Abstract
We investigated the molecular effects of glucosamine supplements, a popular and safe alternative to nonsteroidal anti-inflammatory drugs, for decreasing pain, inflammation, and maintaining healthy joints. Numerous studies have reported an array of molecular effects after glucosamine treatment. We questioned whether the differences in the effects observed in previous studies were associated with the focus on a specific subproteome or with the use of specific cell lines or tissues. To address this question, global mass spectrometry- and transcription array-based glucosamine drug profiling was performed on malignant cell lines from different stages of lymphocyte development. We combined global label-free MS-based protein quantitation with an open search for modifications to obtain the best possible proteome coverage. Our data were largely consistent with previous studies in a variety of cellular models. We mainly observed glucosamine induced O-GlcNAcylation/O-GalNAcylation (O-HexNAcylation); however, we also observed global and local changes in acetylation, methylation, and phosphorylation. For example, our data provides two additional examples of “yin-yang” between phosphorylation and O-HexNAcylation. Furthermore, we mapped novel O-HexNAc sites on GLU2B and calnexin. GLU2B and calnexin are known to be located in the endoplasmic reticulum (ER) and involved in protein folding and quality control. The O-HexNAc sites were regulated by glucosamine treatment and correlated with the up-regulation of the ER stress marker GRP78. The occupancy of O-HexNAc on GLU2B and calnexin sites differed between the cytosolic and nuclear fractions with a higher occupancy in the cytosolic fraction. Based on our data we propose the hypothesis that O-HexNAc either inactivates calnexin and/or targets it to the cytosolic fraction. Further, we hypothesize that O-HexNAcylation induced by glucosamine treatment enhances protein trafficking.
Glucosamine (GlcN)1 induces the posttranslational modification O-linked β-N-acetylglucosamine (O-GlcNAc) on serines and threonines (2, 3). Studies have demonstrated that GlcN treatment can lead to glucose intolerance and the death of pancreatic β-cells. For example, O-GlcNAc plays a role in the chronic complications of diabetes mellitus and insulin resistance (4, 5). The clinical relevance of GlcN, a dietary supplement used in osteoarthritis patients, is unclear (6). Previous investigations of long-term GlcN administration did not reveal risks or concerns (7), which is also consistent with recent studies (8). However, in recent clinical studies, GlcN oral administration (8) did not demonstrate any benefits, and the concentration used in vitro was not comparable with the levels observed in the plasma in vivo after the oral administration of GlcN, which raised skepticism (8, 9). Therefore, several studies have suggested caution for the use of GlcN in the treatment of osteoarthritis and other autoimmune diseases. In contrast, other studies claim that in addition to providing pain relief for osteoarthritis patients, GlcN is beneficial in ischemia/reperfusion injuries. For example, GlcN cardioprotection has been observed in perfused rat hearts (10), demonstrating the feasibility of obtaining an in vivo response to GlcN.
GlcN exhibits anticancer properties in vitro (11, 12), and a recent review by Slawson et al. (13) suggested several molecular mechanisms through which O-GlcNAcylation can play a regulatory role in cancer. In the case of cancer other administration strategies than oral intake are possible. Furthermore, the negative side effect from high drug dosages is more acceptable in the case of terminal cancers.
In general, elevated O-GlcNAc levels elicted by GlcN administration, utilizing in vitro cell lines and animal models, exhibit ER stress (14), changes in calcium signaling (15), modified transcriptional activity (16), and alterations in phospho-signaling cascades (17). Additionally, GlcN causes cell cycle arrest in G1 and/or G2 (11) accompanied by apoptosis (11). More recently, GlcN treatment has been shown to increase hyaluronan synthesis (18) and autophagy (19, 20). Autophagy is considered important in cancer because depending on the drug type, autophagy can either increase sensitivity to a drug or protect cancer cells from a drug (21).
Understanding the molecular mechanisms and pathways activated following GlcN intake could lead to the discovery of novel targets for the treatment of cardiovascular diseases, diabetes, and cancer. LC-MS-based proteomics for drug profiling demonstrates great potential for the elucidation of molecular mechanisms and provides detailed information about how specific cell types compensate for or become resistant to drugs. In this study, we combined, label-free MS-based quantitation with an open search strategy for modifications to obtain deepest possible proteome coverage in drug profiling experiments given the obtained Q-Exactive data.
To date, there is only indirect evidence to explain how GlcN affects calcium signaling and causes ER stress. PLB (phospholamban), a regulator of SERCA (sarco/endoplasmic reticulum calcium ATPase), contains an O-GlcNAc site; however, the regulation of this protein requires further investigation (22, 23). The link between GlcN treatment and ER stress might stem from the O-GlcNAc modification of heat shock proteins; however, this hypothesis needs experimental validation (24). In search of explanations for the above observations, we investigated GlcN-treated malignant cells from different stages of lymphocyte development using mass spectrometry (Q-Exactive) and transcriptional arrays (Affymetrix). We observed the expected phenotypic mRNA and protein changes after GlcN treatment, such as ER stress, cytoplasmic vacuolization, altered regulation of proteins with immunological functions, and apoptosis. We identified novel O-HexNAc sites induced by GlcN treatment, which suggest a link between GlcN treatment and ER stress. Finally, the global up-regulation of O-HexNAcylation caused the expected global down-regulation of phosphorylation levels but also affected methylation and acetylation levels.
EXPERIMENTAL PROCEDURES
Short Summary of Methods
O-GlcNAc levels (probed with antibodies CTD 110.6 and RL2) in four malignant hematopoietic cell lines, KMH2, RAMOS, HDML2, and Jurkat, before and after GlcN (Sigma) treatment, were assayed by Western blot over time and at different concentrations of GlcN (0.1, 2.5, 10, and 20 mm). The viability of the cell lines could be re-established by removing GlcN for all the tested conditions (supplemental Fig. S1). The largest increment in the level of O-GlcNAcylation of proteins was observed in KMH2 cells following treatment with 20 mm GlcN for 24 h. The cell lines were characterized with respect to cell survival, cell cycle arrest, and apoptosis. KMH2 cell line was treated with different concentrations of GlcN and was assayed by blotting with anti-O-GlcNAc antibody at different times. The time point and concentration exhibiting the maximum O-GlcNAc response was globally profiled and compared with the untreated cells with respect to mRNA (microarray) and protein expression (MS-based proteomics, Q-Exactive). The quantitative proteomics results were validated using Western blots and three different MS-based protein quantitation techniques (Tandem Mass Tag labeling (TMT), stable-isotope dimethyl labeling, and label-free quantitation). KMH2 cells were fractionated into nuclear, mitochondrial, and cytosolic crude fractions. The main aim of the fractionation was to obtain a deeper sampling rather than determining the subcellular localization of proteins.
Cell lines and Culture Conditions
The human Hodgkin Lymphoma derived cell lines HDLM-2 and KMH2 and the T cell leukemia-derived cell line Jurkat and Burkitt lymphoma-derived cell line RAMOS were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures. All cell lines were cultured in Invitrogen RPMI medium 1640 GlutaMAX™ (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Invitrogen) in a humid environment of 5% CO2 at 37 °C. For GlcN treatment cells were cultured for 24 h and replated at 5 × 105 cells/ml with or without GlcN at 20 mm for 6, 12, 24, and 48 h. For concentration effect cells were plated at 5 × 105 cells/ml with GlcN at 0.1, 2.5, 10, and 20 mm for 24 h.
For Bortezomib treatment, KMH2 cells were seeded into six well plates at a density of 5 × 105 cells/ml per well (2 ml) and treated with the following: vehicle control (DMSO), Bortezomib (PS-341) (Selleck Chemicals, US) at 1, 5, 10, and 100 nm and GlcN at 20 mm. The cells were treated for 24 h.
Analytical and Biological Reliability
All mRNA expression analysis such as qPCR and expression arrays was performed with three biological replicas. Western blots, cell-cycle, and apoptosis assays were performed with minimum three biological replicas. Each of the quantitative MS methods used were done with three technical replicas on independent biological samples.
Cell-cycle and Apoptosis Assays
Apoptosis was determined by annexin V-FITC and propidium iodide (PI) double staining according to the manufacturer's instructions (BD Biosciences). Cell-cycle fractions were determined by propidium iodide nuclear staining. Briefly, cells were harvested, washed in PBS, fixed in 70% ethanol overnight at 4 °C, and incubated in propidium iodide solution (10 μg/ml propidium iodide, 0.1 mg/ml RNase A in PBS-Tween 20 (0.1% v/v) for 30 min at 37 °C). Data were collected on a FACSCanto II flow cytometer (BD Biosciences) and analyzed with FlowJo Version 7.6.5 software (TreeStar, US). Results represent the mean value of three independent experiments.
RNA Isolation, Reverse Transcription, and Quantitative PCR
Total RNA was isolated from the various cell lines at different conditions using the RNeasy Plus Mini Kit (Qiagen, Stanford, CA) according to the manufacturer's protocol. RNA yield and quality were determined spectrophotometrically.
