Abstract
Oligosaccharyltransferase is a multiprotein complex that catalyzes asparagine-linked glycosylation of diverse proteins. Using yeast genetics and glycoproteomics, we found that transient interactions between nascent polypeptide and Ost3p/Ost6p, homologous subunits of oligosaccharyltransferase, were able to modulate glycosylation efficiency in a site-specific manner in vivo. These interactions were driven by hydrophobic and electrostatic complementarity between amino acids in the peptide-binding groove of Ost3p/Ost6p and the sequestered stretch of substrate polypeptide. Based on this dependence, we used in vivo scanning mutagenesis and in vitro biochemistry to map the precise interactions that affect site-specific glycosylation efficiency. We conclude that transient binding of substrate polypeptide by Ost3p/Ost6p increases glycosylation efficiency at asparagines proximal and C-terminal to sequestered sequences. We detail a novel mode of interaction between translocating nascent polypeptide and oligosaccharyltransferase in which binding to Ost3p/Ost6p segregates a short flexible loop of glycosylation-competent polypeptide substrate that is delivered to the oligosaccharyltransferase active site for efficient modification.
Asparagine (N)-linked glycosylation is an essential post-translational modification of secretory and membrane proteins in eukaryota and also occurs in archaea and some bacteria (1). Oligosaccharyltransferase (OTase)1 is an integral membrane protein that catalyzes N-glycosylation of nascent polypeptides in the lumen of the endoplasmic reticulum (ER) (2). Eukaryotic OTase is physically associated with the translocon (3) and transfers oligosaccharide from a dolichol-pyrophosphate carrier onto asparagine side-chains of substrate polypeptides (2). The efficiency of glycosylation of asparagine residues is dramatically increased if they are present in glycosylation “sequons” (N-x-T/S; x ≠ P), the peptide recognition motifs of the catalytic site of OTase (4). After the transfer of glycan to protein, the presence of N-glycosylation assists efficient glycoprotein folding in the ER intrinsically and by locally recruiting the disulfide isomerase ERp57 through the lectins calnexin and calreticulin (5). Correctly folded glycoproteins are free to traffic through the Golgi, where further modification and extension of glycan structures can occur (6). The precise glycan structures present on mature glycoproteins are often vital for their biological functions, including those involved in immune response, embryonic development, and cancer (6–8).
OTase in Baker's yeast, Saccharomyces cerevisiae, is a multiprotein complex consisting of eight subunits (2). The catalytic site is located in Stt3p, and other protein subunits whose functions have been investigated are required for the integrity of the complex and for regulation of substrate specificity. It has been proposed that the requirement of Ost1p (human ribophorin I) for efficient glycosylation of a subset of integral membrane proteins (9) is due to direct physical association (10) to retain potential substrates in close proximity to Stt3p (11). The details and mechanisms of this association are not known. Ost3p and Ost6p are homologous proteins, and the incorporation of either into OTase defines two isoforms of the enzyme with distinct protein substrate specificities (Fig. 1) (12–14). Efficient glycosylation of some asparagine residues requires Ost3p-OTase, whereas others require Ost6p-OTase (15). A model of Ost3p/Ost6p function in N-glycosylation site selection has been proposed (16) in which the ER-lumenal peptide-binding grooves transiently tether nascent polypeptide non-covalently or through mixed disulfides, inhibiting local protein folding and increasing the efficiency of glycosylation of nearby asparagine residues. Aspects of this model, including mixed-disulfide formation (17) and non-covalent peptide binding (18), have been tested in vitro. Although it has been established that Ost3p-OTase and Ost6p-OTase have different polypeptide substrate preferences in vivo (12–15, 19), the physiological relevance and details of any interactions between Ost3p/Ost6p and substrate polypeptide are unclear.
Fig. 1.

Overview of experimental manipulation of yeast OTase isoforms. A, OTase in wild-type yeast has eight subunits, with two isoforms defined by incorporation of either of the homologous Ost3p or Ost6p subunits. Yeast with only (B) Ost3p-OTase or (C) Ost6p-OTase was constructed via genomic deletion of OST3 and OST6 with overexpression of either Ost3p or Ost6p. D, yeast with a single variant OTase isoform was constructed through overexpression of variant Ost3p or Ost6p, for example, Ost3Q103K,Q106K.
Here, we used in vivo yeast genetics, glycoproteomics, and in vitro biochemistry to identify the precise sites of interaction between Ost3p/Ost6p and substrate polypeptides that affect the efficiency of N-glycosylation at diverse asparagines. Based on these data, we present a model in which transient binding of nascent polypeptide by Ost3p/Ost6p isolates newly translocating polypeptide from pre-translocated polypeptide, resulting in the formation of a short flexible loop of glycosylation-competent polypeptide substrate in close proximity to the active site of OTase for efficient modification.
