Abstract
Ookinete invasion of Anopheles midgut is a critical step for malaria transmission; the parasite numbers drop drastically and practically reach a minimum during the parasite's whole life cycle. At this stage, the parasite as well as the vector undergoes immense oxidative stress. Thereafter, the vector undergoes oxidative stress at different time points as the parasite invades its tissues during the parasite development. The present study was undertaken to reconstruct the network of differentially expressed genes involved in oxidative stress in Anopheles stephensi during Plasmodium development and maturation in the midgut. Using high throughput next generation sequencing methods, we generated the transcriptome of the An. stephensi midgut during Plasmodium vinckei petteri oocyst invasion of the midgut epithelium. Further, we utilized large datasets available on public domain on Anopheles during Plasmodium ookinete invasion and Drosophila datasets and arrived upon clusters of genes that may play a role in oxidative stress. Finally, we used support vector machines for the functional prediction of the un-annotated genes of An. stephensi. Integrating the results from all the different data analyses, we identified a total of 516 genes that were involved in oxidative stress in An. stephensi during Plasmodium development. The significantly regulated genes were further extracted from this gene cluster and used to infer an oxidative stress network of An. stephensi. Using system biology approaches, we have been able to ascertain the role of several putative genes in An. stephensi with respect to oxidative stress. Further experimental validations of these genes are underway.
Introduction
Maintenance of redox homeostasis is critical for proper functioning of cellular processes and disruption of this prooxidant-antioxidant balance in a cell results in oxidative stress. Oxidative stress may be caused by the normal functioning of the cell (mitochondrial respiration) or as an immune response to pathogens [1], [2] and is manifested by an increase in reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the cells. These reactive species are capable of modifying DNA and proteins, inactivating biological activity and causing oxidative injury [3], [4]
Several studies have established that generation of ROS can be endogenous due to the leakage of activated oxygen from mitochondria during oxidative phosphorylation, peroxisomes, and activated inflammatory cells [5] or exogenous inflammatory cytokines, pathogens, and metals [6], [7]. ROS are toxic to cells and there are several detoxifying mechanisms that are employed by the cell to prevent oxidative damage.
Plasmodium, the causative agent of malaria, leads a complex life cycle, alternating between two hosts, vertebrate and invertebrate, with diverse environmental and physiological regimens. Further, within these two hosts, the parasite also exists as intra- and extracellular forms thereby being exposed to extreme surroundings. Several studies have revealed that Plasmodium undergoes immense oxidative stress during their erythrocyte cycle, considering that they live in a pro-oxidant environment in the red blood cells that contains oxygen and iron [8]–[10]. Recent studies have focused on targeting the Plasmodial redox system for anti-malarial therapy [11]. Several drugs have been developed to disrupt the mechanism and balance of ROS and RNS molecules, by targeting the enzymes of the parasite responsible for maintaining the redox balance [12]. During the mosquito cycle, the parasite undergoes tremendous oxidative stress. It can be rightly said that one of the major bottleneck in the parasite life cycle is the dwindling of its numbers during oocyst development in the mosquito stage [13]. However, it has been shown that Plasmodium overcomes this obstacle by using its defense mechanisms to protect against oxidative damage [10], [14], [15].
Just as in the case of Plasmodium, its vector, Anopheles also undergoes tremendous oxidative stress due to the high proliferative rate of the parasite and invasion of several of its organs by the parasite. The zygote transforms into motile ookinetes within 24 hours of ingestion of an infected blood meal and invades the mosquito midgut epithelium. Once inside, the ookinete develops into the oocyst between the basal lamina and the midgut epithelium. Upon maturity, the oocyst produces thousands of sporozoites that are released from the midgut into the hemocoel and finally reach the salivary glands. Here, they invade the salivary glands and mature to form the salivary gland derived sporozoites that are ready to infect the host during the next mosquito bite. During each of the invasion process and subsequent increase in parasite numbers, the mosquito undergoes extreme oxidative stress and several of the signaling pathways and innate immunity pathways are activated to protect the mosquito [16]–[20].
In the post-omics era, it is becoming clear that integration of genome-scale technologies provide better tools for understanding biological function [21]. Any cellular function is a dynamic interaction of several proteins to enforce a highly sensitive and a regulated system. A ‘single gene’-‘single function’ approach is fast being replaced by interaction networks for evaluating the intricacies involved in complex conditions like pathogen infection [22]–[24].
We have undertaken the present study to elaborate perturbations in the redox system of An. stephensi during successive stages of the development and maturation of Plasmodium vinckei petteri. Using next generation sequencing platforms, we obtained the transcriptome of the midgut of An. stephensi during P. vinckei petteri oocyst stage. We identified those transcripts that were differentially expressed and evaluated the dynamics of the An. stephensi redox system during oocyst development. Using Support Vector Machines (SVM) we classified unannotated genes of the transcriptome dataset into oxidative stress pathways. Additionally, we identified microarray datasets from public databases that studied An. gambiae during Plasmodium development, and arrived upon the set of An. gambiae genes involved specifically in oxidative stress during Plasmodium midgut invasion. Using all the above information, we inferred an almost complete network of the oxidative stress of An. stephensi during Plasmodium invasion.
Materials and Methods
Ethics statement
Animal experiments were performed in accordance with National animal ethics guidelines of the Government of India after approval by Institutional Animals Ethics Committees of International Centre for Genetic Engineering & Biotechnology, New Delhi (Permit number: ICGEB/AH/2011/01/IR-8).
Mosquito rearing and Plasmodium vinckei petteri infection
Anopheles stephensi were reared at 28–30°C and humidity maintained at 70–75%. Mosquitoes were maintained by feeding with raisin soaked in 2% sterile glucose solution and water. 4–5 days old female mosquitoes were fed on P. vinckei petteri 279 BY (gametocytemia = ∼0.05%) infected mice. Midguts from the infected mosquitoes were dissected on 5th day post infection and checked for the presence of oocysts for the confirmation of infection.
Anopheles stephensi sample collection and RNA isolation
Anopheles stephensi midgut samples were collected from three different stages, namely, sugar fed (PVpSF), blood fed (PVpBF5D) and blood fed 5 days post P. vinckei petteri infection (PVpiBF5d). In case of infection and blood feed, around 150–200 An. stephensi mosquitoes were fed on P. vinckei petteri 279 BY (gametocytemia = ∼0.05%) infected mice. Fully fed mosquitoes were separated from unfed and partially fed mosquitoes and reared in cages until day 5 post feeding. Midguts were dissected and stored in trizol in −80°C. The feeding experiments were performed for a minimum of three times for both blood and infected blood feeding and a total of 200–300 midguts were collected over a period of time for each sample. Total RNA was isolated separately from each lot and finally pooled during RNA seq library preparation. The libraries were made following manufacturer's instruction. RNA sequencing was performed using Illumina platform. The Total RNA quality was verified using RNA 6000 Nano Kit (Agilent Technologies, USA) on 2100 Bioanalyzer (Agilent Technologies, USA), with a minimum RNA Integrity number (RIN) value of 7.