Reverse transcription was performed using 1 μg total RNA, random oligonucleotides primers, and SuperScript II RT (Invitrogen) in a total volume of 20 μl as described by the manufacturer. For real-time PCR analysis, cDNA samples were diluted 10-fold with water and PCR amplified in triplicate using TaqMan® Gene Expression Assays (Hs00269228_m1-OGT, Hs00201970_m1-MGEA5), Hs03928985_g1-RN18S1, Hs01003267_m1-HRPT1) in an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) according with manufacturer's protocol. Expression of 18S and HRPT1 was used for normalization of target gene abundance.
Microarray Data Acquisition and Analysis
RNA expression profiling was performed using the Affymetrix GeneChip® technology, following the protocols recommended by the manufacturers. mRNA expression in the cell line RAMOS and three biological replicas of KMH2 were analyzed, before and after 24 h of GlcN treatment, by Affymetrix arrays (HuGENE-1_1-st-v1, Probe set annotation, release 32, 9/30/11). The data were collectively analyzed by using the R package “AFFYLMGUI” (http://www.bioconductor.org) (25). Background adjustment was done by using robust multichip average (RMA) (26). Correction for multiple testing was done by the method of Benjamini and Hochberg (27). Microarray data is publicly available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc = GSE49185, Gene Expression Omnibus (GEO) accession number GSE49185.
Cell Fractionation
Cells were homogenized in ice-cold cell homogenization medium (10 mm Tris, pH 6.7, 150 mm MgCl2, and 10 mm KCl) by passing through a 20G syringe. Cell breakage was examined under a phase-contrast microscope. Nuclei were pelleted by centrifuging for 5 min at 1000 × g at 4 °C after addition of cell homogenization medium containing 1 m sucrose (final concentration 250 mm). Mitochondria were isolated by centrifuging the remaining supernatant for 10 min at 5000 × g at 4 °C and resuspending the pellet in ice-cold sucrose/Mg2+ medium (10 mm Tris, pH 6.7, 150 mm MgCl2, and 0.25 m sucrose). The mitochondrial fraction is obtained by recentrifuging the suspension at 5000 × g for 10 min at 4 °C. The supernatant constituted the cytosolic fraction. All samples were stored at −80 °C until use.
SDS-PAGE and Western blot
Nuclei and mitochondria pellet obtained as described above and total cells were lysed using RIPA lysis buffer at 4 °C for 20 min and the lysate cleared by centrifuging 20 min at 15,000 × g at 4 °C. Cytosolic fraction was analyzed without further processing. Samples were loaded into 10% acrylamide SDS-page gels and transferred overnight to PVDF membranes. Membranes were blocked and incubated with primary antibody and secondary HRP labeled antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) according to manufacturer's recommendations. ECL-prime (GE Healthcare) was used for detection.
Immunoprecipitation
After 24 h incubation cells were treated with 20 mm GlcN for 24 h and then lysed with RIPA buffer. Dynabeads Protein G (50 μl of a 50% slurry, Invitrogen) were mixed with anticalnexin, antibody (Thermo Scientific) or control mouse IgG and rotated for 1 h at room temperature. Lysates containing 800 μg proteins were added to the antibody-conjugated dynabeads and rotated overnight at 4 °C. The immunoprecipitates were collected, washed, and eluted before standard Western blot analysis using either anti-O-GlcNAc (both CTD110.6 and RL2 were used) or calnexin antibodies.
In Vitro O-GlcNAc Labeling Assay
Immunoprecipitated calnexin from treated and nontreated cells was labeled with mutated Gal-T1(Y289L) and UDP-GalNAz using the Click-iT O-GlcNAc enzymatic labeling kit, and biotin alkaline from the glycoprotein detection kit (Invitrogen) was used for detection by Western blot.
Peptide Sample Preparation
Protein solution containing SDS and DTT are loaded into filtering columns and washed exhaustively with 8 m urea in HEPES buffer. Proteins are then incubated overnight with trypsin sequencing grade (Promega, Madison, WI) after alkylation with iodoacetamide and reduction with DTT.
Chemical Labeling of Peptides with Stable Isotopes
For stable-isotope dimethyl labeling and Tandem Mass Tag labeling two portions of ∼30 μg peptides from each of the two samples were prepared in 100 mm TEAB. For the stable-isotope dimethyl labeling experiment published protocols were followed (28). “Control Sample” was labeled with (CH2H2)2 and “Treated Sample” was labeled with (13C2H3)2. After labeling the two samples were mixed.
For the Tandem Mass Tag labeling (TMT) labeling experiment a 6-plex TMT kit (cat# 90066, ThermoFisher, IL) was used. Following the manufacturer's protocol “Control Sample” and “Treated Sample” was labeled with the tandem mass tags 126 and 127, respectively.
Both labeling experiments were desalted, concentrated and analyzed. Proper labeling was verified by LC-MS/MS. Hereafter the dimethyl and TMT samples were subjected to optional strong cation exchange fractionation and STAGE tip cleanup. Seven fractions, including flow through, were collected for each stable isotope labeled experiment (29).
Mass Spectrometry
Peptides generated as described above were desalted and concentrated (30) prior to analysis by nano LC-MS/MS using a Q-Exactive (Thermo, San Jose, CA) mass spectrometer coupled to a Dionex NCP3200RS HPLC setup (Thermo, Sunnyvale, CA). A 75 μm ID, 15 cm in length home build reversed phase column (Reprosil-pur C18-AQ 3 μm, Ammerbuch-Entringen, Germany) was used to separate peptides. The analytical gradient was generated at 200 nL/min increasing from 5% Buffer B (0.1% formic acid in acetonitrile)/95% Buffer A (0.1% formic acid) to 35% Buffer B/65% Buffer A over 110 min followed by an increase to 90% Buffer B/10% Buffer in 10 min. MS survey scans were scanned from m/z 350 to m/z 1400 at 70,000 resolution (AGC: 1e6 and Maximum IT: 120 ms). An upper limit of 20 most abundant ions was subjected to MS/MS and measured at a resolution of 35,000 (AGC: 5e4 and Maximum IT: 120 ms) with lowest mass set to m/z 100. All data are available in online repositories and supplemental files.
Preprocessing of MS Data
All Q-Exactive data were calibrated using polycyclodi-methylsiloxane (PCMs—outgassed material from semiconductors) present in the ambient air and Bis(2-Ethylhexyl) (Phthalate) (DEHP—from plastic) (31, 32) using both MaxQuant version 1.3.0.5 (33) and modular VEMS, mVEMS v1.0 (34) (supplemental Fig. S2). mVEMS further allows alternative parent ion annotations for each MS/MS spectrum that is needed if two peptide elution profiles overlap in the m/z and retention time dimension. By allowing alternative parent ion annotation for each MS/MS spectrum, provides a space efficient data format. Furthermore these alternative parent ion annotations were taken into account during the database dependent search of MSMS data.
Database Dependent Search of MS Data
All data were searched with mVEMS (35) and MaxQuant version 1.3.0.5 (33). Mass accuracy was set to 10 ppm for peptides and 10 mDa for peptide fragments. Four missed cleavages were specified and the database UniProtKB/TrEMBL (Release 2013_02) were used including permutated protein sequences, leaving Lys and Arg in place, together with common contaminants such as human keratins, bovine serum proteins, and proteases (36). The total number of protein entries searched was 162,982. Fixed modification of carbamidomethyl cysteine was included in the search parameters. In MaxQuant the following variable modifications were considered: Met-loss (UNIMOD 765), Oxidized methionine (UNIMOD 885), and HexNAc (UNIMOD 43). In mVEMS a list of 36 variable modifications were considered for all MSMS data against the full protein database. Protein N-terminal Met-loss is not specified for mVEMS searches because mVEMS by default checks N-terminal Met-loss. The modifications Phosphate-ribosylation (UNIMOD 1356), ADP-ribose (UNIMOD 213), Hydroxylation (UNIMOD 35), and Myristoylation (UNIMOD 45) were not frequent at a 1% false discovery rate (FDR) threshold. We conclude that these few counts are likely to be false positives and the search was therefore repeated excluding these modifications (see supplemental Table S1 for the final list of modifications that was used for the analysis). For stable-isotope dimethyl labeled data the additional variable modifications: 32.0564/36.0757 m/z on Lys and N-terminal were included. For TMT data the additional variable modifications: 229.1629 on Lys and N-terminal were included. The minimal peptide length was set to six amino acids for MaxQuant and unrestricted for mVEMS. The FDR was set to 0.01 for peptide and protein identifications in both MaxQuant and mVEMS. Only proteins in evidence groups one to three, as defined by Matthiesen et al. (37), were considered in subsequent quantitative analysis.
Quantitative Proteome Analysis
Multiple quantitative proteomic approaches were utilized to measure the changes in protein expression and the modification of peptides in nuclear, mitochondrial, and cytoplasmic preparations of KMH2 cells incubated with or without GlcN for 24 h. Integrated intensity counts were obtained by mziXIC (mass, charge, and isotope-dependent extracted ion chromatograms) (34) profiles extracted from label free, stable-isotope dimethyl labeling, and TMT LC-MS data and used for peptide quantitation. Proteins were quantified by spectral counting (38). To determine significant changes in protein expression or modification upon glucosamine treatment, ANOVA tests on log transformed quantitative values were employed using the R package DanteR (39). p values were corrected for multiple testing by the method of Benjamini and Hochberg (BH) (27) and no imputation for missing values were used.