EXPERIMENTAL PROCEDURES
Cloning and Mutagenesis
DNA encoding OST3 or OST6 including 1 kb up- and downstream were amplified using PCR and cloned into the YEp352 vector using BamHI and SalI restriction enzymes, yielding pOST3 and pOST6. DNA encoding GAS1 including 1 kb up- and downstream was amplified via PCR and cloned into the pRS415 vector using BamHI and SacI restriction enzymes, yielding pGAS1. The thioredoxin-like ER lumenal domain of Ost6p and the mature soluble domain of Gas1p cloned as MBP fusion proteins (pMAL-Ost6L and pMAL-Gas1, respectively) were used as described (18). Variants with amino acid replacements (pOST3Q103K,Q106K, pOST6K96Q,K99Q, pGAS1K34Q, pGAS1R47Q, pGAS1R76Q, pGAS1K82Q,K83Q, pGAS1R90Q, pGAS1K105Q, pMAL-GAS1R90Q) were constructed as described (20).
Yeast Strains
The OST3 and OST6 genes were genomically knocked out in BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) through homologous recombination of transformed PCR products to produce strain Δost3/Δost6 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 Δost3::KanMX Δost6::HisMX). Yeast strain Δost3/Δost6 was transformed with YEp352, pOST3, pOST6, pOST3Q103K,Q106K, or pOST6K96Q,K99Q to produce yeast expressing only a single isoform of OTase (Fig. 1) (14). To determine the precise sites of interaction between Gas1p nascent polypeptide and the Ost6p peptide binding groove in vivo, pGAS1, pGAS1K34Q, pGAS1R47Q, pGAS1R76Q, pGAS1K82Q,K83Q, pGAS1R90Q, or pGAS1K105Q was transformed into strain Δost3/Δost6 pOST6 or Δost3/Δost6 pOST6K96Q,K99Q. Yeast cells were grown to mid-log phase at 30 °C in minimal medium (0.67% yeast nitrogen base and 2% glucose with appropriate supplements to allow growth).
Cell Wall Protein Sample Preparation
Analysis of site-specific glycosylation occupancy of cell wall glycoproteins was performed as described (15). Briefly, cells were mechanically lysed, and insoluble cell wall material was reduced/alkylated and exhaustively washed. The resulting cell wall fraction was highly enriched in glycoproteins covalently linked to the cell wall polysaccharide through glycophosphatidylinositol remnants or alkaline-sensitive linkages. N-glycans were released with endoglycosidase H, leaving a single N-acetylglucosamine (GlcNAc) monosaccharide on previously glycosylated asparagines, and proteins were digested with trypsin. Peptides and glycopeptides were desalted with C18 ZipTips. Strains were analyzed in biological triplicate from independent transformants. Peptides and deglycosylated peptides were detected with LC-ESI-MS/MS.
Peptide Affinity Chromatography
MBP-Ost6L, MBP-Gas1, and MBP-Gas1R90Q were expressed in Escherichia coli carrying plasmids pMAL-OST6L, pMAL-GAS1, and pMAL-GAS1R90Q, respectively, and purified via amylose-agarose affinity chromatography as described (18). MBP-Gas1 and MBP-Gas1R90Q were eluted in column buffer (20 mm Tris HCl, pH 7.4, 200 mm NaCl, 1 mm EDTA) with 1% SDS, and MBP-Ost6L was retained on the amylose-agarose resin. MBP-Gas1 and MBP-Gas1R90Q were reduced, alkylated, precipitated, and digested with AspN as described (21). Residual AspN was inactivated by the addition of EDTA to 1 mm. Peptide affinity chromatography was performed as described (18). Briefly, peptides from equal amounts of MBP-Gas1 and MBP-Gas1R90Q in column buffer were combined and applied to amylose-agarose beads containing bound MBP-Ost6L, with the Ost6p CxxC motif either oxidized or reduced by prior incubation with DTT. Peptides were washed from the affinity matrix by means of sequential gravity flow washing with column buffer, and wash fractions were collected. Peptides in the load and wash fractions were then detected via LC-ESI-MS/MS.
Mass Spectrometry and Data Analysis
Peptides were analyzed via LC-ESI-MS/MS using a Prominence nanoLC system (Shimadzu, Kyoto, Japan) and TripleTof 5600 mass spectrometer with a Nanospray III interface (AB SCIEX, Washington, D.C.) as described (21, 22). Glycosylation occupancy was measured at previously identified glycosylation sites (15, 21) using unmodified and GlcNAc-modified versions of the same peptide. This identification used ProteinPilot (AB SCIEX) searching the LudwigNR database with standard settings: sample type, identification; cysteine alkylation, acrylamide; instrument, TripleTof 5600; species, S. cerevisiae with common contaminants; I.D. focus, biological modifications; enzyme, trypsin or AspN; and search effort, thorough I.D. False discovery rate analysis using ProteinPilot was performed on all searches, and peptides identified with greater than 99% confidence and a local false discovery rate of less than 1% were included for further analysis. The glycosylation occupancy at a given sequon was defined by the abundance as measured by the peak intensity of the GlcNAc-modified peptide as a fraction of the sum of the GlcNAc-modified and unmodified versions of the same peptide (15). For in vitro peptide affinity chromatography analysis, the relative enrichment of each peptide in each wash fraction was determined as described (18) based on the ratio of the abundance of each peptide. Interactions with the peptide-binding groove of Ost6p were determined for each peptide based on the log of the relative abundance of each peptide in each wash fraction between the oxidized and reduced versions of Ost6p. Experiments were performed in biological triplicate.
