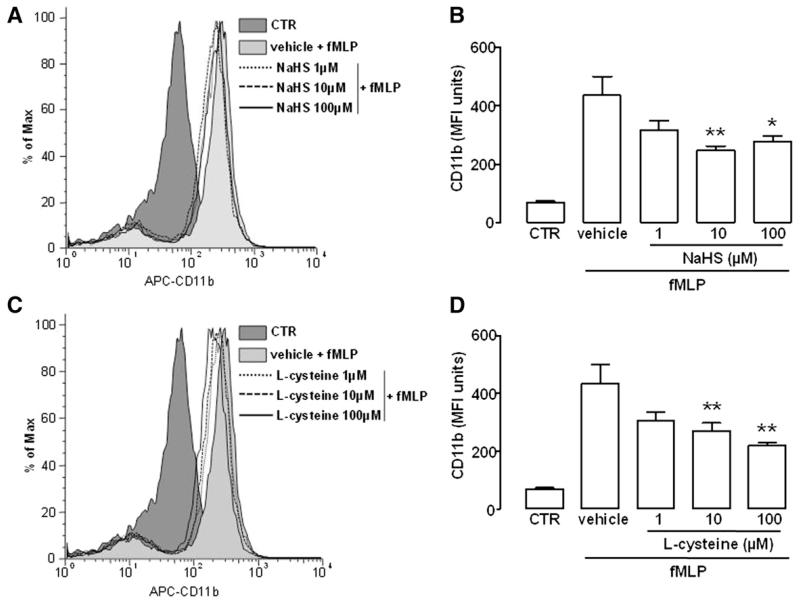
Finally, we aimed to verify whether AnxA1 exposure on PMN cell surface triggered by H2S might generate a functional response underlying the reduced leukocyte transmigration observed in vivo (Fig. 3). For this purpose, we determined PMN surface expression of CD11b, a well established marker for leukocyte activation, following fMLP (0.1 μM) treatment in the presence of NaHS or l-cysteine (1–100 μM). As shown in Fig. 5, flow cytometry data indicated that cell preincubation with either NaHS or l-cysteine reduced CD11b surface expression induced by fMLP in a concentration-dependent manner.
Fig. 5.
Modulation of CD11b surface expression by H2S. (A) Histograms of CD11b surface expression in human PMNs pretreated with vehicle or NaHS (1–100 μM) 10 minutes before challenge with fMLP (0.1 μM, 30 minutes). (B) Expression of CD11b as mean fluorescence intensity (MFI) in human PMN upon NaHS treatment. (C) Histograms of CD11b surface expression in human PMN pretreated with vehicle or l-cysteine (1–100 μM) 10 minutes before challenge with fMLP (0.1 μM, 30 minutes). (D) Expression of CD11b as MFI in human PMN upon l-cysteine treatment. Control (CTR) histograms and bars represent untreated cells. Statistical analysis was made using one-way analysis of variance (*P < 0.05, **P < 0.01 versus vehicle; n = 4).
Discussion
Inflammatory response relies on a complex network of chemically different modulators produced within the body (Stipanuk, 2004; Perretti and D’Acquisto, 2009). Hydrogen sulfide is one of these, although it can promote inflammation in animal models of sepsis (Zhang et al., 2007a), where inhibition of its biosynthesis exerted beneficial effects (Li et al., 2005). Nevertheless, it can also dampen inflammation through different pathways, i.e., reducing cytokine (IL-6, IL-8, tumor necrosis factor α) production or iNOS expression (Ganster et al., 2010; Zeng et al., 2013). Furthermore, additional evidence shows that H2S treatment during ongoing inflammatory reactions attenuates leukocyte infiltration by reducing adhesion molecule expression (Elrod et al., 2007; Sivarajah et al., 2009; Li et al., 2011). Here, we have further investigated the role played by hydrogen sulfide in the control of inflammation, aiming to disclose a possible interplay with the proresolving protein AnxA1. To pursue our aim, we first evaluated whether lack of functional AnxA1 in mice could affect expression of H2S-synthesizing enzymes CBS and CSE. Real-time PCR data showed that mRNA levels of either CBS or CSE were actually augmented in the aorta, spleen, and kidney samples derived from AnxA1−/− mice (Fig. 1). Such an increased expression associated with lack of AnxA1 reflected a parallel increase in plasma H2S levels, indicating the existence of cross-talk between hydrogen sulfide and AnxA1. To verify this aspect in functional terms, we used LPS-challenged BMDMs collected from AnxA1+/+ or AnxA1−/− mice. We first showed that, in AnxA1+/+-derived cells, exposure to hydrogen sulfide prior to LPS challenge significantly reversed the increase in mRNA expression of COX2, iNOS, and CSE. Such an anti-inflammatory response was absent in cells derived from AnxA1−/−. This finding indicates that hydrogen sulfide has no effect when AnxA1 is lacking or not functional, thereby suggesting that H2S requires AnxA1 activation. Nevertheless, we cannot exclude that the lack of effect by H2S in AnxA1−/− cells might derive from the fact that, in AnxA1−/− mice, H2S is already overproduced, generating a possible saturated condition.
