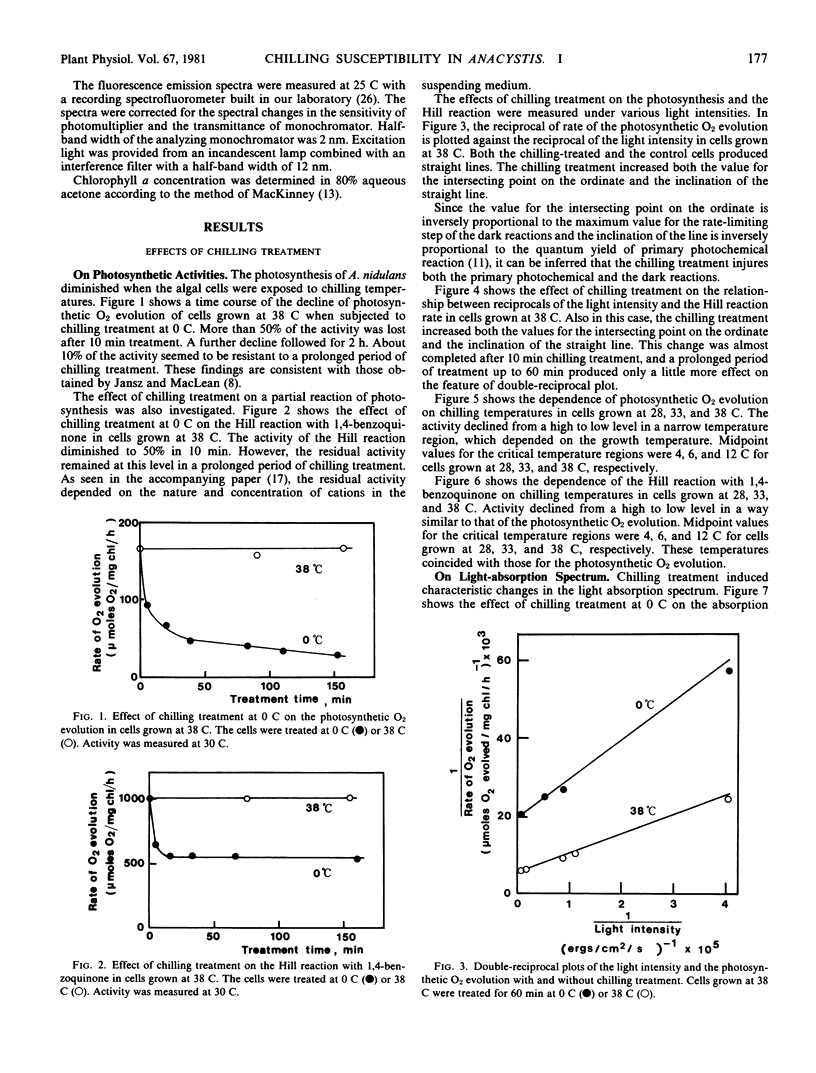
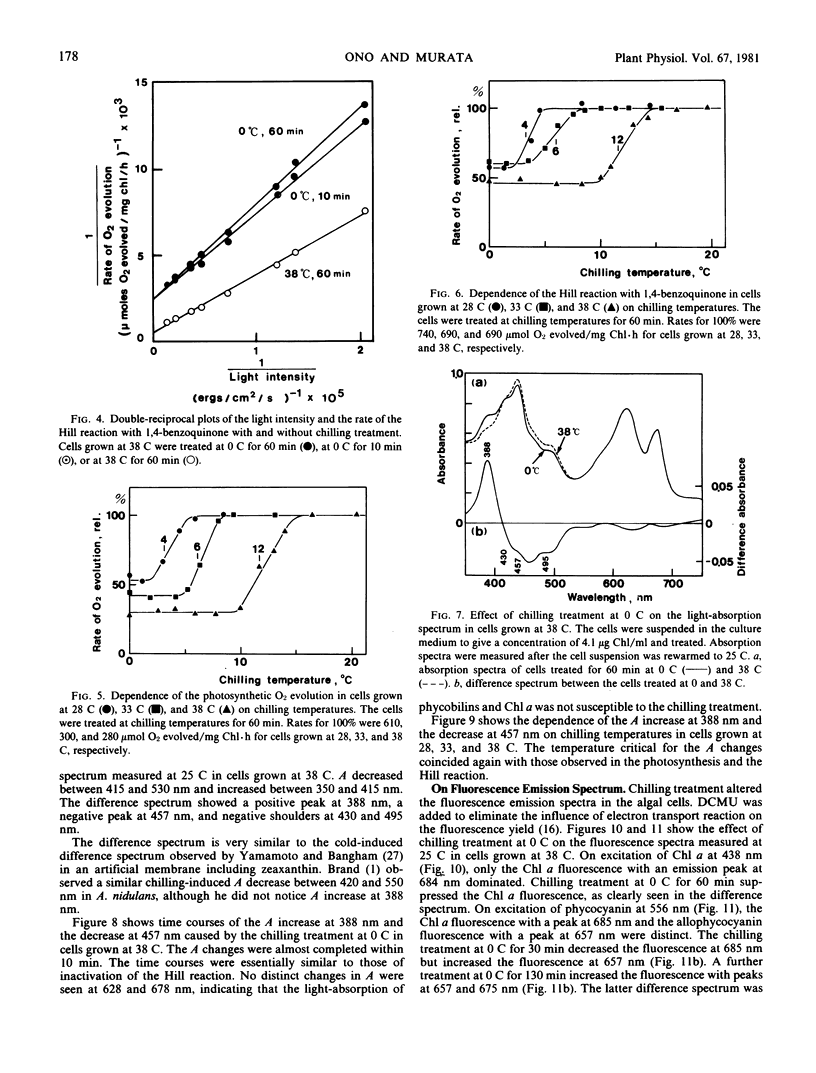
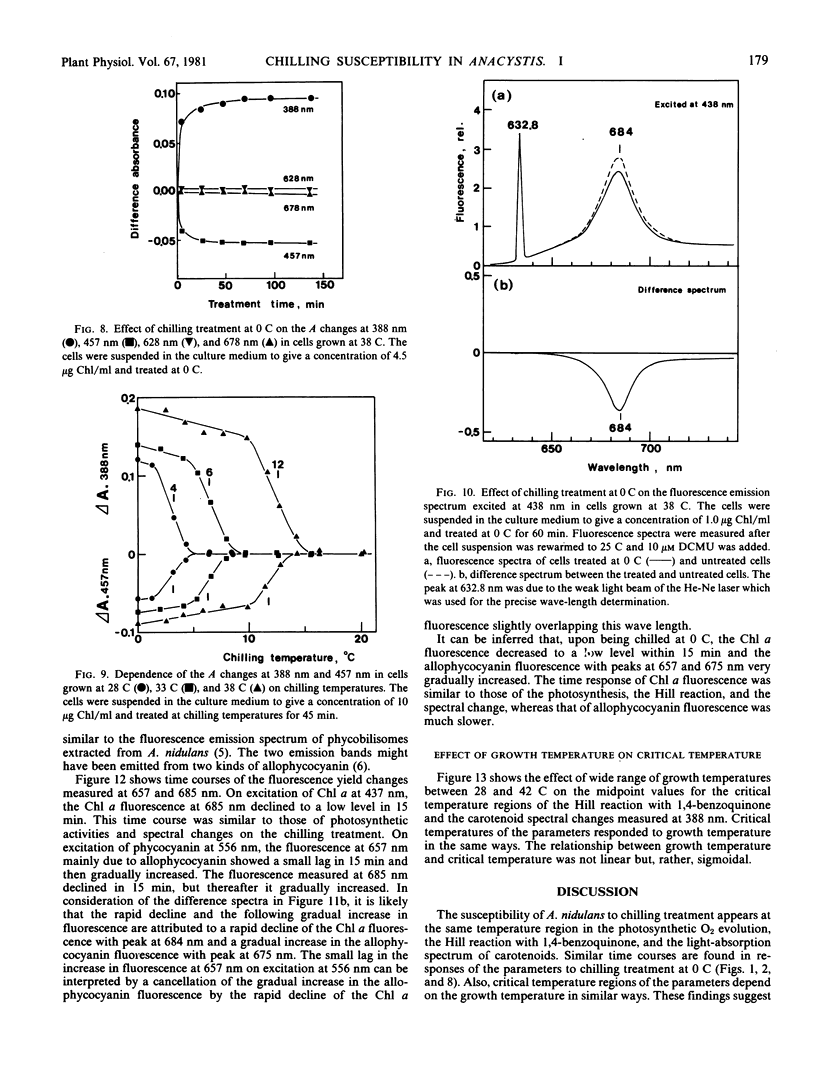
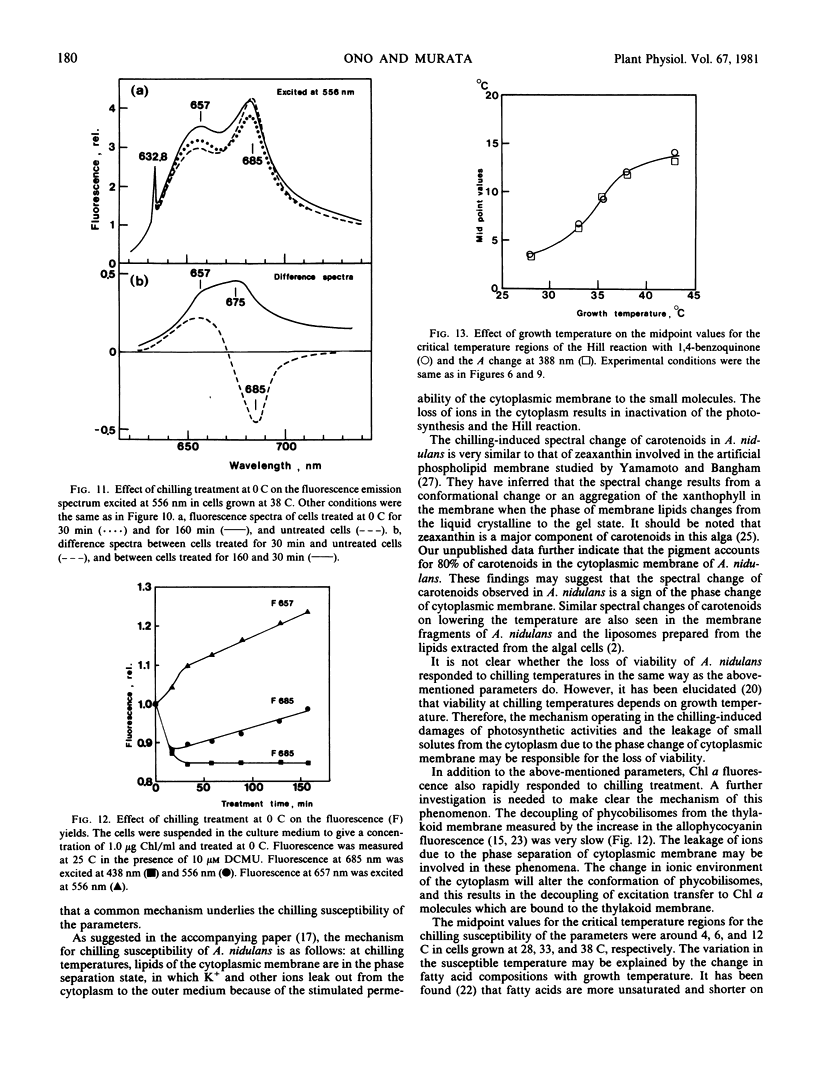
Abstract
Effects of chilling treatment on the photosynthetic activities and the light-absorption and fluorescence spectra were investigated in intact cells of the blue-green alga Anacystis nidulans that were grown at different temperatures. When the algal cells grown at 38 C were treated at 0 C for 10 minutes, the photosynthesis and the Hill reaction with 1,4-benzoquinone were significantly inactivated and the light-absorption spectrum of carotenoids was modified. These parameters showed very similar temperature dependencies in the chilling susceptibility and the temperature regions critical for the susceptibility depended on the growth temperature. The midpoint values for the critical temperature regions were 4, 6, and 12 C in cells grown at 28, 33, and 38 C, respectively. It is proposed that a common mechanism would underlie the chilling susceptibility of the photosynthesis, the Hill reaction, and the carotenoid absorption spectrum. The decoupling of excitation transfer from allophycocyanin to chlorophyll a at the chilling temperatures occurred very slowly and is attributed to a somewhat different mechanism of the chilling susceptibility.