RESULTS AND DISCUSSION
Ost3p and Ost6p Peptide Binding Controls Glycosylation
In vitro, Ost3p and Ost6p can transiently bind selected peptides with complementary characteristics to their peptide-binding grooves (16). The Ost6p groove is hydrophobic and lined by basic residues (K96 and K99), and it binds peptides enriched in hydrophobic and acidic residues. Point mutations removing these basic residues in Ost6p (Ost6K96Q,K99Q) abolish in vitro peptide binding, whereas the addition of basic residues in place of the natively neutral residues at the equivalent positions in the Ost3p groove (Ost3Q103K,Q106K) allows binding of a hydrophobic and acidic peptide that also binds to wild-type Ost6p (18). This demonstrates that mutations to the groove of Ost3p and Ost6p affect peptide binding in vitro. To investigate the physiological relevance of these in vitro interactions to in vivo site-specific N-glycosylation efficiency, we constructed yeast strains expressing only a single isoform of OTase, wild-type Ost3p-OTase, Ost6p-OTase, or variants of each with point mutations at their peptide-binding grooves (Ost3Q103K,Q106K or Ost6K96Q,K99Q). We then used mass spectrometry to measure the site-specific glycosylation occupancy of cell wall glycoproteins from these cells. As this method analyzes mature glycoproteins in the yeast cell wall that have successfully folded and trafficked from the ER, it likely underestimates the effect of deleterious mutations to OTase (15, 16). The analysis used endoglycosidase H to release N-glycans, leaving a single GlcNAc “tag” on previously glycosylated asparagines. LC-ESI-MS/MS analysis could then detect GlcNAc-modified (glycosylated) and unmodified (non-glycosylated) forms of the same peptide to measure site-specific glycosylation occupancy at many different asparagines. For example, this approach detected both GlcNAc-modified and unmodified forms of peptide V91-K105 from Gas1p, which contains a potential glycosylation site at N95 (Figs. 2A and 2B).
Fig. 2.

LC-ESI-MS/MS identification and relative quantification of glycosylation occupancy at N95 in Gas1p. ProteinPilot matched fragmentation of (A) GlcNAc-modified and (B) unmodified peptide V91-K105 from Gas1p. Single ion chromatograms corresponding to GlcNAc-modified (GlcNAc-N, black) and unmodified (N, gray) peptide V91-K105 from Gas1p ([M+3H]3+ ions at m/z of 666.98 and 559.30, respectively) from yeast with only (C) Ost6p-OTase (Δost3/Δost6 pOST6), (D) Ost6K96Q,K99Q-OTase (Δost3/Δost6 pOST6K96Q,K99Q), (E) Ost3p-OTase (Δost3/Δost6 pOST3), or (F) Ost3Q103K,Q106K-OTase (Δost3/Δost6 pOST3Q103K,Q106K). Horizontal gray lines are aligned with the peak height of the unmodified peptide in C between C and D, and in F between E and F.
We first compared yeast with only Ost3p-OTase or with only Ost6p-OTase. Consistent with previous reports (12, 14, 15), this analysis confirmed that certain asparagines required Ost3p-OTase for efficient glycosylation equivalent to that of wild-type cells, whereas others required Ost6p-OTase. For example, glycosylation occupancy at N95 in Gas1p was significantly lower in yeast with only Ost6p-OTase than in yeast with only Ost3p-OTase, indicating a requirement for Ost3p-OTase for efficient glycosylation at this site (Figs. 2C and 2E). Equivalent analyses were performed in biological triplicate for the other robustly detected glycosylation sites in cell wall glycoproteins (Fig. 3A, column “pOST3:pOST6”; supplemental Table S1). This showed that efficient glycosylation at N253 in Gas1p required Ost6p-OTase, as has been previously reported (15). In contrast, Ost3p-OTase was required for efficient glycosylation at N79 in Sag1p; N40, N57, and N95 in Gas1p; N177 in Crh1p; N304 and N328 in Ecm33p; N491 in Plb2p; and N60 in Gas5p. Although qualitatively consistent, our analysis here showed a stronger requirement for Ost3p-OTase than previous reports (15). Indeed, all asparagines that were glycosylated in wild-type cells required the presence of either Ost3p or Ost6p for efficient glycosylation (supplemental Table S1), highlighting the importance of Ost3p/Ost6p-mediated glycosylation. Further, Gas1p contained three sites (N40, N57, and N95) that required Ost3p and one site (N253) that required Ost6p. As all of these sites are efficiently glycosylated in wild-type yeast (with both Ost3p and Ost6p), this result implies that both Ost3p-OTase and Ost6p-OTase isoforms are located concurrently at the same translocon. This is consistent with previous genetic and biochemical studies (13, 15, 16), although split-ubiquitin studies have proposed that Ost3p and Ost6p interact differentially with the Sec61 and Ssh1 translocons (23).
Fig. 3.