Preparation of library and Sequencing
Three Paired-End RNA-seq libraries of An. stephensi were generated, one each from total RNA extracted from sugar fed mosquitoes (PVpSF), mosquitoes 5 days post blood feed, (PVpBF5D) and mosquitoes 5 days post infected blood feed (PVpiBF5D). The RNA-Seq library construction and sequencing was performed by commercial service providers (NxGenBio Life Sciences, New Delhi, India). Total RNA was used to enrich mRNA using Oligotex mRNA midi prep kit (QIAGEN, Germany). 2 µg of total RNA using oligo(dT) magnetic beads and fragmented into 200–500 bp using divalent cations at 94°C for 5 min. The cleaved RNA fragments were copied into first strand cDNA using SuperScript II reverse transcriptase (Life Technologies, Inc.) and random primers. Fragments were A-tailed and end repaired after second strand cDNA synthesis. The cDNA libraries were constructed for the samples using the TruSeq RNA Sample Preparation Kit (Illumina, Inc.) with alternate fragmentation method for generating 200–500 bp fragments, according to manufacturer's instructions. The Paired-End RNA-Seq libraries were diluted and sequenced using TruSeq SBS Kit V3 on HiSeq2000 (Illumina, San Diego, CA) for generating 2×100 bp sequencing reads.
Transcriptome Assembly and Read Mapping
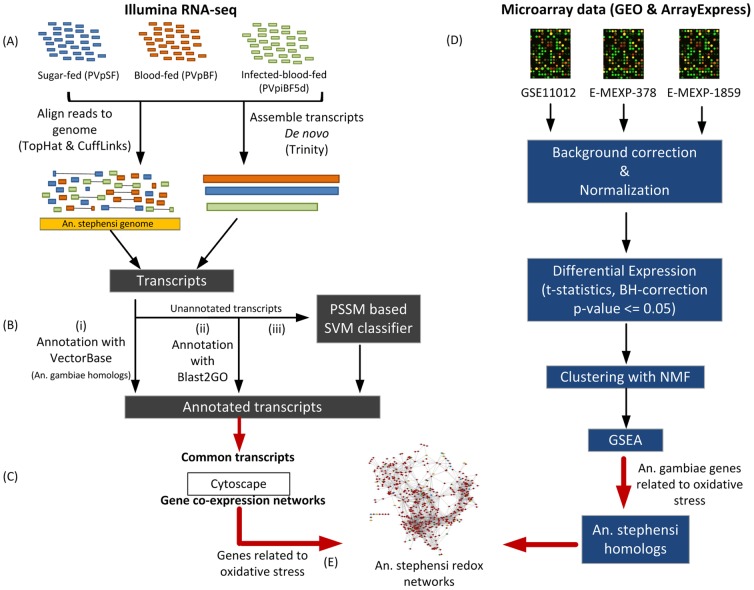
A simplified workflow of transcriptome assembly and analysis performed in this study is shown in Figure 1. The sequence reads of all the libraries were adapter trimmed using fastx toolkit and were subjected to quality check (QC) using FastQC retaining only high quality reads (>Q20) and discarding the rest [25]. The high quality reads were analyzed both by de novo assembly using Trinity [26] (data not shown) and by genome based analysis. TopHat [27] was used for genome mapping and An. stephensi genome was used as the reference genome which was downloaded from VectorBase [27]. Further analysis was performed with Cufflinks [27]. TopHat aligns the reads to the reference sequences using Bowtie tool [28] and realign the unaligned sequences by breaking them into small fragments.
Figure 1. Pictorial representation of the workflow followed for the analysis of RNA-seq data and microarray data.
Functional annotation
One of the critical and essential steps in the analysis of high-throughput sequencing data is proper annotation of the assembled reads. The functional characterization of the assembled transcriptomes of all libraries of An. stephensi consisted of three steps (Figure 1). First, we performed homology based search of VectorBase mapped genes of An. stephensi against An. gambiae, a genome that is very well annotated and belongs to the same subgenus. We used BioMart tool from VectorBase to identify the homologs. Second, we used Blast2GO tool [29] to annotate the genes that were not annotated in the first step. Blast2GO blast the input sequence against NCBI nr database, retrieve the GO terms of the blast hits, assign the score to each GO term and finally select the lowest term from the branch of GO hierarchy tree to assign it to the input sequence. Third, we implemented support vector machine (SVM) to annotate the rest of the genes as described in next section.
Annotation of genes from the transcriptome using Support Vector Machines
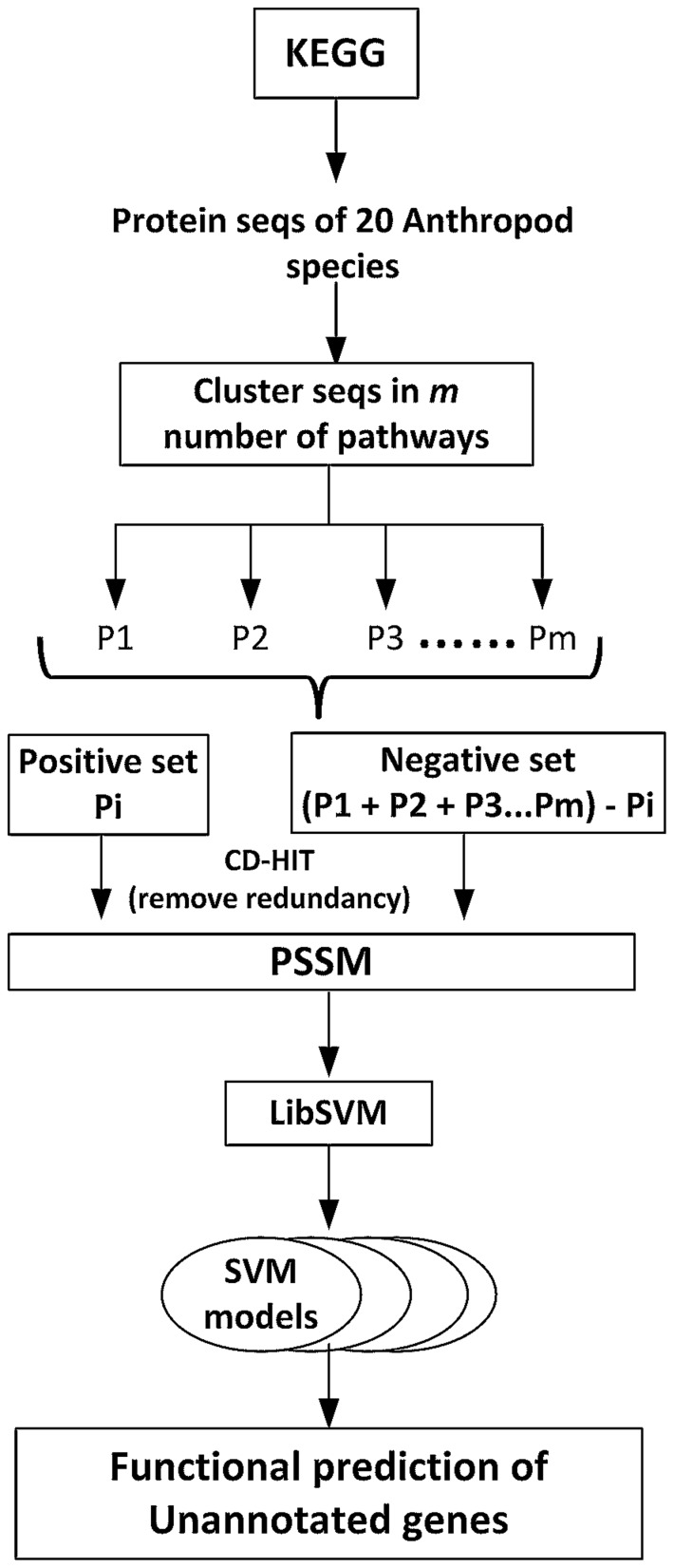
Evolutionary information can be extracted using Position Specific Substitution Matrix (PSSM), hence, for the functional characterization of the An. stephensi transcripts that were not annotated by simple Blast as described in the previous section; we built a predictive model based on PSI-BLAST (Position Specific Iterated BLAST). In order to build the predictive model we used SVM, a powerful supervised machine learning algorithm for prediction, that classifies an object into one or more classes based on the set of input feature vectors. In our study, PSSM generated by PSI-BLAST was used as the input feature vector for SVM. PSSM based classifiers has been reported as the most suited among the SVM classifiers [30]. PSSM generates 20xN matrix, where N is the length of the sequence of the query. To make input feature vectors of fixed length we normalized the matrix using logistic function.