Based on these findings, we then hypothesized that H2S could exert its in vivo anti-inflammatory effect also by engaging the AnxA1 pathway (Li et al., 2011). To validate this hypothesis, we used an in vivo model of inflammation, where IL-1β was injected into the peritoneal cavity of both AnxA1+/+ and AnxA1−/− mice to trigger leukocyte trafficking in the mesenteric microvasculature. Administration of hydrogen sulfide to AnxA1+/+ mice significantly reduced the number of emigrated leukocytes (Fig. 3, A and B). Conversely, its administration failed to counteract in vivo cell trafficking in AnxA1−/− animals. Therefore, absence of functional AnxA1 blunts the H2S in vivo anti-inflammatory effect. This finding is further confirmed by the observation that PAG, used as a CSE inhibitor (Asimakopoulou et al., 2013), exacerbated the inflammatory process when injected before IL-1β (Fig. 3D). These observations further confirm that AnxA1 is somehow involved in controlling PMN trafficking in vivo operated by H2S (Fig. 3, C and D). In addition, the reduction in emigrated cells, following hydrogen sulfide treatment, observed in wild-type animals was consistently independent of the administration time frame. On this basis, we propose that H2S or H2S-based drugs show anti-inflammatory efficacy only during ongoing inflammation. Indeed, there is no effect on leukocyte trafficking in the absence of inflammatory stimulus (Supplemental Fig. 4).
One of the proresolutive actions of AnxA1 occurs through the detachment of adherent leukocytes from the vessel wall (Gavins et al., 2003; Brancaleone et al., 2011), and we found that this effect is also shared by hydrogen sulfide (Supplemental Fig. 5). Indeed, a similar degree of leukocyte detachment between hydrogen sulfide and AnxA1 is evident. Indeed, the observation that such detaching properties by hydrogen sulfide is lost in AnxA1−/− mice adds further evidence to the hypothesis that H2S may trigger activation, and the functional involvement, of the AnxA1 pathway.
Having assessed the importance of AnxA1 in hydrogen sulfide–associated effects, we attempted to translate these observations to humans, using polymorphonuclear cells (mainly neutrophils) harvested from healthy human volunteers. We used human PMNs since AnxA1 is highly abundant in these cells and it is mainly stored in the cytosol as granular or “free” pool. Studies in past years have indicated how distinct stimuli, spanning from lipoxin A4 (Brancaleone et al., 2011) to histone-deacetylase inhibitors (Montero-Melendez et al., 2013), from estrogen (Nadkarni et al., 2011) to glucocorticoids (Yazid et al., 2010), can externalize and/or release the protein in an extracellular environment, leading to the conformational change required to activate its receptors (Perretti and D’Acquisto, 2009). Following hydrogen sulfide treatment, AnxA1 is mobilized from the cytosol onto the plasma membrane and then released in cell supernatant, and this movement does not involve AnxA1 stored within PMN granules (Supplemental Fig. 6). Thus, our data suggest that H2S induces the release of AnxA1 from a free cytosolic pool, although we cannot exclude that such a mobilization might involve microparticle-incorporated AnxA1 (Dalli et al., 2008). Therefore, since H2S has been shown to inhibit PDE (Bucci et al., 2010), we evaluated whether the AnxA1 mobilization process triggered by hydrogen sulfide might involve cAMP release within the cell. Thus, we measured cAMP levels upon NaHS treatment and, in parallel experiments, we also treated PMNs with rolipram, a selective inhibitor of PDE4 (Smolen and Geosits, 1984; Wang et al., 1999). Our results demonstrated that cAMP levels raised upon NaHS stimulation and PDE4 blockade by rolipram caused both elevation of intracellular cAMP and, similar to NaHS, AnxA1 mobilization. In addition, we also observed a synergistic effect when NaHS and rolipram were coadministered at a lower concentration (10 μM). Collectively, our data suggest that hydrogen sulfide can trigger AnxA1 release in inflammatory cells, which in turn activates proresolving pathways to restore tissue homeostasis.