Ost3p and Ost6p peptide-binding groove variants affect in vivo site-specific glycosylation. A, relative occupancy at different glycosylation sites (e.g. N79 in Sag1p) in Δost3/Δost6 cells with pOST3 relative to pOST6 (pOST3:pOST6), pOST6K96Q,K99Q relative to pOST6 (pOST6QQ:pOST6), and pOST3Q103K,Q106K relative to pOST3 (pOST3KK:pOST3). The ratio is mapped from yellow (increase) to blue (decrease) or black (no change). *p < 0.05 ANOVA. Occupancy of (B) N57 in Gas1p, (C) N95 in Gas1p, (D) N253 in Gas1p, and (E) N328 in Ecm33p in various yeast strains. Values are mean. Error bars are S.E. *p < 0.05 ANOVA. F, cartoon surface representation of proposed binding of hydrophobic and neutral (black) but not hydrophobic and basic (blue) stretches of nascent polypeptide by the basic peptide binding groove of Ost6p (PDB I.D. 3G7Y (16, 18)).
We next tested the effect of mutations in the peptide-binding grooves of Ost3p and Ost6p on in vivo site-specific glycosylation. For this, we compared site-specific glycosylation occupancy in yeast with only Ost3Q103K,106K-OTase to that in yeast with only Ost3p-OTase, and we compared that in yeast with only Ost6K96Q,K99Q-OTase to that in yeast with only Ost6p-OTase. These point mutations did affect in vivo glycosylation efficiency at selected sites. For example, glycosylation occupancy at N95 in Gas1p was higher in yeast with only Ost6K96Q,K99Q-OTase than in yeast with only Ost6p-OTase (Figs. 2C and 2D), whereas occupancy at this site was lower in yeast with only Ost3Q103K,Q106K-OTase than in yeast with only Ost3p-OTase (Figs. 2E and 2F). We performed equivalent analyses in biological triplicate for the other robustly detected glycosylation sites in cell wall glycoproteins (Fig. 3A, columns pOST6QQ:pOST6 and pOST3KK:pOST3; supplemental Table S1). N57 and N95 in Gas1p and N328 in Ecm33p required Ost3p for efficient glycosylation and were underglycosylated with Ost6p (Figs. 3A–3C and 3E; supplemental Table S1). However, this requirement for Ost3p was dependent on a neutral Ost3p/Ost6p peptide binding groove, as occupancy at Gas1p N57 and N95 was increased in the Ost6K96Q,K99Q variant and occupancy at all three sites was decreased in the Ost3Q101K,Q103K variant relative to wild-type proteins (Figs. 3A–3C and 3E; supplemental Table S1). In contrast, N253 in Gas1p required Ost6p for efficient glycosylation, and occupancy at this site was increased in the Ost3Q101K,Q103K variant (Figs. 3A and 3D, supplemental Table S1). These effects on site-specific glycosylation occupancy were consistent with growth of the corresponding yeast strains. Whereas the growth defect of Δost3/Δost6 yeast was fully complemented by Ost3p and partially complemented by Ost6p, expression of the Ost3Q101K,Q103K variant reduced growth relative to Ost3p (supplemental Fig. S1), consistent with inefficient glycosylation at several asparagines in this strain (Fig. 3). In summary, glycosylation by Ost6p-OTase at sites requiring Ost3p-OTase could be increased with point mutation to make Ost6p more like Ost3p, and vice versa. These results showed that point mutations that affect in vitro peptide binding by Ost3p and Ost6p also affected in vivo site-specific glycosylation occupancy, consistent with nascent polypeptide interacting directly with the grooves of Ost3p/Ost6p.
Mapping Sites of Interaction between Substrate and Ost6p
Efficient glycosylation at N57 and N95 of Gas1p and N328 of Ecm33p required the absence of basic lysine residues in the peptide-binding groove of Ost3p/Ost6p (Figs. 3A–3C and 3E, supplemental Table S1). This suggested that specific basic amino acid residues in the Gas1p and Ecm33p nascent polypeptides caused electrostatic repulsion with Ost6p or Ost3Q101K,Q103K, which had basic peptide-binding grooves (Fig. 3F). We used this effect to identify the precise sites of interaction between the Gas1p substrate nascent polypeptide and the Ost6p peptide-binding groove in vivo.