We built the training data for SVM model by downloading protein sequences of 20 Arthropod species from KEGG and all the sequences, irrespective of the species, were clustered according to the selected pathways. We built m number of SVM models for m pathways. The training data for each of the ith model consisted of protein sequences of ith pathway of interest as positive set and protein sequences of the m-i pathways as negative set (Figure 2). The redundancy of the positive and negative datasets was removed using CD-HIT [31]. The lowest possible threshold for identity by CD-HIT was 40%; we used this threshold to generate non-redundant training datasets. As a 40% threshold reduced the size of positive dataset quiet a lot and resulted in highly imbalance training datasets, we also generated positive datasets by utilizing thresholds of 50%, 60% and 70%. We used libSVM with radial basis function as the kernel to build SVM model. To handle the class imbalance problem we penalized positive dataset using weight parameter of svm-train. We performed 5-fold cross-validations to estimate the values of cost, gamma and weight parameters. The final training datasets for each SVM model and the selected parameter values are given in Table S3. All the unannotated genes were then subjected to the SVM analysis. The pathway predictions of the genes were performed on the basis of SVM prediction score (Fig. 2).
Figure 2. The figure represents the SVM work flow used in this study.
Evaluation of prediction models
We evaluated the performance of our classifiers by calculating accuracy, Receiver operating characteristic (ROC) curve and Area under Curve (AUC). ROC is a plot of false positive rate (1-specificity) on x-axis and true positive rate (sensitivity) on y-axis. The plot depicts the trade-off between specificity and sensitivity. The mathematical representations of the expressions can be represented as:
Sensitivity = (TP/TP+FN)×100
Specificity = (TN/TN+FN)×100
Accuracy = (TP+TN/TP+FN+TN+FP)×100
MCC = (TP×TN) – (FP×FN)/sq.root [(TP+FN)×(TN+FP)×(TP+FP)×(TN+FN)]
Where TP means True positive, TN means True Negative, FN means False Negative and FP means False Positive.
Differential expression and enrichment analysis
The differential expression analyses of the libraries were performed with Bioconductor package edgeR. edgeR uses TMM (Trimmed mean of M values) approach for the normalization of read counts. We identified differentially expressed genes by comparing all the three libraries with each other i.e. PVpSF vs PVpBF5D, PVpSF vs PVpiBF5D and PVpBF5D vs PVpiBF5D. edgeR analysis were performed by taking disperson value of 0.1 and p value < = 0.05.
Differentially expressed genes from each comparison were taken separately and subjected to pathway enrichment analysis. For the identification of significantly enriched pathways the protein sequences of An. stephensi corresponding to differentially expressed genes were downloaded from VectorBase and were analyzed using KOBAS web server. Hypergeometric test was selected as statistical method and FDR correction was performed using Benjamin and Hochberg method (1995). Pathways with p< = 0.05 were considered as significant pathways.
Microarray Data search
A data search was conducted to identify the relevant data sets to be used for the study. A search was performed using “Mosquito”, “Plasmodium”, “Drosophila”, “Oxidative stress” and “Infection” as key elements and wherever required, the related terms and alternative terms were also used. Online library and databases namely, PubMed, ArrayExpress and Gene Expression Omnibus (GEO) were searched for the data using the key words and the relevant data were downloaded (Table S1).
Microarray Data Analysis
For the purpose of maximizing the information on redox dynamics of Plasmodium invasion in An. stephensi, we incorporated microarray experiment datasets with similar experimental setup from two other dipteran species, one of which belongs to the same subgenus i.e. An gambiae and the other is more closely linked by evolutionary and genetic lineage i.e. Drosophila melanogaster. We downloaded three datasets (Table S1) from public repositories Gene Expression Omnibus (GEO) and ArrayExpress namely (1) E-MEXP-378 : Transcription profiling of mosquitoes fed blood infected with two alternative P. berghei strains; wild type (wt) or an invasion-deficient, CTRP (Circumsporozoite- and TRAP-related protein) knockout (ko) strain (2) E-MEXP-1859: Transcription profiling of Drosophila transformed with two Plasmodium cell surface antigens, circumsporozoite protein (CSP) and Thrombospondin-related adhesive protein (TRAP) and (3) GSE11012: An analysis of the impact of infection by Buchnera aphidicola APS on gene expression of Drosophila S2 cells. All the three datasets were background corrected and normalized using LOESS and Aquantile normalization. The normalized data were further analyzed using Bioconductor packages LIMMA [32], [33]. We used eBayes function to calculate moderated paired t-statistics after fitting the linear model and assessed the genes expressing differentially using p-value cutoff of 0.05. The p-values were corrected for multiple testing with Benjamini and Hochberg's (BH). The differentially expressed genes were further clustered using Non-negative matrix factorization (NMF) [34], which were then subjected to pathway enrichment analysis using gene set enrichment analysis (GSEA) [35].
Generation of An. stephensi gene-gene co-expression network
An. stephensi gene co-expression network was generated using ExpressionCorrelation plugin of Cytoscape (http://apps.cytoscape.org/apps/expressioncorrelation) [36]. ExpressionCorrelation uses Pearson Correlation Coefficient for computing similarity matrix. For generation of the significant network using expression values minimum of four datasets are required. For this purpose, in addition to the gene expression data of the three RNA-seq libraries in this study, namely, PVpSF, PVpBF5D, PVpiBF5D, one more RNA seq datasets, namely, PVxBF5D (generated from An. stephensi fed on human blood) was used to generate the gene co-expression network. Common genes among all the libraries, with their expression values were used as an input for generation of the gene co-expression network using ExpressionCorrelation plugin of Cytoscape. The identified and predicted genes related to oxidative stress were mapped onto the An. stephensi gene interaction network and a sub-network consisting of only these mapped genes was drawn out from the network.
Results and Discussion
Integration of different types of genomic data provided new insights into the interactions that exist between genes that are otherwise not distinguishable while studying single data sets [37]–[39]. In the present study, we inferred gene-gene interaction network of oxidative stress in An. stephensi during P. vinckei petteri development by integrating datasets originating from Illumina RNA-seq technology and gene expression microarrays.
Anopheles stephensi midgut transcriptome during blood feed and P. vinckei petteri infection
In order to understand the dynamics of infection especially during P. vinckei petteri oocyst development in the midgut, we obtained the transcriptome of the midgut during the time point of mature oocyst development in the midgut. Physiologically, at this stage, the oocysts are mature and were ready to invade the midgut with oocyst derived sporozoites which is ideal for our study purpose of studying the oxidative stress in Anopheles during Plasmodium invasion. Analysis of such a cellular state is likely to reveal metabolic homeostasis facilitating parasite maturation and most importantly minimal metabolic penalty on vector. We dissected the midguts at day 5 post infection and processed those midguts that had maximum oocysts [40]. Total RNA was isolated from the midgut of mosquitoes at different time points and at different conditions over several feeding experiments (minimum of three times). The RNA was extracted from each group independently and midguts of around 200–300 mosquitoes for each condition were finally pooled during library preparation and sequenced in one run due to budget constraints. A total of 1.28×108 reads , which includes 4.21×107 reads from PVpSF library, 4.24×107 reads from PVpBF5D library and 4.37×107 reads of PVpiBF5D library were further processed (Table 1). The quality scores of reads were assessed and reads were trimmed by keeping quality score threshold as 20. We got 86.45% reads of PVpSF, 85.64% reads of PVpBF5D and 89.26% reads of PVpiBF5D, which were subjected to de novo assembly (data not shown) as well as reference mapping and assembly using TopHat and Cufflinks. Reference mapping with the newly published An. stephensi genome identified a total of 10496 genes in PVpSF, 9974 genes in PVpBF5D and 9613 genes in PVpiBF5D libraries from a total of 13650 genes present in VectorBase.