Generally, upon membrane exposure, AnxA1 reduces surface expression of adhesion molecules, resulting in leukocyte detachment or reduction of adherent/transmigrated inflammatory cells (Perretti and Flower, 2004). Therefore, we questioned whether hydrogen sulfide treatment could affect PMN surface expression of CD11b, an established marker for leukocyte activation and cell adhesion (Gavins et al., 2003). Interestingly, our data showed that hydrogen sulfide treatment reduced CD11b surface expression in PMNs. Therefore, H2S effect on CD11b expression might occur through mobilization of AnxA1, which results in a reduced cell activation. Noteworthy, this finding was also replicated when H2S precursor l-cysteine was used. This result indicates that, when inflammation occurs, the l-cysteine/H2S pathway contributes to trigger AnxA1 mobilization, which in turn controls leukocyte trafficking.
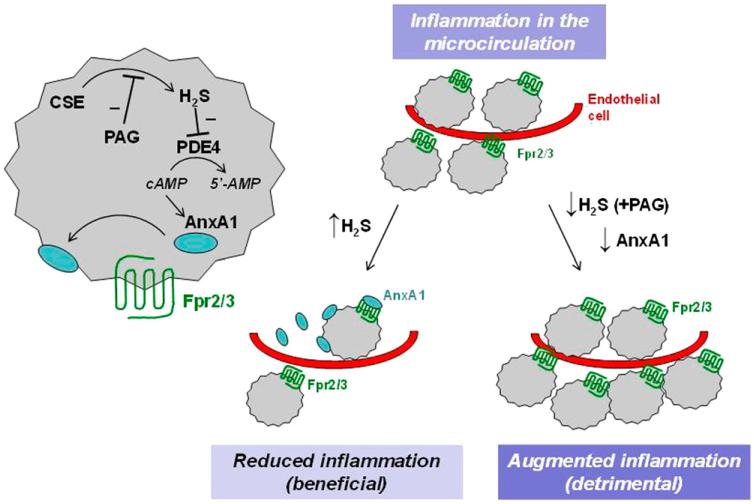
In conclusion, we propose that H2S anti-inflammatory response involves activation of the AnxA1 proresolutive pathway (Fig. 6). Therefore, the increase in H2S levels occurring during inflammation represents a response aiming to restore tissue homeostasis, also based on AnxA1 pathway activation, and to promote resolution and the healing process (Wallace et al., 2012). This novel perspective provides new insights into understanding inflammation-resolution balance and might be crucial in approaching new therapeutic targets that use endogenous hydrogen sulfide to counteract tissue injury.
Fig. 6.
Schematic representation of inflammatory circuit driven by H2S and triggering AnxA1 pathway. Fpr2/3 (formyl-peptide receptor 2/3) represents the murine isoform for human FPR2/ALX.
Supplementary Material
Acknowledgments
This work was partly supported by the William Harvey Research Foundation (M.P.) and forms part of the research themes contributing to the translational research portfolio of Barts and The London National Institute for Health Research Cardiovascular Biomedical Research Unit. This work was also supported by the Wellcome Trust [Grant 086867/Z/08/Z].
ABBREVIATIONS
- AnxA1
annexin A1
- BMDM
bone marrow-derived macrophage
- CBS
cystathionine-β-synthase
- COX2
cyclooxygenase 2
- CSE
cystathionine-γ-lyase
- fMLP
N-formyl-l-methionyl-l-leucyl-l-phenylalanine
- H2S
hydrogen sulfide
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- LXA4
lipoxin A4
- PAG
d,l-propargylglycine
- PCR
polymerase chain reaction
- PDE
phosphodiesterase
- PMN
polymorphonuclear cell
- NaHS
sodium hydrosulfide
Footnotes
This article has supplemental material available at jpet.aspetjournals.org.
This work has been previously presented as an oral presentation at the following conferences: Brancaleone V, Sampaio AL, Cirino G, Flower RJ, Perretti M (2011) A novel cross-talk in resolution: H2S activates the annexin A1 pathway. 10th World Congress of Inflammation; 2011 June 25–29; Paris, France. Vol 60 (Suppl 1), pp S291–S293, International Association of Inflammation Societies, London, UK; and Brancaleone V, Flower RJ, Cirino G, and Perretti M (2013) Annexin A1 mediates hydrogen sulfude effects in the control of inflammation. Second European Conference on the Biology of Hydrogen Sulfide; 2013 Sept 8–11; Exeter, UK. Vol 31 (Suppl 1), pp S21–S22, European Network on Gastrotransmitters, Patras, Greece.