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand J. J. Spectral Changes in Anacystis nidulans Induced by Chilling. Plant Physiol. 1977 May;59(5):970–973. doi: 10.1104/pp.59.5.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand J. J. Spectral changes in membrane fragments and artificial liposomes of Anacystis induced by chilling. Arch Biochem Biophys. 1979 Apr 1;193(2):385–391. doi: 10.1016/0003-9861(79)90044-4. [DOI] [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Bryant D. A. Allophycocyanin B (lambdamax 671, 618 nm): a new cyanobacterial phycobiliprotein. Arch Microbiol. 1975 Jun 20;104(1):15–22. doi: 10.1007/BF00447294. [DOI] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Es G. A., Verkleij A. J., van Deenen L. L. Fragility of the permeability barrier of Escherichia coli. Biochim Biophys Acta. 1972 Oct 23;288(1):43–53. doi: 10.1016/0005-2736(72)90221-0. [DOI] [PubMed] [Google Scholar]
- Jansz E. R., Maclean F. I. The effect of cold shock on the blue-green alga Anacystis nidulans. Can J Microbiol. 1973 Mar;19(3):381–387. doi: 10.1139/m73-062. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- Murata N. Temperature dependence of chlorophyll a fluorescence in relation to the physical phase of membrane lipids algae and higher plants. Plant Physiol. 1975 Dec;56(6):791–796. doi: 10.1104/pp.56.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Chilling Susceptibility of the Blue-green Alga Anacystis nidulans: II. STIMULATION OF THE PASSIVE PERMEABILITY OF CYTOPLASMIC MEMBRANE AT CHILLING TEMPERATURES. Plant Physiol. 1981 Jan;67(1):182–187. doi: 10.1104/pp.67.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., McMurchie E. J., May B. K., Elliott W. H. Effect of growth temperature on membrane fatty acid composition and susceptibility to cold shock of Bacillus amyloliquefaciens. J Bacteriol. 1978 Sep;135(3):754–759. doi: 10.1128/jb.135.3.754-759.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Rao V. S., Brand J. J., Myers J. Cold Shock Syndrome in Anacystis nidulans. Plant Physiol. 1977 May;59(5):965–969. doi: 10.1104/pp.59.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieske J. S., Lumry R., Spikes J. D. The Mechanism of the Photochemical Activity of Isolated Chloroplasts. III. Dependence of Velocity on Light Intensity. Plant Physiol. 1959 May;34(3):293–300. doi: 10.1104/pp.34.