Table 1. Mapping summary of RNA-seq data.
| S. No. | Midgut Samples | Total number of reads | No. of reads after quality trimming | Total number of Anopheles stephensi genes |
| 1. | Sugar-fed mosquito (5 dpi) | 4.21×107 | 3.64×107 | 10496 |
| 2. | Blood-fed mosquito (5 dpi) | 4.24×107 | 3.63×107 | 9974 |
| 3. | P. vinckei petteri infected blood-fed mosquito (5 dpi) | 4.37×107 | 3.90×107 | 9613 |
*RNA-seq libraries were mapped to Anopheles stephensi genome using TopHat and Cufflinks tools.
Diverse An. stephensi genes are impacted during Plasmodium vinckei petteri infection
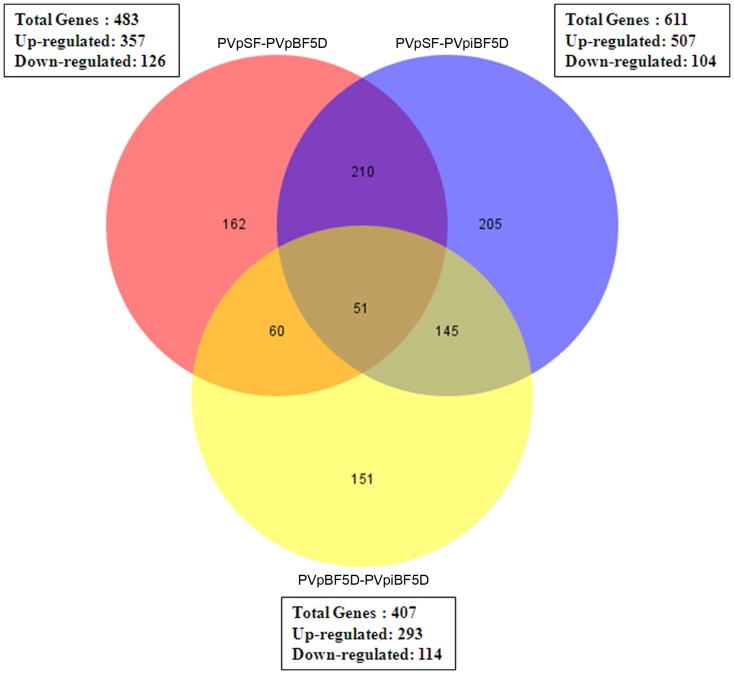
To understand oxidative stress in An. stephensi during P. vinckei petteri infection, it is important to understand the regulation of the An. stephensi transcripts at this stage. For this purpose, we analyzed the expression pattern of the transcripts both at their relative abundance state and the consequence at the related impacted pathways. At the transcript level, within each library, on the basis of fold change in abundance, and P-value, a total 1501 genes were found to be differentially expressed in all the libraries taken together. Upon blood feeding, 483 genes were found to be differentially expressed, out of which 357 genes were found to be up regulated and 126 genes were down regulated. Upon parasitized blood feeding, 611 genes were differentially expressed out of which 507 genes were up regulated and 104 genes were down regulated. When compared between PVpiBF5D and PVpBF5D libraries that would emphasize on role of parasite development in the mosquito, 407 genes were found to be differentially expressed of which 293 genes to be up regulated and 114 genes were down-regulated. (Figure 3).
Figure 3. Venn diagram representing data summary of differentially expressed Anopheles genes from RNA-seq data.
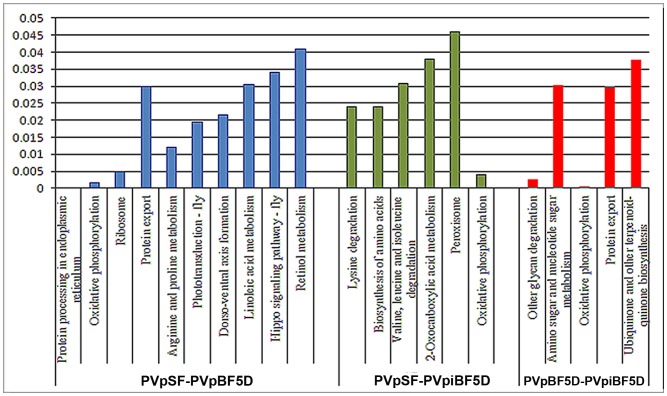
Additionally, the differentially regulated transcripts of all the three libraries were analyzed for their pathway information. The transcripts were clustered into different pathways using KOBAS web server [41] (Fig. 4). Upon blood feeding, ten pathways were found to be significantly regulated (P value <0.05), four to be up-regulated and six found to be down-regulated. Upon parasitized blood feeding, it was found that out of six significant pathways, five pathways were down-regulated, with oxidative phosphorylation (OXPHOS) as the only pathway found to be up-regulated. When the infected blood fed and blood fed libraries were compared to see the impact of parasite development, it was seen that parasite development regulates five pathways significantly of which two were up-regulated and three were down-regulated. It is noteworthy that OXPHOS was up-regulated in both infected PVpSF5D vs PVpiBF5D and PVpiBF5D vs PVpBF5D libraries with high significance (p value <0.005), emphasizing the role of oxidative stress in Anopheles due to the Plasmodium development.
Figure 4. KOBAS analysis of differentially expressed genes.
Graph represents the significant pathways predicted after KOBAS analysis.
This interesting finding prompted us to further investigate those transcripts in these regulated OXPHOS pathways. We found a total of 20 genes to be impacted due to blood feeding and Plasmodium infection with most of the genes being part of the OXPHOS and the electron transport chain (Table 2). Previous studies have established the role of these important pathways in blood feeding in mosquitoes [42], [43]. Similarly, effects of Plasmodium infection in Anopheles OXPHOS have paved the way for better understanding of melanization in Anopheles [44]. Moreover, research has shown conserved nature of the OXPHOS genes within insects [45] and the importance and distribution of mitochondria in the midgut epithelia of mosquitoes [46], [47]. Recent studies have established the existence of dynamic mitochondrial supercomplexes on mammals, plants, yeast and bacteria [48]–[50]. These supercomplexes are categorized into five complexes based on their location and interaction with each other within the inner membrane of mitochondria [51]. In our transcriptome analysis, we observed that Complex I and IV were the most regulated during Plasmodium development while Complex III was also impacted upon blood feeding.