Overall changes of PTMs in subcellular fractions were estimated by summing all PSMs for specific modifications and fraction before and after GlcN treatment. The relative change was calculated as: #PSMs+GlcN/#PSMs-GlcN−1.
Venn diagrams were made with R package “VennDiagram.” To access the level of protein trafficking enrichment analysis of proteins position in the Venn diagram was calculated using the R function dhyper. Heatmaps were generated by using the R package “heatmap.3.” Significant regulated protein isoforms were collapsed to the genes that encode them to facilitate visualization of regulated proteins.
RESULTS
Lymphocyte-derived Cell Lines Respond Differently to GlcN
To define the effects of GlcN at the molecular level, we reasoned that a strong increase in the O-GlcNAcylation of proteins would be needed. Given that the O-GlcNAcylation of proteins is abundant and plays an important role in the differentiation of bone marrow-derived cells, we investigated four malignant cell lines from different stages of lymphocyte development for their response to GlcN treatment: KMH2, RAMOS, Jurkat, and HDML2. KMH2 cells exhibited the strongest response in terms of the O-GlcNAcylation of proteins (supplemental Fig. S1A). The optimal GlcN concentration and time point in terms of an increase in O-GlcNAcylated protein levels for KMH2 cell line was 20 mm and 24 h of treatment (supplemental Fig. 1C).
Experimental Outline of the Global GlcN Profiling of Proteins and mRNA
To investigate the mechanism of the GlcN effects, we examined GlcN-treated malignant cells from different stages of lymphocyte development using mass spectrometry and transcriptional arrays. Total mRNA extracts were used for transcriptional arrays. For quantitative proteome analysis cell fractionation was used to identify localization-dependent responses and to obtain deeper sampling. Nuclear, mitochondrial, and cytosolic fractions were analyzed using a label-free MS approach. The nuclear fraction from nontreated and GlcN-treated cells were analyzed using alternative quantitative methods, such as isobaric tandem mass tags (TMT) and stable-isotope dimethyl labeling providing additional validation for the quantitative findings. The label-free MS data for the nuclear proteins from each condition were analyzed using an open search approach to avoid cumbersome enrichment protocols and to profile several posttranslational modifications simultaneously in a single search.
We used tandem mass tags (TMT), stable-isotope dimethyl labeling and label-free MS-based quantitative methods to analyze the nuclear fraction before and after challenging KMH2 cells with GlcN (supplemental Fig. S3). Label-free quantitation based on spectral counts and ion counts in survey scans provided the deepest profiling (supplemental Fig. S3). The label-free quantitation identified 3397 proteins at 1% FDR threshold after collapsing the proteins to the encoded genes in the nuclear fraction. The label-free approach identified all proteins previously found by alternative quantitative approaches and identified ∼1000 additional proteins. At the peptide level, the label-free method resulted in the identification of twice as many peptide spectral matches (PSMs) per protein, leading to more reliable protein quantitation. High protein sequence coverage generates improved protein quantitation because of problems caused by modifications and partial tryptic cleavages. Therefore, we continued using label-free quantitation for the mitochondrial and cytosolic fractions. However, the TMT and stable-isotope dimethyl labeling methods are still used to validate the differential regulation observed from the label free data. The database-dependent search of the MS/MS spectra from the nuclear, mitochondrial, and cytosolic crude fractions resulted in a set of 18,816 proteins using a 1% FDR cutoff. Of these, 17,745 proteins were placed in evidence groups one to three as defined by Matthiesen et al. (37). Collapsing the proteins into the corresponding coding genes yielded 5181 proteins, 5065 of which were not common contaminants.
GlcN Increases the O-GlcNAcylation of Primarily Cytoplasmic and Nuclear Proteins
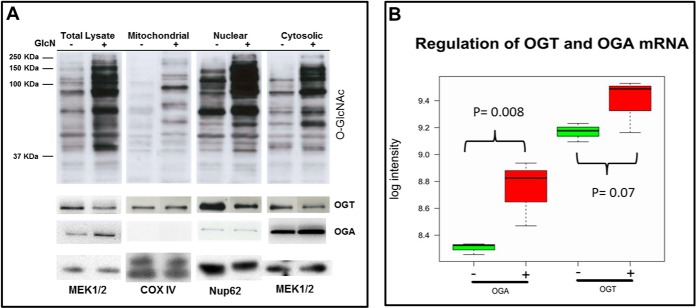
Western blotting of total cell lysate, nuclear, mitochondrial, and cytosolic crude fractions demonstrated abundant O-GlcNAcylation of proteins in the cytosolic and nuclear fractions. Furthermore, the strongest increase in O-GlcNAcylation after the GlcN treatment occurred in the nuclear and cytosolic fractions (Fig. 1A). Indeed, the Western blot analysis using the mitochondrial fractions purified by differential centrifugation were negative for lysosome and peroxisome markers but positive for mitochondrial markers and demonstrated no detectable level of O-GlcNAcylated proteins in KMH2 cells (data not shown). Furthermore, OGA (O-GlcNAcase) was not detected in the crude mitochondrial fraction using MS and Western blot analysis (Fig. 1A). According to the Western blot analysis, O-GlcNAc transferase (OGT) was down-regulated and OGA was up-regulated in all fractions at the protein level after the GlcN treatment (Fig. 1A and supplemental Fig. S1B). Based on the MS data OGT was detected and significantly down-regulated (pBH value<0.05, BH: Benjamini and Hochberg correction) in the cytosolic (more than fourfold), and nuclear fraction (more than 3-fold) and OGA was only detected significantly up-regulated (more than 9-fold) by MS in the cytosolic fraction thereby confirming the Western blots in cases where OGT and OGA were identified by MS (Fig. 2A–2C). OGT and OGA were not detected in mitochondrial fraction by MS. The microarray data showed mRNA up-regulation of OGT with a ratio of 1.14 and a marginal significant p value, whereas OGA (MGEA5) was significantly up-regulated with small effect size (ratio of 1.35) at the mRNA level that was confirmed by qPCR (Fig. 1B, t test followed by Benjamini and Hochberg correction).
Fig. 1.
GlcN causes increased O-GlcNAcylation primarily in the cytosolic and nuclear fractions. A, The effect of GlcN treatment on O-GlcNAc, OGT, and OGA protein levels in different subcellular fractions. CTD110.6 (anti-O-GlcNAc) Western blot of total lysate, crude mitochondrial, nuclear, and cytosolic fractions for control (−) and GlcN-treated (+) samples. Loading controls MEK1/2, COX IV and Nup 62 correspond to cytosolic, mitochondrial, and nuclear markers, respectively. B, mRNA regulation of OGT and OGA determined using Affymetrix arrays.
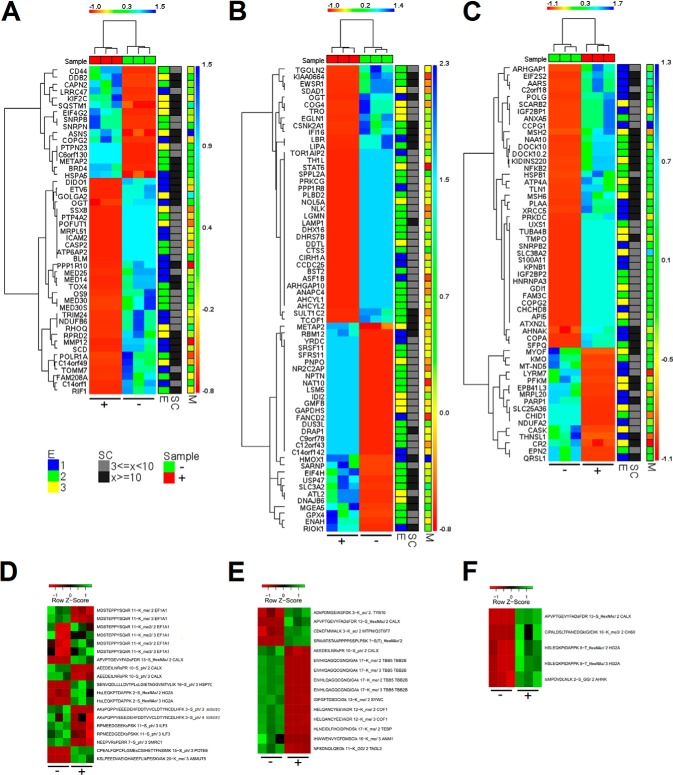
Fig. 2.