We performed scanning mutagenesis of basic to neutral residues through the Gas1p sequence surrounding N57 and N95 and measured the occupancy of Gas1p glycosylation sites in each of these variant proteins in cells expressing Ost6p-OTase (see supplemental Fig. S2). We predicted that mutagenesis of specific basic residues in Gas1p would eliminate the electrostatic repulsion and allow binding to the basic peptide-binding groove of Ost6p, which would then increase the extent of glycosylation in a site-specific manner. Analysis of these cells showed a large and significant increase in occupancy at N95 in Gas1p only with mutation at R90 (Figs. 4A and 4C; supplemental Table S2). This indicated that R90 in wild-type Gas1p limited binding of this stretch of polypeptide to wild-type Ost6p through electrostatic repulsion with the Ost6p peptide-binding groove, and that binding of Gas1p nascent polypeptide proximal to R90 with Ost6p was required for efficient glycosylation of N95. In contrast, occupancy at N57 was increased with mutation at R47, R76, or R90 (Figs. 4A and 4B; supplemental Table S2). We interpreted this result as indicating that increased glycosylation of N57 could be achieved through binding at any one of these positions in Gas1p to Ost6p, or alternatively that some of these mutations destabilized Gas1p, leading to increased glycosylation independent of Ost6p binding. To distinguish between these possibilities, we analyzed the Gas1p variants in yeast with Ost6K96Q,K99Q-OTase. Electrostatic repulsion between Ost6p and a section of Gas1p would require that each be positively charged, and point mutation to remove the charge on either one or on both would eliminate the repulsion. Therefore, increased occupancy with Ost6K96Q,K99Q relative to Ost6p for a particular Gas1p R variant would indicate that binding of that position in Gas1p to Ost6p was not required for glycosylation, whereas no change in occupancy would be consistent with that site in Gas1p binding to Ost6p to increase site-specific glycosylation occupancy. Analysis of these cells showed that in the Gas1R90Q variant, occupancy at N95 did not increase with Ost6K96Q,K99Q relative to Ost6p (Fig. 4F and supplemental Table S3), consistent with the initial scanning mutagenesis (Figs. 4A and 4C; supplemental Table S2) and with Gas1p nascent polypeptide proximal to R90 binding Ost6p to enhance glycosylation at N95. For N57 in Gas1p, occupancy increased with Ost6K96Q,K99Q relative to Ost6p for Gas1R76Q and Gas1R90Q but not for Gas1R47Q (Figs. 4D and 4E; supplemental Table S3). This indicated that binding near R47 of Gas1p to Ost6p was required for efficient glycosylation of N57. Consistent with point mutations at R76 and R90 increasing glycosylation of N57 by destabilizing Gas1p, N57 is in a surface exposed loop of Gas1p, in contrast to N95, which is relatively inaccessible (24) (supplemental Fig. S3). Together, the data showed that transient binding to Ost3p/Ost6p of stretches of Gas1p nascent polypeptide proximal and N-terminal to N57 and N95 allowed efficient glycosylation at these sites (Fig. 4G).
Fig. 4.

Mapping sites of interaction between Ost6p and Gas1p. A, relative occupancy at N40, N57, N95, and N253 in Gas1p variants compared with wild-type Gas1p expressed in Δost3/Δost6 pOST6 cells. Color as in Fig. 3A. *p < 0.05 ANOVA. Occupancy of (B) N57 in Gas1p and (C) N95 in Gas1p in various yeast strains. Values are mean. Error bars are S.E. *p < 0.05 ANOVA. D, relative occupancy at N57 and N95 in Gas1p variants expressed in Δost3/Δost6 pOST6K96Q,K99Q compared with Δost3/Δost6 pOST6. Color as in Fig. 3A. Occupancy of (E) N57 in Gas1p and (F) N95 in Gas1p in various Gas1p variants expressed in Δost3/Δost6 pOST6 (black) or Δost3/Δost6 pOST6K96Q,K99Q (white). Values are mean. Error bars are S.E. *p < 0.05 ANOVA. G, position of basic-neutral point mutations (blue) in Gas1p that increased occupancy at specific glycosylation sites (green).
In Vitro Validation of in Vivo Peptide Binding by Ost6p
To confirm that the increased glycosylation we observed in the Gas1p variants was due to increased physical association of stretches of Gas1p with Ost6p, we performed in vitro peptide binding experiments. We expressed and purified Gas1p and Gas1R90Q as MBP-fusion proteins in E. coli and digested the proteins with AspN to obtain unglycosylated peptides similar to nascent polypeptide entering the ER lumen, which is the physiological substrate of OTase (Fig. 5A). Peptides were applied to resin containing bound MBP-Ost6L, with the active-site CxxC motif either oxidized or reduced through pre-incubation with DTT. The peptide-binding groove is only present when the CxxC motif is oxidized, and peptides do not bind when this motif is reduced (16, 18). We measured the MBP-Gas1 peptides present in each wash fraction via LC-ESI-MS/MS and observed increased retention of the robustly detected Gas1p77–108 peptide from the Gas1R90Q variant relative to wild-type Gas1p with oxidized (Fig. 5D) but not reduced (Fig. 5C) Ost6p, relative to the load (Fig. 5B). This indicated that Gas1R90Q mutation increased in vitro interaction with the Ost6p peptide-binding groove (Fig. 5E), consistent with our in vivo results (Figs. 3 and 4).
Fig. 5.

Non-covalent peptide binding by Ost6p in vitro. A, aligned AspN peptides from MBP-Gas1 and MBP-Gas1R90Q detected via LC-ESI-MS/MS. Extracted ion chromatograms of peptide from MBP-Gas1 (solid) and MBP-Gas1R90Q (dashed) in (B) load, and second wash fraction with (C) reduced MBP-Ost6 and (D) oxidized MBP-Ost6. E, relative enrichment of the Gas177–108 versus the Gas1R90Q 77–108 peptide by columns with attached oxidized MBP-Ost6. Data are average of triplicates. Error bars indicate range.