Table 2. The table shows the genes impacted due to blood feeding and Plasmodium infection.
| Gene stable ID | Gene description | PVpSF | PVpBF5D | PVpiBF5D |
| ASTE000780 | cytochrome c oxidase subunit 6b | 239.485 | 64.1909 | 66.4754 |
| ASTE001936 | V-type H+-transporting ATPase S1 subunit | 809.598 | 495.414 | 1122.98 |
| ASTE002173 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4 | 53473.4 | 62413.1 | 177530 |
| ASTE003681 | F-type H+-transporting ATPase subunit f | 29635.9 | 35116.4 | 127126 |
| ASTE004313 | cytochrome c oxidase subunit VIIa | 19671.2 | 16596.3 | 54387.4 |
| ASTE004686 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 4 | 704.049 | 378.93 | 877.12 |
| ASTE006397 | Mitochondrial cytochrome c oxidase subunit VIC | 4825.3 | 1808.03 | 4017.93 |
| ASTE006537 | NADH dehydrogenase (ubiquinone) Fe-S protein 5 | 1672.32 | 551.183 | 1042.74 |
| ASTE006599 | V-type H+-transporting ATPase subunit G | 5330.65 | 10551.3 | 20947.1 |
| ASTE007332 | Cytochrome b-c1 complex subunit 6, mitochondrial | 2365.17 | 4372.13 | 15279.9 |
| ASTE007457 | cytochrome c oxidase subunit 6a, mitochrondrial | 720.388 | 262.231 | 398.623 |
| ASTE007459 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 1 | 1149.47 | 2322.19 | 4313.28 |
| ASTE007790 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 1 | 2571.73 | 755.536 | 530.959 |
| ASTE007986 | V-type H+-transporting ATPase subunit G | 1152.51 | 2491.39 | 4094.67 |
| ASTE009530 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 5 | 969.942 | 672.816 | 1840.72 |
| ASTE009682 | V-type H+-transporting ATPase subunit D | 821.749 | 421.051 | 1207.4 |
| ASTE010674 | NADH dehydrogenase (ubiquinone) 1 subcomplex unknown 2 | 599.622 | 426.454 | 1739.31 |
| ASTE011398 | F-type H+-transporting ATPase subunit g | 14815.4 | 22686.2 | 53015.7 |
| ASTE011779 | cytochrome c oxidase assembly protein subunit 17 | 180.156 | 319.84 | 489.181 |
| ASTE014405 | ubiquinol-cytochrome c reductase subunit 9 | 1604.72 | 95.9353 | 160.348 |
Most of the genes play role in the OXPHOS and the electron transport chain.
Anopheles genes prediction in oxidative stress pathways using SVM
Comparative genomics can be used for the functional annotation of genomes that are not annotated completely. We annotated assembled transcriptome of the An. stephensi genome by identifying homologs of An. gambiae genes using VectorBase and Blast2GO tool. However, from a total of 13650 genes, 2516 genes were remained unannotated. We utilized PSSM based SVM classifier to annotate these genes and predicted putative genes that may be playing a role in the redox system of the mosquito during Plasmodium development. A total of 1352 non-redundant transcripts were classified into 8 different pathways according to their SVM scores (Table S3). The robust prediction performance of the SVM models is assured by ROC analysis (Figure S1). These genes were further utilized in generation of the oxidative stress network of Anopheles. The accuracy for Citric acid cycle was found to be ∼84%, PPP∼100%, Oxidative phosphorylation ∼98%, Jak ∼96%, MAPK ∼98%, Glycolysis ∼96% , TGF ∼96% and WNT ∼90%. The AUC (Area under Curve) values of these pathways supported the accuracy of the pathway models. The AUC value for the Glycolysis ∼0.9801, Citric acid cycle with AUC value of 0.8571, PPP with highest value 1, oxidative phosphorylation ∼0.9704, TGF ∼0.9978, WNT ∼0.944, for MAPK it is 0.9821 and for JAK is predicted to be 0.9762.
Analysis of An. stephensi gene-gene co-expression network
In the last decade, importance of gene interaction network using integrated data sets in providing insights to gene functions and their interactions is evident from several studies [52]–[54]. The purpose of the study was to generate a network of the redox system of An. stephensi during P. vinckei petteri development and to understand the interactions of these participating genes during Plasmodium invasion of the midgut. For this purpose, it was important first to generate a broad interaction network of An. stephensi before segregating the redox related sub-set. For this purpose, we utilized the annotated genes of our transcriptome dataset to arrive upon a reference network on to which the oxidative stress genes identified and predicted in our study were mapped to infer the oxidative stress sub-network network of An. stephensi upon P. vinckei petteri infection with 516 nodes and 2904 edges (Fig. 4). The reference network was generated keeping default correlation threshold of −0.95 and 0.95. The final reference network was made up of 8871 non-redundant genes.
Oxidative stress gene clusters using microarray data analysis
Anopheles data set E-MEXP-378 was an exhaustive microarray experiment involving time-points during the invasion of ookinete into the midgut of Anopheles. The original study was a functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion [55] using a MMC1 (or 20 K) platform having total of 19,680 EST clones. The elegant experiment setup consisted of 53 sample data sets at three time points of ookinete invasion, namely 18–22 hrs post infection, 24–28 hrs post infection and 40–44 hrs post infection. A significant outcome of this study was the identification of remodeling/restructuring of the actin and microtubule cytoskeleton network due to Plasmodium infection. In the present study, we identified differentially expressed An. stephensi genes in oxidative stress during the early stages of P. vinckei petteri invasion.
Since Anopheles gambiae genome is poorly annotated, we extrapolated orthologs/paralogs of redox system from a Drosophila microarray dataset. A previous study has utilized the robustness of the Drosophila system to identify genes that regulate Plasmodium growth in the mosquito [56]. For our analysis, we utilized the dataset from E-MEXP-1859 which originally was a Drosophila dataset with Plasmodial genes knocked in to understand the function of two important Plasmodium invasion molecules [57]. The study used Drosophila, a model system, to understand the role of Plasmodial surface antigens in Plasmodium invasion. The authors were able to provide evidence of the role of immunity genes in this process using 21 chips of 13,614 Drosophila genes. In our study, in addition to identifying genes affected upon Plasmodium maturation, this dataset was selected as reference data to identify genes playing role in oxidative stress but not yet annotated in Anopheles.
Previous studies have highlighted the impact of bacterial infection on the redox status in mosquitoes [19]. In order to identify those redox genes that are specific to Plasmodium invasion, another Drosophila microarray dataset (GSE11012) involving bacterial infection was selected as a control [58]. The study was performed over different time points of bacterial infection using 13842 unique gene IDs. The genes common to bacterial infections were excluded from analysis.
For the purpose of identifying genes that may play a role in oxidative stress of Anopheles during Plasmodium development, detailed computational analysis was performed on the selected microarray datasets (see Materials and Methods). The entire probe Ids of the datasets were converted to their respective gene Ids separately, using an in-house Perl script. Cluster analysis using Non-negative matrix factorization (NMF) algorithm was performed those clusters with co-phenetic coefficient of 0.9611 and six clusters were selected for further analysis.
Gene Set Enrichment Analysis (GSEA) analysis of data E-MEXP-378 and E-MEXP-1859 revealed six pathways each having significant P-values and OXPHOS pathway was found to be common among them. GSEA results showed cytoskeleton organization and biogenesis, Wnt signaling pathway, JAK-STAT, p53 signaling pathway, pentose phosphate pathway and OXPHOS as significant pathways for E-MEXP-378 and likewise oxidative phosphorylation, response to oxidative stress, Toll pathway, response to ROS, melanization defense response and hydrogen peroxide catabolic response as significant pathways for E-MEXP-1859 dataset. The 13 common differentially expressed significant genes in dataset E-MEXP-1859 and GSE11012 were removed from the further analysis so as to reduce the false positive results. Detailed analysis of these datasets revealed 40 genes of An. gambiae (Table S2) and 145 genes of D. melanogaster to play a key role in oxidative stress response.
Redox system of Anopheles is a complex network of gene interactions
For the purpose of inferring the redox system of Anopheles, a three prong approach was used. Our aim was to integrate different types of data sets and extract information for most of the genes that are playing role in oxidative stress in Anopheles including unannotated genes, information of genes in other insects, extract information available in public domain and our own experimental data. Previous studies performed by integrating such information have yielded much information in predicting gene function [59], [60].