Global protein and PTM regulation observed in the three subcellular fractions and corresponding transcriptional regulation. Z normalized spectral count data for significant regulated proteins in A, nuclear, B, cytosolic, and C, mitochondrial fractions. Z normalized mziXIC values of significantly regulated PTMs in D, nuclear, E, cytosolic, and F, mitochondrial fractions. “C” indicates control and “T” treated. “SC” is the range of total number of spectral counts. “M” is the log ratio for mRNA expression. “E” is the evidence group: For a given protein “E = 1” means that the protein has at least one peptide spectra assignment matching uniquely, “E = 2” means that the protein belongs to a group of proteins that cannot be distinguished based on the MS data and have at least one peptide spectra assignment matching uniquely to the group, and “E = 3” means a protein that belongs to a group of proteins that share peptide spectra assignments of which none belongs to evidence groups one or two.
Global Regulation of Proteins, PTMs, and mRNA Expression in Cells Challenged with GlcN
The combined MS-based quantitative proteomics and transcriptomics data allowed the calculation of the ratios between untreated and treated cells, the p values and the abundance values for the mRNA and proteins in the nuclear, mitochondrial, and cytosolic fractions. Fig. 2A–2C summarizes Z normalized spectral count data for the significant changed proteins observed in the three fractions and correlated the proteomics data with the observed mRNA log ratios (M values). The data presented in Fig. 2A–2C were filtered to include proteins that are not common contaminants seen by MS and with evidence groups 1 to 3. This means that proteins not required to explain the MS data, that is, evidence groups 4 to 5, were filtered out and only peptide spectra assignments with maximum scores were taken into account for protein inference (37). Furthermore, to provide a more compact view and because we have incomplete knowledge of how specific protein isoforms function, the protein isoforms were collapsed into the corresponding coding genes for the presented heatmaps. Most of the significantly regulated proteins were in the cytosolic fraction (Fig. 2A–2C). The percentage of significantly regulated proteins that were up-regulated was considerably higher for the mitochondrial fraction (71% compared to 40 and 45% for the nuclear and cytosolic fractions, respectively).
To identify PTM regulation, statistical analysis of mziXIC (mass, charge, and isotope-dependent extracted ion chromatograms) values were performed as previously described (34) (Fig. 2D–2F). Based on the mziXIC values, a large number of unmodified peptides were observed significantly regulated; however, only significantly regulated peptides exhibiting lysine acetylation, lysine methylation, phosphorylation, or O-HexNAcylation modifications are shown in Fig. 2D–2F (after BH correction of p values). Figures of all raw spectra annotations of modified and significant regulated peptides are provided in supplementary file regulated.pdf. Annotation of raw spectra from all modified peptides and extracted mziXIC values are provided in supplementary file PTMpep.zip as obtained directly from the VEMS modules v1.0. File HexNAc.zip contains raw spectra annotation and quantitative values of all O-HexNAc peptides with ambiguous modifications sites specified. It is unrealistic to determine sites of O-HexNAc based on HCD data because of the intense neutral loss of O-HexNAc.
GlcN Causes ER Stress and Vacuolization Affecting Cell Cycle Progression
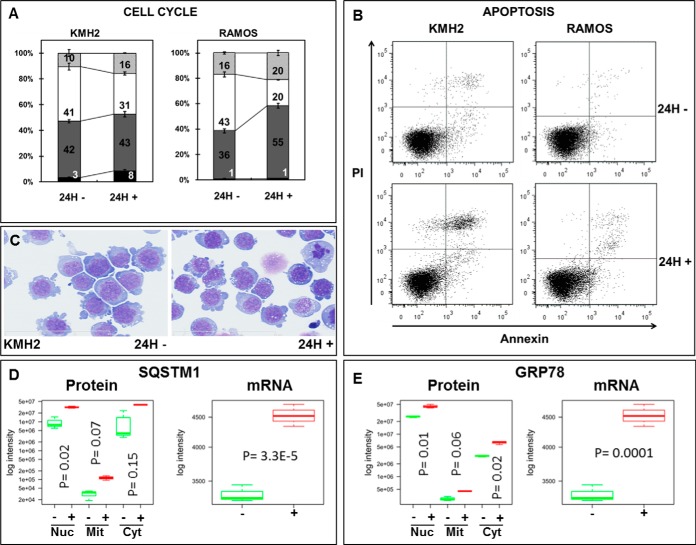
GlcN caused an increase in sub-G1 KMH2 population, G1 RAMOS population and G2/M KMH2 and RAMOS populations (Fig. 3A). KMH2 cells doubling time are longer than RAMOS cells that can account for the smallest decrease in S phase population for KMH2 compared with RAMOS and no significant increase in G0/G1 population for KMH2. GlcN hindered KMH2 and RAMOS cell cycle progression and furthermore a 10% decreased in cell viability was observed for KMH2 compared to 6% for RAMOS cells (data not shown). Increased apoptosis was observed in KMH2 compared with the RAMOS cells, and in both cases, the level of apoptosis could not fully explain the decrease in viable cells (Fig. 3B). In conclusion, the inhibition of cell progression together with apoptosis caused an overall decrease in total number of viable cells upon GlcN treatment for KMH2 and RAMOS. KMH2 (Fig. 3C) and RAMOS (data not shown) cells treated with GlcN accumulated vacuoles in the cytoplasm. The GlcN treatment caused an approximately twofold up-regulation of SQSTM1 (a protein described to interact with MAP1 LC3 family members) in the three subcellular fractions based on spectral counting (Fig. 3D). SQSTM1 in the nuclear fraction (see Fig. 2) and its transcriptional level (Fig. 3D) appeared to be significantly regulated (p < 0.05, t test followed by Benjamini and Hochberg).
Fig. 3.
GlcN causes cell cycle arrest, apoptosis, ER stress and increased autophagy. A, Cell cycle phase distribution for KMH2 and RAMOS cells (black: sub-G1, dark gray: G0/G1, white: S-phase, and light gray: G2/M). B, Apoptosis assay for KMH2 and RAMOS cells. C, Increased vacuolization of KMH2 cells upon GlcN treatment. D, SQSTM1 was up-regulated after GlcN treatment (green depicts untreated and red depicts after the GlcN treatment) at both the (a) protein and (b) mRNA level after GlcN treatment. E, GRP78/HSPA5 was up-regulated following the GlcN treatment (green depicts untreated and red depicts after the GlcN treatment) at both the (a) protein and (b) mRNA level after GlcN treatment.
GlcN also led to the up-regulation of GRP78/HSPA5 (a marker for ER stress) at both the protein and mRNA level (Fig. 3E) based on a t test followed by Benjamini and Hochberg correction (p < 0.05).
GlcN Increases the O-HexNAcylation of Calnexin and Decreases Calnexin Phosphorylation
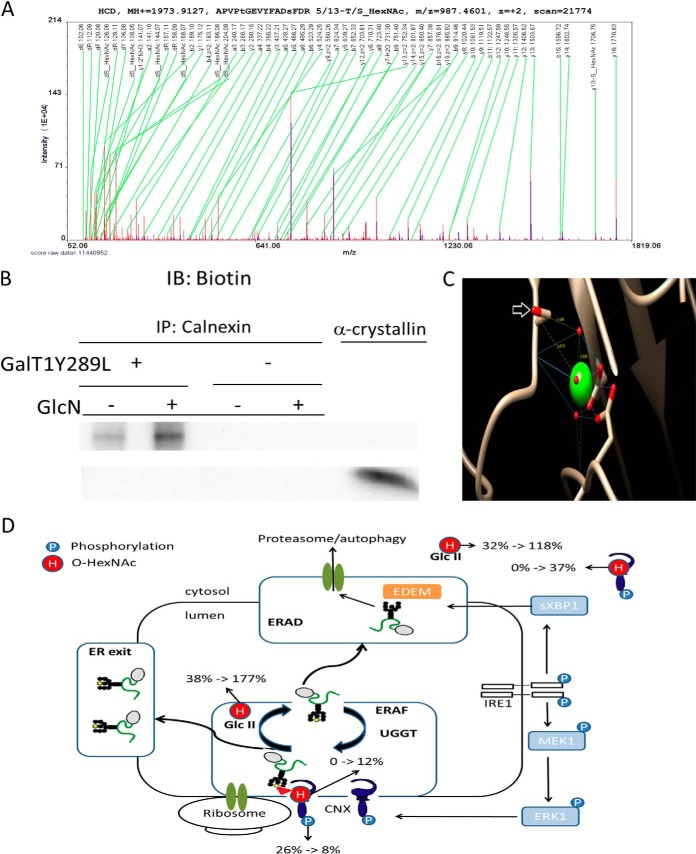
The MS data presented here is unable to distinguish between O-GlcNAc and O-GalNAc and therefore “O-HexNAc” is used for MS spectra annotation. The analysis described in Fig. 2D–2E suggested the novel regulation of O-HexNAc on calnexin. The O-HexNAc-modified peptide “APVPTGEVYFADSFDR” mapped only to calnexin isoforms encoded by a single gene, CANX (supplemental Fig. S4). The label-free (Fig. 4A and supplemental Fig. S5), TMT, and stable-isotope dimethyl labeling data (supplemental Fig. S6A and S6B) confidently identified both the unmodified and O-HexNAc-modified peptide. Annotation of the raw spectrum pinpointed the O-HexNAc site to be Thr-66 or Ser-74 in calnexin (UniProtKB P27824, Version 153 and supplemental Fig. S4) with approximately equal probability for the two sites. GalNAc O-linked as a single residue in the ER has not been previously reported in nonengineered cells because it is normally extended. However, a nonextended GalNAc (the authors assume O-GalNAc because of the ER location of calnexin) at Thr-66 has been described in engineered cells in which the core extending glycosyltransferase responsible for “mucin-type” (GalNAc-type) O-linked glycosylation or its essential chaperone has been knocked out (40). Compared with O-GalNAc, GlcN is a more direct precursor for O-GlcNAc. Furthermore, Sakaidani and coworkers recently reported an alternative O-GlcNAc transferase (EOGT) that localizes to the lumen of the endoplasmic reticulum and transfers GlcNAc to epidermal growth factor-like domains in an OGT-independent manner (41). However, to provide further evidence for the O-GlcNAc modification of calnexin, calnexin IP (immunoprecipitation) from KMH2 cell extracts was performed before and after the GlcN treatment (supplemental Fig. S7A and S7B), followed by Gal-T1 and Click-iT™ Biotin Alkyne Detection (Fig. 4B). The enzyme Gal-T1 is described to be specific for O-GlcNAc, and an increased Biotin Alkyne Detection signal was observed in the treated cells. The negative controls lacking Gal-T1 were blank, and the positive control using alpha-crystallin was positive (Fig. 4B). Positive detection of O-GlcNAc on Western blot of calnexin IP with RL2 and CTD110.6 could not be confirmed (supplemental Fig. S7B). This does, however, not exclude the possibility that calnexin is O-GlcNAc modified because antibodies targeting glycan modified peptides may partially recognize part of the protein as well.