To gauge the generality of our results, we inspected the sequence N-terminal and proximal to glycosylation sites dependent on the presence of basic residues lining the peptide binding groove of either Ost3p or Ost6p (Fig. 3A) and compared the characteristics of these sequences (Fig. 6). Although Ost3p and Ost6p have different charge preferences, both require the presence of short stretches of hydrophobic amino acids in peptides for efficient binding (18, 25). The closest hydrophobic sequence stretches N-terminal to N57 and N95 in Gas1p and N328 in Ecm33p were flanked by basic amino acids, consistent with efficient glycosylation of these sites requiring neutral peptide-binding grooves in Ost3p or Ost6p. In contrast, N253 in Gas1p had a hydrophobic stretch with acidic amino acids nearby, consistent with its preference for basic Ost3p or Ost6p.
Fig. 6.

Conservation of sequence characteristics at sites of Ost3p/Ost6p interaction. Validated and predicted stretches of substrate proteins Gas1p and Ecm33p that bind to Ost3p or Ost6p. Underlined, glycosylation sequon. Gray shading, extended hydrophobic sequence. Blue, basic amino acid. Red, acidic amino acid.
Model of Ost3p/Ost6p Function in Vivo
Together, our data showed that Ost3p/Ost6p transiently bound hydrophobic stretches of nascent polypeptide substrate proximal and N-terminal to specific asparagines to increase their glycosylation efficiency. Based on these findings, we propose a mechanistic model of Ost3p/Ost6p function in N-glycosylation (Fig. 7). In this model, short hydrophobic stretches of nascent polypeptide exiting the translocon into the ER lumen (Fig. 7A) bind to the peptide-binding groove of Ost3p/Ost6p independent of the location of nearby glycosylation sites (Fig. 7B). This binding is non-covalent (18) or through a mixed disulfide (17) and depends on complementarity between the stretch of nascent polypeptide and the peptide-binding groove of Ost3p/Ost6p (Figs. 4–6). Mutations at the thioredoxin-like CxxC active site motif of Ost3p/Ost6p in vivo in yeast affect glycosylation at only ∼10% of sites (16), suggesting that the non-covalent mode of binding is dominant, and mixed disulfides form only when cysteines are appropriately positioned in stretches of bound nascent polypeptide. In either mode, this sequestration physically separates polypeptide that has already translocated into the ER lumen from newly translocating sequence. Continued translocation therefore produces a loop of nascent polypeptide with its ends constrained by the translocon exit and the Ost3p/Ost6p groove (Fig. 7C). As OTase is physically associated with the translocon (3) and Ost3p/Ost6p is adjacent to Stt3p in the OTase complex (2, 26), this configuration produces a flexible loop of nascent polypeptide in close proximity to the acceptor peptide-binding site of Stt3p. This presents asparagines in glycosylation sequons to the active site of OTase in the form of short, flexible peptides that are optimal substrates for efficient glycosylation (4, 27, 28) (Figs. 7D and 7E). Ost3p/Ost6p peptide binding is transient (16–18) (Fig. 7F), allowing continued translocation and folding of the glycosylated polypeptide (Fig. 7G). Incorporation of Ost3p/Ost6p into OTase would therefore enhance the glycosylation of diverse proteins, and their distinct peptide-binding specificities (17, 18, 25) would further increase the range of efficiently modified substrates.
Fig. 7.
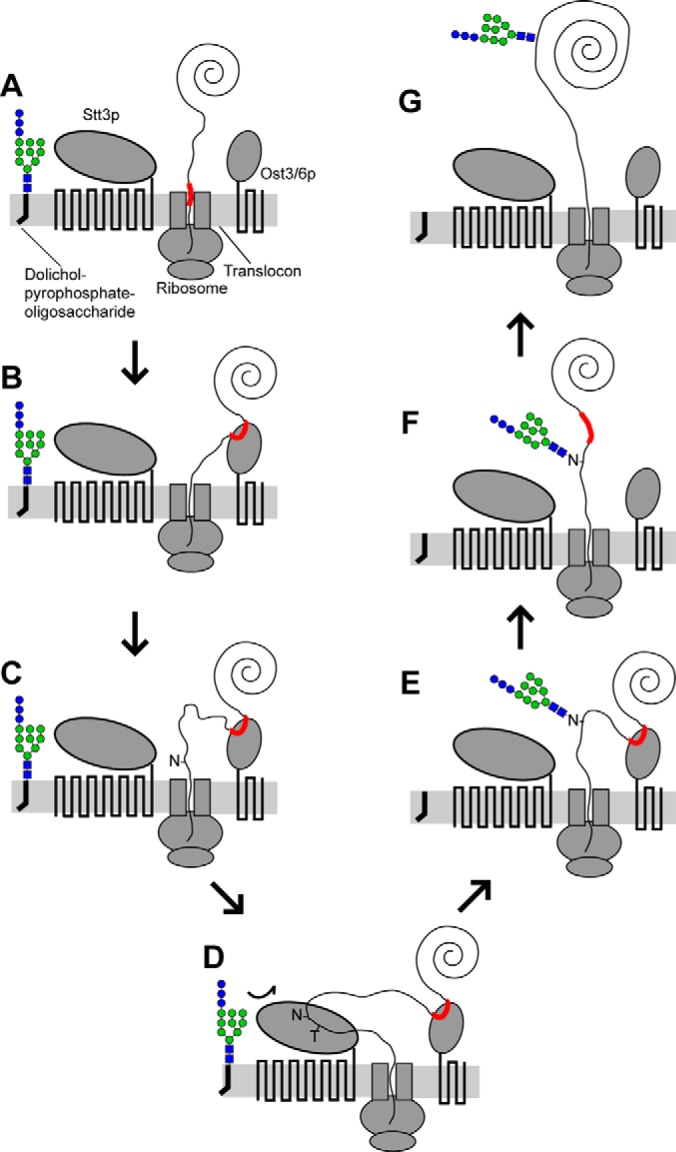
Mechanistic model of Ost3p/Ost6p function in N-glycosylation. A, partially translocated and folded nascent polypeptide enters the ER lumen from the translocon. B, a stretch of nascent polypeptide (red) with affinity for Ost3p/Ost6p is sequestered at the peptide-binding groove. C, continued translocation produces a flexible loop of nascent polypeptide kept physically separate from previously translocated polypeptide. D, glycosylation sequons in the loop are presented to the acceptor-binding site of Stt3p in a glycosylation-competent form and (E) efficiently glycosylated. F, non-covalent or mixed-disulfide binding of nascent polypeptide to Ost3p/Ost6p is transient, and (G) translocation and folding continues. Ost3p/Ost6p and Stt3p are physically adjacent in OTase (2) but are shown separately for clarity. See text for details.