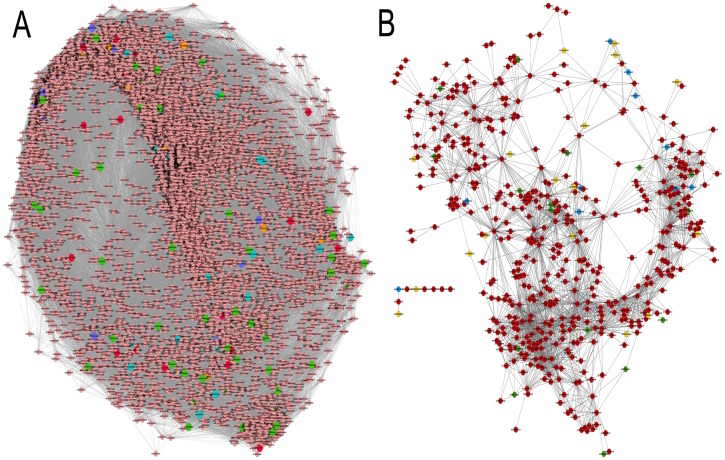
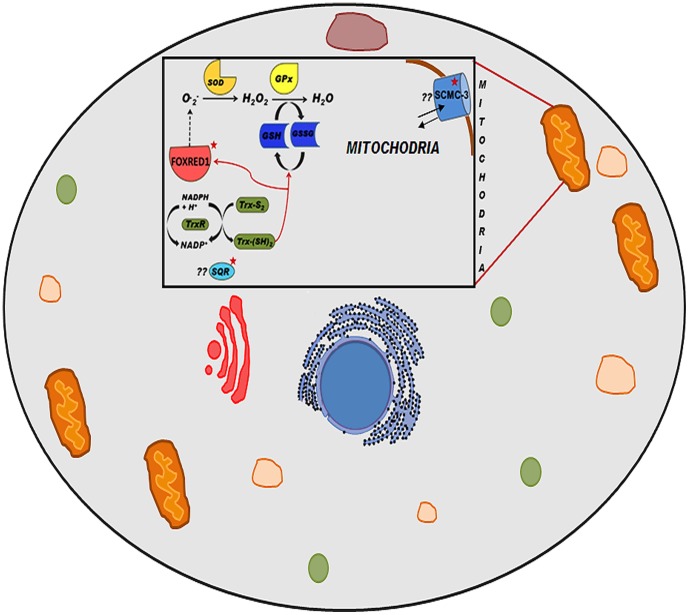
The different methodologies finally resulted in a cluster of transcripts playing role in oxidative pathways (Table 3). Furthermore, those transcripts that were significantly regulated were mapped onto the reference network (Figure 5a) and a sub-network was extracted out of the meta-network (Figure 5b). Ookinete invasion of the midgut is triggered off by the adhesion of the parasite ookinete onto the epithelium of the mosquito midgut resulting in activation of processes that produce reactive oxygen species. Through our study including both de-novo study and reference mapping study using TopHat and Cufflinks, we hypothesize the involvement of some new molecules predicted by SVM and gene ontology in oxidative stress in Anopheles (Figure 6). Maintenance of ROS is accomplished by reduction of O2 to H2O while maximizing ATP synthesis [61] which is accomplished by the action of several enzymes like super oxide dismutase (SOD) and glutathione peroxidases. It is known that superoxide that is formed in mitochondria is produced by respiratory complexes and is detoxified by the action of the several species of SODs. Network generated in our study propose a possible role of FAD-dependent oxidoreductase domain-containing protein 1 (FOXRED1) in this conversion. It is known that FOXRED1 is localized in mitochondria and known to have chaperonic functionality in mitochondria complex 1 [62]. However, this has not been identified in midguts of mosquito prior to our study. Another possible involvement is that of Sulfide: Quinone oxidoreductase (SQR) in the reduction of thioredoxin in the thioredoxin-proxiredoxin pathway to limit accumulation of peroxides [63]. In addition to the above molecules, our study has proposed the role of small calcium binding mitochondrial carrier protein 3 (SCMC 3) in Anopheles redox homeostasis. Role of calcium in maintaining mitochondrial function and combating oxidative stress has been well documented [64].
Table 3. Expression pattern of the RNA-seq and microarray predicted genes related to oxidative stress.
| Gene | Status | SF | BF5D | iBF5D |
| ASTE000971 | Annotated | 381.968 | 319.061 | 405.627 |
| ASTE003004 | Annotated | 47.9869 | 40.0328 | 27.2383 |
| ASTE003715 | Annotated | 27.2428 | 23.6363 | 13.0793 |
| ASTE004575 | Annotated | 500.691 | 356.958 | 332.761 |
| ASTE004646 | Annotated | 50.6197 | 42.9242 | 72.3875 |
| ASTE006040 | Annotated | 12.7964 | 21.5695 | 31.2505 |
| ASTE006069 | Annotated | 598.912 | 490.404 | 334.513 |
| ASTE006760 | Annotated | 287.084 | 180.579 | 341.974 |
| ASTE007589 | Annotated | 3.80819 | 4.85993 | 16.6953 |
| ASTE008571 | Annotated | 68.1175 | 28.406 | 42.6152 |
| ASTE009039 | Annotated | 0.423056 | 0.170206 | 0.229036 |
| ASTE010206 | Annotated | 332.959 | 593.219 | 303.61 |
| ASTE010699 | Annotated | 10.8378 | 8.66985 | 12.0489 |
| ASTE010862 | Annotated | 26.6517 | 20.8814 | 22.1117 |
| ASTE001492 | Annotated;GO | 4.81434 | 3.51005 | 5.44027 |
| ASTE003100 | Annotated;GO | 686.938 | 848.5 | 1261.81 |
| ASTE006711 | Annotated;GO | 174.985 | 203.972 | 182.272 |
| ASTE008907 | Annotated;GO | 1812.43 | 1262.74 | 2702.9 |
| ASTE009813 | Annotated;GO | 350.706 | 72.1614 | 73.289 |
| ASTE010772 | Annotated;GO | 527.803 | 391.384 | 471.926 |
| ASTE011022 | Annotated;GO | 3193.95 | 7203.98 | 22057.8 |
| ASTE000131 | GO | 47.282 | 41.9047 | 96.9806 |
| ASTE000143 | GO | 9.26815 | 5.86659 | 6.20112 |
| ASTE000912 | GO | 86.6084 | 130.991 | 80.9358 |
| ASTE001043 | GO | 210.914 | 102.15 | 147.858 |
| ASTE001249 | GO | 153.995 | 102.33 | 201.927 |
| ASTE001371 | GO | 128.209 | 100.369 | 85.1941 |
| ASTE001567 | GO | 242.662 | 628.562 | 512.411 |
| ASTE001773 | GO | 7351.09 | 5017.66 | 6439.2 |
| ASTE002909 | GO | 115.25 | 121.663 | 32.7457 |
| ASTE002914 | GO | 162.207 | 122.774 | 58.1257 |
| ASTE002991 | GO | 13.0357 | 27.1839 | 34.4705 |
| ASTE003073 | GO | 3.95207 | 5.35709 | 6.64083 |
| ASTE003130 | GO | 111.493 | 70.7715 | 125.698 |
| ASTE003223 | GO | 18.1754 | 14.4909 | 19.7527 |
| ASTE003848 | GO | 3.05716 | 3.12325 | 2.94652 |
| ASTE004135 | GO | 107.759 | 112.337 | 60.473 |
| ASTE004515 | GO | 179.896 | 135.373 | 139.161 |
| ASTE004690 | GO | 38.7047 | 30.4935 | 43.1306 |
| ASTE004709 | GO | 4.87481 | 7.14552 | 13.1994 |
| ASTE004710 | GO | 8.93586 | 10.5808 | 22.1294 |
| ASTE005121 | GO | 3.3418 | 19.4879 | 20.4617 |
| ASTE005165 | GO | 412.232 | 743.759 | 654.745 |
| ASTE005387 | GO | 8.67074 | 55.0254 | 4.73109 |
| ASTE005445 | GO | 251.465 | 152.927 | 107.231 |
| ASTE005602 | GO | 371.038 | 314.707 | 204.804 |
| ASTE005696 | GO | 77.6152 | 347.119 | 236.1 |
| ASTE006080 | GO | 106.617 | 41.7707 | 47.5019 |
| ASTE006277 | GO | 143.891 | 219.73 | 109.634 |
| ASTE006361 | GO | 48.7165 | 43.3185 | 23.9857 |
| ASTE006397 | GO | 4825.3 | 1808.03 | 4017.93 |
| ASTE006501 | GO | 180.108 | 296.007 | 79.8115 |
| ASTE007457 | GO | 720.388 | 262.231 | 398.623 |
| ASTE007781 | GO | 138.289 | 138.146 | 182.986 |
| ASTE007782 | GO | 144.385 | 111.405 | 62.9876 |
| ASTE007973 | GO | 152.821 | 126.328 | 81.7444 |
| ASTE008071 | GO | 161.366 | 90.8716 | 84.8784 |
| ASTE008211 | GO | 3.89653 | 2.99567 | 6.48923 |
| ASTE008276 | GO | 481.106 | 321.01 | 140.308 |
| ASTE008687 | GO | 111.786 | 72.2075 | 115.313 |
| ASTE008731 | GO | 37.1639 | 200.549 | 160.301 |
| ASTE008825 | GO | 16.4813 | 30.7846 | 5.32786 |
| ASTE008900 | GO | 99.0236 | 81.4236 | 78.6687 |
| ASTE009014 | GO | 18.4728 | 16.4796 | 18.2949 |
| ASTE009146 | GO | 217.069 | 185.006 | 178.595 |
| ASTE009370 | GO | 86.3904 | 77.4245 | 120.952 |
| ASTE009555 | GO | 24.3915 | 36.7647 | 33.2701 |
| ASTE009860 | GO | 70.0946 | 49.8105 | 9.94767 |
| ASTE010293 | GO | 343.966 | 322.758 | 399.022 |
| ASTE010572 | GO | 26.2349 | 20.7426 | 17.9355 |
| ASTE010775 | GO | 7.49661 | 7.50756 | 7.59294 |
| ASTE011003 | GO | 11.0987 | 8.78333 | 5.66391 |