Fig. 4.
GlcN increases the O-GlcNAcylation of calnexin. A, MS/MS raw spectrum of the assigned peptide “APVPtGEVYFADsFDR 5/13-T/S_HexNAc/.” B, Purified calnexin from untreated and treated cells labeled using the Click-iT™ O-GlcNAc Enzymatic Labeling System. Detection was performed using the Click-iT™ Biotin Alkyne Detection Reagent (+). Purified calnexin (vide Supplemental Fig. S7) was subjected to the same procedure without the addition of the Gal-T1 (Y289L) enzyme (–). C, One of the mapped O-HexNAc site is in close proximity to the Ca2+ pocket in calnexin. The modified serine residues are indicated by a white arrow, and the backbone carbonyl group is involved in the metal coordination of Ca2+. D, Regulation of ER quality control under protein misfolding conditions (schematic modified from (44)). Subcellular quantitative levels of O-HexNAc and phosphorylation on calnexin. % indicates the ratio between modified and unmodified residues (in percentage); black and yellow circles represent mannose residues, and the triangle represents a glucose residue; H, O-HexNAc; P, phosphorylation; ERAF, ER-assisted folding; ERAD, ER-associated protein degradation; Glc II, glucosidase II; UGGT, UDP-glucose:glycoprotein glucosyltransferase.
Ser-74 in calnexin is required for Ca2+ binding. The Ca2+-binding residues are highly conserved, and calnexin undergoes Ca2+-dependent conformational changes (42). The peptide “APVPTGEVYFADSFDR HexNAc” in calnexin was up-regulated more than 73-fold compared with the control (supplemental Fig. S8), providing a potential explanation for how GlcN can cause ER stress directly (Fig. 4D).
GlcN decreased the calnexin phosphorylation from 26% to 8% (ratio of modified/unmodified protein in the nuclear fraction, supplemental Fig. S9 and S10) on Ser-583. The modification of Ser-583 is known to regulate calnexin activity levels during the unfolding protein response (43) and is a potential target of PKC (protein kinase C) (44, 45). The ratio between the unmodified and the O-HexNAc-modified “APVPTGEVYFADSFDR” form of calnexin changed from ∼0% to ∼12% in the nuclear fraction after the GlcN treatment (Fig. 4D). Interestingly, the ratio between the unmodified and O-HexNAc-modified “APVPTGEVYFADSFDR” form of calnexin changed from ∼0% to ∼37% in the cytosolic fraction (Fig. 4D).
The ratio between unmodified and O-HexNAc-modified “SEALPTDLPAPsAPDLTEPK” forms of GLU2B (glucosidase 2 subunit beta), an ER heterodimeric enzyme, increased from 38 to 177% (increase of ∼fivefold compared with the canonical peptide) in the nuclear fraction after GlcN treatment (supplemental Fig. S11 and Fig. 5D). In the cytosolic fraction, the ratio increased from 32% to 118%, which represents a ∼fourfold increase compared with the canonical peptide.
Fig. 5.
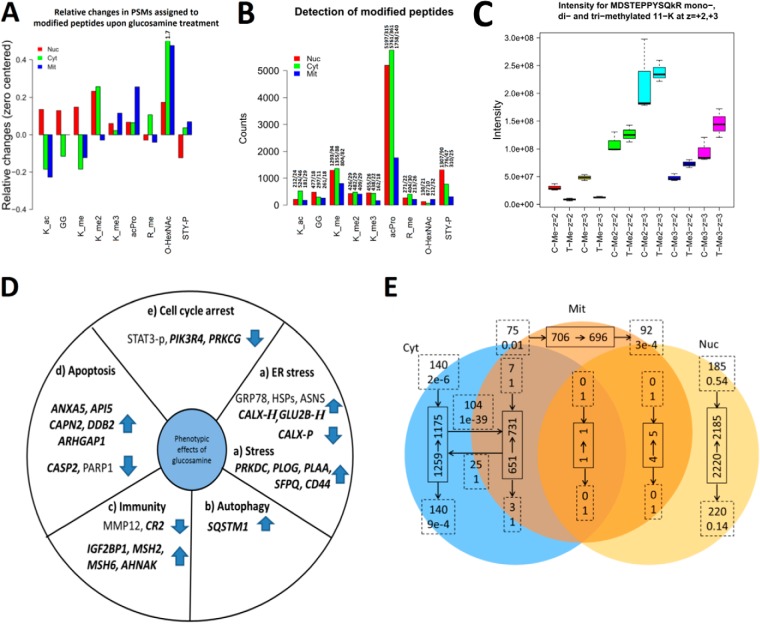
Effect of GlcN treatments on detected posttranslational modifications. In A, B, and C, _ac corresponds to acetylation; GG, diglycine from ubiquitin; P, phosphorylation; _me, mono-, me2 di-, and me3 is tri- methylation. z is charge state. A, Relative changes in modifications observed after GlcN treatment for the nuclear, cytosolic and mitochondrial fractions based on spectral counting. Relative changes of O-HexNAc for cytosolic fraction went out of scale indicated by the number 1.7. B, Number of PSMs for modified peptides in the nuclear, cytosolic, and mitochondrial fractions. Bar labels indicate: “number of PSMs for modified peptides/number of unique spectra assignments for modified peptides.” C, GlcN increased the levels of EF1A1 methylation in the nuclear fraction. Intensity counts from survey scans for the mono-, di-, and tri- methylated peptide MDSTEPPYSQkR (on 11-K) from elongation factor “EF1A1.” D, Summary of the most relevant markers for (a) ER stress, (b) autophagy, (c) immunity, (d) apoptosis, and (e) cell cycle arrest that were significantly regulated at the protein level after the GlcN treatment. Novel findings are highlighted in bold and italics. “H” indicates O-HexNAcylation, and “P” indicates phosphorylation. E, Protein overlap between subcellular fractions using a 1% FDR threshold and a minimum of five PSMs assigned per protein in a specific subcellular fraction. Full line boxes indicate the number of proteins before (start of arrow) and after (end of arrow) the GlcN treatment. Dashed line boxes indicate the exchange of proteins after the GlcN treatment (upper text is the number of proteins and lower text is the likelihood such an exchange of proteins occurred by chance). Dashed line boxes located at the start of an arrow are the number of new proteins observed only after GlcN treatment and those located at the end of an arrow are the number of proteins only observed before GlcN treatment.
Link between GlcN Metabolism and Posttranslational Modifications
The GlcN treatment lead to alterations in acetylation, methylation, and phosphorylation in addition to O-HexNAcylation, as evaluated by the relative change in the number of assigned PSMs on a 1% FDR threshold (Fig. 5). The largest change in O-HexNAcylation in terms of the assigned PSMs occurred in the cytosolic fraction (Fig. 5A). Interestingly only phosphorylation in the nucleus decreased overall following the GlcN treatment. The GlcN caused acetylation to increase in the nucleus and decrease in the cytosolic and mitochondrial fractions. Although monomethylation in the cytosolic and mitochondrial fractions and dimethylation in the mitochondrial fraction decreased, trimethylation increased in all fractions (Fig. 5A). N-terminal protein acetylation was prominent as the posttranslational modification detected most often (Fig. 5B). In this analysis, we disregarded modifications, such as deamidation and oxidation, which were more abundant but difficult to interpret because they are often the result of an artifact of sample handling. GlcN treatment increased the levels of EF1A1 methylation in the nuclear fraction (Fig. 5C). The label-free MS data set identified the unmodified, mono-, di-, and tri-methylated versions of the peptide MDSTEPPYSQkR (lowercase indicates site of methylation). Furthermore, these peptides were both observed as double- and triple-charged. A standard statistical analysis (t test in combination with Benjamini and Hochberg correction) identified only the mono-methylated peptide as significantly down-regulated, giving the general impression that methylation decreased. However, a careful analysis of the label-free quantitative data indicated that di- and tri-methylated versions of the peptide were significantly up-regulated before the Benjamini and Hochberg correction. In addition, if the combined up-regulation of the di- and tri-methylated peptides were considered in the calculation of the p value, the interpretation would have changed and reflected a higher degree of methylation rather than decreased methylation (Fig. 5C). Furthermore, up-regulation was observed for both the double- and triple-charged versions of the peptides. This example not only demonstrates a data analysis pitfall but also a potential pitfall in analyzing data enriched for a single modification.