Transient binding of short, hydrophobic stretches of nascent polypeptide by Ost3p and Ost6p is reminiscent of the activity of co-translational Hsp70 chaperones such as cytoplasmic SSA/SSB (29) and ER lumenal Kar2p (BiP) (30), which assist co-translational folding (31, 32). As binding to Ost3p or Ost6p is independent of the presence of a nearby glycosylation site, all stretches of nascent polypeptides with sufficient affinity to either Ost3p or Ost6p are likely transiently sequestered. This may therefore facilitate proteome-wide co-translational folding of proteins translocated into the ER, independent of their glycosylation status.
Supplementary Material
Footnotes
Author contributions: M.F.J., U.B., and B.L.S. designed research; M.F.J., U.B., and B.L.S. performed research; M.F.J., U.B., and B.L.S. analyzed data; M.F.J., U.B., and B.L.S. wrote the paper.
* This work was supported in part by a project grant to B.L.S. from the National Health and Medical Research Council (631615). B.L.S. was supported in part by a Career Development Fellowship from the National Health and Medical Research Council (APP1031542).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- OTase
- oligosaccharyltransferase
- ER
- endoplasmic reticulum
- GlcNAc
- N-acetylglucosamine
- ESI
- electrospray ionization
- ANOVA
- analysis of variance.
REFERENCES
- 1. Schwarz F., Aebi M. (2011) Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 [DOI] [PubMed] [Google Scholar]
- 2. Kelleher D. J., Gilmore R. (2006) An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 16, 47R–62R [DOI] [PubMed] [Google Scholar]
- 3. Harada Y., Li H., Li H., Lennarz W. J. (2009) Oligosaccharyltransferase directly binds to ribosome at a location near the translocon-binding site. Proc. Natl. Acad. Sci. U.S.A. 106, 6945–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lizak C., Gerber S., Numao S., Aebi M., Locher K. P. (2011) X-ray structure of a bacterial oligosaccharyltransferase. Nature 474, 350–355 [DOI] [PubMed] [Google Scholar]
- 5. Helenius A., Aebi M. (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 6. Ohtsubo K., Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 [DOI] [PubMed] [Google Scholar]
- 7. Marth J. D., Grewal P. K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schulz B. L., Laroy W., Callewaert N. (2007) Clinical laboratory testing in human medicine based on the detection of glycoconjugates. Curr. Mol. Med. 7, 397–416 [DOI] [PubMed] [Google Scholar]
- 9. Wilson C. M., High S. (2007) Ribophorin I acts as a substrate-specific facilitator of N-glycosylation. J. Cell Sci. 120, 648–657 [DOI] [PubMed] [Google Scholar]
- 10. Wilson C. M., Kraft C., Duggan C., Ismail N., Crawshaw S. G., High S. (2005) Ribophorin I associates with a subset of membrane proteins after their integration at the sec61 translocon. J. Biol. Chem. 280, 4195–4206 [DOI] [PubMed] [Google Scholar]
- 11. Wilson C. M., Roebuck Q., High S. (2008) Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core. Proc. Natl. Acad. Sci. U.S.A. 105, 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karaoglu D., Kelleher D. J., Gilmore R. (1995) Functional characterization of Ost3p. Loss of the 34-kD subunit of the Saccharomyces cerevisiae oligosaccharyltransferase results in biased underglycosylation of acceptor substrates. J. Cell Biol. 130, 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwarz M., Knauer M., Lehle L. (2005) Yeast oligosaccharyltransferase consists of two functionally distinct sub-complexes, specified by either the Ost3p or Ost6p subunit. FEBS Lett. 579, 6564–6568 [DOI] [PubMed] [Google Scholar]
- 14. Spirig U., Bodmer D., Wacker M., Burda P., Aebi M. (2005) The 3.4-kDa Ost4 protein is required for the assembly of two distinct oligosaccharyltransferase complexes in yeast. Glycobiology 15, 1396–1406 [DOI] [PubMed] [Google Scholar]