These genes were selected on the basis of GO terms, and microarray analysis. RPKM values of all the genes are also shown in the table.
*The analysis was performed using Anopheles gambiae genes. Anopheles gambiae IDs were then converted into Anopheles stephensi IDs using BioMart tool of VectorBase.
Figure 5. Gene interaction Co-expression network of Anopheles stephensi.
(a) Meta-network showing interaction between the genes. The oxidative stress genes are highlighted in different colors. (b) A sub-network representing the interaction between oxidative stress related genes. Red color nodes shows genes predicted using SVM, Green color represents the GSEA predicted genes, Dark yellow node represent GO predicted genes, Light blue shows already annotated genes.
Figure 6. Figure representing the hypothetical model of oxidative stress pathway.
This model includes 3 predicted proteins namely, FOXRED1, SCMC3 and SQR represented by red star.
The cluster of genes involved in oxidative stress in Anopheles in our study is quite exhaustive. We have utilized both computational and high throughput data generation platforms to infer an almost complete redox system of Anopheles and proposed a model where new molecules could be playing important roles in oxidative stress of the malaria vector. Our proposed equilibrium awaits experimental validation.
Supporting Information
ROC plot showing the performance of the SVM models of different pathways.
(TIF)
Table showing the data used in the study.
(DOCX)
Table representing the GSEA predicted Anopheles genes (microarray data) which may play role in oxidative stress.
(DOCX)
The excel sheet shows the SVM summary and the predicted SVM scores of unannotated transcripts.
(XLS)
Acknowledgments
The RNA-seq library construction and sequencing were performed by NxGenBio Life Sciences, New Delhi, India. We acknowledge the technical assistance of Ms. Shanu Jain in An. stephensi RNA isolation.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All the files are available from the SRA database (accession number SRX671574).
Funding Statement
SS is a recipient of Ramanujan fellowship, Department of Science and Technology, Government of India, and their support is duly acknowledged. The study was funded through grant number "SR/S2/RJN-53/2009". The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beutler B, Moresco EMY (2008) The forward genetic dissection of afferent innate immunity. Immunology, Phenotype First: How Mutations Have Established New Principles and Pathways in Immunology: Springer. pp.3–26. [DOI] [PubMed]
- 2. Iwanaga S, Lee B-L (2005) Recent advances in the innate immunity of invertebrate animals. BMB Reports 38:128–150. [DOI] [PubMed] [Google Scholar]
- 3. Jomova K, Lawson M, Gal'a L (2012) PROOXIDANT EFFECT OF LYCOPENE ON TRIGLYCERIDE OXIDATION. Journal of Microbiology, Biotechnology and Food Sciences 1 [Google Scholar]
- 4. Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, et al. (2003) Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424:561–565. [DOI] [PubMed] [Google Scholar]
- 5. Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267. [DOI] [PubMed] [Google Scholar]
- 6. Gostner JM, Becker K, Fuchs D, Sucher R (2013) Redox regulation of the immune response. Redox Report 18:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Current medicinal chemistry. pp.1161–1208. [DOI] [PubMed]
- 8. Hunt NH, Stocker R (1989) Oxidative stress and the redox status of malaria-infected erythrocytes. Blood cells 16:499–526 discussion 527–430. [PubMed] [Google Scholar]
- 9. Jortzik E, Becker K (2012) Thioredoxin and glutathione systems in Plasmodium falciparum. International Journal of Medical Microbiology 302:187–194. [DOI] [PubMed] [Google Scholar]
- 10. Muller S (2004) Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Molecular microbiology 53:1291–1305. [DOI] [PubMed] [Google Scholar]
- 11. Nepveu Fo, Turrini F (2013) Targeting the redox metabolism of Plasmodium falciparum. Future medicinal chemistry 5:1993–2006. [DOI] [PubMed] [Google Scholar]
- 12. Pal C, Bandyopadhyay U (2012) Redox-active antiparasitic drugs. Antioxidants & redox signaling 17:555–582. [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos G (2003) Insect immunity and its implication in mosquito-malaria interactions. Cellular microbiology 5:3–14. [DOI] [PubMed] [Google Scholar]
- 14. Duran-Bedolla J, Rodriguez MH, Saldana-Navor V, Rivas-Arancibia S, Cerbon M, et al. (2013) Oxidative stress: production in several processes and organelles during Plasmodium sp development. Oxidants and Antioxidants in Medical Science 2:93–100. [Google Scholar]
- 15. Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, et al. (2009) The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS pathogens 5:e1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dessens JT, Siden-Kiamos I, Mendoza J, Mahairaki V, Khater E, et al. (2003) SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Molecular microbiology 49:319–329. [DOI] [PubMed] [Google Scholar]
- 17. Han YS, Thompson J, Kafatos FC, Barillas-Mury C (2000) Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. The EMBO journal 19:6030–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327:1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, et al. (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. Journal of biological chemistry 283:3217–3223. [DOI] [PubMed] [Google Scholar]
- 20. Peterson TML, Gow AJ, Luckhart S (2007) Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radical Biology and Medicine 42:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kogenaru S, Yan Q, Guo Y, Wang N (2012) RNA-seq and microarray complement each other in transcriptome profiling. BMC genomics 13:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, et al. (2008) Hepatitis C virus infection protein network. Molecular systems biology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forst CV (2006) Host-pathogen systems biology. Infectious Disease Informatics: Springer. pp.123–147.