DISCUSSION
Global MS-based Drug Profiling Reconciles Results from Previous Studies
In general, our results reconcile observations from a broad range of published studies on GlcN-induced O-HexNAcylation and the effect of this modification on cancer and other diseases in both in vitro and in vivo models. We observed little correlation between protein and mRNA levels (Fig. 2 and supplemental Fig. S12), suggesting that O-HexNAc not only functions in transcriptional regulation but also in protein homeostasis. The MS data provided evidence in the form of regulated protein markers for all previously described phenotypic effects of GlcN treatment, including: (1) ER stress, (2) autophagy, (3) immunity, (4) apoptosis, and (5) cell cycle arrest (Fig. 2 and 5D). Enrichment analysis of down-regulated genes in the transcription array data showed a consistent and highly significant enrichment of replication dependent histones (supplemental Fig. S13A and S13C). This result is consistent with the significant decrease of cells in S phase for both RAMOS and KMH2 cells upon GlcN treatment (see Fig. 3A). GlcN induced ER stress (46, 47), as confirmed by the up-regulation of the ER stress markers GRP78, HSPs, ASNS, etc. (Fig. 1E, 3, and 5D-a). Recent studies have demonstrated that GlcN-induced ER stress causes autophagy, as evidenced by Western blots for the autophagy marker LC3 (19). In this study, we have observed an increase in the number of vacuoles in the cytoplasm of malignant cell lines from different stages of lymphocyte development such as KMH2 (Fig. 1C) and RAMOS (data not shown) and the up-regulation of SQSTM1/p62 (Fig. 1D and 5D-b).
GlcN protects chondrocytes isolated from the cartilage of rats from the arthritogenic effects of IL-1beta, and this effect correlates with the down-regulation of matrix metalloproteinase (48). Wang et al. reported that the overexpression of matrix metalloproteinase 12 (MMP12) in inflammatory arthritis in transgenic rabbits exacerbates arthritic lesions (49). We observed the down-regulation of MMP12 in KMH2 lymphoma cell line after challenge with GlcN, which might be relevant in terms of limiting cancer cell invasiveness (Fig. 5D-c). We also observed AHNAK up-regulation after the GlcN treatment (Fig. 2). AHNAK regulates calcium channels in T lymphocytes, and this functionality is critical in autoimmune diseases, such as lupus and arthritis (50). Furthermore, AHNAK is down-regulated in Burkitt's lymphoma (51), providing an additional link between GlcN and cancer. The literature contains contradictory information on GlcN-induced apoptosis. Our data from cancer cells from different stages of lymphocyte development were consistent with the recent finding that GlcN induces limited apoptosis (Fig. 3B) and instead causes cell death via an autophagy-mediated pathway (19). For example, GlcN down-regulated caspase-2 (CASP2), a positive regulator of apoptosis, and up-regulated apoptosis inhibitor 5 (API5) (Fig. 2 and 5D-d). Finally, we observed cell cycle arrest (Fig. 3A), which was consistent with previous findings (11). We observed the up-regulation of ANXA5, a known inhibitor of PKC (52), which was consistent with the down-regulation of PRKCG (PKC), STAT3-p, and PIK3R4, and the inhibition of the cell cycle (Fig. 5D-e). Host Cell factor-1 (HCF1), a transcriptional co-regulator of human cell cycle regulation was significantly identified and modified by O-HexNAc (supplemental Table “HexNAc.zip”). O-HexNAc modified peptides for HCF1 showed an overall ∼25% down-regulation; however, this overall down-regulation was not significant.
We observed little overlap of significantly regulated proteins among the subcellular fractions; however, METAP2 was up-regulated in both the nuclear and cytosolic fraction, OGT was down-regulated in both the nuclear and cytosolic fraction, and COPG2 was up-regulated in the nuclear and mitochondrial fractions. These results are consistent with the analysis of identified proteins in each of the subcellular fractions before and after the GlcN treatment (Fig. 5E). After the GlcN treatment, the altered proteins (1% FDR and a minimum of five peptide spectra matches per protein) were located predominantly in the parts of the Venn diagram that were unique to specific subcellular fractions (Fig. 5E). For example, 140 new proteins were observed in cytosolic fraction after GlcN treatment (p value = 2e-6, using the hypergeometric distribution). This analysis was repeated with 1% FDR and a minimum of 0, 5, and 10 peptide spectra matches per protein, and in all cases the pattern persisted. Using all the proteins identified at 1% FDR resulted in more protein overlap between fractions. Furthermore, the analysis suggested a significant trafficking of proteins between the cytoplasm and the mitochondria. For example, 104 proteins that were uniquely identified in the cytosolic fraction without GlcN treatment were observed in both the mitochondrial and cytosolic fraction upon GlcN treatment (p value = 1e-39 using the hypergeometric distribution). This finding is consistent with our hypothesis that O-GlcNAc is involved in protein trafficking. It will be interesting to determine in future studies whether this is a general trend for drug-affected proteomes. A number of the observed regulated proteins are not in the anticipated canonical subcellular fraction (Fig. 2A, 2B, and 2C). For example, IGFBP1 and IGFBP2, known to be secreted, end up in the mitochondrial fraction after GlcN treatment. This might be the result of the gross changes in cellular architecture as indicated by the formation of vacuoles and cells undergoing apoptosis. Furthermore, subtypes of KMH2 cells are multinucleated, which potentially can influence proteins canonical subcellular location.
The identification of significantly regulated novel key protein markers obtained by MS-based drug profiling can be used to define innovative combined treatment strategies. Furthermore, these data provide novel evidence for how GlcN affects cells at the molecular level. MS-based drug profiling experiments, demonstrated in this study using GlcN, provide detailed information regarding the effects of drugs at a cost similar to mRNA expression arrays.
Glucosamine, ER Stress, and Autophagy
To date, no mechanisms linking GlcN to ER stress and autophagy have been described. The O-GlcNAcylation of heat shock proteins is a viable hypothesis; however, there is no direct evidence to support this hypothesis. We propose that the O-HexNAcylation of Ser-74 in calnexin (located on the luminal side of the ER), a residue involved in the Ca2+ binding pocket, inactivates the protein or disturbs ion coordination (Fig. 4C). Ca2+ regulates the activity of calnexin, and in protein data bank (PDB) structures, Ca2+-binding pockets in general exhibit a hydrophobic environment. Placing O-HexNAcylation on Ser-74 increases the hydrophilicity of the local environment. Therefore a possible hypothesis is that Ser-74 O-HexNAc lowers calnexin's affinity for Ca2+. Furthermore, the MS data support this model. The GlcN decreased calnexin phosphorylation on Ser-583 (from 26% to 8% modified/unmodified), a modification that should increase following ER stress (44), and based on the increase of the ER marker GRP78, ER stress was clearly generated. The decrease in Ser-583 phosphorylation (in the cytoplasmic domain of calnexin), together with the increased ratio of O-HexNAc-modified to unmodified calnexin in the cytosolic fraction (presumably from autophagy vesicles) compared with the nuclear fraction (presumably of ER origin), suggests that autophagy clears the impaired calnexin (with O-HexNAcylation on Ser-74) from the ER. However, O-HexNAcylation of calnexin Ser-74 or Thr-66 could be involved in regulating vesicle transport, providing an alternative hypothesis in which the O-HexNAcylation constitutes a consequence instead of a cause of ER stress.
GLU2B (glucosidase 2, subunit beta), is an ER luminal protein involved in protein quality control, and the ratio between the O-HexNAc-modified and unmodified GLU2B in the nuclear fraction (presumably of ER origin) increased from 38% to 177% after GlcN treatment. The GlcN down-regulated PKC (Fig. 2 and 5D-e, PRKCG) and the level of O-HexNAcylation of GLU2B, a known substrate of PKC, increased. This observation is consistent with the “yin-yang” hypothesis for phosphorylation and O-GlcNAcylation proposed by Hart and co-authors (53).
Previous studies have demonstrated functional roles for O-GlcNAc in the nucleus and cytoplasm. The identification of two regulated O-HexNAc (potentially O-GlcNAc) sites on proteins known to be ER residents suggests that O-GlcNAc may play a functional role in the ER in addition to O-GalNAc.