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring K. The effect of low temperatures of permeability in Streptomyces hydrogenans. Biochem Biophys Res Commun. 1965 May 18;19(5):576–581. doi: 10.1016/0006-291x(65)90377-3. [DOI] [PubMed] [Google Scholar]
- Sato N., Murata N., Miura Y., Ueta N. Effect of growth temperature on lipid and fatty acid compositions in the blue-green algae, Anabaena variabilis and Anacystis nidulans. Biochim Biophys Acta. 1979 Jan 29;572(1):19–28. [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Stransky H., Hager A. Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. IV. Cyanophyceae und Rhodophyceae. Arch Mikrobiol. 1970;72(1):84–96. [PubMed] [Google Scholar]
- Sugiyama K. I., Murata N. Analyses of absorption and fluorescence spectra of water-soluble chlorophyll proteins, pigment system II particles and chlorophyll a in diethylether solution by the curve-fitting method. Biochim Biophys Acta. 1978 Jul 6;503(1):107–119. doi: 10.1016/0005-2728(78)90165-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Bangham A. D. Carotenoid organization in membranes. Thermal transition and spectral properties of carotenoid-containing liposomes. Biochim Biophys Acta. 1978 Feb 2;507(1):119–127. doi: 10.1016/0005-2736(78)90379-6. [DOI] [PubMed] [Google Scholar]