- 24. Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, et al. (2003) A protein interaction map of Drosophila melanogaster. Science 302:1727–1736. [DOI] [PubMed] [Google Scholar]
- 25. Andrews S (2010) FastQC: A quality control tool for high throughput sequence data. Reference Source [Google Scholar]
- 26. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature biotechnology 29:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, et al. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols 7:562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. [DOI] [PubMed] [Google Scholar]
- 30. Kalita MK, Nandal UK, Pattnaik A, Sivalingam A, Ramasamy G, et al. (2008) CyclinPred: a SVM-based method for predicting cyclin protein sequences. PloS one 3:e2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Niu B, Gao Y, Fu L, Li W (2006) CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26:680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315. [DOI] [PubMed] [Google Scholar]
- 33.Smyth GK (2005) Limma: linear models for microarray data. Bioinformatics and computational biology solutions using R and Bioconductor: Springer. pp.397–420.
- 34.Lee DD, Seung HS. Algorithms for non-negative matrix factorization; 2000. pp.556–562.
- 35. Shi J, Walker MG (2007) Gene set enrichment analysis (GSEA) for interpreting gene expression profiles. Current Bioinformatics 2:133–137. [Google Scholar]
- 36.Niissalo A (2007) Cytoscape and its Plugins.
- 37. Joyce AR, Palsson B (2006) The model organism as a system: integrating'omics' data sets. Nature Reviews Molecular Cell Biology 7:198–210. [DOI] [PubMed] [Google Scholar]
- 38. Mohien CU, Colquhoun DR, Mathias DK, Gibbons JG, Armistead JS, et al. (2012) A bioinformatics approach for integrated transcriptomic and proteomic comparative analyses of model and non-sequenced anopheline vectors of human malaria parasites. Molecular & Cellular Proteomics 12:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu X, Gerstein M, Snyder M (2007) Getting connected: analysis and principles of biological networks. Genes & development 21:1010–1024. [DOI] [PubMed] [Google Scholar]
- 40. Montalvo Alvarez AM, Landau I, Baccam D (1991) Plasmodium vinckei petteri: some aspects of its sporogony and exoerythrocytic schizogony. Revista do Instituto de Medicina Tropical de Sao Paulo 33:421–426. [PubMed] [Google Scholar]
- 41. Wu J, Mao X, Cai T, Luo J, Wei L (2006) KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic acids research 34:W720–W724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Das S, Radtke A, Choi Y-J, Mendes AM, Valenzuela JG, et al. (2010) Transcriptomic and functional analysis of the Anopheles gambiae salivary gland in relation to blood feeding. BMC genomics 11:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanders HR, Evans AM, Ross LS, Gill SS (2003) Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect biochemistry and molecular biology 33:1105–1122. [DOI] [PubMed] [Google Scholar]
- 44. Kumar S, Christophides GK, Cantera R, Charles B, Han YS, et al. (2003) The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proceedings of the National Academy of Sciences 100:14139–14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tripoli G, D'Elia D, Barsanti P, Caggese C (2005) Comparison of the oxidative phosphorylation (OXPHOS) nuclear genes in the genomes of Drosophila melanogaster, Drosophila pseudoobscura and Anopheles gambiae. Genome biology 6:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark TM, Hutchinson MJ, Huegel KL, Moffett SB, Moffett DF (2005) Additional morphological and physiological heterogeneity within the midgut of larval Aedes aegypti(Diptera: Culicidae) revealed by histology, electrophysiology, and effects of Bacillus thuringiensis endotoxin. Tissue and Cell 37:457–468. [DOI] [PubMed] [Google Scholar]
- 47.Lehane M, Billingsley P (1996) Biology of the insect midgut: Springer.
- 48. Dudkina NV, Kouil R, Peters K, Braun H-P, Boekema EJ (2010) Structure and function of mitochondrial supercomplexes. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1797:664–670. [DOI] [PubMed] [Google Scholar]
- 49. Nubel E, Wittig I, Kerscher S, Brandt U, Schagger H (2009) Two-dimensional native electrophoretic analysis of respiratory supercomplexes from Yarrowia lipolytica. Proteomics 9:2408–2418. [DOI] [PubMed] [Google Scholar]
- 50. Wittig I, Schagger H (2009) Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1787:672–680. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Cruz O, Sanchez-Paz A, Garcia-Carreao F, Jimenez-Gutierrez L Invertebrates Mitochondrial Function and Energetic Challenges.
- 52. Linksvayer TA, Fewell JH, Gadau J, Laubichler MD (2012) Developmental evolution in social insects: regulatory networks from genes to societies. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 318:159–169. [DOI] [PubMed] [Google Scholar]
- 53. Seyres D, Rider L, Perrin L (2012) Genes and networks regulating cardiac development and function in flies: genetic and functional genomic approaches. Briefings in functional genomics 11:366–374. [DOI] [PubMed] [Google Scholar]
- 54. Zmasek CM, Godzik A (2013) Evolution of the animal apoptosis network. Cold Spring Harbor perspectives in biology 5:a008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC (2005) Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol 15:1185–1195. [DOI] [PubMed] [Google Scholar]
- 56. Brandt SM, Jaramillo-Gutierrez G, Kumar S, Barillas-Mury C, Schneider DS (2008) Use of a Drosophila model to identify genes regulating Plasmodium growth in the mosquito. Genetics 180:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yan J, Yang X, Mortin MA, Shahabuddin M (2009) Malaria sporozoite antigen-directed genome-wide response in transgenic Drosophila. genesis 47:196–203. [DOI] [PubMed] [Google Scholar]
- 58. Douglas AE, Bouvaine S, Russell RR (2011) How the insect immune system interacts with an obligate symbiotic bacterium. Proceedings of the Royal Society B: Biological Sciences 278:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Costello JC, Dalkilic MM, Beason SM, Gehlhausen JR, Patwardhan R, et al. (2009) Gene networks in Drosophila melanogaster: integrating experimental data to predict gene function. Genome Biol 10:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee I, Lehner B, Crombie C, Wong W, Fraser AG, et al. (2008) A single gene network accurately predicts phenotypic effects of gene perturbation in Caenorhabditis elegans. Nature genetics 40:181–188. [DOI] [PubMed] [Google Scholar]
- 61. Inoue M, Sato EF, Nishikawa M, Park A-M, Kira Y, et al. (2003) Mitochondrial generation of reactive oxygen species and its role in aerobic life. Current medicinal chemistry 10:2495–2505. [DOI] [PubMed] [Google Scholar]
- 62. Calvo SE, Tucker EJ, Compton AG, Kirby DM, Crawford G, et al. (2010) High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nature genetics 42:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Theissen U, Martin W (2008) Sulfide: quinone oxidoreductase (SQR) from the lugworm Arenicola marina shows cyanide and thioredoxin-dependent activity. FEBS journal 275:1131–1139. [DOI] [PubMed] [Google Scholar]
- 64. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. American Journal of Physiology-Cell Physiology 287:C817–C833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC plot showing the performance of the SVM models of different pathways.
(TIF)
Table showing the data used in the study.
(DOCX)
Table representing the GSEA predicted Anopheles genes (microarray data) which may play role in oxidative stress.
(DOCX)
The excel sheet shows the SVM summary and the predicted SVM scores of unannotated transcripts.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All the files are available from the SRA database (accession number SRX671574).