Anticancer Properties of GlcN
Our data indicate that the induced overload of internal UDP-GlcNAc, probably because of some cancer cells inability to regulate GlcN transporters in cell membrane, leads to saturation of O-glycans pathways in the ER. We speculate that improper and uncontrolled initiation of O-glycosylation lead to ER stress, which is partly resolved by transporting the affected proteins to internal vacuoles rather than the normal secretory pathways. Specifically targeting the proteome of vacuoles formed upon GlcN treatment by MS-based methods could resolve this issue in the future. We believe this is important because one possibility is that increased short O-glycans on the cell surface potentially can lead to increase migration potential and invasiveness of the cancer cells (54). Cell migration assays could address this question.
Other issue to consider in terms of anticancer properties is the observation that KMH2 cells become more resistance to Bortezomib treatment (Supplemental Fig. S14), if combined with GlcN treatment. We observe that GlcN has an additive effect in terms of causing cell death at low Bortezomib concentration. However, at higher Bortezomib concentrations GlcN protects the cells. This suggests that a clinical study on the combined effect of bortezomib treatment and GlcN intake or diet is relevant.
Analyzing the global regulation of mRNA and proteins in KMH2 and RAMOS cell lines shows a clear trend that mitochondrial genes and proteins are in general down-regulated (supplemental Fig. S15). This could indicate a shift to glucose metabolism as a response to the metabolic burden.
The ER stress activates signaling pathways that leads to cell cycle arrest as indicated by the decrease in S-phase, replication dependent histones (both mRNA and protein level), and deactivation of STAT3 (pTyr705 and pSer727). STAT3 activation can be restored by releasing the cells from GlcN media (see supplemental Fig. S1). The STAT3 dephosphorylation is unlikely to be caused by steric hindrance from STAT3 O-GlcNAcylation because STAT3 IP followed by Western blot for O-GlcNAc displayed no signal for O-GlcNAcylation but positive for STAT3 enrichment (Click-IT, CTD110.6, and RL2 assays for O-GlcNAc were attempted). We therefore speculate that the “yin-yang” relationship between phosphorylation and O-HexNAcylation that we observe occurs not by steric hindrance but rather by regulation of kinases and phosphatase activity.
GlcN-induced O-GlcNAcylation Affects Modifications other than the Global Levels of Phosphorylation
Several reports support the hypothesis of a “yin-yang” relationship between phosphorylation and O-GlcNAcylation (53, 55, 56). These reports include examples ranging from a global, overall “yin-yang” relationship between phosphorylation, and O-GlcNAcylation to a “yin-yang” on specific protein targets. In this study, we identified two additional examples of a “yin-yang” relationship between O-HexNAcylation and phosphorylation. For example, the relationship between O-HexNAcylation and phosphorylation on calnexin, observed in our study, in which the modification sites were in two different subcellular compartments. Interestingly, our subcellular fractionation in combination with GlcN-induced O-HexNAcylation down-regulated the phosphorylation level of primarily nuclear proteins (Fig. 5A). This example supports further the hypothesis of global “yin-yang” between O-GlcNAcylation and phosphorylation. The “yin-yang” between O-GlcNAcylation and phosphorylation observed upon GlcN treatment could provide an alternative hypothesis to how GlcN cause cell cycle arrest and displays anticancer properties.
Finally, GlcN caused an overall shift from monomethylation to di- and tri-methylation and altered the acetylation pattern of proteins (Fig. 5A), suggesting the need for studies examining PTMs in combination.
CONCLUSION
The data presented in this study demonstrate that two important protein factors, calnexin and GLU2B, which function in the protein folding and quality control pathway in ER, are O-HexNAc-regulated after GlcN treatment. Furthermore, different ratios of the O-HexNAc modified and canonical peptide from GLU2B and calnexin were observed in different cellular compartments. Because GLU2B and calnexin are involved in ER stress this demonstrates a strong evidence for a direct link between O-HexNAc and GlcN-induced ER stress as either a cause or a consequence and suggests that O-HexNAc potentially in the O-GlcNAc form plays a functional role in the ER in terms of subcellular localization.
Supplementary Material
Acknowledgments
We thank Dr. Jorg Becker and João Sobral (Instituto Gulbenkian de Ciência, Gene Expression Unit, Oeiras) for microarray service. We thank Catarina Leitão (Advanced Flow Cytometry Unit at Institute for Molecular and Cell Biology, Porto) for flow cytometry technical assistance.
Footnotes
Author contributions: A.S.C. and R.M. designed research; A.S.C., H.R., P.V., and R.M. performed research; A.S.C., O.N.J., H.M., and R.M. contributed new reagents or analytic tools; A.S.C. and R.M. analyzed data; A.S.C. and R.M. wrote the paper; D.P. commented the manuscript.
* Funding: RM is supported by PTDC/EIA-EIA/099458/2008 Fundacçã para a Ciência e a Tecnologia (FCT), program FCT investigator 2012. ASC is supported by grant SFRH / BPD / 85569 / 2012 funded by Fundacçã para a Ciência e Tecnologia. IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education and is partially supported by FCT. HR, PV, ASC, and RM are further supported by FCT grants (PTDC/QUI-BIQ/099457/2008).
 This article contains Supplemental Figs. S1 to S16 and Table S1.
This article contains Supplemental Figs. S1 to S16 and Table S1.
Data and materials availability: Mass spectrometry raw data including help files, processed spectra and tables containing the identified peptides, raw spectra number, processed spectra number, peptide scores, FDR statistics, extracted ion counts for peptides, spectra counts for proteins and statistical analysis have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository (1) with the dataset identifier PXD000380. Microarray data is publicly available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSE49185, Gene Expression Omnibus (GEO) accession number GSE49185.
1 The abbreviations used are:
- GlcN
- Glucosamine
- API5
- apoptosis inhibitor 5
- CANX
- calnexin
- CASP2
- caspase-2
- DEHP
- Bis (2-Ethylhexyl)(Phthalate)
- FDR
- false discovery rate
- GEO
- Gene Expression Omnibus
- GLU2B
- glucosidase 2 subunit beta
- M
- log ratios
- MMP12
- matrix metalloproteinase 12
- mziXIC
- mass, charge and isotope-dependent extracted ion chromatograms
- O-GlcNAc
- O-linked β-N-acetylglucosamine
- OGA
- O-GlcNAcase
- OGT
- O-GlcNAc transferase
- PCMs
- polycyclodi-methylsiloxane
- PI
- propidium iodide
- PKC
- protein kinase C
- PLB
- Phospholamban
- PSMs
- peptide spectral matches
- qPCR
- quantitative polymerase chain reaction
- RIPA
- radioimmune precipitation assay
- RMA
- robust multichip average
- SERCA
- sarco/endoplasmic reticulum calcium ATPase
- UDP-GalNAz
- Uridine 5′-diphospho-N-azidoacetylgalactosamine.