- 15. Schulz B. L., Aebi M. (2009) Analysis of glycosylation site occupancy reveals a role for Ost3p and Ost6p in site-specific N-glycosylation efficiency. Mol. Cell. Proteomics 8, 357–364 [DOI] [PubMed] [Google Scholar]
- 16. Schulz B. L., Stirnimann C. U., Grimshaw J. P. A., Brozzo M. S., Fritsch F., Mohorko E., Capitani G., Glockshuber R., Grütter M. G., Aebi M. (2009) Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc. Natl. Acad. Sci. U.S.A. 106, 11061–11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohd Yusuf S. N., Bailey U. M., Tan N. Y. J., Jamaluddin M. F. B., Schulz B. L. (2013) Mixed disulfide formation in vitro between a glycoprotein substrate and yeast oligosaccharyltransferase subunits Ost3p and Ost6p. Biochem. Biophys. Res. Commun. 432, 438–443 [DOI] [PubMed] [Google Scholar]
- 18. Jamaluddin M. F. B., Bailey U. M., Tan N. Y. J., Stark A. P., Schulz B. L. (2011) Polypeptide binding specificities of Saccharomyces cerevisiae oligosaccharyltransferase accessory proteins Ost3p and Ost6p. Protein Sci. 20, 849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knauer R., Lehle L. (1999) The oligosaccharyltransferase complex from Saccharomyces cerevisiae. Isolation of the OST6 gene, its synthetic interaction with OST3, and analysis of the native complex. J. Biol. Chem. 274, 17249–17256 [DOI] [PubMed] [Google Scholar]
- 20. Imai Y., Matsushima Y., Sugimura T., Terada M. (1991) A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 19, 2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey U. M., Jamaluddin M. F. B., Schulz B. L. (2012) Analysis of congenital disorder of glycosylation-Id in a yeast model system shows diverse site-specific under-glycosylation of glycoproteins. J. Proteome Res. 11, 5376–5383 [DOI] [PubMed] [Google Scholar]
- 22. Bailey U. M., Punyadeera C., Cooper-White J. J., Schulz B. L. (2012) Analysis of the extreme diversity of salivary alpha-amylase isoforms generated by physiological proteolysis using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 911, 21–26 [DOI] [PubMed] [Google Scholar]
- 23. Yan A., Lennarz W. J. (2005) Two oligosaccharyl transferase complexes exist in yeast and associate with two different translocons. Glycobiology 15, 1407–1415 [DOI] [PubMed] [Google Scholar]
- 24. Hurtado-Guerrero R., Schüttelkopf A. W., Mouyna I., Ibrahim A. F., Shepherd S., Fontaine T., Latgè J. P., van Aalten D. M. (2009) Molecular mechanisms of yeast cell wall glucan remodeling. J. Biol. Chem. 284, 8461–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan N. Y., Bailey U. M., Jamaluddin M. F., Binte Mahmud S. H., Raman S. C., Schulz B. L. (2014) Sequence-based protein stabilization in the absence of glycosylation. Nat. Commun. 5, 3099. [DOI] [PubMed] [Google Scholar]
- 26. Yan Q., Lennarz W. J. (2002) Studies on the function of oligosaccharyl transferase subunits. Stt3p is directly involved in the glycosylation process. J. Biol. Chem. 277, 47692–47700 [DOI] [PubMed] [Google Scholar]
- 27. Gerber S., Lizak C., Michaud G., Bucher M., Darbre T., Aebi M., Reymond J. L., Locher K. P. (2013) Mechanism of bacterial oligosaccharyltransferase: in vitro quantification of sequon binding and catalysis. J. Biol. Chem. 288, 8849–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holst B., Bruun A. W., Kielland-Brandt M. C., Winther J. R. (1996) Competition between folding and glycosylation in the endoplasmic reticulum. EMBO J. 15, 3538–3546 [PMC free article] [PubMed] [Google Scholar]
- 29. Willmund F., del Alamo M., Pechmann S., Chen T., Albanèse V., Dammer E. B., Peng J., Frydman J. (2013) The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152, 196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. (1991) Peptide-binding specificity of the molecular chaperone BiP. Nature 353, 726–730 [DOI] [PubMed] [Google Scholar]
- 31. Kim Y. E., Hipp M. S., Bracher A., Hayer-Hartl M., Hartl F. U. (2013) Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 [DOI] [PubMed] [Google Scholar]
- 32. Kampinga H. H., Craig E. A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