References
- Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- Brancaleone V, Dalli J, Bena S, Flower RJ, Cirino G, Perretti M. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J Immunol. 2011;186:4905–4914. doi: 10.4049/jimmunol.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived micro-particles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- d’Emmanuele di Villa Bianca R, Mitidieri E, Di Minno MN, Kirkby NS, Warner TD, Di Minno G, Cirino G, Sorrentino R. Hydrogen sulphide pathway contributes to the enhanced human platelet aggregation in hyperhomocysteinemia. Proc Natl Acad Sci USA. 2013;110:15812–15817. doi: 10.1073/pnas.1309049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Villa Bianca Rd, Coletta C, Mitidieri E, De Dominicis G, Rossi A, Sautebin L, Cirino G, Bucci M, Sorrentino R. Hydrogen sulphide induces mouse paw oedema through activation of phospholipase A2. Br J Pharmacol. 2010;161:1835–1842. doi: 10.1111/j.1476-5381.2010.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganster F, Burban M, de la Bourdonnaye M, Fizanne L, Douay O, Loufrani L, Mercat A, Calès P, Radermacher P, Henrion D, et al. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care. 2010;14:R165. doi: 10.1186/cc9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastardelo TS, Damazo AS, Dalli J, Flower RJ, Perretti M, Oliani SM. Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am J Pathol. 2009;174:177–183. doi: 10.2353/ajpath.2009.080342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte anti-adhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- Grady PG, Thomas LL. Characterization of cyclic-nucleotide phosphodiesterase activities in resting and N-formylmethionylleucylphenylalanine-stimulated human neutrophils. Biochim Biophys Acta. 1986;885:282–293. doi: 10.1016/0167-4889(86)90243-0. [DOI] [PubMed] [Google Scholar]
- Hui Y, Du J, Tang C, Bin G, Jiang H. Changes in arterial hydrogen sulfide (H(2)S) content during septic shock and endotoxin shock in rats. J Infect. 2003;47:155–160. doi: 10.1016/s0163-4453(03)00043-4. [DOI] [PubMed] [Google Scholar]
- Leoni G, Patel HB, Sampaio AL, Gavins FN, Murray JF, Grieco P, Getting SJ, Perretti M. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB J. 2008;22:4228–4238. doi: 10.1096/fj.08-113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- Montero-Melendez T, Dalli J, Perretti M. Gene expression signature-based approach identifies a pro-resolving mechanism of action for histone deacetylase inhibitors. Cell Death Differ. 2013;20:567–575. doi: 10.1038/cdd.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni S, Cooper D, Brancaleone V, Bena S, Perretti M. Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arterioscler Thromb Vasc Biol. 2011;31:2749–2759. doi: 10.1161/ATVBAHA.111.235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Sinclair LK, Dougas I, Liang CM, Lawton P, Browning JL. Monoclonal antibodies to lipocortin-1 as probes for biological function. FEBS Lett. 1990;261:247–252. doi: 10.1016/0014-5793(90)80564-y. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24:163–174. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76:25–29. doi: 10.1189/jlb.1103552. [DOI] [PubMed] [Google Scholar]
- Qu K, Chen CP, Halliwell B, Moore PK, Wong PT. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke. 2006;37:889–893. doi: 10.1161/01.STR.0000204184.34946.41. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Ernst JD. Calcium-dependent neutrophil secretion: characterization and regulation by annexins. J Immunol. 1997;159:6195–6202. [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- Smolen JE, Geosits SJ. Human neutrophil phosphodiesterase. Calmodulin insensitivity and other properties. Inflammation. 1984;8:193–199. doi: 10.1007/BF00916094. [DOI] [PubMed] [Google Scholar]
- Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel non-genomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Vong L, D’Acquisto F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, Perretti M. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem. 2007;282:29998–30004. doi: 10.1074/jbc.M702876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Ferraz JG, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Ohleth KM, Egan RW, Billah MM. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- Yazid S, Leoni G, Getting SJ, Cooper D, Solito E, Perretti M, Flower RJ. Antiallergic cromones inhibit neutrophil recruitment onto vascular endothelium via annexin-A1 mobilization. Arterioscler Thromb Vasc Biol. 2010;30:1718–1724. doi: 10.1161/ATVBAHA.110.209536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zeng J, Lin X, Fan H, Li C. Hydrogen sulfide attenuates the inflammatory response in a mouse burn injury model. Mol Med Rep. 2013;8:1204–1208. doi: 10.3892/mmr.2013.1610. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2007a;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J Leukoc Biol. 2007b;82:894–905. doi: 10.1189/jlb.0407237. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Liu SJ, Liu YJ, Wang S, Ni X. Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell Mol Life Sci. 2010;67:1119–1132. doi: 10.1007/s00018-009-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.