REFERENCES
- 1. Vizcaino J. A., Cote R. G., Csordas A., Dianes J. A., Fabregat A., Foster J. M., Griss J., Alpi E., Birim M., Contell J., O'Kelly G., Schoenegger A., Ovelleiro D., Perez-Riverol Y., Reisinger F., Rios D., Wang R., Hermjakob H. (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 3. Jones S. P., Zachara N. E., Ngoh G. A., Hill B. G., Teshima Y., Bhatnagar A., Hart G. W., Marban E. (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117, 1172–1182 [DOI] [PubMed] [Google Scholar]
- 4. Vosseller K., Wells L., Lane M. D., Hart G. W. (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U. S. A. 99, 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akimoto Y., Hart G. W., Hirano H., Kawakami H. (2005) O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med. Mol. Morphol. 38, 84–91 [DOI] [PubMed] [Google Scholar]
- 6. McCarty M. F. (1994) The neglect of glucosamine as a treatment for osteoarthritis–a personal perspective. Med. Hypotheses 42, 323–327 [DOI] [PubMed] [Google Scholar]
- 7. Reginster J. Y., Deroisy R., Rovati L. C., Lee R. L., Lejeune E., Bruyere O., Giacovelli G., Henrotin Y., Dacre J. E., Gossett C. (2001) Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357, 251–256 [DOI] [PubMed] [Google Scholar]
- 8. Silbert J. E. (2009) Dietary glucosamine under question. Glycobiology 19, 564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muniyappa R. (2011) Glucosamine and osteoarthritis: time to quit? Diabetes Metab. Res. Rev. 27, 233–234 [DOI] [PubMed] [Google Scholar]
- 10. Fulop N., Zhang Z., Marchase R. B., Chatham J. C. (2007) Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am. J. Physiol. Heart Circ. Physiol. 292, H2227–H2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chesnokov V., Sun C., Itakura K. (2009) Glucosamine suppresses proliferation of human prostate carcinoma DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int. 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quastel JH, C. A. (1953) Inhibition of tumor growth by D-glucosamine. Nature 171, 252–254 [DOI] [PubMed] [Google Scholar]
- 13. Slawson C., Hart G. W. (2011) O-GlcNAc signaling: implications for cancer cell biology. Nat. Rev. Cancer 11, 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beriault D. R., Werstuck G. H. (2012) The role of glucosamine-induced ER stress in diabetic atherogenesis. Exp. Diabetes Res. Epub 2012, Feb 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagy T., Champattanachai V., Marchase R. B., Chatham J. C. (2006) Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am. J. Physiol. Cell Physiol. 290, C57–C65 [DOI] [PubMed] [Google Scholar]
- 16. Ranuncolo S. M., Ghosh S., Hanover J. A., Hart G. W., Lewis B. A. (2012) Evidence of the involvement of O-GlcNAc-modified human RNA polymerase II CTD in transcription in vitro and in vivo. J. Biol. Chem. 287, 23549–23561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z., Gucek M., Hart G. W. (2008) Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc. Natl. Acad. Sci. U. S. A. 105, 13793–13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jokela T. A., Makkonen K. M., Oikari S., Karna R., Koli E., Hart G. W., Tammi R. H., Carlberg C., Tammi M. I. (2011) Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1. J. Biol. Chem. 286, 33632–33640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hwang M. S., Baek W. K. (2010) Glucosamine induces autophagic cell death through the stimulation of ER stress in human glioma cancer cells. Biochem. Biophys. Res. Commun. 399, 111–116 [DOI] [PubMed] [Google Scholar]
- 20. Carames B., Kiosses W. B., Akasaki Y., Brinson D. C., Eap W., Koziol J., Lotz M. K. (2013) Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheum. 65(7), 1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma Y., Hendershot L. M. (2004) The role of the unfolded protein response in tumour development: friend or foe? Nat. Rev. Cancer 4, 966–977 [DOI] [PubMed] [Google Scholar]
- 22. Laczy B., Hill B. G., Wang K., Paterson A. J., White C. R., Xing D., Chen Y. F., Darley-Usmar V., Oparil S., Chatham J. C. (2009) Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 296, H13–H28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokoe S., Asahi M., Takeda T., Otsu K., Taniguchi N., Miyoshi E., Suzuki K. (2010) Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology 20, 1217–1226 [DOI] [PubMed] [Google Scholar]
- 24. Ngoh G. A., Hamid T., Prabhu S. D., Jones S. P. (2009) O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 297, H1711–H1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wettenhall J. M., Simpson K. M., Satterley K., Smyth G. K. (2006) affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22, 897–899 [DOI] [PubMed] [Google Scholar]
- 26. Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y. H., Yosef (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 57, 289–300 [Google Scholar]
- 28. Boersema P. J., Raijmakers R., Lemeer S., Mohammed S., Heck A. J. (2009) Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 4, 484–494 [DOI] [PubMed] [Google Scholar]
- 29. Wisniewski J. R., Zougman A., Mann M. (2009) Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J. Proteome Res. 8, 5674–5678 [DOI] [PubMed] [Google Scholar]
- 30. Rappsilber J., Mann M., Ishihama Y. (2007) Protocol for micro-purification, enrichment, prefractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 31. Schlosser A., Volkmer-Engert R. (2003) Volatile polydimethylcyclosiloxanes in the ambient laboratory air identified as source of extreme background signals in nanoelectrospray mass spectrometry. J. Mass Spectrom. 38, 523–525 [DOI] [PubMed] [Google Scholar]
- 32. Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 33. Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 34. Matthiesen R. (2013) LC-MS spectra processing. Methods Mol. Biol. 1007, 47–63 [DOI] [PubMed] [Google Scholar]
- 35. Matthiesen R. (2013) Algorithms for database-dependent search of MS/MS data. Methods Mol. Biol. 1007, 119–138 [DOI] [PubMed] [Google Scholar]
- 36. Bunkenborg J., Garcia G. E., Paz M. I., Andersen J. S., Molina H. (2010) The minotaur proteome: avoiding cross-species identifications deriving from bovine serum in cell culture models. Proteomics 10, 3040–3044 [DOI] [PubMed] [Google Scholar]
- 37. Matthiesen R., Prieto G., Amorim A., Aloria K., Fullaondo A., Carvalho A. S., Arizmendi J. M. (2012) SIR: Deterministic protein inference from peptides assigned to MS data. J. Proteomics 75, 4176–4183 [DOI] [PubMed] [Google Scholar]
- 38. Matthiesen R., Carvalho A. S. (2013) Methods and algorithms for quantitative proteomics by mass spectrometry. Methods Mol. Biol. 1007, 183–217 [DOI] [PubMed] [Google Scholar]
- 39. Polpitiya A. D., Qian W. J., Jaitly N., Petyuk V. A., Adkins J. N., Camp D. G., 2nd, Anderson G. A., Smith R. D. (2008) DAnTE: a statistical tool for quantitative analysis of -omics data. Bioinformatics 24, 1556–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vakhrushev S. Y., Steentoft C., Vester-Christensen M. B., Bennett E. P., Clausen H., Levery S. B. (2013) Enhanced mass spectrometric mapping of the human GalNAc-type O-glycoproteome with SimpleCells. Mol. Cell. Proteomics 12, 932–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakaidani Y., Nomura T., Matsuura A., Ito M., Suzuki E., Murakami K., Nadano D., Matsuda T., Furukawa K., Okajima T. (2011) O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat. Commun.2, 583. [DOI] [PubMed] [Google Scholar]
- 42. Schrag J. D., Bergeron J. J., Li Y., Borisova S., Hahn M., Thomas D. Y., Cygler M. (2001) The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 8, 633–644 [DOI] [PubMed] [Google Scholar]
- 43. Bollo M., Paredes R. M., Holstein D., Zheleznova N., Camacho P., Lechleiter J. D. (2013) Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS One 5, e11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chevet E., Smirle J., Cameron P. H., Thomas D. Y., Bergeron J. J. (2010) Calnexin phosphorylation: linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin. Cell Dev. Biol. 21, 486–490 [DOI] [PubMed] [Google Scholar]
- 45. Wong H. N., Ward M. A., Bell A. W., Chevet E., Bains S., Blackstock W. P., Solari R., Thomas D. Y., Bergeron J. J. (1998) Conserved in vivo phosphorylation of calnexin at casein kinase II sites as well as a protein kinase C/proline-directed kinase site. J. Biol. Chem. 273, 17227–17235 [DOI] [PubMed] [Google Scholar]
- 46. Qiu W., Kohen-Avramoglu R., Mhapsekar S., Tsai J., Austin R. C., Adeli K. (2005) Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation: evidence for Grp78-mediated targeting to proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 25, 571–577 [DOI] [PubMed] [Google Scholar]
- 47. Matthews J. A., Belof J. L., Acevedo-Duncan M., Potter R. L. (2007) Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol. Cell. Biochem. 298, 109–123 [DOI] [PubMed] [Google Scholar]
- 48. Gouze J. N., Gouze E., Popp M. P., Bush M. L., Dacanay E. A., Kay J. D., Levings P. P., Patel K. R., Saran J. P., Watson R. S., Ghivizzani S. C. (2006) Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta. Arthritis Res. Ther. 8, R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X., Liang J., Koike T., Sun H., Ichikawa T., Kitajima S., Morimoto M., Shikama H., Watanabe T., Sasaguri Y., Fan J. (2004) Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. Am. J. Pathol. 165, 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Komuro A., Masuda Y., Kobayashi K., Babbitt R., Gunel M., Flavell R. A., Marchesi V. T. (2004) The AHNAKs are a class of giant propeller-like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proc. Natl. Acad. Sci. U. S. A. 101, 4053–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shtivelman E., Cohen F. E., Bishop J. M. (1992) A human gene (AHNAK) encoding an unusually large protein with a 1.2-microns polyionic rod structure. Proc. Natl. Acad. Sci. U. S. A. 89, 5472–5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mira J. P., Dubois T., Oudinet J. P., Lukowski S., Russo-Marie F., Geny B. (1997) Inhibition of cytosolic phospholipase A2 by annexin V in differentiated permeabilized HL-60 cells. Evidence of crucial importance of domain I type II Ca2+-binding site in the mechanism of inhibition. J. Biol. Chem. 272, 10474–10482 [DOI] [PubMed] [Google Scholar]
- 53. Hart G. W., Greis K. D., Dong L. Y. D., Blomberg M. A., Chou T. Y., Jiang M. S., Roquemore E. P., Snow D. M., Kreppel L. K., Cole R. N., Comer F. I., Arnold C. S., Hayes B. K. (1995) O-linked N-acetylglucosamine: the “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv. Exp. Med. Biol. 376, 115–123 [PubMed] [Google Scholar]
- 54. Gill D. J., Tham K. M., Chia J., Wang S. C., Steentoft C., Clausen H., Bard-Chapeau E. A., Bard F. A. (2013) Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. U. S. A. 110, E3152–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wells L., Kreppel L. K., Comer F. I., Wadzinski B. E., Hart G. W. (2004) O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J. Biol. Chem. 279, 38466–38470 [DOI] [PubMed] [Google Scholar]
- 56. Slawson C., Hart G. W. (2003) Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr. Opin. Struct. Biol. 13, 631–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.